Abstract
Since the first cell therapeutic study to repair articular cartilage defects in the knee in 1994, several clinical studies have been reported. An overview of the results of clinical studies did not conclusively show improvement over conventional methods, mainly because few studies reach level I of evidence for effects on middle or long term. However, these explorative trials have provided valuable information about study design, mechanisms of repair and clinical outcome and have revealed that much is still unknown and further improvements are required. Furthermore, cellular and molecular studies using new technologies such as cell tracking, gene arrays and proteomics have provided more insight in the cell biology and mechanisms of joint surface regeneration. Besides articular cartilage, cartilage of other anatomical locations as well as progenitor cells are now considered as alternative cell sources. Growth Factor research has revealed some information on optimal conditions to support cartilage repair. Thus, there is hope for improvement. In order to obtain more robust and reproducible results, more detailed information is needed on many aspects including the fate of the cells, choice of cell type and culture parameters. As for the clinical aspects, it becomes clear that careful selection of patient groups is an important input parameter that should be optimized for each application. In addition, the study outcome parameters should be improved. Although reduced pain and improved function are, from the patient's perspective, the most important outcomes, there is a need for more structure/tissue-related outcome measures. Ideally, criteria and/or markers to identify patients at risk and responders to treatment are the ultimate goal for these more sophisticated regenerative approaches in joint surface repair in particular, and regenerative medicine in general.
Keywords: cartilage repair, autologous chondrocyte implantation, clinical study, growth factors, cell types, inflammation
Introduction: the impaired repair capacity of cartilage and the beginning of cell therapy
‘…from Hippocrates down to the present age, we shall find, that an ulcerating cartilage is found to be a very troublesome disease……and that, when destroyed, it is never recovered.’ This statement by Hunter in 1743 is probably the most quoted sentence in the field of cartilage repair. But is it still true? Did developments in Tissue Engineering and Regenerative Medicine not change this paradigm?
Partial-thickness cartilage defects, even very small ones, do not heal spontaneously whereas full-thickness osteochondral lesions below a critical size do, albeit with fibrous cartilage tissue of inferior functional quality [1–3]. This very limited healing capacity of cartilage is partially due to its avascularity. Therefore, the classical wound healing response, where a set of complex biochemical events takes place to repair the damage, does not occur in cartilage. When cartilage is wounded, the tissue at the wound edge is damaged and cells die, resulting in debris similar to wounds in other tissues. However, this damaged and avital cartilage tissue will not be removed. Wound healing is prevented by avascularity in combination with an impaired migration capacity of cartilage cells through the dense extracellular matrix [3–5]. In full-thickness ICRS (International Cartilage Repair Society) grade IV (superficial osteochondral) defects, bleeding from the underlying bone marrow will, to some extent, trigger a natural wound healing reaction. Non-vital tissue will be partially resorbed and cells are attracted to the wound area, with proliferation, differentiation and new matrix production [3]. However, the resulting fibrocartilaginous repair tissue is of inferior quality, rather classified as scar tissue, a fact which is associated with an increased risk for gradual development of secondary osteoarthritis. Besides surgically punching or drilling holes through the subchondral plate, called marrow stimulation techniques, there was no other regenerative treatment option for symptomatic joint surface defects available until 1994 when the first cell therapeutic approach for cartilage repair, autologous chondrocyte implantation (ACI), found its way into the clinic [6].
Autologous chondrocyte implantation
The original ACI procedure involves the isolation of chondrocytes from a cartilage biopsy harvested during an arthroscopic procedure from a less load-bearing area of the knee with the defect. After expansion in monolayer culture, the cells in solution are shipped back to the orthopaedic surgeon. The cell solution is injected during a surgical procedure under a flap of periosteum harvested from the tibia that has been fixed with sutures, fibrin glue (or both) to the edges of the cartilage lesions.
Since the first report by Brittberg et al. on the first 23 patients in 1994 [6], ACI has been performed in more than 30,000 patients throughout the world (personal estimation by MB based on cases reported in literature and information from different companies using ACI). The clinical results have been reported from different centres worldwide. In a prospective clinical evaluation (evidence level II) of 244 patients with a 2–10 year follow-up [7], a high percentage of good to excellent clinical results (84–90%) was reported in patients with different types of single femoral condyle lesions, while other types of lesions had a lower degree of success (mean 74%). The reported histology mostly shows a mixed tissue repair of hyaline-fibrocartilaginous appearance. The total failure rate was 16% (10/61) at 7.4 years mean follow-up. All ACI failures occurred in the first 2 years and patients showing good to excellent improvement at 2 years had a high percentage of good results at long-term follow-up [7].
