Type 1 diabetes mellitus (T1DM) is characterized by the destruction of the insulin-producing β-cells in the pancreas. Exogenous insulin administration is therefore required to regulate the blood glucose concentration. The goal of diabetes management is to maintain homeostasis and blood glucose near normal levels (80 mg/dL – 140 mg/dL) and thus to avoid immediate life-threatening situations, such as severe hypoglycemia and ketoacidosis, and long-term complications, such as cardiovascular disease, nephropathy, neuropathy, and retinopathy.
Treatment of T1DM requires either multiple daily insulin injections (MDI) or continuous subcutaneous insulin infusion (CSII) delivered via an insulin infusion pump. Both treatment modes necessitate frequent blood glucose measurements (8 – 10 times/day, including fasting, pre-and post-prandial, before bedtime and in the middle of the night) to determine the daily insulin requirements for maintaining near normal blood glucose levels [1–3]. With the advent of continuous glucose sensing, which reports interstitial glucose concentrations (that reflect the blood glucose) approximately every minute, and the development of hardware and algorithms to communicate with and control insulin pumps, the vision of closed-loop control of blood glucose is approaching reality. In individuals without diabetes, blood glucose is controlled by various neural and hormonal inputs from the brain, gut, liver, and pancreas that respond to various situations such as meals, stress, and exercise. A closed-loop system for T1DM must be responsive to all daily challenges in life and be able to accurately predict blood glucose levels in advance. This closed-loop system, or artificial pancreas, would include an amalgam of features necessary in order to bring a person with diabetes as close as possible to normoglycemia using subcutaneous (SC) insulin therapy.
The artificial pancreas can be seen as a puzzle, represented in Figure 1, in which several integral pieces form the core, including communication, modeling, control algorithms, learning, meal detection and safety algorithms. Optional features such as telemedicine can further improve the lives of people with T1DM. With current technology and development, the pieces, as described in detail in the following sections, are coming together, though many still need further refinement. The remaining challenges include detecting and overcoming irregular or variable features in the life of a person with T1DM such as stress, illness, and exercise.
Figure 1.
The essential pieces of the artificial pancreas puzzle.
Communication: Artificial Pancreas System
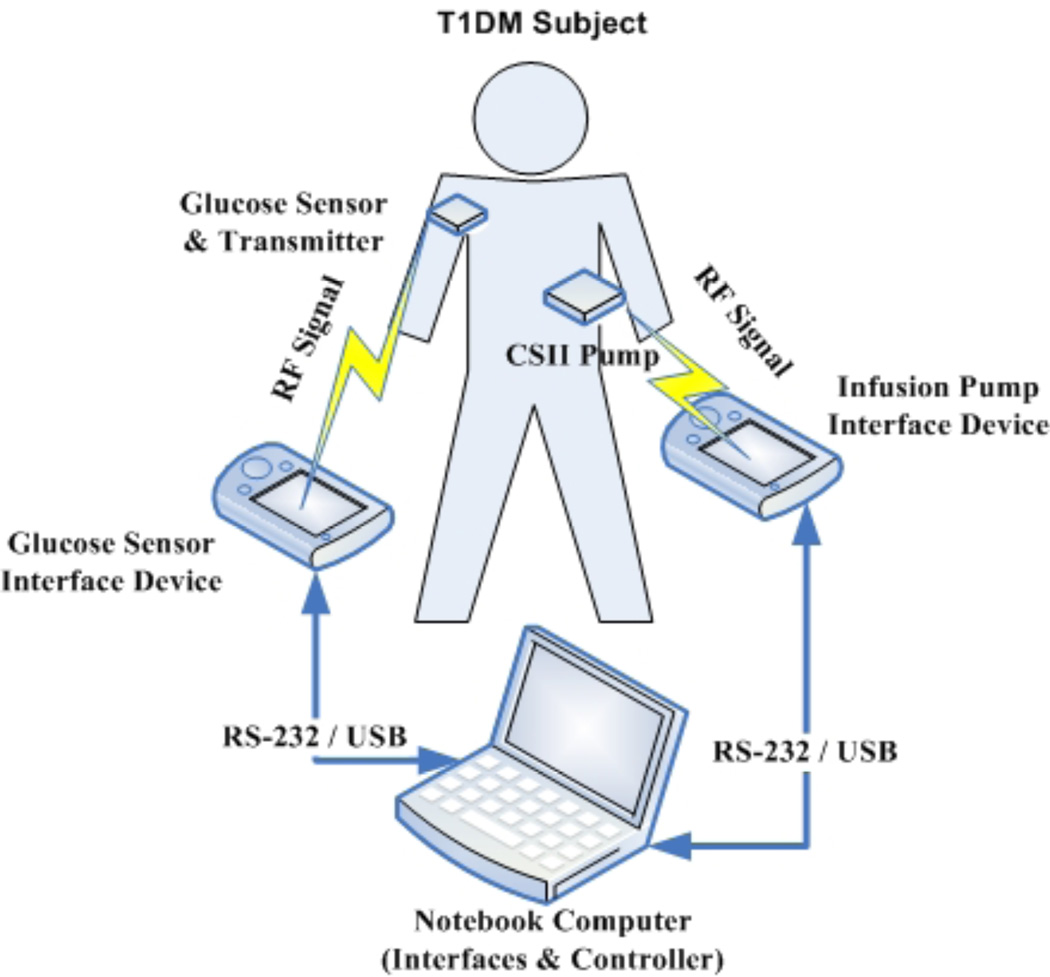
The primary piece of the puzzle involves reliable communication and secure data transfer between the glucose sensor, a control algorithm, and the insulin delivery pump. This research team has developed a novel platform for use in clinical trials and made progress toward a portable unit. This prototype encapsulates communication between the control algorithm and the pump and sensors as illustrated in Figure 2. The Artificial Pancreas System (APS) facilitates communication and provides a simple and clear human interface that presents all the information to the physician. Additionally, the system allows all events to be logged electronically [4]. It also ensures safety by integrating interlocks, checklists, and alarms. Furthermore, the plug-and-play concept allows the APS to serve as a test bed for various control algorithms without regard to the specific sensors or pumps involved.
Figure 2.
The components of a closed-loop system with RF communication links. RF is radio frequency, RS-232 is a standard for serial binary data transfer, and USB is Universal Serial Bus.
The APS has been validated and evaluated using a testing platform known as “hardware in the loop” [5]. A simulated subject with T1DM, instead of a human volunteer, is connected to the Continuous Glucose Monitor (CGM) and a CSII pump with the APS and control algorithm in the same fashion as during a clinical trial. Such a test allows researchers to conduct a full system validation and verification including extreme scenarios. The current version of the APS (v. 2.6) has received the approval of the Food and Drug Administration (FDA) through a Master File (MAF-1625) to be used in human clinical trials [6]. The APS allows investigators to conduct clinical trials using the Insulet OmniPod (Bedford, MA) pump and two CGMs, the DexCom Seven® system (San Diego, CA) and the Abbott FreeStyle Navigator® system (Alameda, CA), with their control algorithms. Additional pumps and sensors will be added as they become available.
