Abstract
Infection during pregnancy triggers inflammation, which can increase myometrial contractions and the risk of premature labor and delivery. In this study, we assessed the effects of vitamin D, an anti-inflammatory ligand on cytokines, chemokines, toll-like receptors, and contractile-associated proteins on immortalized human myometrial smooth muscle (UtSM) cells stimulated with lipopolysaccharide (LPS), a bacterial endotoxin, or interleukin (IL)-1β and measured Toll-like receptor (TLR)-10 expression in pregnant myometrial tissues. A superarray analysis revealed downregulation of the chemokines monocyte chemoattractant protein (MCP)-1, Chemokine (C-X-C motif) ligand (CXCL)-10, CXCL-11, and chemokine (C-X3-C motif) ligand (CX3CL)-1; the proinflammatory cytokines IL-13 and tumor necrosis factor (TNF)-α; the TLR-4 and -5 and triggering receptor expressed on myeloid cells (TREM)-2 and upregulation of the anti-inflammatory cytokine IL-10, as well as Toll interacting protein (TOLLIP) and TREM-1 in vitamin D-treated UtSM cells. In the presence of LPS, vitamin D caused dose-dependent decreases in the messenger RNA expression of MCP-1, IL-1β, IL-13, TNF-α, TLR-4, and TLR-5, the contractile-associated proteins connexin 43, the oxytocin receptor, and the prostaglandin receptor but caused increases in IL-10 and TLR-10 in UtSM cells. The TLR-10 expression was higher in human myometrial tissue obtained from women at term not in labor compared to labor. Vitamin D also attenuated IL-1β-induced MCP-1, IL-6, connexin 43, cyclooxygenase (COX)-2, and prostaglandin receptor expression. Western analysis showed that vitamin D decreased MCP-1, TLR-4, and connexin 43 in the presence of LPS and decreased connexin 43 in the presence of IL-1β. Our results suggest that vitamin D can potentially decrease infection-induced increases in cytokines and contractile-associated proteins in the myometrium.
Keywords: myometrial smooth muscle cells, UtSM cells, vitamin D, cytokines, contractile-associated proteins, toll-like receptors
Preterm delivery is a major problem in obstetrics. Deaths among premature infants account for 70% of all perinatal mortalities.1 In addition, premature babies are more likely to have long-term pulmonary, neurologic, mental, and behavioral health problems.2 Infection is reported to be responsible for 40% of preterm births3 and is a potentially preventable cause of preterm birth. Vitamin D is a potent modulator of human immune responses and is reported to play a protective role against infectious diseases.4–6 African Americans have lower levels of serum vitamin D than caucasians7 and hence are more susceptible to infection. The efficient upregulation of the antimicrobial protein cathelicidin in the serum of African Americans after vitamin D supplementation suggests that vitamin D promotes innate immunity8 and can potentially reduce infection-associated inflammation.
The expression levels of the proinflammatory cytokines interleukin (IL)-1β, IL-13, and chemokine Chemokine (C-X-C motif) ligand (CXCL)-10 were reported to be higher in the myometrium obtained from women at term in labor compared with women at term not in labor.9,10 Monocyte chemoattractant protein (MCP)-1 was reported to increase in gravid uterine horn of unilateral pregnant rat suggesting that MCP-1 contribute to inflammation at term pregnancy.11 Gene expressions of MCP-1 and IL-1β were also reported to be high in the amnion and choriodecidua membranes obtained from women at term in labor compared with women at term not in labor,12 confirming a role for proinflammatory cytokines in the process leading to labor. Lipopolysaccharide (LPS), a bacterial endotoxin, has been reported to trigger the secretion of inflammatory markers in myometrial tissue,9 myometrial smooth muscle (UtSM) cells,13 and macrophages.14 Additionally, proinflammatory markers increase gene expression and the synthesis of various contractile-associated proteins, such as prostaglandins,15–19 connexin 20 and oxytocin receptors21 in bovine endometrial cells and human myometrium. Lipopolysaccharide and IL-1β administration caused preterm delivery in rodent models,22,23 indicating that inflammation increases the risk of preterm labor by enhancing the expression of contractile-associated proteins. Because vitamin D is involved in the regulation of innate immunity4,5 and its receptor expression is reported in myometrium,24 vitamin D might exert an anti-inflammatory effect in human myometrium and help in the maintenance of myometrial quiescence during gestation. In this work, we studied the effects of vitamin D on the expression of various inflammatory markers and several contractile-associated proteins in immortalized human UtSM cells subjected to LPS and IL-1β treatment. We measured messenger RNA (mRNA) expression of TLR-10 in nonlaboring and laboring pregnant human myometrium tissues obtained at cesarean section.
Materials and Methods
Cell Lines and Reagents
Immortalized human myometrial cells (UtSM cells) were a generous gift from Dr Darlene Dixon (National Institute of Environmental Health Sciences, Research Triangle Park, NC). Smooth muscle basal medium (SmBM), growth factors, and antibiotics—gentamicin sulfate and amphotericin B were obtained from Lonza (Walkersville, Maryland). Charcoal-treated fetal bovine serum (cFBS) was purchased from Life Technologies (Grand Island, New York). Lipopolysaccharide, 1,25-dihydroxyvitamin D3 (vitamin D), ethanol, and β-actin antibody were obtained from Sigma (St Louis, Missouri). Interleukin 1β was purchased from BD Pharmingen (San Diego, California). SYBR green was obtained from Bio-Rad (Hercules, California). Antibodies against MCP-1 and TLR-4 were purchased from Santa Cruz Biotechnology (Santa Cruz, California), and connexin 43 antibody was purchased from Life Technologies.
Cell Culture
Human myometrial (UtSM) cells were cultured in SmBM supplemented with 5% FBS, human epidermal growth factor (0.1%), recombinant human fibroblast growth factor (0.2%), 0.1% insulin and 0.1% gentamicin sulfate, and amphotericin B. These cells were maintained at 37°C in a humidified atmosphere of air and 5% CO2. At 90% confluence, the cells were starved for 24 hours in SmBM medium containing 5% charcoal-treated FBS and treated with vitamin D or LPS. Cells treated with IL-1β were starved for 24 hours in SmBM medium containing 0.1% bovine serum albumin (Sigma), as suggested by the supplier.
Treatment of UtSM Cells With Vitamin D
To assess, by superarray analysis, whether vitamin D induced changes in gene expression profile of human cytokines, their receptors, and toll-like receptors, UtSM cells were treated in triplicate with 100 nmol/L of vitamin D dissolved in ethanol in the presence of 5% charcoal-treated FBS. Equal volume of ethanol was added to the media of the cells in the control (vehicle-treated) group. To assess mRNA expression of chemokines, cytokines, and contractile-associated proteins in response to vitamin D and LPS, the UtSM cells were treated in triplicate with vitamin D (0, 10, 100, and 500 nmol/L) alone or LPS (0, 10, 100, and 1000 ng/mL) alone for 12 hours. To test whether vitamin D regulates LPS and IL-1β-induced effects, the cells were treated with vitamin D for 24 and 48 hours in the presence of LPS (1000 ng/mL) and IL-1β (10 ng/mL) separately.
Participants
Myometrial tissues were collected at cesarean delivery from healthy term pregnant women—6 each from not in labor and in labor to assess TLR-10 mRNA expression. Gestational age was determined by the last menstrual period and corroborated by ultrasound dating. Participants with preeclampsia, premature rupture of membranes, placental previa, fetal anomalies, gestational diabetes, poly and oligohydramnios, and other complications such as surgeries during pregnancies were excluded. The study was approved by the institutional review board of Meharry Medical College, Nashville, Tennessee. All participants provided informed consent.