Reports on results with ACI from other centres [8–10] show similar figures with a high degree of success, however the evidence level of these reports is II or lower. In order to properly position this new treatment in an algorithm, and to establish its relevance for daily clinical practice, ACI needs to be evaluated in direct comparison to other cartilage repair techniques in prospective randomized trials [11]. The findings described above are related to what one defines as the first generation of ACI with cells in suspension covered with a periosteal flap. In a so-called second generation of an ACI procedure, the periosteum has been replaced with a collagen membrane. This approach was mainly developed to improve the surgical and patient friendliness, but it is still unclear how far this is affecting outcome. The third generation of cartilage repair products involves so-called combination products (CP) being either cells grown on a carrier membrane such as matrix-induced autologous chondrocyte implantation (MACI) or cells seeded and grown in a scaffold such as hyaluronic acid (Figure 1) or collagen.
1.
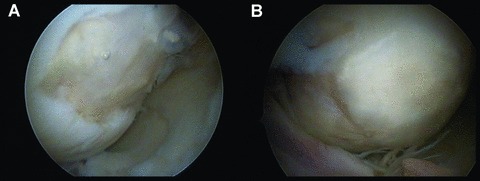
Clinical view on femoral condylar cartilage lesion treated by autologous chondrocyte cultured in a hyaluronic scaffold (hyalograft-C). (A) The scaffold with cells has just been implanted and glued to the defect site transarthroscopically. (B) The same lesion at a second look arthroscopy 1 year post-surgery.
Combination products
Behrens et al.[12] studied 38 patients with localized cartilage defects treated with a cell seeded collagen membrane who where evaluated up to 5 years after the intervention. Eleven (73.3%) of the 15 patients that were followed for at least 5 years after surgery had significant improvement of clinical/functional results.
Zheng and coworkers [13] analysed histological samples from a cohort of 56 MACI patients to examine the phenotype of chon-drocytes seeded on type I/III collagen scaffold over a period of 6 months. Biopsies showed the formation of cartilage-like tissue as early as 21 days, and in 75% of the biopsies taken from a group of 11 patients after 6 months, hyaline-like cartilage regeneration was seen.
Most of the ACI techniques are still performed as an open procedure. The use of a biological implant with cells seeded in a scaffold allows arthroscopic implantation. Marcacci et al.[14, 15] have worked with a biodegradable, hyaluronan-based biocompatible scaffold seeded with autologous chondrocytes. They presented a prospective study with 70 consecutive patients treated by arthroscopic ACI that were followed at least 24 months. Clinical improvement was statistically significant at 24 months and the second-look arthroscopy demonstrated a complete coverage of the grafted area with a hyaline cartilage-like tissue in 12 of the 15 analysed patients (evidence level II). A better clinical outcome was observed in young, well-trained patients and in the treatment of traumatic lesions.
Randomized controlled studies
All the above-mentioned studies are limited in their scope as most of them do not reach the level of evidence needed to position these approaches in a treatment algorithm. Therefore, randomized prospective multi-centre comparative trials are required. To date, seven clinical randomized trials have been published in peer-reviewed papers, representing short-term and, at most, mid-term studies.
Horas et al.[16] performed a prospective clinical study to investigate the 2-year outcomes in 40 patients with an articular cartilage lesion of the femoral condyle who had been randomly allocated to be treated with either ACI or transplantation of an autologous osteochondral cylinder. Both treatments alleviated symptoms. However, the improvement provided by ACI lagged behind that obtained using osteochondral cylinder transplantation.
Bentley et al.[9] studied a total of 100 patients with symptomatic chondral and osteochondral lesions of the knee (mean age 31.3 years; mean defect size 4.66 cm2) randomized to undergo either ACI (58 patients) or mosaicplasty (42 patients) with a mean follow-up of 19 months (12 to 26). Functional assessment using the modified Cincinnati and Stanmore scores and objective clinical assessment showed that 88% had excellent or good results after ACI and 69% after mosaicplasty. Structural assessment by arthroscopy at 1 year demonstrated excellent or good repair in 82% after ACI and in 34% after mosaicplasty. This study has been criticized for a few reasons, including the lesions in this study were larger than the optimum size cartilage defect for mosaic arthroplasty is 1–4 cm2 and the postoperative rehabilitation differed from that recommended for mosaic arthroplasty.