Control Algorithms
There is a rich control literature with numerous examples of algorithms applied to diabetes [7]. However, regulation of blood glucose is different from conventional process control, such as temperature regulation by a thermostat or cruise control in a car. Due to the complexity of human physiology, traditional control algorithms need to be customized to meet the challenges of controlling glucose concentrations in people with T1DM.
The effects of exogenous insulin and carbohydrate on blood glucose concentrations are affected by diverse factors. For control purposes, these variations can be addressed at two levels. During 24 hour periods, the process is, effectively, continuous. A controller can be tuned to the individual subject based on their insulin needs, thus accounting for inter-subject variations. In contrast, when considering variations on a day-to-day basis, the process can be viewed as a batch operation, and the control algorithms should be updated as such, in order to account for intra-subject variations. For this reason, we have focused on model predictive control for the continuous process, and iterative learning control for the batch process.
Model Predictive Control
The control of blood glucose concentrations using SC insulin delivery, without administering counter-regulatory hormone or hormones, is at a distinct disadvantage compared with the non-diabetic state, because SC insulin administration is not able to be absorbed fast enough to balance the rapid gut absorption of recently ingested carbohydrates [8]. Although a change in the basal infusion rate may improve the post meal glucose excursion, if food is not ingested, an automatic change in basal rate can be dangerous.
The inter-subject variation in insulin pharmacokinetics is relatively large, requiring a personalized control algorithm [9]. These factors motivate the use of MPC using a subject-specific model of SC insulin and carbohydrate effects on blood glucose concentrations. Basic MPC formulations can be solved analytically, and in some cases are equivalent to classical PID controllers [10].
In addition to physical limitations on the SC insulin pump, further constraints should be considered, such as safety constraints that explicitly consider active insulin, and soft constraints designed to achieve a specific set of hierarchical objectives [11]. With the addition of constraints there is not typically an analytical solution and a constrained quadratic program (QP) must be solved. The solution of such a QP involves an iterative optimization procedure with no guarantee of convergence. Additionally, such problems are computationally expensive, and in the context of a portable system, this could be an undesirable drain on battery power.
The application of multi-parametric programming techniques to MPC (mpMPC) offers a means by which to retain optimal control while minimizing on-line computation [12]. This technique involves the reformulation of the MPC problem via a multi-parametric variable; all the optimal solutions are then obtained offline, for the expected operating conditions. Within this expected operating space, there are a finite number of critical regions, within which an affine function of the state vector defines the optimal control law. The online problem therefore reduces to the evaluation of an affine function contained within a lookup table. This technique has been shown to be feasible for intravenous (IV) insulin delivery [13] and SC insulin delivery [14].
Learning Patterns for Delivery
It is evident that there exist repetitive cycles in glucose-insulin dynamics. For example, people generally consume meals at similar times from day to day, and the corresponding meal sizes are similar from one day to the next. In addition, insulin sensitivity changes in a predictable manner throughout the day due to circadian variation of hormone levels.
To exploit the repetitive nature of glucose-insulin dynamics, run-to-run (R2R) control has been used to update the meal bolus for outpatient subjects [15–17]. Iterative learning control (ILC) can be considered an enhanced version of R2R in a 2-dimensional sense; hence, ILC has been successfully implemented in silico for glucose regulation. The general structure of ILC can be represented as follows:
| (1) |
where u is the control signal designed by ILC, r is termed as the updating law for ILC, t denotes the time step, and k is the batch index. Equation indicates that the control signal for the current batch is composed of the control signal from the previous batch and the updating law.
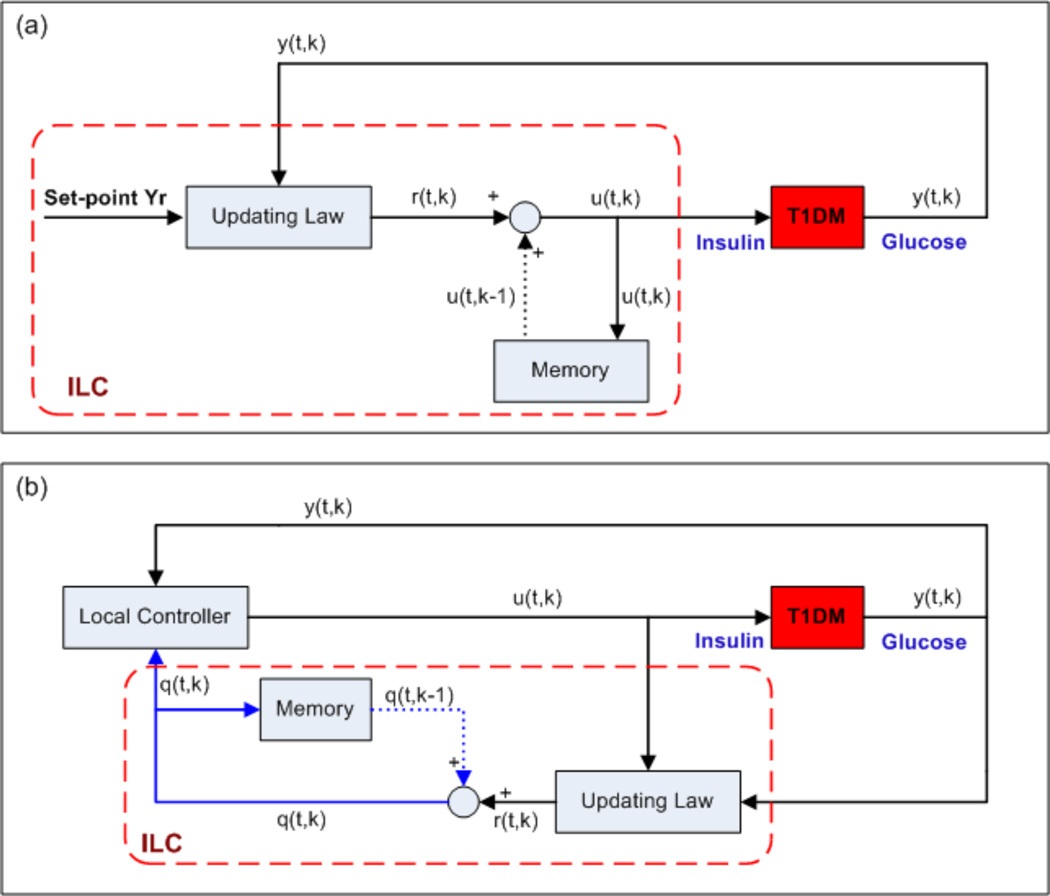
In general, there are two ways to adjust the insulin delivery rate using ILC, as shown in Figure 3 [18]: first, ILC can be utilized to determine the insulin delivery rate directly, a method that is called direct ILC; second, the delivery rate can be determined by a local controller and ILC is used to adjust the local controller, a combination that is known as indirect ILC.
Figure 3.
Block diagrams for the direct and indirect ILC, where t is the time index and k denotes different days: (a) direct ILC; (b) indirect ILC. The solid lines denote the real-time information; the dotted lines denote the information in the previous batch; the components in the dashed frames comprise the ILC.