Isolation of Total RNA and Reverse Transcription
Total RNA was extracted from the treated UtSM cells and human myometrial tissues using RNeasy kit (Qiagen, Maryland) and subjected to DNAse I to remove genomic DNA contamination, per the supplier’s instructions. For reverse transcription, 1 µg of total RNA was briefly mixed with 3.0 nmol of oligo deoxythymine, 200 µmol/L deoxyribonucleotide triphosphate, 10 U of avian myeloblastosis virus reverse transcriptase, and 5 U RNase inhibitor in a total volume of 20 µL. Complementary DNA (cDNA) was prepared by reverse transcription in a thermal cycler at 25°C for 5 minutes and at 42°C for 60 minutes and stored at −20°C.
StellARray Quantitative Polymerase Chain Reaction
The cDNA obtained from the control and vitamin D (100 nmol/L)-treated UtSM cells were subjected to StellARray quantitative polymerase chain reaction (qPCR) using human cytokine and related receptors and toll-like receptor pathway array panels, per the supplier’s specification (Lonza).
Quantitative PCR
Quantitative PCR was performed to assess the mRNA expression levels of chemokines, cytokines, TLRs, and contractile-associated proteins in control and treated UtSM cells and TLR-10 in human myometrial tissues. Primers used were obtained from published literature. The sequences of the forward (F) and reverse (R) primers are listed in Table 1. All primer sets used for qPCR generated a single amplicon. Briefly, 50 ng of cDNA were added to the master mix containing appropriate primers and SYBR green. The qPCR was performed in a Bio-Rad MyiQ5. After an initial denaturation step, all genes are amplified at 95°C for 15seconds and 60°C for 1 minute for 40 cycles except for cyclooxygenase (COX)-2, for which the annealing temperature was 50°C. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) values of the relevant samples were used to normalize the data.
Table 1.
Human Forward and Reverse Primers for Quantitative RT-PCR.
| Gene | Primer Sequence | Amplicon Length | NCBI Accession # |
|---|---|---|---|
| MCP-1 forward | 5′-GCG GAG CTA TAG AAG AAT CAC-3′ | 174 | NM_002982 |
| MCP-1 reverse | 5′-TTG GGT TGT GGA GTG AGT GT-3′ | ||
| CXCL-10 forward | 5′-CCA GAA TCG AAG GCC ATC AA-3′ | 49 | NM_001565 |
| CXCL-10 reverse | 5′-CAT TTC CTT GCT AAC TGC TTT CAG-3′ | ||
| CXCL-11 forward | 5′-ATG AGT GTG AAG GGC ATG GC-3′ | 121 | NM_005409 |
| CXCL-11 reverse | 5′-TCA CTG CTT TTA CCC CAG GG-3′ | ||
| TNF-α forward | 5′-CAG AGG GAA GAG TTC CCC AG-3′ | 84 | NM_000594 |
| TNF-α reverse | 5′-CCT TGG TCT GGT AGG AGA CG-3′ | ||
| IL-1β forward | 5′-ACA GAT GAA GTG CTC CTT CCA-3′ | 73 | NM_000576 |
| IL-1β reverse | 5′-GTC GGA GAT TCG TAG CTG GAT-3′ | ||
| IL-6 forward | 5′-CAA ATT CGG TAC ATC CTC GAC-3′ | 339 | NM_000600 |
| IL-6 reverse | 5′-GTC AGG GGT GGT TAT TGC ATC-3′ | ||
| IL-10 forward | 5′-CAT CGA TTT CTT CCC TGT GAA-3′ | 74 | NM_000572 |
| IL-10 reverse | 5′-TCT TGG AGC TTA TTA AAG GCA TTC-3′ | ||
| IL-13 forward | 5′-TGA GGA GCT GGT CAA CAT CA-3′ | 76 | NM_002188 |
| IL-13 reverse | 5′-CAG GTT GAT GCT CCA TAC CAT-3′ | ||
| TLR-4 forward | 5′-GCA GTG AGG ATG ATG CCA GGA T-3′ | 67 | NM_138554 |
| TLR-4 reverse | 5′-GCC ATG GCT GGG ATC AGA GT-3′ | ||
| TLR-5 forward | 5′-TGC CTT GAA GCC TTC AGT TAT G-3′ | 77 | NM_003268 |
| TLR-5 reverse | 5′-CCA ACC ACC ACC ATG ATG AG-3′ | ||
| TLR-10 forward | 5′-TTA TGA CAG CAG AGG GTG ATG C-3′ | 150 | NM_001017388 |
| TLR-10 reverse | 5′-GGA GTT GAA AAA GGA GGT TAT AGG-3′ | ||
| Connexin 43 forward | 5′-CCT ATG TCT CCT CCT GGG TA-3′ | 176 | NM_000615 |
| Connexin 43 reverse | 5′-GGG AAA TCA AAA GGC TGT G-3′ | ||
| Prostaglandin (FP) receptor forward | 5′-GCA GCT GCG CTT CTT TCA A-3′ | 81 | NM_000959 |
| Prostaglandin (FP) receptor reverse | 5′-CAC TGT CAT GAA GAT TAC TGA AAA AAA TAC-3′ | ||
| Oxytocin receptor forward | 5′-CTG AAC ATC CCG AGG AAC TG-3′ | 84 | NM_000916 |
| Oxytocin receptor reverse | 5′-CTC TGA GCC ACT GCA AAT GA-3′ | ||
| COX-2 forward | 5′-TTC AAA TGA GAT TGT GGA AAA AT-3′ | 305 | NM_000963 |
| COX-2 reverse | 5′-AGA TCA TCT CTG CCT GAG TAT CTT-3′ | ||
| GAPDH forward | 5′-TGA TGA CAT CAA GAA GGT GGT-3′ | 240 | NM_002046 |
| GAPDH reverse | 5′-TCC TTG GAG GCC ATG TGG GCC-3′ |
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IL, interleukin; MCP-1, monocyte chemoattractant protein 1; RT-PCR, reverse transcription–polymerase chain reaction; CXCL, chemokine (C-X-C motif) ligand; TLR, Toll-like receptor; TNF, tumor necrosis factor; COX, cyclooxygenase.
Western Blot Analysis
Whole cell lysates were prepared from UtSM cells after 24 and 48 hours of treatment with vitamin D (100 nmol/L) in the presence of either LPS (1000 ng/mL) or IL-1β (10 ng/mL). Briefly, monolayer cells in 60-mm dishes were placed on ice and incubated for 10 minutes in radioimmunoprecipitation assay buffer containing 1× protease inhibitor cocktail, 1 mmol/L sodium vanadate, and 1 mmol/L sodium fluoride (Sigma). The lysates were disrupted using a sonicator (Misonix Incorporated, Farmingdale, New York). Cell debris was removed by centrifugation at 10 000 rpm on a benchtop centrifuge for 10 minutes at 4°C, and the supernatant was aliquoted and stored at −80°C. Equal amounts of protein were resolved in 10% Tris-Bis gels and transferred onto polyvinylidene difluoride membranes. Western blot analysis was performed using primary antibodies against MCP-1 (1:500; Santa Cruz), TLR-4 (1:500; Santa Cruz), connexin 43 (1:500; Life Technologies), and β-actin (1:5000; Sigma). The protein signal intensity was quantified using an image documentation system (Proteinsimple, Santa Clara, California) and normalized with the corresponding β-actin values.
Statistical Analysis
StellARray data were analyzed using software obtained from Lonza. Cycle threshold (Ct) values obtained were analyzed using the Global Pattern Recognition Analysis tool. A 2-fold change in gene expression compared to controls is considered statistically significant. Quantitative PCR data analysis was performed using the Bio-Rad iQ5 Optical System Software Version 2.0. One-way analysis of variance and Student t test were used to evaluate the differences between the controls and treatments. The data are represented as the mean ± standard error of the mean of 3 separate experiments.