Dozin et al.[17] studied 47 patients that were randomly assigned to ACI or mosaicplasty and subjected to arthroscopic debridement of the lesion at the time of enrolment. Notable, and not unexpected, was the fact that 14 patients (31.8%) experienced substantial improvement following the initial debridement alone and, being clinically asymptomatic, received no further treatment. Among the 23 patients (52.3%) who could effectively be evaluated, a complete recovery was observed based on clinical examination in 88% of the mosaicplasty-treated patients and in 68% of the ACI-treated patients (P= 0.093). Knutsen et al.[18] studied 80 patients who needed local cartilage repair because of symptomatic lesions on the femoral condyles between 2 and 10 cm2 . Patients were randomized into ACI or microfracture treatment. At 2 and 5 years, both groups had significant clinical improvement compared with their preoperative status. At the 5-year follow-up, there were nine failures (23%) in both groups. Younger patients did better in both groups. The authors did not find a correlation between histological quality of the repair tissue and clinical outcome. However, none of the patients with the best-quality cartilage (predominantly hyaline) at the 2-year mark had subsequent failure. Saris et al.[19] conducted a prospective multi-centre trial to compare ACI with microfracture in patients with single ICRS grade III (>50% penetration to the cartilage, may be full thickness lesions, but not into the subchondral bone) to IV (penetrates sub-chondral bone, superficial osteochondral lesions) symptomatic cartilage defects of the femoral condyle. Patients aged 18–50 years were randomized to ACI (n= 57) or microfracture (n= 61). One year after treatment, ACI was associated with a tissue regenerate that was histologically superior to that after microfracture. Short-term clinical outcome was similar for both treatments. Saris et al.[19] used a well-characterized chondrocyte cell population (called ChondroCelect). In this expansion method of chondrocytes, care was taken to prevent as much as possible dedifferentiation, and was verified by the analysis of a gene marker profile that is predictive of the capacity to form hyaline-like cartilage in vivo and considered a potency assay. A specific gene marker cut-off score is used as the criterion for implantation. In the study by Saris et al.[19], 6 of the 57 patients did not receive ACI treatment because the gene expression profile was too low. So far, it is not known if low gene scores are indeed associated with less successful outcomes, and if high gene scores are potentially predictive of better clinical outcomes. Results of this analysis at 24 and 36 months have been partially reported (Saris et al., AAOS February 2009, P422) and will be submitted soon (FP Luyten, personal communication).
Furthermore, there are two randomized studies comparing two types of ACI: ACI with collagen membrane (patch) versus MACI for osteochondral defects of the knee [20] and a prospective, randomized study comparing periosteum versus type I/III collagen membrane covered ACI [21]. There were no differences in the outcome of collagen covered ACI and MACI. A significant number of patients who had the periosteum covered ACI required shaving of a hypertrophied graft. It was concluded that there was no advantage in using periosteum.
Wasiak and Villaneuva published in 2006 [22] a review in the Cochrane database that included four randomized controlled trials (266 participants). They concluded that at that time there was no evidence of a significant difference in the outcomes between ACI and other cartilage repair interventions. They stated that additional good quality randomized controlled trials with long-term functional outcomes were required to provide clear guidelines for practice. The last randomized study from 2008 by Saris et al.[19] was not evaluated in this Cochrane review [22]. Future reports of the long-term results of that study and other ongoing studies will be of great interest.
Vavken et al.[23] studied clinical effectiveness and cost effectiveness of ACI in a review of six randomized controlled studies that assessed the effectiveness of ACI compared with microfrac-ture or mosaicplasty. Four of these studies reported no or only insignificant differences between the procedures whereas two studies observed better results with ACI. The authors stated that the long-term results were good throughout, but the high quality of the regenerative tissue is a clear potential advantage of ACI. Cost-effectiveness models support ACI for the durability of its results and thus potentially lower costs in the long-term.