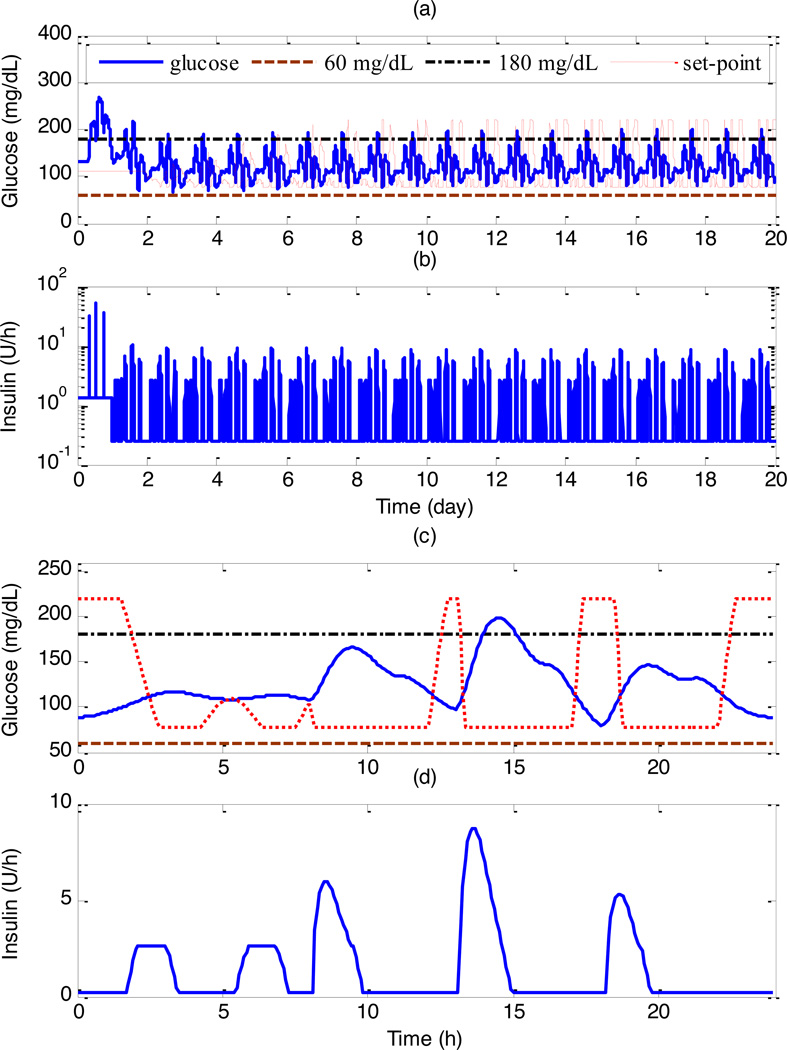
A key concern for direct ILC is the design of the updating law. In our previous studies, e.g., [19], the updating law of ILC was designed by MPC. On the other hand, for indirect ILC, two essential issues need to be established: what algorithms are used to design the local control, and which parameters of the local controller are updated by ILC. In our previous studies [20, 21], the local control was designed by MPC, and the set-point for MPC was updated by ILC. This novel combination is referred to as learning- type MPC (L-MPC). L-MPC has been tested on eleven adult in silico subjects from the UVa/Padova diabetes simulator, which has been accepted by the FDA as a surrogate for animal studies in developing a future artificial pancreas [22, 23]. The tracking performance under L-MPC is superior to that with MPC, with an average reduction of the tracking error of 21.1%. Specifically, the closed-loop control results for the Adult Average subject under L-MPC are shown in Figure 4. After twenty days, the blood glucose concentrations can be kept within 68–145 mg/dL.
Figure 4.
Control performance under L-MPC in 20 days for Adult Average, where the subject consumes three meals a day at 7:00, 13:00, and 18:00 with fixed amounts of carbohydrate, 60g, 100g, and 70g, respectively: (a) glucose concentration; (b) insulin delivery rate, where logarithmic scale was used for the Y-axis; (c) last day’s glucose; (d) last day’s insulin [20].
Patient Modeling
Different mathematical models can serve as candidates for the model in an MPC-based artificial pancreas; however, MPC will work best with a personalized model since the glucose-insulin dynamics differ greatly between subjects. The mathematical model for MPC, for example, could be based on a simplified two differential equation system such as the Bergman "minimal" model [24]. More detailed models use several subsystems including a subcutaneous phase, resulting in higher order models that also take into account exogenous delivery of meals and insulin [25–27]. However, complicated models often contain variables difficult to measure physiologically and parameters that need to be reevaluated in vivo Moreover, methodological models that include physiological insights include nonlinear terms that will transform the linear-MPC problem into a much more difficult nonlinear-MPC problem that is less robust and more difficult to adjust than linear-MPC. Alternatively, input/output black-box models, such as autoregressive, exogenous input (ARX)-models, have demonstrated the ability to capture the blood glucose concentration dynamics of living subjects without taking into account physiological insights [28]. The ARX models are a more reasonable choice for control, being easy to personalize, computationally efficient to estimate, and readily updated with well-known recursive identification methods [29]. The ARX-models are based on standard linear regression that provides a straightforward way to personalize them to different subjects.
Meal Detection Algorithm
A fully functioning artificial pancreas must regulate glucose levels during fasting periods as well as compensate for meals. In control terminology, meals can be considered as disturbances for a feedback control algorithm. An additional challenge is that an automated system that is responsible for regulating the blood glucose concentration of people with T1DM needs to be able to perform under different compliance levels without relying on the ability of the user to announce the meal or to correctly estimate its content. The concept of reliable and robust meal detection was presented by Dassau, et al., as a way to overcome compliance issues and to allow a secondary line of defense [30].
The meal detection algorithm (MDA) combines four ways to analyze glucose values and its rate of change to flag a meal event. A set of binary inputs from the algorithm is evaluated by a voting algorithm that issues an alarm to the controller in case that predetermined majority is reached. Evaluation of the algorithm on historical clinical data has indicated that a meal can be detected at a mean time of 30 min from the onset of the meal and, importantly, the mean serum glucose was on average only 21 mg/dL higher at detection than at the meal time. This rapid detection allows an automated control algorithm to intervene and compensate for the meal disturbance. The MDA can be used by any type of control algorithm to inform the controller of a meal disturbance and then issue the appropriate meal response, as demonstrated by simulation with MPC controller [31]. The use of MDA can improve overall blood glucose control by minimizing the likelihood of hyperglycemia due to no control action and, later, hypoglycemia due to aggressive control action.
Safety Considerations
A functional artificial pancreas will be required to demonstrate both efficacy and safety in controlling blood glucose concentrations. As part of the development of such a system, various alarms, interlocks and algorithms are used to provide a safe device for the end user. The main objective is to prevent the over-dosing of insulin that can result in immediate life-threatening consequences that are associated with severe hypoglycemia.
Insulin on Board
Understanding the dynamics and kinetics of exogenous insulin in the human body can be utilized to insure that the correct dosage of insulin is administered without erroneous over-dosing. Insulin on Board (IOB) is an empirical estimation technique that predicts the amount of available insulin over a period of time following exogenous insulin admissions.