Results
Vitamin D Downregulates Gene Expression for Several Chemokines, Inflammatory Cytokines, and Their Receptors and Members of Toll-Like Receptor Pathway in UtSM Cells
Quantitative PCR array analysis was performed for human myometrial cells treated with 100 nmol/L vitamin D using StellARray Human cytokine and receptors and toll-like receptor pathway panels. Vitamin D treatment of human myometrial cells downregulated the inflammatory cytokines IL-2, IL-9, IL-13, and tumor necrosis factor (TNF)-α and the chemokines MCP-1, chemokine (C-X3-C motif) ligand (CX3CL)-1, CXCL-10, and CXCL-11 (Table 2). In addition, vitamin D treatment downregulated toll-like receptor (TLR)-4, TLR-5, and triggering receptor expressed on myeloid cells (TREM)-2; upregulated Toll interacting protein (TOLLIP), CD-80, TREM-1, and the anti-inflammatory cytokine IL-10.
Table 2.
Human Myometrial Smooth Muscle Cells Were Treated With Vitamin D3 (100 nmol/L) and Subjected to qPCR Array Using StellARray Cytokine and Receptors and Toll-Like Receptor Panels.a
| Gene Symbol | Reference Sequence No. | Fold Change | Comments |
|---|---|---|---|
| Genes downregulated | |||
| LY-86 | NM 004271 | 2.02 | lymphocyte antigen 86 |
| MCP-1 | NM 002982 | 2.12 | Chemokine (C-C motif) ligand 2 |
| CSF-2 | NM 000758 | 2.15 | Colony-stimulating factor 2 (granulocyte-macrophage) |
| IFN-β1 | NM 002176 | 2.18 | Interferon, beta 1, fibroblast |
| TREM-2 | NM 018965 | 2.55 | Triggering receptor expressed on myeloid cells 2 |
| CXCL-10 | NM 001565 | 2.66 | Chemokine (C-X-C motif) ligand 10 |
| IRAK-3 | NM 007199 | 3.37 | Interleukin-1 receptor-associated kinase 3 |
| CXCL-11 | NM 005409 | 4.29 | Chemokine (C-X-C motif) ligand 11 |
| CX3CL-1 | NM 002996 | 2.06 | Chemokine (C-X3-C motif) ligand 1 |
| TNF-α | NM 000594 | 2.18 | tumor necrosis factor |
| IL-2 | NM 000586 | 6.77 | Interleukin 2 |
| IL-9 | NM 000590 | 2.00 | Interleukin 9 |
| SYK | NM 001135052 | 8.11 | Spleen tyrosine kinase |
| IL-13 | NM 002188 | 41.00 | Interleukin 13 |
| TLR-4 | NM 003266 | 2.00 | Toll-like receptor 4 |
| TLR-5 | NM 003268 | 2.40 | Toll-like receptor 5 |
| Genes upregulated | |||
| TOLLIP | NM 019009 | 2.11 | Toll interacting protein |
| CD-80 | NM 005191 | 3.44 | CD-80 molecule |
| IL-10 | NM 000572 | 4.80 | Interleukin 10 |
| TREM-1 | NM 001242590 | 5.10 | Triggering receptor expressed on myeloid cells 1 |
Abbreviation: qPCR, quantitative polymerase chain reaction.
aGenes with expression fold changes ≥2 relative to the untreated control are shown.
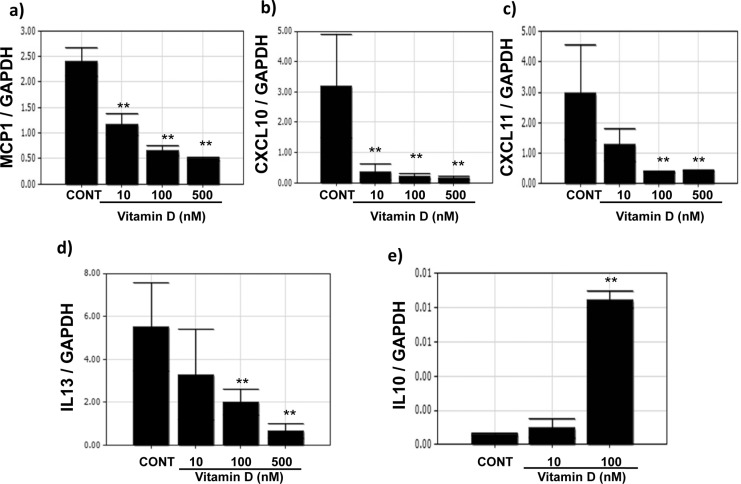
To confirm the results of StellARray, we performed qPCR using mRNA obtained from UtSM cells treated with different concentrations (1-500 nmol/L) of vitamin D. The quantitative PCR analysis showed dose-dependent decreases (P < .01) in the mRNA levels of MCP-1, CXCL-10, CXCL-11, and IL-13 in UtSM cells treated with vitamin D compared to vehicle-treated control (Figure 1A-D). The expression of the anti-inflammatory IL-10 significantly increased with vitamin D treatment at 100 nmol/L compared to vehicle-treated control (P < .01; Figure 1E).
Figure 1.
The effects of vitamin D on the mRNA expression of cytokine and chemokine genes in immortalized human myometrial (UtSM) cells treated with vitamin D for 12 hours. The UtSM cells were treated with different concentrations of vitamin D and the effects on (a) MCP-1, (b) CXCL-10, (c) CXCL-11, (d) IL-13, and (e) IL-10 were assessed using reverse transcription and qPCR analysis. The data are normalized with respective GAPDH values. The bars represent the mean ± SEM from 3 replicates in each group. Groups with asterisks (**P < .01) are significantly different from the vehicle-treated controls. GAPDH indicates glyceraldehyde 3-phosphate dehydrogenase; mRNA, messenger RNA; IL, interleukin; MCP, monocyte chemoattractant protein; SEM, standard error of the mean; qPCR, qualitative polymerase chain reaction; UtSM, myometrial smooth muscle; CXCL, Chemokine (C-X-C motif) ligand.
Vitamin D Treatment Reduces Contractile-Associated Proteins in UtSM Cells
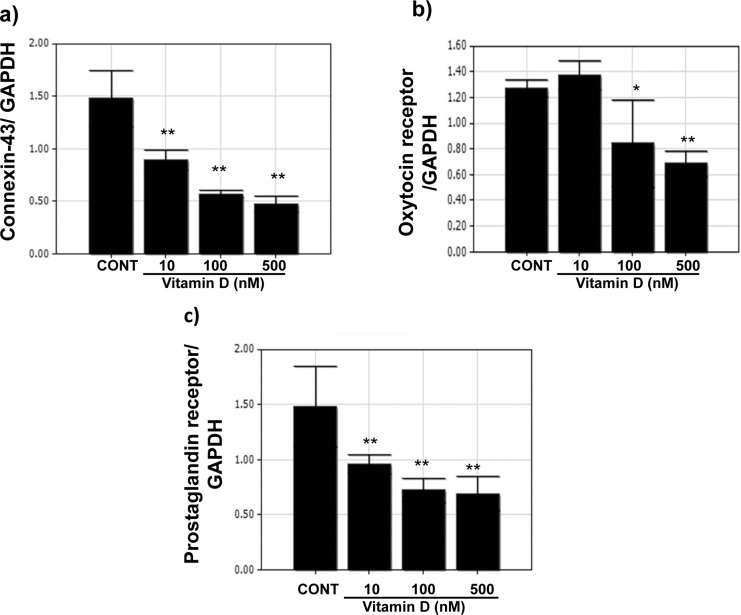
To test the effects of vitamin D treatment on the expression of various contractile-associated proteins, RNA obtained from UtSM cells treated with different concentrations of vitamin D and vehicle-treated controls were subjected to qPCR analysis. As shown in Figure 2 (A-C), compared to vehicle-treated control, vitamin D treatment significantly decreased the gene expression of connexin 43, oxytocin receptor, and prostaglandin receptor (P < .01) in human myometrial cells.