Taken together, these more recent randomized studies have given valuable information about what can and should be done in clinical cell therapy studies. They showed that the field is seriously exploring therapeutic options of earlier developed procedures. The clinical studies also demonstrate that much is still unknown and further improvement is required. Future cell therapeutic applications should give rise to a durable and well-integrated repair tissue, result in clinical improvement, eliminate or significantly postpone the need for subsequent arthroplasty, and be suited for both focal defects and early degenerative joint disease.
Choice of cell types
The first critical issue in the development and improvement of a cell therapeutic strategy for cartilage repair is selection of the cell type. Articular cartilage was the first source of cells to be used in an FDA-approved method (ACI) to repair focal cartilage defects. There are now several tissue sources and cell types considered, each of which has certain advantages and disadvantages. Among the several factors to be considered are ease of harvest, cell yield, purity, effective proliferative capacity, and the phenotype of the cells and the cartilage produced, also regarded by the regulators as potency assays.
One of the main drawbacks to the use of expanded articular chondrocytes is their dedifferentiation and loss of re-differentiation potential after expansion [24–26], as significant chondrocyte expansion appeared to be needed to make tissue engineering approaches feasible. Studies looking into the underlying molecular mechanism of this loss of chondrogenic potential have shown that stable in vivo chondrogenic potential correlates positively with a number of gene markers including Col2A1 and FGFR3 expression and its loss is marked by up-regulation of ALK-1 expression [11]. Furthermore, Sox5, Sox6 and Sox9 co-expression correlates with a stable chondrogenic phenotype in concert with a lack of osteogenic or hypertrophic chondrocyte markers [27].
In addition to articular cartilage, chondrocytes isolated from other cartilaginous structures (i.e. auricle, nasal septum, rib) have also been considered for cartilage repair procedures [28–30]. Chondrocytes from these different anatomic locations have distinctive growth and differentiation characteristics. Several studies have shown differences in growth and re-differentiation of chondrocytes of samples from different anatomical regions of rabbit [31, 32], bovine [33] and porcine origin [34, 35]. Similar demonstrations of site-specific differences were also noted in a study of human auricular, nasal and costal chondrocytes where auricular and nasal chondrocytes showed greater proliferative and re-differentiation capacity than costal chondrocytes [36], and in other studies which disclosed significant differences in cartilage-forming capacity between human articular- and auricular-derived chondrocytes [37, 38]. The overriding question for all of these studies using chondrocytes from different tissues and locations is whether or not the matrix elaborated by these chondrocytes will have the biomechanical properties necessary to withstand the compression and shear forces that will be experienced in the joint. However, this is also the question when articular chondrocytes are used for implantation. At the moment, we do not know the exact biomechanical properties needed and what the body can provide or compensate for. We also know little about tissue formation, organization (a tissue is more than an assembly of cells in a matrix) and integration into the environment after transplantation. These subtle aspects of tissue engineering biology are likely to be critical for long-term clinical outcome. Some hypothesize that to create a typical zonal structure, the cells from different cartilage zones should be seeded in a construct separately [39, 40]. Regardless of the correctness of this approach, it seems difficult to translate this into a robust manufacturing process, unless progenitor cells are used and principles of developmental tissue formation can be mimicked in vitro (Lenas P, Moos M Jr, and Luyten FP, manuscript in revision).
Cartilaginous structures, with the exception of articular cartilage, are covered by a perichondrium layer that contains cartilage progenitor cells. Perichondrial cells have been investigated for their utility as cell sources for tissue engineering as early as 1972, when whole perichondrial grafts were used to generate repair cartilage [41]. The clinical results of perichondrium transplantations were initially reported to be good [42], however no differences were observed between debridement and drilling and perichondrium transplantation in the treatment of isolated cartilage defects after 10 years of follow-up, although slightly more problems were reported in the perichondrium transplantation group [43]. More recent studies report cartilage formation by cells isolated from perichondrium [44] and evidence for a subset of cells in the perichondrium with stem cell-like characteristics. This was based on long-term retention of BrdU label, extensive expansion in culture, and ability upon induction to develop to cells expressing either chondrogenic or adipogenic phenotypes in vitro[45]. It is an intriguing observation that perichondrium may contain a stem cell population that may be able to be expanded extensively in culture under the proper conditions.