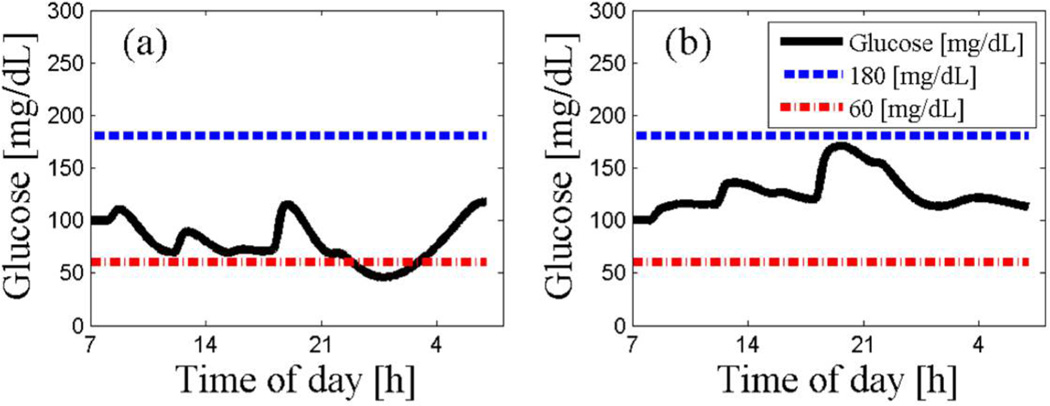
The IOB concept was introduced in modern CSII pumps with different decay insulin curves, both linear and curvilinear, with a duration of action of 2 – 8 hours, as part of the “smart” pump bolus wizard [32]. The use of the IOB concept was demonstrated as a practical dynamic constraint in preventing over-delivery of insulin by the artificial pancreas; see Figure 5 for an in silico example [33]. The synergistic effect of IOB with MPC will result in an aggressive but safe therapy.
Figure 5.
Example of a glucose tracing with an ARX-based MPC with (b) and without (a) the IOB constraint [33]. Note that with the IOB constraint, glucose remains well above the hypoglycemia threshold of 60 mg/dL.
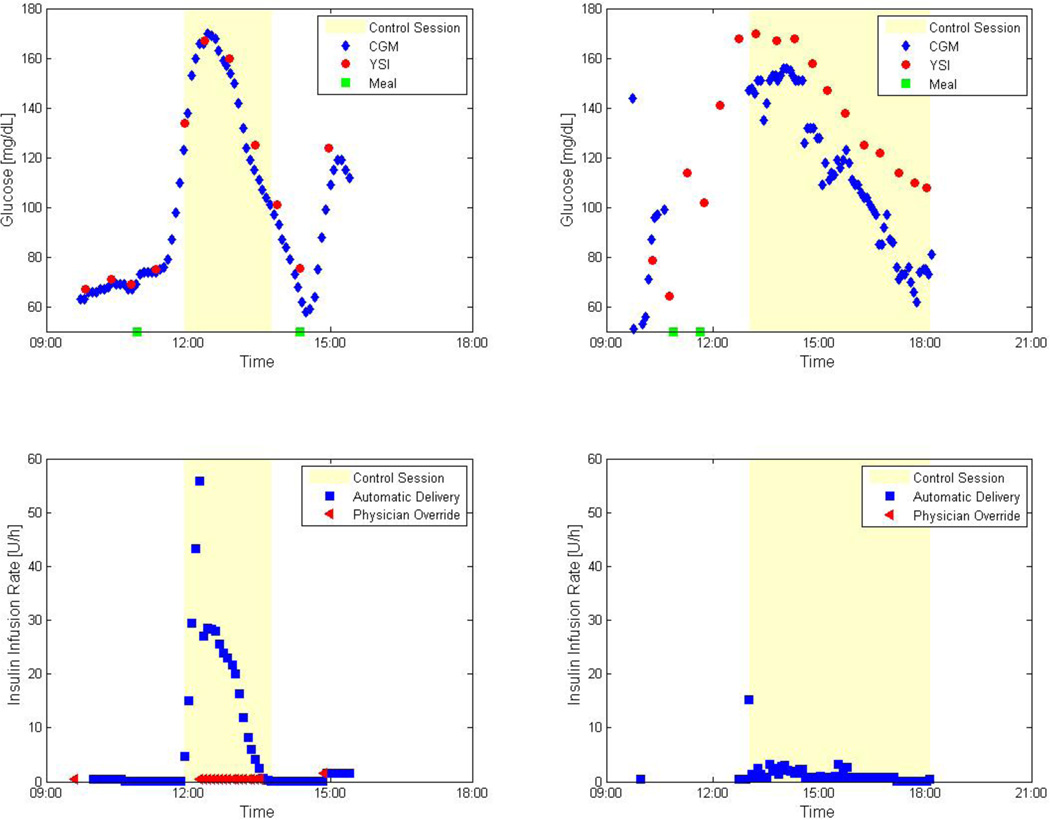
The motivation for including IOB constraints was demonstrated in a fully automated closed-loop clinical study at Sansum Diabetes Research Institute in 2007. Initially, no constraint was included, and trials had to be interrupted by the physician in order to correct for excessive insulin delivery (Figure 6, left). The IOB constraint was tested and found to be effective in the avoidance of excessive insulin delivery, as shown in the right panel of Figure 6.
Figure 6.
Clinical results of a fully automated closed-loop control from a clinical study conducted at Sansum Diabetes Research Institute using MPC with and without IOB. The left panels represent a trial without IOB where excessive insulin was delivered and resulted in physician override of the controller. The right panels represent the results of MPC with IOB conducted on the same subject. Note that there were no physician overrides in the right panel. In both trials the control session started at the hyperglycemia state to challenge the control algorithm.
Hypoglycemia Alarming
A major goal of diabetes therapy is to ameliorate the long-term microvascular and macrovascular complications associated with the disease, i.e. retinopathy, nephropathy, neuropathy, cardio-and cerebrovascular disease [34]. The Diabetes Control and Complications Trial Research Group demonstrated that intensive insulin therapy slows the onset and progression of these complications, although intensive insulin therapy carries an elevated risk of severe hypoglycemia, or low blood glucose concentrations [34]. In order to best improve the life of people with T1DM, therapy to relieve long-term complications must be supplemented with features to resolve more immediate problems such as hypoglycemia.
One of the most feared risks for a person with T1DM is the occurrence of hypoglycemia. Hypoglycemia causes symptoms which range from sweating, confusion, and dizziness in the short term, to seizure, coma and death if severe and prolonged [35]. Nocturnal hypoglycemia is especially critical, as meals are less likely to be ingested. Additionally, evidence shows that the counterregulatory response to hypoglycemia is blunted during sleep, along with a marked suppression auditory response by the sleeping person [36].