Figure 2.
Quantitative RT-PCR analysis of contractile-associated proteins in immortalized human myometrial (UtSM) cells treated with vitamin D. The UtSM cells were treated with different concentrations of vitamin D for 12 hours and the RNA obtained was subjected to qPCR analysis using specific primers for (A) connexin 43, (B) oxytocin receptor, and (C) prostaglandin receptor. The data are normalized with their respective GAPDH values. The bars represent the mean ± SEM from 3 replicates in each group. Groups with asterisks (*P < .05; **P < .01) are significantly different from vehicle-treated controls. GAPDH indicates glyceraldehyde 3-phosphate dehydrogenase; RT-PCR, reverse transcription–polymerase chain reaction; SEM, standard error of the mean; UtSM, myometrial smooth muscle.
Vitamin D Reduces LPS-Induced Inflammatory Markers and Contractile-Associated Proteins in UtSM Cells
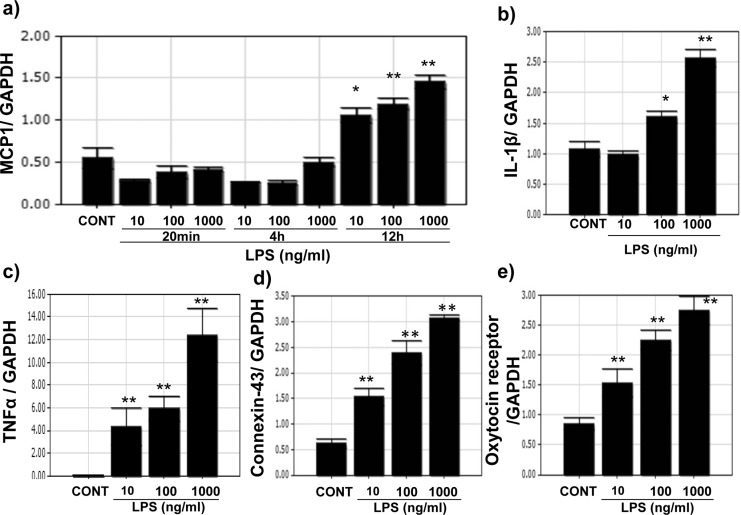
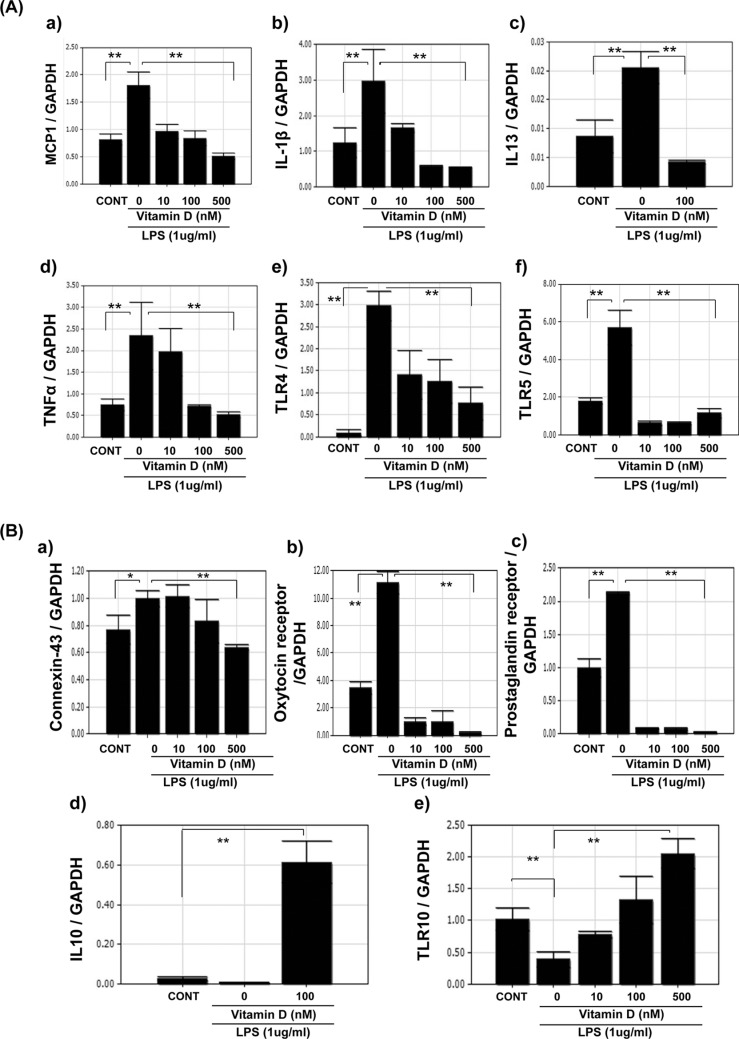
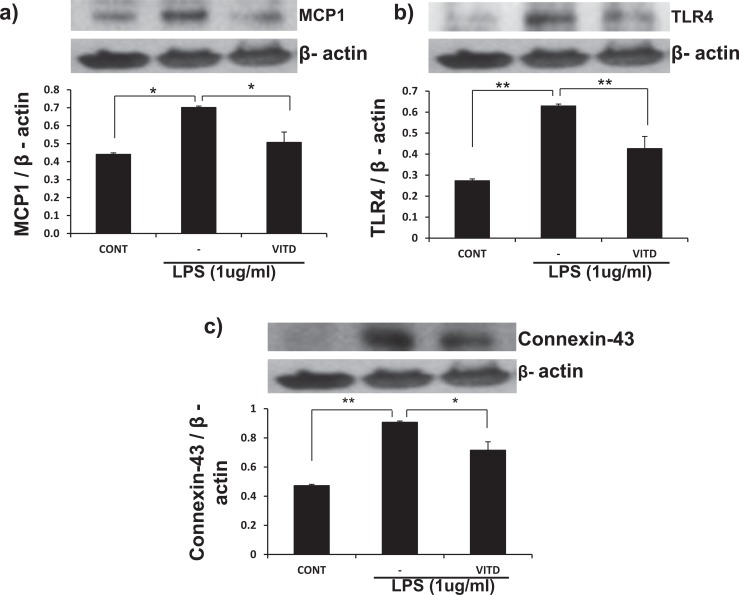
Because infection increases inflammatory markers in the myometrium, UtSM cells treated with LPS, a bacterial endotoxin were evaluated for the expression of MCP-1 and IL-1β and contractile-associated proteins. Quantitative PCR analysis showed that LPS significantly increased (P < .01) the mRNA expression of MCP-1, IL-1β, TNF-α (Figure 3A-C), and the contractile-associated proteins connexin 43 and oxytocin receptor in UtSM cells compared to vehicle-treated control (Figure 3D and E). To test whether vitamin D can reverse LPS-induced inflammatory markers and contractile-associated proteins, UtSM cells were treated with vitamin D in the presence of LPS. Vitamin D significantly decreased (P < .05) the LPS-induced mRNA expression of MCP-1, IL-1β, IL-13, TNF-α, TLR-4, and TLR-5 (Figure 4A, a-f) as well as the contractile-associated proteins connexin 43, oxytocin receptor, and prostaglandin receptor (Figure 4B, a-c). In contrast, vitamin D significantly increased (P < .01) the anti-inflammatory marker IL-10 and TLR-10 in the presence of LPS in UtSM cells (Figure 4B d and e). Western blot analysis performed using whole-cell lysates showed that vitamin D significantly decreased (P < .05) LPS-induced MCP-1, TLR-4, and connexin 43 expression in UtSM cells (Figure 5A-C).
