Abstract
Immune responses are pathologically sustained in several common diseases, including asthma. To determine endogenous pro-resolving mechanisms for adaptive immune responses, we used a murine model of self-limited allergic airway inflammation. After cessation of allergen exposure, eosinophils and T-cells were cleared concomitant with the appearance of increased numbers of natural killer (NK) cells in the lung and mediastinal lymph nodes (MLN). The MLN NK cells were activated, expressing CD27, CD11b, CD69, CD107a and IFN-γ. NK cell depletion disrupted the endogenous resolution program, leading to delayed clearance of airway eosinophils and antigen-specific CD4+ T cells. NK cell trafficking to inflamed tissues for resolution was dependent upon CXCR3 and CD62L. During resolution, eosinophils and antigen-specific CD4+ T cells expressed NKG2D ligands and a blocking antibody for the NKG2D receptor delayed clearance of these leukocytes. Of interest, NK cells expressed CMKLR1, a receptor for the pro-resolving mediator resolvin E1, and depletion of NK cells decreased resolvin E1-mediated resolution of allergic inflammation. Resolvin E1 regulated NK cell migration in vivo and NK cell cytotoxicity in vitro. Together, these findings indicate new functions in catabasis for NK cells that can also serve as targets for pro-resolving mediators in the resolution of adaptive immunity.
Introduction
Resolution of inflammation is fundamental to health and tissue homeostasis. Failure of inflammatory responses to resolve in a timely manner can lead to pathological inflammation, a feature of several common human diseases (1-2). Asthma is a chronic inflammatory disease of the airways that is characterized by infiltration of eosinophils and TH2 cells and release of pro-inflammatory cytokines and lipid mediators (3). The cellular and molecular regulators of adaptive immunity are of great interest.
Resolution of acute tissue inflammation is now appreciated to entail an active process with distinct cellular effectors and biochemical signaling pathways that are regulated by a new genus of specialized pro-resolving mediators, including resolvins (4). Resolvin-E1 (RvE1; 5S, 12R, 18R-tri-hydroxy-6Z, 8E, 10E, 14Z, 16E-eicosapaentanoic acid) (RvE1) is an endogenous pro-resolving mediator for allergic airway responses (5) that serves as an agonist at CMKLR1 receptors (6). In contrast to pathogenic roles for T cells in chronic inflammation, select populations of lymphocytes can display counter-regulatory actions to dampen inflammation (7).
Here, we utilized a murine model of asthma exacerbation with self-limited adaptive inflammation to determine the natural time course for leukocyte trafficking during tissue catabasis (as defined in reference (8)) and identified an integral role for NK cells in mediating resolution. During resolution, NK cells accumulated in the lung draining lymph nodes and either depleting NK cells, blocking their ability to interact with target cells or disrupting their migration to target tissues delayed resolution of allergic inflammation, implicating these innate lymphocytes as pivotal cellular, pro-resolving effectors for adaptive immune responses.
Materials and Methods
Sensitization and challenge protocols
Allergic airway inflammation and resolution was modeled in allergen (ovalbumin (OVA) sensitized and aerosol challenged FVB and BALB/cj mice (as in (5), Fig. 1a) using a protocol approved by the Harvard Medical Area IRB (Protocol #03618). Select mice were given RvE1 methyl-ester (100ng, intravenous) once a day for 3 consecutive days (as in (5)). To measure the impact of perturbations on the pace of resolution, the time from maximal to half-maximal responses was determined; defined as the resolution interval (Ri) (Supplementary Fig. 1).
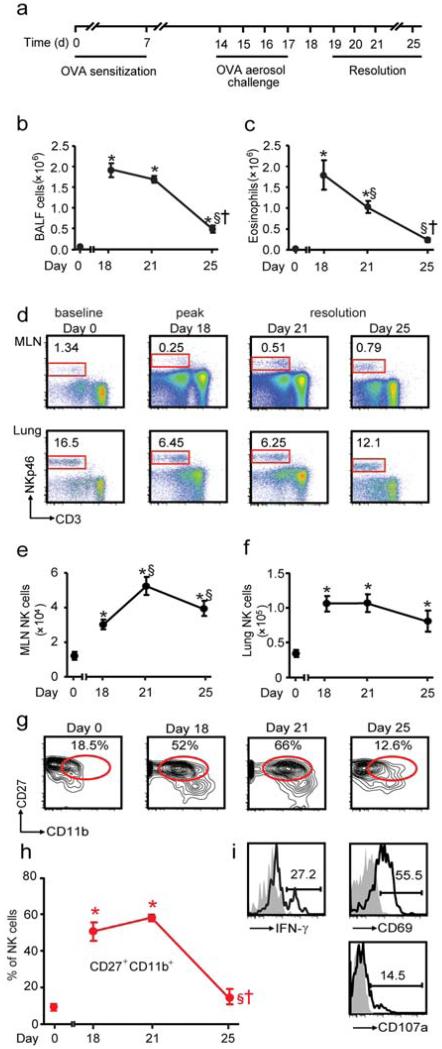
Figure 1. NK cells increase in local lymph nodes during resolution.