A robust alarm, which can awaken the person or another household member, is essential to minimize hypoglycemia and avoid its complications. According to Buckingham, et al. [36], seizure occurs two to four hours after the onset of severe hypoglycemia, providing a window of opportunity in which corrective action can be taken to avoid seizures. Corrective actions may include manual correction or, after sufficient time with no response, pump suspension [36]. Ideally, an alarm will be sounded sufficiently far in advance of the onset of severe hypoglycemia in order to avoid it altogether or at least “soften the landing,” or blunt the rate of change and ultimate nadir of blood glucose. Such an alarm is essential to provide a layer of safety to the artificial pancreas.
An alarm suite which can easily be implemented into current CGM systems has been developed by the authors along with several collaborators. The Hypoglycemia Prediction Algorithm (HPA) includes five predictive algorithms incorporated into a scheme in which a voting threshold must be reached in order to set off an alarm [37]. Validation followed, using a historical set of 22 Clinical Research Center admissions of T1DM subjects with induced hypoglycemia. With a voting threshold of 4 out of 5, 82% of events were predicted from 35–55 minutes before a glucose threshold of 80 mg/dL [38]. The HPA suite appears to be an effective system to enable alarming and eventual pump suspension prior to a hypoglycemia event. This safety feature is a crucial fail-safe for automatic glucose control in the artificial pancreas.
Clinical Results
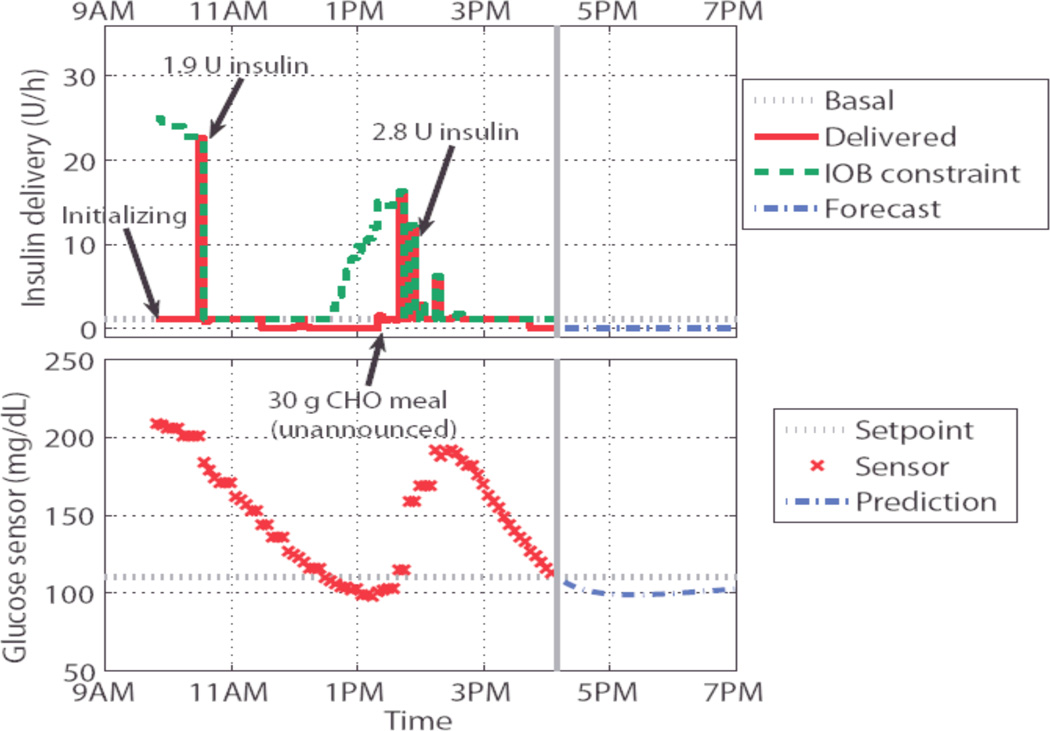
The authors conducted a series of clinical trials at Schneider Children’s Medical Hospital, Israel, consisting of open-loop model identification and closed-loop insulin delivery in people with T1DM. ARX models were identified from ambulatory data comprising CGM measurements, CSII pump records, and subject estimated carbohydrate content (CHO). The best ARX model was chosen based on model validation performance. The mpMPC control law was formulated with this model and included the personalized IOB safety constraint, resulting in a lookup table representing optimal control. This lookup table comprised 10–100 entries and required approximately 100 KB of RAM [39, 40].
Hyperglycemia was induced prior to the initiation of closed-loop control. During the closed-loop trial, the subject consumed unannounced meals of up to 30 g CHO. The control algorithm restored euglycemia in under three hours. No hypoglycemic events occurred. In no case did the physician override the controller. Exemplary results are given in Figure 7 [41].
Figure 7.
In this clinical trial insulin was delivered in quantities commensurate with the subject’s insulin requirements, thus normalizing blood glucose concentrations after clinically induced hyperglycemia and an unannounced meal. The controller delivered an appropriate amount of insulin without overdosing due to the IOB constraint [41].
Telemedicine
The other piece of the puzzle that can both improve the safety of the system and assist with system maintenance is telemedicine. Telemedicine, the use of information and communication technology to support medical care and decision-making, has been broadly used to enhance medical care and to provide up-to-date medical information [42]. Although telemedicine is not a required piece in the evolution of the artificial pancreas, it should and will play an integral role in this emerging technological treatment. Telemedicine will allow both data logging and monitoring of artificial pancreas users by their medical providers and even remote tuning of their control algorithm.
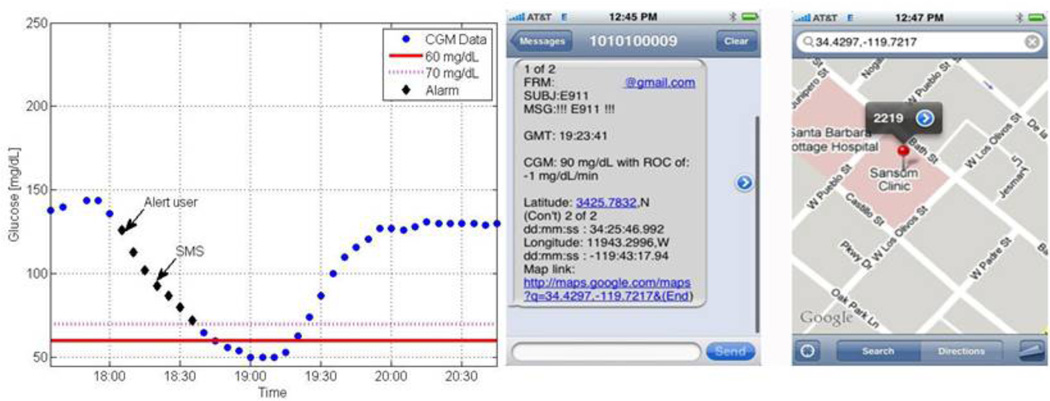
From a safety point of view, the telemedicine feature of the artificial pancreas will bring not only a means to remotely monitor glucose, as is the case for parents of children with T1DM, but also will include an active layer of defense. This safeguard can be embedded such that the artificial pancreas will switch from closed-loop mode to open-loop or suspend insulin delivery in cases of a hypoglycemia event or system failure [43]. The use of GPS technology with a monitoring system within a telemedicine application can add essential security to the artificial pancreas. Such an application, termed E911, has been suggested by the authors as a way to monitor, alert and locate people with T1DM that are experiencing a hypoglycemia event. The proposed system would issue a short message service (SMS), or text message, to a predefined list in case of a pending event. An example of a blood glucose tracing including a hypoglycemia event with the visualization of an automatic SMS with GPS coordinates is in Figure 8.