Figure 3.
The dose effect of lipopolysaccharide (LPS) on mRNA expression of cytokines and contractile-associated proteins in immortalized human myometrial (UtSM) cells. The UtSM cells were treated with different concentrations of LPS for 12 hours, and the effects on (A) MCP-1, (B) IL-1β, (C) TNF-α, (D) connexin 43, and (F) oxytocin receptor were assessed with reverse transcription and qPCR analysis. The data were normalized with their respective GAPDH values. The bars represent the mean ± SEM from 3 replicates in each group. Groups with asterisks (*P < .05; **P < .01) are significantly different from the vehicle-treated controls. GAPDH indicates glyceraldehyde 3-phosphate dehydrogenase; IL, interleukin; MCP, monocyte chemoattractant protein; TNF, tumor necrosis factor; mRNA, messenger RNA; qPCR, quantitative polymerase chain reaction; SEM, standard error of the mean; UtSM, myometrial smooth muscle.
Figure 4.
The effects of vitamin D on LPS-induced mRNA expression of cytokines, chemokines, and contractile-associated proteins in immortalized human myometrial (UtSM) cells. The UtSM cells were treated with different concentrations of vitamin D for 12 hours in the presence of lipopolysaccharide (1 μg/mL). The RNA was subjected to quantitative RT-PCR analysis. The data were normalized with their respective GAPDH values. Vitamin D decreased (A) LPS-induced (a) MCP-1, (b) IL-1β, (c) IL-13, (d) TNF-α, (e) TLR-4, and (f) TLR-5; (B) LPS-induced (a) connexin 43, (b) oxytocin receptor, and (c) prostaglandin receptor mRNA expression. Vitamin D increased (d) IL-10 and (e) TLR-10 mRNA expression in the presence of LPS. The bars represent the mean ± standard error of the mean (SEM) from 3 replicates in each group. Groups with asterisks (*P < .05; **P < .01) are significantly different from the vehicle treated control or LPS. GAPDH indicates glyceraldehyde 3-phosphate dehydrogenase; IL, interleukin; LPS, lipopolysaccharide; MCP, monocyte chemoattractant protein; TLR, Toll-like receptor; mRNA, messenger RNA; RT-PCR, reverse transcription–polymerase chain reaction; UtSM, myometrial smooth muscle.
Figure 5.
The effects of vitamin D on LPS-induced protein expression of MCP-1, TLR-4, and connexin 43 in immortalized human myometrial (UtSM) cells. The UtSM cells were treated with 100 nmol/L of vitamin D for 48 hours in the presence of LPS (1 μg/mL). The prepared cell lysates were subjected to Western blot analysis using (A) anti-MCP-1, (B) anti-TLR-4, and (C) anti-connexin 43. β-Actin was used as a loading control. The bars represent the mean ± SEM from 3 replicates in each group. Groups with asterisks (*P < .05; **P < .01) are significantly different from the vehicle-treated control or LPS. LPS indicates lipopolysaccharide; MCP, monocyte chemoattractant protein; SEM, standard error of the mean; TLR, Toll-like receptor; UtSM, myometrial smooth muscle.
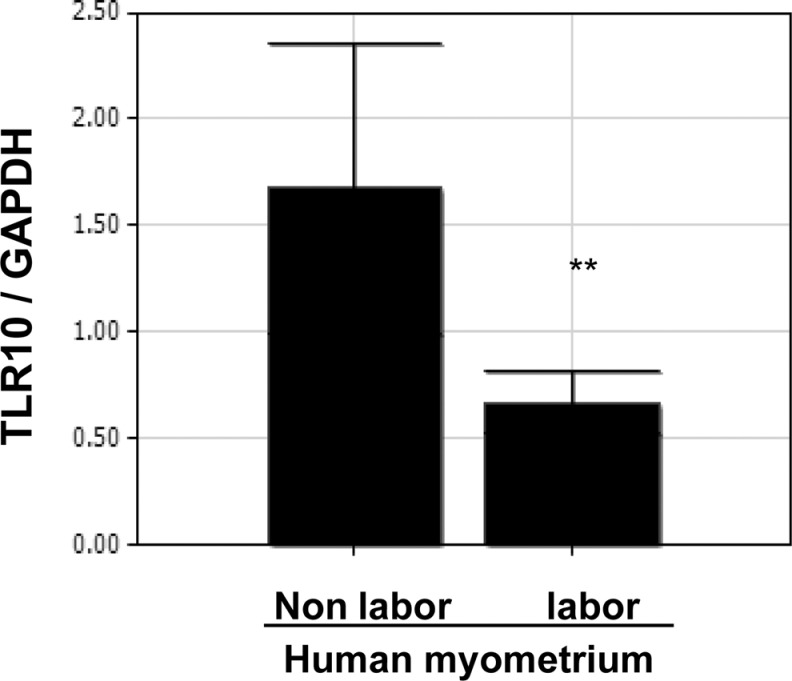
TLR-10 Expression is Higher in the Human Myometrial Tissues of Term Nonlaboring Versus Term Laboring Women
Because TLR-10 expression increased in UtSM cells treated with vitamin D in the presence of LPS, we also assessed its expression in human myometrial tissues obtained from term nonlaboring and laboring women at cesarean delivery. Reverse transcription-PCR analysis showed that TLR-10 expression is significantly higher (P < .01) in myometrial tissues from term nonlaboring compared to those from term laboring women (Figure 6).
Figure 6.
Differential mRNA expression of Toll-like receptor 10 in term human myometrial tissues. Myometrial tissues were obtained from term pregnant women in labor and not in labor. The complementary DNA obtained was subjected to qPCR analysis using primers specific for TLR-10. The data were normalized with respect to GAPDH values. The bars represent the mean ± SEM from 6 replicates in each group. Group with asterisks (**P < .01) is significantly different from the other. GAPDH indicates glyceraldehyde 3-phosphate dehydrogenase; mRNA, messenger RNA; qPCR, quantitative polymerase chain reaction; SEM, standard error of the mean; TLR, Toll-like receptor.