a Mice were sensitized and aerosol challenged with OVA (as in 5) and the extent of inflammation was determined on protocol days 18 (peak inflammation), 21 and 25 (resolution). b Total number of BALF cells and c BALF eosinophils were enumerated. d Representative flow cytometry plots from MLNs and lung at baseline (day 0), peak inflammation (protocol day 18) and resolution (protocol days 21 and 25). Inserts show percentage of lymphocytes that are NK cells (NKp46+ CD3−). Time course for total NK cells in the (e) MLNs and (f) lung. g Flow cytometry plots for NK cell CD27+ CD11b+ subset during inflammation and resolution. Insets show percentages; h Percentage of CD27+ CD11b+ NK cells during the onset and resolution of allergic airway inflammation. i Representative flow cytometry plots showing the expression of IFN-γ, CD69 and CD107a on NKp46+ CD3− NK cells from the MLNs obtained in early resolution (day 21). Inset: Numbers indicate the percentage positive. Data (mean ± s.e.m) are representative of more than 3 independent experiments with n≥4 FVB mice in each group * P <0.05 (day 0) § P <0.05 (day 18), † P <0.05 (day 21).
In vivo depletion of NK cells
To deplete NK cells, mice were given anti-asialo GM1 antibody (aGM1) (9) (Wako, 50μl/mouse, i.p.) or control IgG (rabbit) at the peak of allergic inflammation (protocol day 18) (Supplementary Fig. 2,3). Although aGM1 can interact with other cell types, such as T cells (10-11), only NK cells were significantly decreased with aGM1 here, as the numbers of CD4+ T cells from the BALF were increased (see Results) and no significant changes in CD8+ T cells were observed with aGM1.
Tracking OVA-specific (KJ1-26) CD4+ T cells in vivo
Antigen-specific CD4+ T cells were isolated (Miltenyi Biotech) from DO11.10 BALB/cj mice (Jackson Labs) and ~2 ×106 cells were injected into recipient BALB/cj mice (as in reference (12)). The next day, mice started the sensitization and challenge protocol. CD4+ KJ1-26+ T cells were detected using an antibody against the DO11.10 T cell receptor (KJ1-26), and CD4+ KJ1-26+ T cell numbers were calculated as % KJ1-26 cells × total CD4+ T cells (% CD4+ T cells × total number of lymphocytes).
Adoptive transfer of NK cells
As in (13), NK cells were isolated (Miltenyi Biotech) from spleens of donor mice at protocol day 19 (to coincide with the recipient mice), labeled with CFSE (5μM, Invitrogen) according to manufacturer’s instructions and incubated (30 min, 37°C) with 10μg of anti-mouse CXCR3, anti-mouse NKG2D blocking antibodies or IgG control (rat IgG) before washing twice with RPMI 1640 medium supplemented with 10% FCS, antibiotics and 50μM β-mercaptoethanol (Sigma). The NKG2D antibody clone (C7) blocks the actions of NKG2D in vivo (14). On protocol day 19, NK cell depleted recipient mice were reconstituted (i.v.) with ~2 × 106 donor NK cells. After aGM1, endogenous NK cells are decreased for approximately 48h, providing a window for administration of NK cells labeled with CFSE, which were readily detected in inflamed tissues and draining lymph nodes on day 21. The percentage of CFSE+ cells was determined in tissues at day 21 (Supplementary Fig. 4).
Antibodies and Flow cytometry
Single-cell suspensions were generated with a 70μm cell strainer (Fisher). Lung and peripheral blood (PB) lymphocytes were enriched using Ficoll (Sigma). NK cells were identified as being NKp46+ CD3− (15). Antibodies were obtained from eBioscience; CD4 (L3T4), CD8 (53-6.7), CD3ε (145-2C11), NKp46 (29A1.4), CD27 (LG 7F9),, CD69 (H12F3), CXCR3 (CXCR3-173), CD62L (MEL-14), CMKLR1 (BZ194), KJ1-26 (KJI-26), NKG2D (C7), CD107a (1D4B); Invitrogen; CD11b (M1/70.15), Biolegend; CD11b (M1/70), CD27 (LG.3A10) and BD Pharmingen; IFN-γ (XMG1.2). Blocking Abs were obtained for anti-mouse NKG2D (C7; eBioscience), anti-mouse CXCR3 (CXCR3-173; Biolegend) and anti-CD62L (MEL-14; Biolegend). Rat IgG (Biolegend) and Hamster IgG (eBioscience) were used as controls. To detect NKG2D ligands, recombinant mouse NKG2D-human Fc fusion protein (R&D) was used followed by an anti-human-IgG Fc (eBioscience). As a control, the secondary Ab was used alone. FACS Canto II (BD) and FloJo software (Tree Star) were used for analyses.
Measurement of peptide and lipid mediators
Select mediators were measured in aliquots of cell-free BALFs (2000×g, 10 min, 4°C) by protein bead array (Aushon Biosystems) or ELISA (LXA4 (Neogen), PGE2 and LTB4 (Cayman)).
Immunohistology
Lungs were fixed, sectioned and stained by H&E or PAS. Select images were acquired using a Leica (model DMLB) microscope.
Gene expression
MLNs and lungs were obtained and snap frozen. RNA was extracted with Trizol and reverse transcribed. The cDNA was used as a template for the amplification of cxcl9 [GeneID: 17329], cxcl10 [GeneID: 15945], cxc11 [GeneID: 56066] and a control gene ppia [GeneID: 268373] by real-time PCR using a Stratagene real-time PCR machine (model #Mx 3005). Fold change was calculated as 2−ΔΔCT for the difference between the CT value for the gene of interest and the respective CT value for ppia (ΔCT) compared to day 0.