Figure 8.
Blood glucose tracing in which a hypoglycemia event is forecasted (left). The user is alerted first, and after not responding for 15 minutes (evident by the unchanged trajectory), an SMS text message is sent (right), including GPS coordinates of the subject [43].
Present Challenges
The successful operation of an artificial pancreas will require careful monitoring to detect underlying changes in the subject’s glucose-insulin dynamics due to factors such as stress, illness or unusual exercise. If such abnormal conditions can be detected and diagnosed in a timely manner, the AP can make the appropriate adjustments.
Automatic Detection of Stress States
A recent study by the co-authors has demonstrated that a statistically-based monitoring technique, principal component analysis (PCA), can detect stress states in type 1 diabetes subjects for ambulatory conditions [44]. Nine adults (six men and three women) participated in the study. The data consisted of continuous (5-min) glucose measurements, insulin pump records of basal rates and bolus amounts and times, and subject-recorded estimates of the times and CHO content of meals.
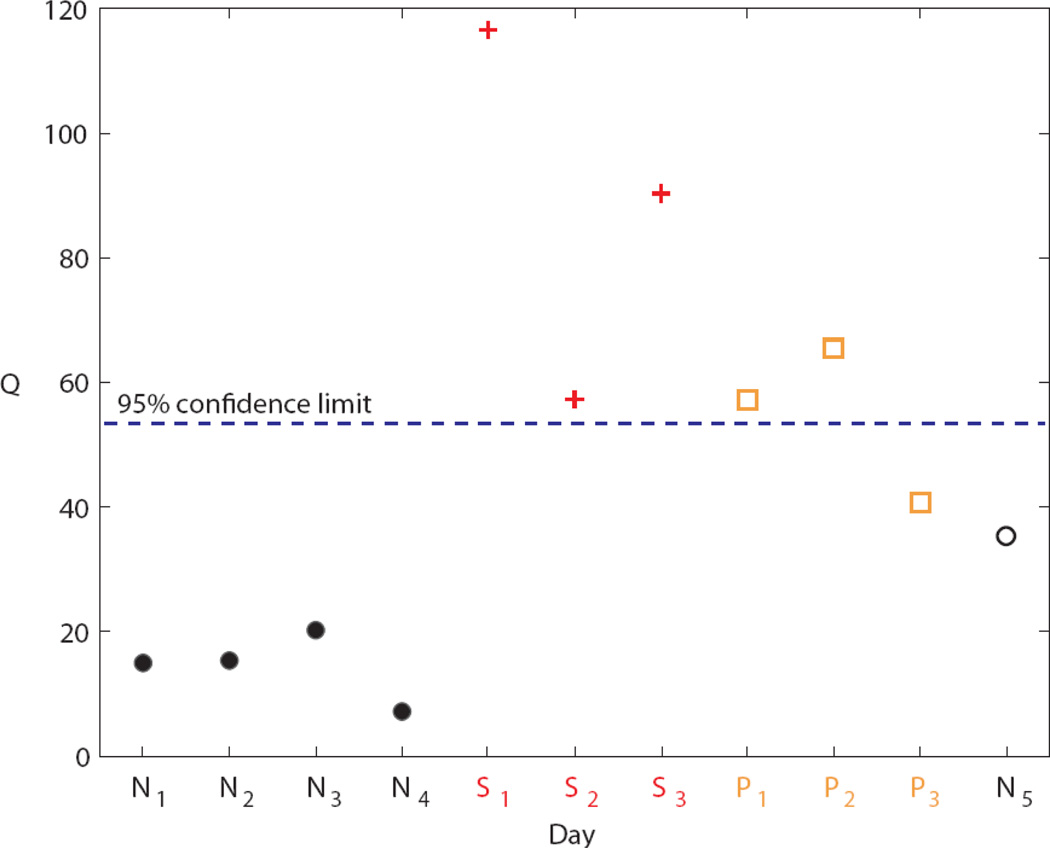
An empirical PCA model was calculated for each subject from several days of “normal” data (i.e., the training data). The subjects were then administered prednisone for three consecutive days, causing decreased insulin sensitivity and thereby simulating a physiological stress state. Then new data for both stress days and normal days (i.e., the test data) were evaluated. A total of 37 test days for the 9 subjects were available.
Of the 37 test days, 33 (89%) were classified correctly. Thus the proposed monitoring technique was able to differentiate between normal days and stress days with a high degree of accuracy. Typical monitoring results for a single subject are shown in Figure 9 [44]. The Q statistic is a standard metric of PCA model accuracy. A Q value above the 95% confidence limit indicates an abnormal (i.e., stress) day.
Figure 9.
PCA results for Subject 2 using the Q statistic. The PCA model was developed from the normal training days N1 – N4. All subsequent days are test data. The stress days are denoted by S1 – S3. The post-stress days are denoted by P1 – P3. Post-stress days are not included in the results due to the unknown residual effects of the prednisone during these days. Day N5 is a normal test day [44].
Exercise
Another major impediment to closed-loop glucose control is the physiological change related to glucose control induced by exercise. In order to achieve automatic control, exercise must be detected without the user informing the controller of impending exercise. This is especially important for young patients, as they are generally very active and would be unable to inform a controller of every exercise session.
The challenge in detecting exercise is that glucose monitoring alone may not give an accurate picture of the influence of exercise. The effect of exercise on glucose-insulin dynamics changes over time, as opposed to the transient perturbation of a meal or insulin bolus [45]. Adding to the complexity of detecting exercise is the subject’s fitness and the duration and intensity (mild, moderate, or strenuous) of exercise, all of which influence the body’s response differently. For example, mild to moderate exercise can cause hypoglycemia in people with T1DM, with the added complication of a blunted autonomic nervous system response [46]; on the other hand, strenuous activity may cause hyperglycemia after exercise without recovery even when insulin is delivered [47]. As a result, the duration and intensity of exercise must be detected with a certain degree of confidence to maintain glycemia within a safe range. In order to do this accurately, a second metric must be used to supplement blood glucose monitoring. The chosen measurement must be both convenient for the patient and representative of the effect of exercise on glucose-insulin dynamics.
Challenges to Commercializing the Artificial Pancreas
It is envisioned that the artificial pancreas will be produced in stages, each one a step closer toward full automation. Initial devices, such as those currently available in Europe, consist of overnight pump suspension to prevent hypoglycemia. Intermediate models may require user defined inputs for frequent occurrences such as meals and exercise. As physiological sensing technology improves, user defined inputs may be eliminated entirely, thus effectively delivering automated glucose control of similar performance to that found in nature. Market availability of any of these products will depend upon regulatory body approval, which will require extensive clinical trials. Product use will ultimately rely upon a cost-benefit analysis by health insurance companies.