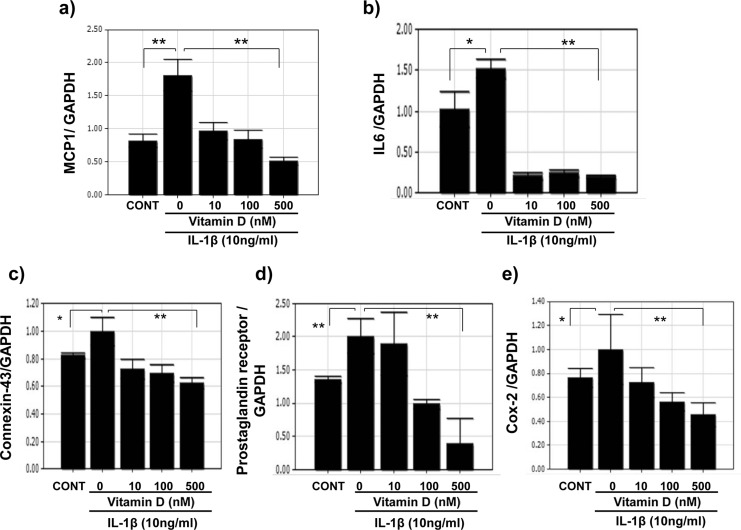
Vitamin D Reduces IL-1β-Induced Inflammatory Markers and Contractile-Associated Proteins in UtSM Cells
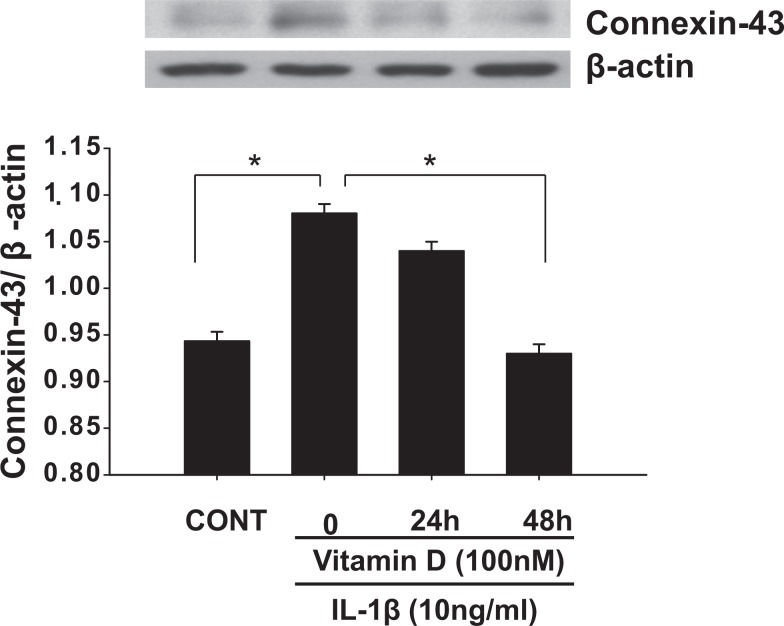
Interleukin 1β was shown to cause preterm myometrial contractions and delivery in rodent models. Therefore, we assessed the effects of IL-1β on the expression of cytokines and contractile-associated proteins and vitamin D regulation on the IL-1β-induced increases in these factors in UtSM cells. Interleukin 1β significantly increased (P < .05) the mRNA expression of MCP-1 and IL-6 (Figure 7A and B) and the contractile-associated proteins connexin 43, prostaglandin receptor, and COX-2 compared to vehicle-treated control (Figure 7C-E). Vitamin D significantly decreased (P < .01) the IL-1β-induced mRNA expression of MCP-1 and IL-6 (Figure 7A and B) and the contractile-associated proteins connexin 43, prostaglandin receptor, and COX-2 in UtSM cells (Figure 7C-E). Western blot analysis of whole-cell lysates showed that vitamin D significantly decreased (P < .05) the IL-1β-induced increase in connexin 43 expression in UtSM cells (Figure 8).
Figure 7.
The quantitative RT-PCR analysis of the expression of inflammatory markers and contractile-associated factors in immortalized human myometrial (UtSM) cells treated with vitamin D in the presence of IL-1β. The UtSM cells were treated with different concentrations of vitamin D for 12 hours, in the presence of IL-1β (10 ng/mL). The prepared RNA was subjected to quantitative RT-PCR analysis using specific primers for (A) MCP-1, (B) IL-6, (C) connexin 43, (D) prostaglandin receptor, and (E) COX-2. The data were normalized with their respective GAPDH values and are shown as changes in gene expression relative to the vehicle-treated control or IL-1β alone. The bars represent the mean ± SEM from 3 replicates in each group. Groups with asterisks (*P < .05; **P < .01) are significantly different from the vehicle-treated control or IL-1β. GAPDH indicates glyceraldehyde 3-phosphate dehydrogenase; IL, interleukin; MCP, monocyte chemoattractant protein; RT-PCR, reverse transcription–polymerase chain reaction; SEM, standard error of the mean; UtSM, myometrial smooth muscle.
Figure 8.
The effects of vitamin D on the protein expression of connexin 43 in immortalized human myometrial (UtSM) cells treated with interleukin 1β. The UtSM cells were treated with 100 nmol/L of vitamin D for 24 and 48 hours in the presence of interleukin 1β (10 ng/mL). The prepared cell lysates were subjected to Western blot analysis using anti-connexin 43. β-Actin was used as a loading control. The bars represent the mean ± SEM from 3 replicates in each group. Groups with asterisks (*P < .05) are significantly different from the vehicle-treated control or interleukin 1β. SEM indicates standard error of the mean; UtSM, myometrial smooth muscle.
Discussion
In this study, we analyzed the effects of vitamin D on the expression of inflammatory markers and contractile-associated proteins in immortalized normal human myometrial (UtSM) cells treated with either the bacterial endotoxin LPS or IL-1β and assessed mRNA expression of TLR10 in term human myometrium. Our results showed that (i) vitamin D decreases the basal, LPS- and IL-1β-induced expression of proinflammatory markers and contractile-associated proteins in UtSM cells, (ii) vitamin D increases the anti-inflammatory marker IL-10 and TLR-10 in the presence of LPS in UtSM cells, and (iii) myometrial tissue obtained from term nonlaboring women have higher expression of TLR-10 compared to the laboring women. These results suggest that vitamin D may play a role in mitigating the inflammation associated with preterm labor.
Studies from our laboratory25 and others26,27 showed that serum levels of vitamin D are low in preterm participants compared to term participants, suggesting a potential role for vitamin D in myometrial quiescence and the maintenance of pregnancy. Therefore, deficient or low levels of vitamin D during pregnancy might be a novel risk factor for preterm birth. Chemokines, proinflammatory cytokines, and many genes involved in the inflammatory pathway have been reported to play a role in delivery at term.28,29 However, excessive and untimely cytokine productions can lead to infection-induced preterm labor.30–32 In this study, the StellARray analysis performed using RNA obtained from vitamin D-treated UtSM cells showed decreases in the chemokines MCP-1, CXCL-10, CXCL-11, CX3CL-1 and the cytokines IL-9, IL-13, TNF-α, and TLR-5 as well as marked increases in the anti-inflammatory marker IL-10. The changes observed in some of these genes were confirmed with qPCR. A similar decrease in cytokine synthesis and increase in cathelicidin antimicrobial peptide expression were reported in human decidual natural killer cells treated with vitamin D.33 Vitamin D also caused a marked decrease in IL-13 expression in UtSM cells in our study. Recent studies have shown that gene polymorphisms in IL-13 are associated with preterm birth,34 and transgenic mice overexpressing IL-13 were more susceptible to infection.35 In addition, IL-13 has been reported to enhance oxytocin-induced calcium in airway smooth muscle cells, suggesting that it plays a role in muscle contraction36 and at higher levels could be a risk factor for preterm birth. Cytokines and chemokines are reported to increase the expression of COX-2 and contractile-associated proteins in human myometrium and COX-2, prostaglandin E synthase, and prostaglandin F synthase in cultured myometrial cells.37,38 In addition, nuclear factor κB (NFκB) activation was also reported to increase COX-2 in human UtSM cells.39 These results clearly suggest that inflammation increases the expression of contractile-associated proteins in human myometrium. In addition to decreasing inflammatory markers, vitamin D treatment also decreased connexin 43, prostaglandin receptor, and oxytocin receptor in UtSM cells, suggesting that vitamin D regulates the expression of both inflammatory and contractile-associated proteins. Cytokines induced during infection are reported to phosphorylate IKBα and activate the NFκB pathway.40 In addition to the direct effect on myometrial cells described in this work, vitamin D was also reported to increase IKBα levels in macrophages.41 These results suggest that vitamin D regulates the expression of contractile-associated proteins at least in part by regulating inflammatory markers in the UtSM cells.