NK cell cytotoxicity assay
NK cells were isolated from mouse spleens (Miltenyi-Biotech) and incubated with vehicle or RvE1 free-acid (10nM) for 15 minutes prior to the addition of RMA/S target cells labelled with cell tracker orange™ (Invitrogen). Cytotoxicity was assessed after 4h (37°C, 5% CO2) using 7-amino-actinomycin (7-AAD) (BD Pharmingen).
Statistical analysis
Results are expressed as the mean ± s.e.m. Statistical significance of differences was assessed by a one tailed unpaired Student’s t-test and a one-way ANOVA with Bonferroni’s multiple comparison post test to compare selected pairs. P <0.05 was set as the level of significance.
Results
NK cells increase in tissue draining lymph nodes during resolution of allergic inflammation
To identify pro-resolving mechanisms in adaptive inflammation, we utilized a self-limited model of allergic airway inflammation (Fig. 1a). After four daily aerosol allergen challenges, sensitized mice developed marked lung inflammation with increased airway eosinophils and lymphocytes. After cessation of allergen exposure, the lung inflammation largely resolved over a 7 day interval (Fig. 1b and Supplementary Fig. 1a-b). During this natural resolution process, the number of eosinophils (Fig. 1c) and T cells (Supplementary Fig. 1b) decreased in a linear trend in bronchoalveolar lavage fluids (BALFs) with a resolution interval (Ri) of ~4 days (Supplementary Fig. 1a,b). T cell numbers also decreased in mediastinal lymph nodes (MLNs), but with a longer Ri (~5 days for CD8+ and ~6 days for CD4+ T cells) (Supplementary Fig. 1c). In contrast, natural killer (NK) cells (NKp46+ CD3−) in the lung draining MLNs and lung displayed markedly different kinetics (Fig. 1d-f). The number of NK cells in MLNs increased ~2.5 fold at peak inflammation (24 hours after last challenge on protocol day 18) (P≥0.01) (Fig 1d). There was a further accumulation of NK cells in MLNs during early resolution (day 21) (~1.7 fold increase compared to peak inflammation) (P≥0.01) that persisted in late resolution (day 25) (~1.3 fold increase compared to peak inflammation) (P=0.05) (Fig. 1e). NK cell numbers in the lung were similarly increased at day 18 compared to day 0 (Fig. 1f). The increased MLN NK cells in resolution temporally overlapped with stable to declining NK cell numbers in the lung (Fig. 1e,f), suggesting that NK cells were recruited to MLNs. In addition, MLN NK cells did not show evidence of proliferation by BrdU incorporation (data not shown).
To determine if MLN NK cell phenotype was dynamically regulated during resolution, we next examined NK cell subsets defined by CD27 and CD11b (Fig. 1g). CD27+ CD11b+ NK cells have been reported to display increased cytotoxicity (16) and there was a transient increase in the CD27+ CD11b+ population in MLNs during peak inflammation and early resolution (day 21) (Fig. 1g,h). During early resolution, MLN NK cells also expressed the cell activation markers CD69 and CD107a (lysosomal associated membrane protein-1; LAMP-1) and could produce IFN-γ upon stimulation with PMA and ionomcycin (Fig. 1i). In addition to increased cell number (Fig. 1d), expression of these molecules indicated that MLN NK cells were activated during resolution and suggested their involvement in the clearance of adaptive inflammation.
Decreased NK cells delays resolution
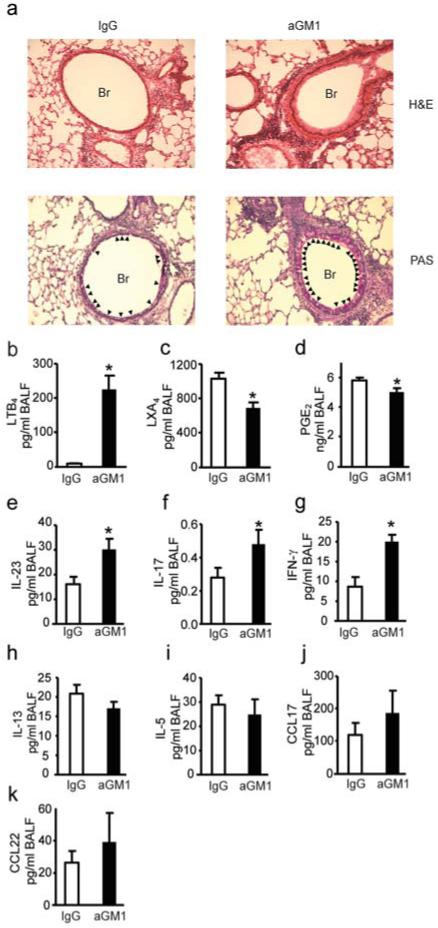
To investigate if these changes in NK cell number and phenotype had an impact on resolution, NK cells were decreased in number after lung inflammation was established using anti-asialo GM1 antibody (aGM1) (Supplementary Fig. 2a-d). Administration of aGM1 significantly reduced NK cells in MLNs, lung and spleen during resolution (Supplementary Fig. 2c, d). NK cell depletion led to persistent allergic airway inflammation with a relative increase in peribronchial leukocyte infiltration and inflammatory changes to the airway epithelium with associated mucus metaplasia (Fig. 2a and Supplementary Fig. 3). Levels of BALF lipid mediators were impacted by aGM1, including significant increases in leukotriene B4 (LTB4) (P<0.001) (Fig. 2b) and decreases in lipoxin A4 (LXA4) (P=0.001) (Fig. 2c) and prostaglandin E2 (PGE2) (P=0.022) (Fig. 2d). aGM1 also led to increases in BALF levels of IL-23 and IL-17 (Fig. 2e, f) and modest increases in IFN-γ (Fig. 2g). No significant changes were noted in BALF levels of the TH2 cytokines IL-5 and IL-13 (Fig. 2h,i). Of note, MLN and lung NK cells generated IL-13 and the percentage of IL-13 producing NK cells in both sites significantly decreased from peak inflammation to resolution (MLN, 38.6 + 2.3% (day 18) to 24.4 + 2.8% (day 21); lung, 36.7 + 1.9% (day 18) to 16.6 + 2.7 (day 21); mean + SEM for n > 6, *P<0.01). There was no significant change in the BALF levels of T cell chemokines CCL17 (TARC) and CCL22 (MDC) (Fig. 2j,k).