Bone marrow is the best-known source of human adult mesenchymal progenitor cells (BMSC) that have been shown to have chondrogenic potential [46]. BMSCs have been tested for repair of articular cartilage in a rabbit model as early as 1994 [47], wherein it was shown that defects implanted with a type I collagen/MSC mixture showed slightly improved histologic scores for cartilage healing compared to a type I collagen/periosteal stem cell mixture, although variability in scores precluded reaching statistical significance. Since then, many experimental studies in rabbits have been performed where BMSCs have been combined with different biomaterials and or growth factors [48–52] often with promising results. Some clinical studies using BMSCs to repair cartilage lesions have been reported. Most of these are case studies using bone marrow cells in an ACI-like procedure where cells are seeded in collagen and implanted in a cartilage defect covered with periosteum [42, 53, 54] or where the cells are percutaneously injected in the knee joint [55]. At this point, it is not known whether these procedures are able to reduce clinical symptoms, repair cartilage defects or slow down the progression of osteoarthritis.
One potential concern about the use of BMSCs is the question as to whether BMSCs produce stable hyaline cartilage. Several in vitro studies have shown that BMSCs produce type X collagen, a marker for hypertrophic cartilage [46, 56, 57] and chondrocytes from BMSCs implanted into SCID mice recapitulate endochondral bone formation, resulting in the loss of cartilage that is replaced by bone [58]. It may be that BMSCs are not destined to a hyper-trophic chondrocyte fate based on a recent study by Liu et al.[59] who showed that a stable hyaline cartilage is formed when BMSCs are first allowed to mature in vitro for and extended time (12 weeks) prior to implantation. If true, this study makes bone marrow derived MSCs a viable source of cells for cartilage repair, with the caveat that the BMSCs will need to be pre-differentiated prior to use to differentiate into permanent cartilage after implantation. The molecular basis of stable hyaline cartilage is not yet completely understood. Therefore, it is unlikely that in the immediate future cartilage repair using BMSCs will be sufficiently robust to allow large-scale clinical use with predictable outcomes.
Next to production of matrix, BMSCs have been extensively documented to secrete trophic factors and in this way enhance wound healing or to act as immunomodulators [60–62]. By secretion of these factors, they might influence the catabolic conditions in a joint with cartilage lesions and prevent progression of degeneration. This hypothesis is under investigation.
Another promising source of multi-potent progenitor cells is adipose tissue. The advantages of using adipose tissue as a source are the ease of collection and the large yield of cells that can be obtained [63]. Zuk et al.[63, 64] demonstrated that adipose-derived stromal cells (ADSCs) are multi-potent for several mesenchymal phenotypes, including chondrocytes, and thus are a potential source of cells for tissue engineering. The expression of hypertrophic chondrocyte markers, such as Runx2, Osterix and type X collagen, was observed in ADSCs [64] as was noted for BMSCs [57, 64]. In addition, ADSCs were reported to have a reduced potential for chondrogenic differentiation that may be a result of a lack of TGFβ1-receptor expression and reduced expression of mRNAs for bone morphogenetic proteins (BMPs) [65]. This study also suggested that MSC of different anatomical origins may require different stimulant cocktails for their chondrogenic differentiation. ADSCs had a lot of attention in experimental studies the past years, however no studies in human beings have been reported yet.
Cells from the synovium have chondrogenic potential [66]. The study by Sakaguchi et al.[67] showed that human synovial-derived cells had greater chondrogenic potential than BMSCs, ADSCs, periosteal- or muscle-derived cells from the same patients, and a follow-up study showed that synovial-derived cells produced consistently larger cartilage aggregates than BMSCs from the same patients [68]. The synovial fluid of arthritic knees of patients has been reported to contain MSCs [69]. However knowledge about these synovial fluid MSCs, their possible role in the aetiology of arthropathies and their potency for therapy is still limited. Additional support for the chondrogenic capacity of synovium-derived cell populations are developmental studies indicating that the precursor cells of the articular cartilage, marked by Gdf5, also end up in the postnatal synovium. Thus, the synovium appears to be a potential reservoir of cell populations develop-mentally destined to make articular cartilage and meniscal tissue [70]. This may be critical in the tissue organization and tissue integration aspects, important for long-term clinical durability.
Furthermore, multi-potent cells have been isolated from a variety of different sources like human amniotic fluid [71], umbilical cord [72] and tendon [73]. The potency of these cells for cartilage repair has not been demonstrated and no studies using these cells for human cartilage repair have been published until now.