Summary
The various components of the artificial pancreas puzzle are being put into place. Features such as communication, control, modeling, and learning are being realized presently. Steps have been set in motion to carry the conceptual design through simulation to clinical implementation. The challenging pieces still to be addressed include stress and exercise; as integral parts of the ultimate goal, effort has begun to shift toward overcoming the remaining hurdles to the full artificial pancreas. The artificial 15 pancreas is close to becoming a reality, driven by technology and the expectation that lives will be improved.
Acknowledgments
This work was supported by the Otis Williams Fund at the Santa Barbara Foundation, the National Institutes of Health (grants R21-DK69833 and R01-DK068683), and the Juvenile Diabetes Research Foundation, which provided funding from 2006 to the present for the Sansum Diabetes Research Institute and the University of California, Santa Barbara. Dr. Cesar Palerm is acknowledged for his role in some of the clinical studies discussed in this article.
References
- 1.Jovanovič L. Rationale for Prevention and Treatment of Postprandial Glucose-Mediated Toxicity. The Endocrinologist. 1999;vol. 9:87–92. [Google Scholar]
- 2.Jovanovič L, Peterson CM. Insulin and glucose requirements during the first stage of labor in insulin-dependent diabetic women. Am J Med. 1983 Oct;vol. 75:607–612. doi: 10.1016/0002-9343(83)90441-2. [DOI] [PubMed] [Google Scholar]
- 3.Peterson CM, Jovanovič L, Chanoch LH. Randomized trial of computer-assisted insulin delivery in patients with type I diabetes beginning pump therapy. Am J Med. 1986 Jul;vol. 81:69–72. doi: 10.1016/0002-9343(86)90184-1. [DOI] [PubMed] [Google Scholar]
- 4.Dassau E, Zisser H, Palerm CC, Buckingham BA, Jovanovič L, Doyle FJ., III Modular Artificial β-Cell System: A Prototype for Clinical Research. J Diabetes Sci Technol. 2008;vol. 2:863–872. doi: 10.1177/193229680800200518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dassau E, Palerm CC, Zisser H, Buckingham BA, Jovanovič L, Doyle FJ., III In silico evaluation platform for artificial pancreatic beta-cell development--a dynamic simulator for closed-loop control with hardware-in-the-loop. Diabetes Technol Ther. 2009 Mar;vol. 11:187–194. doi: 10.1089/dia.2008.0055. [DOI] [PubMed] [Google Scholar]
- 6.Dassau E, Zisser H, Doyle FJ, III, Jovanovič L. Sansum / UCSB Artificial Pancreas Software (APS) Food and Drug Administration Master File MAF-1625. 2009 [Google Scholar]
- 7.Hovorka R. The future of continuous glucose monitoring: closed loop. Curr Diabetes Rev. 2008 Aug;vol. 4:269–279. doi: 10.2174/157339908785294479. [DOI] [PubMed] [Google Scholar]
- 8.Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabet Med. 2006 Jan;vol. 23:1–12. doi: 10.1111/j.1464-5491.2005.01672.x. [DOI] [PubMed] [Google Scholar]
- 9.Fischer U, Schenk W, Salzsieder E, Albrecht G, Abel P, Freyse EJ. Does Physiological Blood Glucose Control Require an Adaptive Control Strategy? IEEE Trans Biomed Eng. 1987;vol. BME-34:575–582. doi: 10.1109/tbme.1987.326068. [DOI] [PubMed] [Google Scholar]
- 10.Percival MW, Zisser H, Jovanovič L, Doyle FJ., III Closed-loop control and advisory mode evaluation of an artificial pancreatic β cell: Use of proportional-integral-derivative equivalent model-based controllers. J Diabetes Sci Technol. 2008;vol. 2:636–644. doi: 10.1177/193229680800200415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dua P, Doyle FJ, III, Pistikopoulos EN. Multi-objective blood glucose control for type 1 diabetes. Med Biol Eng Comput. 2009;vol. 47:343–352. doi: 10.1007/s11517-009-0453-0. [DOI] [PubMed] [Google Scholar]
- 12.Pistikopoulos EN, Dua V, Bozinis NA, Bemporad A, Morari M. On-line optimization via off-line parametric optimization tools. Comput Chem Eng. 2000;vol. 24:188–188. [Google Scholar]
- 13.Dua P, Doyle FJ, III, Pistikopoulos EN. Model-based blood glucose control for type 1 diabetes via parametric programming. IEEE Trans Biomed Eng. 2006;vol. 53:1478–1491. doi: 10.1109/TBME.2006.878075. [DOI] [PubMed] [Google Scholar]
- 14.Percival MW, Dassau E, Zisser H, Jovanovič L, Doyle FJ., III Closed-loop control of an artificial pancreatic beta cell using multi-parametric model predictive control; 2008 AIChE Annual Meeting; Philadelphia, PA. 2008. [Google Scholar]
- 15.Zisser H, Jovanovic L, Doyle FJ, III, Ospina P, Owens C. Run-to-run control of meal, related insulin dosing. Diabetes Technol Ther. 2005;vol. 7:48–57. doi: 10.1089/dia.2005.7.48. [DOI] [PubMed] [Google Scholar]
- 16.Zisser H, Palerm CC, Bevier WC, Doyle FJ, III, Jovanovič L. Clinical Update on Optimal Prandial Insulin Dosing Using a Refined Run-to-Run Control Algorithm. J Diabetes Sci Technol. 2009;vol. 3:487–491. doi: 10.1177/193229680900300312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palerm CC, Zisser H, Bevier WC, Jovanovic L, Doyle FJ., III Prandial insulin dosing using run-to-run control: application of clinical data and medical expertise to define a suitable performance metric. Diabetes Care. 2007 May;vol. 30:1131–1136. doi: 10.2337/dc06-2115. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Gao F, Doyle FJ., III Survey on iterative learning control, repetitive control, and run-to-run control. J Process Control. 2009;vol. 19:1589–1600. [Google Scholar]
- 19.Wang Y, Dassau E, Doyle FJ., III Closed-loop control of artificial pancreatic β-cell in type 1 diabetes mellitus using model predictive iterative learning control. IEEE Trans Biomed Eng. 2009 doi: 10.1109/TBME.2009.2024409. (in press) [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Zisser H, Dassau E, Jovanovič L, Doyle FJ., III Model predictive control with learning-type reference: application in artificial pancreatic β-cell. AIChE J. 2009 (in press) [Google Scholar]
- 21.Wang Y, Doyle FJ., III Indirect iterative learning control: application on artificial pancreatic β-cell; 21st Chinese Control & Descision Conference; Guilin, China. 2009. pp. 1739–1744. [Google Scholar]