Figure 2. NK cells contribute to tissue catabasis and inflammatory mediator release.
a Representative (n≥3) lung tissue sections from day 21 FVB mice given aGM1 antibody or IgG were stained with hematoxylin and eosin (H&E) or Periodic Acid Schiff (PAS). Original magnification × 200. Arrowheads denote mucus (magenta) containing Goblet cells. Br; bronchus. b-d BALF lipid mediators; LTB4, LXA4 and PGE2 e-k BALF cytokines and chemokines. Data (mean ± s.e.m) are representative of more than 3 independent experiments with n≥3 mice in each group. * P<0.05.
NK cell depletion reduces resolvin E1 mediated resolution of airway inflammation
As NK cells appeared to have a pivotal role in the endogenous resolution program for adaptive immune responses, their contribution to RvE1 mediated resolution was next determined. Administration of RvE1 at the peak of inflammation, (Supplementary Fig. 4a) accelerated resolution by day 21 with decreased BALF total cells and eosinophils by ~40% (Fig. 3a,b). These protective actions for RvE1 were blocked by administration of aGM1 (Fig. 3a,b). At peak inflammation (day 18), the percentage of NK cells decreased relative to baseline (day 0) in both lung and PB (Fig. 3c,d). Administration of RvE1 hastened a return of NK cells to baseline levels in these tissues (Fig. 3c,d). No significant changes in the already increased MLN NK cell numbers were observed with RvE1 (Fig. 3e).
Figure 3. RvE1 mediated resolution of airway inflammation is blocked by NK cell depletion.
a BALF total cells and (b) eosinophils at day 21 from mice given rabbit IgG or aGM1 plus vehicle or RvE1 (100ng; inset). The percentage of NK cells in (c) lung, (d) PB and (e) total NK cell number in MLNs from mice given vehicle or RvE1 (100ng). Data (mean ± s.e.m) are representative of more than 3 independent experiments with n≥3 mice in each group. * P<0.05.
NK cells clear eosinophils and antigen specific T cells during resolution
Depletion of NK cells with aGM1 during resolution significantly increased the number of inflammatory cells in BALFs at day 21 (Fig. 4a,b). The total number of BALF cells and eosinophils significantly increased (P<0.001) in mice depleted of NK cells (Fig. 4a). Total CD4+ T lymphocytes were increased in BALFs (3.06 ± 0.45 × 104 (IgG) vs 4.5 ± 0.59 × 104 (aGM1), P<0.05) and MLN (3.9 ± 0.26 × 106 (IgG) vs 4.8 ± 0.32 × 106 (aGM1), P<0.05) and the Ri for BALF lymphocytes nearly doubled from ~3 days to ~6 days (P<0.001) (Fig. 4b). In view of this increase in T cells, interactions between NK cells and CD4+ T cell responses in vivo were investigated using an adoptive transfer model of allergic airway inflammation with CD4+ T cells from transgenic DO11.10 mice with a T cell receptor that is specific for chicken ovalbumin (OVA) (KJ1-26+ T cells) (12). Recipient BALB/cj mice given CD4+ KJ1-26+ T cells were sensitized and aerosol challenged with OVA, and then aGM1 or an IgG control antibody was administered to investigate the impact of NK cells on clearance of antigen specific CD4+ KJ1-26+ T cells. NK cell depletion led to increased CD4+ KJ1-26+ T cells in MLNs (P=0.03), lung (P=0.01) and BALFs (P=0.03) (Fig. 4c,d). During resolution, RvE1 accelerated the removal of antigen specific CD4+ KJ1-26+ T cells in the MLNs and lung in an NK cell dependent manner (Fig. 4e-g).
Figure 4. Depletion of NK cells delays resolution of allergic inflammation.