Because 40% of preterm births are infection related,3 we used LPS, a bacterial endotoxin, to stimulate myometrial cells and potentially mimic the in vivo microenvironment of the human myometrium undergoing premature contractility. In our study of UtSM cells, LPS increased the expression of MCP-1, TNF-α, IL-1β, and IL-13 and the contractile-associated proteins connexin 43, oxytocin, and prostaglandin receptor. Lipopolysaccharide has been reported to trigger the secretion of inflammatory markers in the myometrium and UtSM cells.9,13 In addition, LPS was also reported to increase prostaglandins in human myometrium9 and human myometrial cells.18 These reports not only support our findings but also suggest an involvement of inflammatory markers and contractile-associated proteins in LPS-induced preterm deliveries. In this study, treating UtSM cells with vitamin D decreased the LPS-induced MCP-1, IL-1β, IL-13, and TNF-α expression and increased IL-10, an anti-inflammatory marker. Our findings also support a role for vitamin D in innate immunity and suggest that vitamin D inhibits the expression of inflammatory markers and contractile-associated proteins in the myometrium. In addition, the low levels of IL-10 reported in the placental tissue of 1, α hydroxylase knockout mice42 suggest that IL-10 expression is directly correlated with vitamin D levels. Our findings that vitamin D decreases LPS-induced inflammatory markers and contractile-associated proteins suggest that vitamin D can potentially prevent preterm birth by halting infection-induced contractions in the myometrium.
Toll-like receptor 4 has been reported to play a role in infection-induced preterm delivery.43 In addition, gene polymorphism in TLR-10 was also reported to have an association with preterm birth.34 Therefore, we assessed the effects of vitamin D on TLR-4 and TLR-10 in UtSM cells. Vitamin D decreased TLR-4 in this study, suggesting that vitamin D can inhibit preterm deliveries mediated by TLR-4 during infection.43 On the other hand, vitamin D increased TLR-10 in the presence of LPS in UtSM cells, suggesting an anti-inflammatory role for TLR-10. TLR-10 expression was reported in B cells, plasmacytoid dendritic cells, and monocytes.44,45 The literature suggests that TLR-10 appears to cooperate with TLR-2 in the sensing of microbes and fungi.46 Furthermore, the upregulation of TLR-10 expression reported in human monocytes cultured in hypoxia or in the presence of hydrogen peroxide suggests a protective role for TLR-10 during infection-induced inflammation.45 In this study, we also assessed the expression of TLR-10 in the myometrium obtained from term pregnancies. The TLR-10 expression was higher in myometrial tissue from nonlaboring term women than in myometrial tissue from laboring term women. Results from in vitro and in vivo experiments in this study suggest that (1) TLR-10 may have an anti-inflammatory role during pregnancy and (2) its temporal downregulation might contribute to the shift from a quiescent to a contractile phenotype at the end of the pregnancy. However, TLR-10 remains the only mammalian toll-like receptor to which ligand has not yet been characterized. Therefore, additional studies are needed to understand the role of TLR-10 in the myometrium during human pregnancy and parturition.
Because IL-1 has been reported to cause preterm deliveries in rodents,23 we treated UtSM cells with vitamin D in the presence of IL-1β and assessed its effects on the expression of proinflammatory markers and contractile-associated proteins. Interleukin 1β treatment increased the expression of the inflammatory cytokines MCP-1 and IL-6 and the contractile-associated proteins connexin 43, COX-2, and prostaglandin receptor in UtSM cells. Interleukin 1β has been reported to increase IL-6 expression in uterine smooth muscle cells47 and prostaglandin production in human myometrium through increases in COX-2.19 Furthermore, IL-1β expression has been reported to be high in the myometrium,10 amnion, and choriodecidua12 of women in active labor compared with its expression during term nonlabor, suggesting a role for IL-1β in labor and delivery. In our study of UtSM cells, vitamin D significantly decreased the IL-1β-induced expression of the inflammatory cytokines MCP-1 and IL-6 and the contractile-associated proteins connexin 43, COX-2, and prostaglandin receptor. Vitamin D was also reported to decrease prostaglandin E2 production following IL-1β stimulation of rheumatoid synovial fibroblasts,48 suggesting that vitamin D can potentially prevent preterm deliveries by inhibiting IL-1β-induced labor. In summary, our results clearly demonstrate that vitamin D decreases basal and both LPS- and IL-1β-induced inflammatory and contractile-associated proteins in UtSM cells. These findings suggest a potential role for vitamin D in attenuating the inflammation-induced expression of contractile-associated proteins in UtSM cells. In turn, vitamin D could have a beneficial effect in modulating inflammation-associated changes that lead to myometrial contractions and, therefore, preventing infection-/inflammation-induced preterm labor. Further studies are needed to evaluate in vivo the role of vitamin D in regulating preterm delivery, especially in relation to infection-induced inflammation during pregnancy. The changes in TLR-10 expression observed in UtSM cells treated with vitamin D and its differential expression in nonlaboring and laboring myometrial tissues warrant additional studies to understand the role of TLR-10 during pregnancy and parturition.
Acknowledgments
We would like to thank Dr Veera Rajaratnam for her critical reading, editing of the manuscript, and helping with the figures.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: in part by research grants from Regional Centers for Minority Institution 5G12 RR03032-27 and ARRAp20 to C.T. and RO1 HD046228 to A.A.
References
- 1.Goldenberg Robert L, Hauth John C, Andrews William W. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507. [DOI] [PubMed] [Google Scholar]
- 2.Trachtenbarg DE, Golemon TB. Care of the premature infant: Part I. Monitoring growth and development. Am Fam Physician. 1998;57(9):2123–2130. [PubMed] [Google Scholar]
- 3.Lamont RF, Sawant SR. Infection in the prediction and antibiotics in the prevention of spontaneous preterm labour and preterm birth. Minerva Ginecol. 2005;57(4):423–433. [PubMed] [Google Scholar]
- 4.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176(2):208–213. [DOI] [PubMed] [Google Scholar]
- 5.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4(2):80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nesby-O'Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. 2002;76(1):187–192. [DOI] [PubMed] [Google Scholar]
- 8.Albert MA, Glynn RJ, Buring J, Ridker PM. C-reactive protein levels among women of various ethnic groups living in the United States (from the Women's Health Study). Am J Cardiol. 2004;93(10):1238–1242. [DOI] [PubMed] [Google Scholar]
- 9.Sehringer B, Schafer WR, Wetzka B, et al. Formation of proinflammatory cytokines in human term myometrium is stimulated by lipopolysaccharide but not by corticotropin-releasing hormone. J Clin Endocrinol Metab. 2000;85(12):4859–4865. [DOI] [PubMed] [Google Scholar]
- 10.Breuiller-Fouche M, Germain G. Gene and protein expression in the myometrium in pregnancy and labor. Reproduction. 2006;31(5):837–850. [DOI] [PubMed] [Google Scholar]
- 11.Shynlova O, Tsui P, Dorogin A, Lye SJ. Monocyte chemoattractant protein-1 (CCL2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol. 2008;181(2):1470–1479. [DOI] [PubMed] [Google Scholar]
- 12.Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Mol Hum Reprod. 2002;8(4):399–408. [DOI] [PubMed] [Google Scholar]