Finally, pluripotent cells are also considered as a cell source for cartilage repair. Embryonic stem cells (ESCs) have potential as a source of cartilage due to their ability to be expanded almost indefinitely. Next to ethical concerns, the critical issues to overcome with any ESC application are to direct the cells along the differentiation pathway of interest to the exclusion of other pathways. Directing ESCs down the chondrogenic pathway in significant numbers has been a major challenge, but co-culture of human ESCs with adult-derived chondrocytes [74], hypoxic culture conditions and BMP-2 and BMP-4 have been shown to be chondro-inductive [75]. A hypertrophic phenotype and calcification of the matrix have been described when ESCs are induced into the chondrogenic lineage [76, 77], indicating that ESCs may also be preprogrammed towards the hypertrophic phenotype. Genetic engineering is one option that could be used to direct ESCs towards hyaline cartilage, such as the transduction of ESCs with SOX5, SOX6 and SOX9 which induced stable production of hyaline cartilage in mouse ESCs [27]. It should be noted that this same combination of transcription factors was able to induce hyaline cartilage formation in human MSCs and dermal fibroblasts, which negates most advantages gained from using ESCs as a cell source. Perhaps the most promising result for ESCs to date is a study where 85% of the hESCs incubated with TGFβ1 and β1-integrin activating antibodies and subsequently co-cultured in transwells with bovine chondrocytes, differentiated towards chondrocytes, without signs of teratoma formation in nude mouse implants and no expression of type X collagen by RT-PCR [78].
Thus, although ESCs have chondrogenic potential and advances are being made in producing larger numbers of chondrogenic cells, the technology has not reached a level that is practical for tissue engineering purposes and ethical considerations still remain. Technological developments in stem cell biology may provide alternatives to ESCs. Successful cloning and reprogramming of adult animal cell nuclei by somatic cell nuclear transplantation (SCNT) or nuclear transfer (NT) provides stem cells tailored to the donor organism. In 2006, adult somatic cells were reprogrammed to regain pluripotency by transduction of four transcription factor to produce the so-called induced pluripotent stem cells (iPS) [79]. This breakthrough technique was further improved [80–82] and by the end of 2007 Yamanaka and his team managed to make the first human iPS [83]. Even though this is a great achievement, the random insertion of the dedifferentiation-initiating genes into the host cell's DNA remains a problem, and the method itself increases the risk of initiating cancer. Before these cells can be used as patient-specific cell therapy it will be crucial to characterize the cells exhaustively with respect to purity, potency and toxicity. Once implanted, cells cannot be removed anymore, and some may reside in the host lifelong.
Regulating cellular activities
Immediately after the choice of cell source comes the question of cell regulation. How should we stimulate cells in vitro or in vivo or can we trust that the cells know what to do themselves such that they will be directed by the local environment?
There are several options to investigate and optimize these critical issues. First, the selection of specific cell populations characterized by preferably in vivo function relevant to the clinical application deserves more attention. For instance, cells that are able to produce stable hyaline-like cartilage can be selected from a pool of isolated cells by different approaches such as adapting cell culture conditions or using membrane markers. Dell’Accio et al.[24] published that the capacity to form stable cartilage by articular chondrocytes was lost upon passaging and that this can be monitored using molecular markers such as FGF receptor 3. This approach to select a subset of cells from a more heterogeneous population to optimize the outcome deserves attention for other cell sources as well.
Furthermore, cells (either progenitor cells, or dedifferentiated chondrocytes) can be stimulated to differentiate towards the chondrogenic lineage and/or to produce cartilaginous matrix by addition of growth and differentiation factors or by application on or in biomaterial scaffolds. This approach can be taken in vitro ex vivo during expansion procedures or the manufacturing of the implant or directly in vivo. In the latter case, the combination implant is more complex in many aspects as it contains both cells and growth factors.