Mice were given aGM1 antibody or control IgG at peak inflammation (protocol day 18) and a, the total number of BALF cells (left panel) and eosinophils (right panel) were enumerated during resolution (protocol day 21). b The Ri was determined for BALF lymphocytes from FVB mice given aGM1 (red) or control IgG (white). c Representative flow cytometry plots showing percentage of CD4+ KJ1-26+ cells (inserts) from MLNs, lung, BALFs and spleen from peak inflammation (day 18) and early resolution (day 21) from mice given control IgG or aGM1. d The total number of CD4+ KJ1-26+ T cells in MLNs, lung, BALFs and spleen at peak inflammation (day 18) and following aGM1 or control IgG (day 21). e Flow cytometry plots of KJ1-26 CD4+ T cells from MLNs and lung from mice given rabbit IgG and RvE1 (100ng) or vehicle control or aGM1 and RvE1(100ng). f Total number of CD4+ KJ1-26+ T cells in MLNs and (g) lung from day 21 BALB/cj mice. Data (mean ± s.e.m) are representative of 3 experiments with n≥3 BALB/cj mice in each group. § P <0.05 (day 18) † P <0.05 (day 21).
Blocking NK cell homing disrupts resolution
To determine if NK cell recruitment was an active step in resolution, the time course for expression of the NK cell homing receptors CXCR3 and CD62L (13) was measured by flow cytometry. During resolution, CXCR3 expression was selectively up-regulated on NK cells from MLNs and PB (Fig. 5a). In contrast, CD62L was down-regulated on NK cells from the MLNs and spleen during resolution and up-regulated on lung NK cells (Fig. 5a,b). To test if CXCR3 and CD62L were required for NK cell recruitment to MLNs and lung during resolution, NK cells were labelled ex vivo with CFSE and incubated with a blocking antibody for CXCR3, CD62L, a combination of both or control antibody prior to reconstitution by adoptive transfer into mice depleted of endogenous NK cells with aGM1 (Supplementary Fig. 4b,c). Blocking NK cell CXCR3 markedly inhibited recruitment into MLNs (P=0.01) by approximately 50% (Fig. 5c) and disrupted resolution, leading to increased MLN (P=0.03) and BALF total cells (P=0.02) and BALF eosinophils (P=0.007) (Fig. 5d).
Figure 5. NK cells are recruited to MLNs and lung during resolution.
a Histograms show representative expression of CXCR3 and CD62L on NK cells (NKp46+CD3−) from MLNs, lung, PB and spleen at peak inflammation (day 18, gray), and resolution (day 21 (red). b Median fluorescence intensity (MFI) of CXCR3 and CD62L expression on NK cells from the MLNs, lung, PB and spleen. c After depletion of endogenous NK cells with aGM1, CFSE labelled donor NK cells were adoptively transferred (day 19) after exposure ex vivo to anti-CXCR3, anti-CD62L, a combination of both or control IgG and enumerated in recipient mouse tissues during resolution (day 21). d MLN total cells and BALF total cell counts and eosinophils from mice reconstituted with adoptively transferred NK cells. Fold change in cxcl9 expression in (e) MLNs and (f) lung during peak inflammation (day 18) and resolution (day 21) of airway inflammation in mice given vehicle or RvE1. Data (mean ± s.e.m) are representative of more than 3 independent experiments with n≥3 FVB mice in each group. * P <0.05 (day 18); # P <0.05 (percentage compared to IgG).
The CXCR3 ligands cxcl9, cxcl10 and cxcl11 were all expressed in murine lung and MLNs (cxcl10 and cxcl11 data not shown). There was a marked decrease in MLN cxcl9 and increase in lung cxcl9 at peak inflammation that returned towards basal expression during resolution (day 21) (Fig. 5e,f). RvE1 increased cxcl9 expression in both tissues during resolution (Fig. 5e,f). In contrast, RvE1 did not significantly change NK cell CD62L or CXCR3 in MLNs or lung at day 21 (data not shown). In conjunction with the findings with the CXCR3 blocking antibody (Fig. 5c), these results indicate that CXCL9-CXCR3 interactions are important to timely resolution and that RvE1 can enhance NK cell trafficking to target organs by up-regulation of cxcl9 expression during resolution. Together, these findings emphasize the importance of NK cell trafficking to target tissues and secondary lymphoid organs for resolution of adaptive inflammation.
NK cell recognition of eosinophils and antigen specific CD4+ T cells
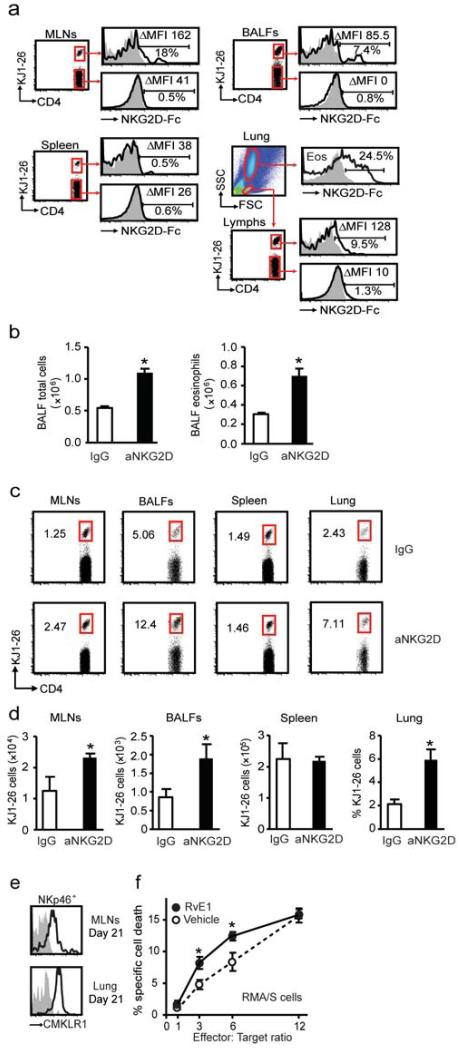
NK cells can target autologous CD4+ T cells via their NKG2D receptor (17), so the impact of this receptor system on leukocyte clearance was next determined. Because multiple ligands for the NKG2D receptor are up-regulated during inflammation and cellular stress (18), their expression was measured using a NKG2D-Fc fusion protein (19). During resolution, NKG2D ligands were expressed on antigen specific CD4+ KJ1-26+ T cells from MLNs, BALFs and lung, but not spleen (Fig. 6a). NKG2D ligands were not evident on KJ1-26− T cells (Fig. 6a). Of interest, NKG2D ligands were also detected on lung eosinophils (CD11b+ CCR3+) (Fig. 6a). The functional impact of signaling at this receptor was determined by blocking the NKG2D receptor on NK cells. Endogenous NK cells were depleted with aGM1 and reconstituted from donor mice with NK cells that were exposed ex vivo to an NKG2D blocking or IgG control antibody (Supplementary Fig. 4b). Mice given NK cells incubated with anti-NKG2D (aNKG2D) had significantly more inflammation at protocol day 21 with increased BALF total cells (P= 0.02) and eosinophils (P=0.02) (Fig. 6b). In addition, blocking NK cell NKG2D impaired the clearance of KJ1-26+ CD4+ T cells from MLNs (P=0.04), BALFs (P=0.04) and lung (P=0.02) during resolution (Fig. 6c,d).