- 13.Helmer H, Tretzmuller U, Bruanbauer M, Kaider A, Husslein P, Knofler M. Production of oxytocin receptor and cytokines in primary uterine smooth muscle cells cultivated under inflammatory conditions. J Soc Gynecol Investig. 2002;9(1):15–21. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee S, Chen LY, Papadimos TJ, Huang S, Zuraw BL, Pan ZK. Lipopolysaccharide-driven Th2 cytokine production in macrophages is regulated by both MyD88 and TRAM. J Biol Chem. 2009;284(43):29391–29398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen WR, Keelan JA, Skinner SJ, Mitchell MD. Key enzymes of prostaglandin biosynthesis and metabolism. Coordinate regulation of expression by cytokines in gestational tissues: a review. Prostaglandins Other Lipid Mediat. 1999;57(4):243–257. [DOI] [PubMed] [Google Scholar]
- 16.Olson DM. The role of prostaglandins in the initiation of parturition. Best Pract Res Clin Obstet Gynaecol. 2003;17(5):717–730. [DOI] [PubMed] [Google Scholar]
- 17.Skarzynski DJ, Miyamoto Y, Okuda K. Production of prostaglandin f(2alpha) by cultured bovine endometrial cells in response to tumor necrosis factor a: cell type specificity and intracellular mechanisms. Biol. Reprod. 2000;62(5):1116–1120. [DOI] [PubMed] [Google Scholar]
- 18.Pollard JK, Mitchell MD. Intrauterine infection and the effects of inflammatory mediators on prostaglandin production by myometrial cells from pregnant women. Am J Obstet Gynecol. 1996;174(2):682–686. [DOI] [PubMed] [Google Scholar]
- 19.Rauk PN, Chiao JP. Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am J Reprod Immunol. 2000;43(3):152–159. [DOI] [PubMed] [Google Scholar]
- 20.Tattersall M, Engineer N, Khanjani S, et al. Pro-labour myometrial gene expression: are preterm labour and term labour the same? Reproduction. 2008;135(4):569–579. [DOI] [PubMed] [Google Scholar]
- 21.Amrani Y, Syed F, Huang C, et al. Expression and activation of the oxytocin receptor in airway smooth muscle cells: regulation by TNFalpha and IL-13. Respir Res. 2010;11:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buhimschi IA, Buhimschi CS, Weiner CP. Protective effect of N-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. Am J Obstet Gynecol. 2003;188(1):203–208. [DOI] [PubMed] [Google Scholar]
- 23.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165(4 pt 1):969–971. [DOI] [PubMed] [Google Scholar]
- 24.Vienonen A, Miettinen S, Bläuer M, et al. Expression of nuclear receptors and cofactors in human endometrium and myometrium. J Soc Gynecol Investig. 2004;11(2):104–112. [DOI] [PubMed] [Google Scholar]
- 25.Thota C, Menon R, Fortunato SJ, Al-Hendy A. Vitamin D3 deficiency is a novel and important risk factor for preterm labor in African American and Caucasian women. 57th Annual Meeting of Society of Gynecologic Investigation; March 24-27, 2010; SAGE Publication: Orlando, Florida. [Google Scholar]
- 26.Arora CP, Huynh P, Bergman A, Sarafin D, Hobel CJ. Vitamin D and IL-6 levels in pregnancies with subsequent spontaneous preterm birth. 57th Annual meeting of Society of Gynecologic Investigation; March 24-27, 2010; SAGE Publication: Orlando, Florida. [Google Scholar]
- 27.Bodnar LM, Simhan HN. Early pregnancy maternal vitamin D status and preterm birth in black and whilte women. 57th Annual Meeting of Society of Gynecologic Investigation. March 24-27, 2010; SAGE Publication: Orlando, Florida. [Google Scholar]
- 28.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11(5):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girotti M, Zingg HH. Gene expression profiling of rat uterus at different stages of parturition. Endocrinology. 2003;144(6):2254–2265. [DOI] [PubMed] [Google Scholar]
- 30.Charpigny G, Leroy MJ, Breuiller-Fouché M, et al. A functional genomic study to identify differential gene expression in the preterm and term human myometrium. Biol. Reprod. 2003;68(6):2289–2296. [DOI] [PubMed] [Google Scholar]
- 31.Hirsch E, Filipovich Y, Mahendroo M. Signaling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am J Obstet Gynecol. 2006;194(5):1334–1340. [DOI] [PubMed] [Google Scholar]
- 32.Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intra amniotic infusions of interleukin-1β and tumor necrosis alpha but not by of interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195(6):1578–1589. [DOI] [PubMed] [Google Scholar]
- 33.Evans KN, Nguyen L, Chan J, et al. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol. Reprod. 2006;75(6):816–822. [DOI] [PubMed] [Google Scholar]
- 34.Heinzmann A, Mailaparambil B, Mingirulli N, Krueger M. Association of interleukin-13/-4 and toll-like receptor 10 with preterm births. Neonatology. 2009;96(3):175–181. [DOI] [PubMed] [Google Scholar]
- 35.Müller U, Stenzel W, Köhler G, et al. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with cryptococcus neoformans. J Immunol. 2007;179(8):5367–5377. [DOI] [PubMed] [Google Scholar]
- 36.Amrani Y, Syed F, Huang C, et al. Expression and activation of the oxytocin receptor in airway smooth muscle cells: regulation by TNFα and IL-13. Respir Res. 2010;11(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J. Reprod Immunol. 2008;79:50–57. [DOI] [PubMed] [Google Scholar]
- 38.Phillips RJ, Al-Zamil H, Hunt LP, Fortier MA, López Bernal A. Genes for prostaglandin synthesis, transport and inactivation are differentially expressed in human uterine tissues, and the prostaglandin F synthase AKR1B1 is induced in myometrial cells by inflammatory cytokines. Mol Hum Reprod. 2011;17:1–13. [DOI] [PubMed] [Google Scholar]
- 39.Duggan SV, Lindstrom T, Iglesias T, Bennett PR, Mann GE, Bartlett SR. Role of atypical protein kinase C isozymes and NF-kappaB in IL-1beta-induced expression of cyclooxygenase-2 in human myometrial smooth muscle cells. J Cell Physiol. 2007;210(3):637–643. [DOI] [PubMed] [Google Scholar]
- 40.Liu W, Kato M, Itoigawa M, et al. Distinct involvement of NF-kappaB and p38 mitogen-activated protein kinase pathways in serum deprivation-mediated stimulation of inducible nitric oxide synthase and Its inhibition by 4-hydroxynonenal. J Cell Biochem. 2001;83(2):271–280. [DOI] [PubMed] [Google Scholar]
- 41.Cohen-Lahav M, Shany S, Tobvin D, Chaimovitz C, Douvdevani A. Vitamin D decreases NFκB activity by increasing IκBα levels. Nephrol Dial Transplant. 2006;21(4):889–897. [DOI] [PubMed] [Google Scholar]
- 42.Liu NQ, Kaplan AT, Lagishetty V, et al. Vitamin D and the regulation of placental inflammation. J Immunol. 2011;186(10):5968–5974. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Kang J, Lei W. Role of Toll-like receptor 4 in inflammation-induced preterm delivery. Mol Hum Reprod. 2010;16(4):267–272. [DOI] [PubMed] [Google Scholar]
- 44.Hasan U, Chaffois C, Gaillard C, et al. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J Immunol. 2005;174(5):2942–2950. [DOI] [PubMed] [Google Scholar]
- 45.Kim D, Kim YJ, Koh HS, Jang TY, Park HE, Kim JY. Reactive oxygen species enhance TLR10 expression in the human monocytic cell line THP-1. Int J Mol Sci. 2010;11:3769–3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guan Y, Ranoa DR, Jiang S, et al. Human TLRs 10 and 1 share common mechanisms of innate immune sensing but not signaling. J Immunol. 2010;184(9):5094–5103. [DOI] [PubMed] [Google Scholar]
- 47.Chevillard G, Derjuga A, Devost D, Zingg HH, Blank V. Identification of interleukin-1β regulated genes in uterine smooth muscle cells. Reproduction. 2007;134:811–822. [DOI] [PubMed] [Google Scholar]
- 48.Tetlow LC, Woolley DE. The effects of 1 alpha,25-dihydroxyvitamin D(3) on matrix metalloproteinase and prostaglandin E(2) production by cells of the rheumatoid lesion. Arthritis Res. 1999;1(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]