- 22.Kovatchev BP, Breton C, Dalla Man C, Cobelli C. In Silico Preclinical Trials: A Proof of Concept in Closed-Loop Control of Type 1 Diabetes. J Diabetes Sci Technol. 2009;vol. 3:44–55. doi: 10.1177/193229680900300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovatchev BP, Breton DM, Dalla Man C, Cobelli C. In Silico model and computer simulation environment approximating the human glucose/insulin utilization. Food and Drug Administration Master File MAF-1521. 2008 [Google Scholar]
- 24.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981 Dec;vol. 68:1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hovorka R, Canonico V, Chassin LJ, Haueter U, Massi-Benedetti M, Federici MO, Pieber TR, Schaller HC, Schaupp L, Vering T, Wilinska ME. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas. 2004;vol. 25:905–920. doi: 10.1088/0967-3334/25/4/010. [DOI] [PubMed] [Google Scholar]
- 26.Wilinska ME, Chassin LJ, Schaller HC, Schaupp L, Pieber TR, Hovorka R. Insulin kinetics in type-I diabetes: continuous and bolus delivery of rapid acting insulin. IEEE Trans Biomed Eng. 2005 Jan;vol. 52:3–12. doi: 10.1109/TBME.2004.839639. [DOI] [PubMed] [Google Scholar]
- 27.Dalla Man C, Rizza RA, Cobelli C. Meal simulation model of the glucose-insulin system. IEEE Trans Biomed Eng. 2007 Oct;vol. 54:1740–1749. doi: 10.1109/TBME.2007.893506. [DOI] [PubMed] [Google Scholar]
- 28.Finan DA, Zisser H, Jovanovic L, Bevier WC, Seborg DE. Practical issues in the identification of empirical models from simulated type 1 diabetes data. Diabetes Technol Ther. 2007 Oct;vol. 9:438–450. doi: 10.1089/dia.2007.0202. [DOI] [PubMed] [Google Scholar]
- 29.Finan DA, Doyle FJ, III, Palerm CC, Bevier WC, Zisser HC, Jovanovič L, Seborg DE. Experimental Evaluation of a Recursive Model Identification Technique for Type 1 Diabetes. J Diabetes Sci Technol. 2009;vol. 3:1192–1202. doi: 10.1177/193229680900300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dassau E, Bequette BW, Buckingham BA, Doyle FJ., III Detection of a Meal Using Continuous Glucose Monitoring (CGM): Implications for an Artificial β-cell. Diabetes Care. 2008;vol. 31:295–300. doi: 10.2337/dc07-1293. [DOI] [PubMed] [Google Scholar]
- 31.Dassau E, Herrero P, Zisser H, Buckingham BA, Jovanovič L, Dalla Man C, Cobelli C, Vehí J, Doyle FJ., III Implications of Meal Library & Meal Detection to Glycemic Control of Type 1 Diabetes Mellitus through MPC Control; Proc 17th IFAC World Congress; Seoul, Korea. 2008. pp. 4228–4233. [Google Scholar]
- 32.Zisser H, Robinson L, Bevier W, Dassau E, Ellingsen C, Doyle FJ, III, Jovanovič L. Bolus Calculator: A Review of Four "Smart" Insulin Pumps. Diabetes Technol Ther. 2008;vol. 10:441–444. doi: 10.1089/dia.2007.0284. [DOI] [PubMed] [Google Scholar]
- 33.Ellingsen C, Dassau E, Zisser H, Grosman B, Percival MW, Jovanovič L, Doyle FJ., III Safety constraints in an artificial pancreatic β-cell: an implementation of model predictive control with insulin-on-board. J Diabetes Sci Technol. 2009;vol. 3:536–544. doi: 10.1177/193229680900300319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N Engl J Med. 1993;vol. 329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 35.Widmaier EP, Raff H, Strang KT. Vander's human physiology: the mechanisms of body function. 11th ed. Boston: McGraw-Hill Higher Education; 2008. [Google Scholar]
- 36.Buckingham B, Wilson DM, Lecher T, Hanas R, Kaiserman K, Cameron F. Duration of nocturnal hypoglycemia before seizures. Diabetes Care. 2008 Nov;vol. 31:2110–2112. doi: 10.2337/dc08-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dassau E, Cameron FM, Lee H, Bequette BW, Doyle FJ, III, Niemeyer G, Chase P, Buckingham B. Real-time Hypoglycemia Prediction Using Continuous Glucose Monitoring (CGM), A Safety Net to the Artificial Pancreas; 68th American Diabetes Association Meeting; San Francisco CA, USA. 2008. p. A13. Diabetes. [Google Scholar]
- 38.Dassau E, Cameron F, Lee H, Bequette BW, Zisser H, Jovanovič L, Chase HP, Wilson DM, Buckingham BA, Doyle FJ., III Real-time Hypoglycemia Prediction Suite Using Continuous Glucose Monitoring (CGM), A Safety Net for the Artificial Pancreas. Diabetes Care. 2009 doi: 10.2337/dc09-1487. (in Submission) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dassau E, Zisser H, Percival MW, Grosman B, Jovanovič L, Doyle FJ., III Design, Validation And Clinical Evaluation Of A Fully Automated Artificial Pancreatic β-Cell With Unannounced Meal Using mpMPC And IOB; 3ed Conference on Advanced Technologies & Treatments for Diabetes; Basel, Switzerland. 2010. [Google Scholar]
- 40.Dassau E, Zisser H, Percival MW, Grosman B, Jovanovič L, Doyle FJ., III Clinical Evaluation of Fully Automated Artificial Pancreatic β-Cell With Unannounced Meal Using mpMPC And Insulin-On-Board. Diabetes Care. 2009 (In Submission) [Google Scholar]
- 41.Percival MW, Grosman B, Dassau E, Zisser H, Jovanovič L, Doyle FJ., III Automated Insulin Delivery System Demonstrates Safe and Efficacious Control of Glycemia; 69th American Diabetes Association Meeting; New Orleans, LA. 2009. [Google Scholar]
- 42.Bellazzi R. Telemedicine and Diabetes Management: Current Challenges and Future Research Directions. J Diabetes Sci Technol. 2008;vol. 2:98–104. doi: 10.1177/193229680800200114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dassau E, Jovanovič L, Doyle FJ, III, Zisser H. Enhanced 911/GPS Wizard: a Telemedicine application for the Prevention of Severe Hypoglycemia - Monitor, Alert and Locate. J Diabetes Sci Technol. 2009;vol. 3:1501–1506. doi: 10.1177/193229680900300632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finan DA. Department of Chemical Engineering. Ph.D. dissertation. UC-Santa Barbara; 2008. Modeling and Monitoring Strategies for Type 1 Diabetes. [Google Scholar]
- 45.Breton M. Physical Activity-The Major Unaccounted Impediment to Closed Loop Control. J Diabetes Sci Technol. 2008;vol. 2:169–174. doi: 10.1177/193229680800200127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ertl AC, Davis SN. Evidence for a vicious cycle of exercise and hypoglycemia in type 1 diabetes mellitus. Diabetes Metab Res Rev. 2004 Mar-Apr;vol. 20:124–130. doi: 10.1002/dmrr.450. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell TH, Abraham G, Schiffrin A, Leiter LA, Marliss EB. Hyperglycemia after intense exercise in IDDM subjects during continuous subcutaneous insulin infusion. Diabetes Care. 1988 Apr;vol. 11:311–317. doi: 10.2337/diacare.11.4.311. [DOI] [PubMed] [Google Scholar]