Figure 6. Antigen specific T cells and eosinophils express NKG2D ligands for clearance from inflamed lung.
a Expression of NKG2D ligands on KJ1-26+ and KJ1-26− CD4+ T cells from MLNs, BALFs, spleen and lung eosinophils (Eos) was determined with NKG2D-Fc fusion protein. Histograms show secondary alone (gray) and expression of ligands (black). Inserts show the delta (Δ) median fluorescence intensity (MFI-MFI control) and the percentage of cells positive for NKG2D ligands. Mice were depleted of endogenous NK cells with aGM1 and reconstituted with donor NK cells that were exposed ex vivo to anti-NKG2D (aNKG2D). b BALF total cells and eosinophils were enumerated after aNKG2D or IgG control antibody. c Flow cytometry plots from CD4+ T cells in MLNs, BALFs, spleen and lung. Inserts show percentages of CD4+ KJ1-26+ T cells. d The number of CD4+ KJ1-26+ T cells in MLNs, BALFs, spleen and lung (percentage) after aNKG2D or control antibody. e Representative histograms show expression of the RvE1 receptor CMKLR1 on NK cells (NKp46+ CD3−) from MLNs and lung (day 21). f NK cell cytotoxicity towards RMA/S target cells was determined in the presence of RvE1 (10 nM) or vehicle control * P <0.05 (vehicle), § P <0.05 (RvE1). Data (mean ± s.e.m) are representative of more than 3 independent experiments with n≥4 BALB/cj mice in each group.
Of interest, day 21 NK cells expressed the RvE1 receptor CMKLR1 (Fig. 6e). Because RvE1 can accelerate catabasis by clearance of antigen specific CD4+ KJ1-26+ T cells (Fig. 4), the direct impact of RvE1 on in vitro NK cell cytotoxicity was determined. Exposure of NK cells to RvE1 (10nM) significantly increased their ability to kill target RMA/S cells at effector to target ratios of 3:1 and 6:1 (Fig. 6f). Together, these results suggest that NK cells were activated in vivo to utilize the NKG2D receptor to clear antigen specific targets of allergic inflammation, such as CD4+ lymphocytes, to promote the timely resolution of adaptive immune responses.
Discussion
Clearance of leukocytes from inflamed tissues is fundamental to resolution and restoration of tissue homeostasis, and can be impaired in diseases of chronic inflammation (1, 20). Here, we investigated endogenous mechanisms for resolution of adaptive inflammation by using a self-limited experimental model of allergic airway inflammation. At peak inflammation in this model, eosinophils and activated T cells infiltrated the lung, and after cessation of allergen exposure, the leukocytes were cleared from the lung within approximately one week. During this one week resolution phase, there was an increase in the numbers of NK cells in the lung draining MLNs that temporally overlapped decrements in BALF eosinophils and T cells. In addition to accumulating in MLNs during resolution, NK cells acquired markers consistent with cell activation. Administration of aGM1 antibody that principally depletes NK cells led to delayed resolution for both eosinophils and CD4+ T cells. In addition to limiting the development of adaptive immune responses (21), the present results provide evidence that NK cells are important cellular effectors for promoting the resolution of established adaptive inflammation.
NK cells express the RvE1 receptor CMKLR1 (also known as ChemR23) (6, 22). RvE1 is a potent pro-resolving mediator for allergic airway inflammation (5) and NK cell depletion markedly impaired RvE1’s protective actions. RvE1 regulated NK cell homing and in vivo clearance of antigen specific CD4+ T cells. In addition to RvE1’s in vivo actions, RvE1 increased NK cell cytotoxicity in vitro. The RvE1 mediated increase in PB and lung NK cells suggests that this mediator also increased NK cell transit through the MLNs. CXCL9 was originally identified as a chemokine regulated by IFN-γ (23). Blocking CXCL9 decreases allergic airway responses (24) and here a neutralizing antibody for CXCL9’s cognate receptor CXCR3 on NK cells impeded their ability to reach the MLNs and delayed resolution. These findings are consistent with a role for IFN-γ during resolution. In this model, RvE1 increases IFN-γ (5), and here increased both MLN and lung expression of cxcl9. Of note, CXCR3 deficient mice have delayed wound healing (25). Together, these results indicate that RvE1 selectively regulates tissue chemokines to target NK cell homing for catabasis.
NK cells are innate lymphocytes that can play diverse roles in immunity, in particular in anti-viral and anti-tumor host responses via the regulated killing of transformed cells and the release of immunomodulatory cytokines (26). They are also capable of influencing adaptive immunity (reviewed in (27)), including for the development of allergic airway inflammation (28), contact hypersensitivity (29) and memory responses to murine cytomegalovirus infection (30). When provided in a therapeutic dosing strategy, NK cells can diminish allergic airway responses in mice (31). Moreover, NK cells can regulate pathogen-mediated inflammation in the lung to facilitate the clearance of acute bacterial pneumonia, and their activation in murine pneumonia is dependent upon recognition of NKG2D ligands in the lung (32). Here, the NKG2D receptor contributed to inflammatory cell removal in vivo. Eosinophils and antigen specific CD4+ T cells expressed NKG2D ligands and blocking the NK cell NKG2D receptor delayed clearance of these cells. In addition to these leukocyte effectors of adaptive immune responses, structural cells in the lung can also use the NKG2D system to regulate airway immune responses, as airway epithelial cells express low levels of NKG2D ligands that are increased upon exposure to oxidative stress (33).
During resolution, the CD27+ CD11b+ NK cell population transiently increased and was associated with expression of IFN-γ, CD69 and CD107a (LAMP-1), demonstrating that the resolution NK cells were activated and not passively trafficking to the lymph nodes. These activated NK cells were potent regulators of the levels of inflammatory mediators in the lung. In this murine experimental model of asthma, TH2 cytokines, such as IL-5 and IL-13, are pivotal for the development of allergic airway responses (34), but in the resolution phase, IL-23 and IL-17 serve non-redundant roles (5). Administration of aGM1 depleted NK cells and delayed resolution. With aGM1, airway inflammation and mucus metaplasia persisted, and levels of the TH2 cytokines IL-5 and IL-13 and the T cell chemokines CCL17 (TARC) and CCL22 (MDC) were not significantly changed. In contrast, IL-23 levels were increased and there was a marked increase in LTB4. These mediators can increase recruitment of CD4+ and CD8+ effector T cell populations; (35) and LTB4 in particular is also a chemoattractant and secretagogue for eosinophils (34, 36). In addition to changes in these pro-phlogistic signals, levels of PGE2 and LXA4, an endogenous anti-inflammatory/pro-resolution lipid mediator, were decreased. The distinct mechanisms for the development of inflammation and its resolution is further emphasized by depletion of NK cells during OVA challenge (rather than resolution) that ameliorates allergic airway responses (37), indicating the potential of NK cells to play significant roles in both the onset and down stroke of inflammatory responses.
In summary, findings presented here provide evidence for transient NK cell activation during the resolution of self-limited adaptive inflammation and that NK cells play an integral role in RvE1 mediated catabasis. Regulation of NK cell trafficking to the target tissue and draining lymph nodes contributes to a resolution program for inflamed tissue via regulation of inflammatory mediators and clearance of eosinophils and antigen-specific T cells. Together, these results demonstrate pivotal pro-resolving roles for NK cells in tissue catabasis for adaptive immune responses.
Supplementary Material
Acknowledgements
The authors thank Charles N. Serhan (BWH-HMS) for providing RvE1 methyl-ester, helpful discussion and critical review of this manuscript and Michael A. Pfeffer for technical assistance.
This research was supported in part by the US National Institutes of Health grant AI068084 (B.D.L) and an American Lung Association Senior Research Training Fellowship (O.H).
Footnotes
Author contributions
O.H designed and performed experiments, analyzed data and wrote the manuscript. M.C provided reagents, analyzed data and was involved in experimental design. B.D.L designed and performed experiments, analyzed data and wrote the manuscript.
Competing interest statement
BDL is a co-inventor on patents assigned to Brigham and Women’s Hospital and Partners HealthCare on the uses and clinical development of anti-inflammatory and proresolving lipid mediators. These are licensed for clinical development.
References
- 1.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature Reviews. Immunology. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan C, Ding A. Nonresolving inflammation. Cell. 140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 3.Busse WW, Lemanske RF., Jr. Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 4.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 5.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajakariar R, Lawrence T, Bystrom J, Hilliard M, Colville-Nash P, Bellingan G, Fitzgerald D, Yaqoob MM, Gilroy DW. Novel biphasic role for lymphocytes revealed during resolving inflammation. Blood. 2008;111:4184–4192. doi: 10.1182/blood-2007-08-108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 9.Kasai M, Yoneda T, Habu S, Maruyama Y, Okumura K, Tokunaga T. In vivo effect of anti-asialo GM1 antibody on natural killer activity. Nature. 1981;291:334–335. doi: 10.1038/291334a0. [DOI] [PubMed] [Google Scholar]
- 10.Stitz L, Baenziger J, Pircher H, Hengartner H, Zinkernagel RM. Effect of rabbit anti-asialo GM1 treatment in vivo or with anti-asialo GM1 plus complement in vitro on cytotoxic T cell activities. Journal of Immunology. 1986;136:4674–4680. [PubMed] [Google Scholar]
- 11.Habu S, Kasai M, Nagai Y, Tamaoki N, Herzenberg LA, Okumura K. The glycolipid asialo GM1 as a new differentiation antigen of fetal thymocytes. J Immunol. 1980;125:2284–2288. T. A. T. [PubMed] [Google Scholar]
- 12.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunological Reviews. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol. 2002;3:1150–1155. doi: 10.1038/ni857. [DOI] [PubMed] [Google Scholar]
- 15.Walzer T, Blery M, Chaix J, Fuseri N, Chasson L, Robbins SH, Jaeger S, Andre P, Gauthier L, Daniel L, Chemin K, Morel Y, Dalod M, Imbert J, Pierres M, Moretta A, Romagne F, Vivier E. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3384–3389. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. Journal of Immunology. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 17.Rabinovich BA, Li J, Shannon J, Hurren R, Chalupny J, Cosman D, Miller RG. Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. Journal of Immunology. 2003;170:3572–3576. doi: 10.4049/jimmunol.170.7.3572. [DOI] [PubMed] [Google Scholar]
- 18.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nature Reviews. Immunology. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 19.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 21.Hao J, Liu R, Piao W, Zhou Q, Vollmer TL, Campagnolo DI, Xiang R, La Cava A, Van Kaer L, Shi FD. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J Exp Med. 207:1907–1921. doi: 10.1084/jem.20092749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parolini S, Santoro A, Marcenaro E, Luini W, Massardi L, Facchetti F, Communi D, Parmentier M, Majorana A, Sironi M, Tabellini G, Moretta A, Sozzani S. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood. 2007;109:3625–3632. doi: 10.1182/blood-2006-08-038844. [DOI] [PubMed] [Google Scholar]
- 23.Liao F, Rabin RL, Yannelli JR, Koniaris LG, Vanguri P, Farber JM. Human Mig chemokine: biochemical and functional characterization. J Exp Med. 1995;182:1301–1314. doi: 10.1084/jem.182.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas MS, Kunkel SL, Lukacs NW. Regulation of cockroach antigen-induced allergic airway hyperreactivity by the CXCR3 ligand CXCL9. J Immunol. 2004;173:615–623. doi: 10.4049/jimmunol.173.1.615. [DOI] [PubMed] [Google Scholar]
- 25.Yates CC, Whaley D, Kulasekeran P, Hancock WW, Lu B, Bodnar R, Newsome J, Hebda PA, Wells A. Delayed and deficient dermal maturation in mice lacking the CXCR3 ELR-negative CXC chemokine receptor. Am J Pathol. 2007;171:484–495. doi: 10.2353/ajpath.2007.061092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nature Immunology. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 27.Di Santo JP. Natural killer cells: diversity in search of a niche. Nat Immunol. 2008;9:473–475. doi: 10.1038/ni.f.201. [DOI] [PubMed] [Google Scholar]
- 28.Korsgren M, Persson CG, Sundler F, Bjerke T, Hansson T, Chambers BJ, Hong S, Van Kaer L, Ljunggren HG, Korsgren O. Natural killer cells determine development of allergen-induced eosinophilic airway inflammation in mice. Journal of Experimental Medicine. 1999;189:553–562. doi: 10.1084/jem.189.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 30.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsubara S, Takeda K, Kodama T, Joetham A, Miyahara N, Koya T, Swasey CH, Okamoto M, Dakhama A, Gelfand EW. IL-2 and IL-18 attenuation of airway hyperresponsiveness requires STAT4, IFN-gamma, and natural killer cells. American Journal of Respiratory Cell & Molecular Biology. 2007;36:324–332. doi: 10.1165/rcmb.2006-0231OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wesselkamper SC, Eppert BL, Motz GT, Lau GW, Hassett DJ, Borchers MT. NKG2D is critical for NK cell activation in host defense against Pseudomonas aeruginosa respiratory infection. J Immunol. 2008;181:5481–5489. doi: 10.4049/jimmunol.181.8.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borchers MT, Harris NL, Wesselkamper SC, Vitucci M, Cosman D. NKG2D ligands are expressed on stressed human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L222–231. doi: 10.1152/ajplung.00327.2005. [DOI] [PubMed] [Google Scholar]
- 34.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, Serhan CN. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 35.Miyahara N, Ohnishi H, Miyahara S, Takeda K, Matsubara S, Matsuda H, Okamoto M, Loader JE, Joetham A, Tanimoto M, Dakhama A, Gelfand EW. Leukotriene B4 release from mast cells in IgE-mediated airway hyperresponsiveness and inflammation. Am J Respir Cell Mol Biol. 2009;40:672–682. doi: 10.1165/rcmb.2008-0095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, Friedrich EB, Carafone AD, Gerszten RE, Luster AD. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol. 2003;4:982–990. doi: 10.1038/ni970. [DOI] [PubMed] [Google Scholar]
- 37.Ple C, Barrier M, Amniai L, Marquillies P, Bertout J, Tsicopoulos A, Walzer T, Lassalle P, Duez C. Natural killer cells accumulate in lung-draining lymph nodes and regulate airway eosinophilia in a murine model of asthma. Scand J Immunol. 72:118–127. doi: 10.1111/j.1365-3083.2010.02419.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.