Influence of growth factors on chondrogenic differentiation
Growth factors can be used to induce and enhance chondrogenic differentiation. Members of the TGF-β superfamily have been demonstrated to be important in chondrogenic differentiation of expanded chondrocytes [84]. To avoid cartilage donor site morbidity, replicative chondrocyte dedifferentiation and aging (senescence) during in vitro expansion, attempts were made to stimulate mesenchymal stem cells (MSCs) to differentiate to cartilage during 3–4 weeks in the presence of cell culture medium containing transforming growth factor-β (TGF-β) [85–88]. TGF-β was also used to induce chondrogenic differentiation of progenitor cells derived from sources such as adipose tissue, synovial tissue and periosteum. BMPs, other members of the TGF-β superfamily are also known to maintain the chondrogenic phenotype and induce chondrogenic differentiation of progenitor cells [88–90] and synergistic effects of BMPs and TGF-β on chondrogenic differentiation were also reported [88]. TGF-β has also been used together with FGF-2 [91], growth and differentiation factor-5 [92], IGF-I [93], or as part of platelet-rich plasma (PRP) [94], although IGF-I [95] and BMP-2 [96] and -7 [97] may also work alone.
Addition of growth factors during the expansion phase has been shown to be able to significantly improve the chondrogenic potential. Expansion of articular chondrocytes in the presence of various growth factors, such as a combination of TGF-β1 [90, 98], TGF-β, FGF-2 and PDGF (BB) [99], TGF-β1 and FGF-2 [100], or 100 ng/ml of FGF-2 in defined medium [101] supported the preservation and re-expression of the cartilage phenotype better than culture conditions without these growth factors. A similar effect of FGF-2 on preservation of chondrogenic potential after culture expansion was demonstrated in BMSCs [102], while a similar combination of TGF-β1, FGF-2 and PDGF (BB) that was effective for increased expansion of human articular chondrocytes (above) was also useful for human auricular and nasal chondrocytes, but not for costal chondrocytes [36].
Influence of growth factors on matrix deposition
Many studies have attempted to improve the quality of the extracellular matrix produced by chondrocytes using growth factors or other soluble compounds. Growth factors typically used in cartilage tissue engineering and matrix repair include IGF-I, FGF-2, TGF-β, BMP-2, BMP-4, BMP-5 and BMP-7, the latter also known as OP1. Other less commonly used growth factors are FGF-9 and FGF-18.
IGF-I (or somatomedin-C) was described to have beneficial effects on cartilage matrix generation, it stimulated chondrocytes to deposit more collagen and proteoglycans [103, 104] and it is known to inhibit matrix degrading enzymes [105, 106]. IGF-I was even more potent in inducing matrix production when combined with BMP-7/OP-1 [107]. The use of IGF-I has been studied in in vivo models for cartilage repair, where it improved cartilage morphology, including increased collagen type II staining [95, 108].
FGF-2 inhibited the stimulatory effects of IGF-I [109] and stimulated production of matrix degrading enzymes [104, 109]. In in vivo models, FGF-2 appeared to have beneficial effects on cartilage repair [110, 111]. Even though cartilage morphology improved when FGF-2 was administered to cartilage lesions in vivo, analysis of matrix produced in vitro showed the opposite effect. This might be explained by the positive effect FGF-2 has on the phenotype of chondrocytes as mentioned earlier [112, 113] or by inhibition of aggrecanase activity as result of IL-1 [114]. Less is known about FGF-18 but evidence exists that it had beneficial effects on matrix production and cartilage repair, both in vitro and in vivo[115, 116].
TGF-β is pleiotrophic and its effect strongly depends on the micro-environment. Addition of TGF-β to chondrocytes in alginate did not affect matrix deposition but lowered the number of crosslinks per collagen molecule [104]. When added to chondrocytes in monolayer, TGFβ stimulated the production of collagen that was heavily cross-linked [117]. In addition, it was shown to lower the production of cytokine-induced matrix degrading enzymes [48, 105, 118]. In general, intra-articular injection of TGF-β led to an increase in proteoglycan content in articular cartilage although contrasting results also have been reported (see [119] for review). This raises the important issue discussed below that the effects of the growth factors could differ depending on the context and microenvironment, e.g. different repair results could ensue in traumatic and degenerative cartilage lesions.
BMP-2, -4, -5 and -7 influence matrix deposition by inducing collagen and proteoglycan production in cartilage explants [90] and in chondrocytes cultured in alginate [120–124]. Overexpression of BMP-2 in vivo induces matrix turnover and thereby contributes to the intrinsic repair capacity of damaged cartilage [125]. In addition to its strong pro-anabolic activity, BMP-7 has very prominent anti-catabolic properties [123]. Animal studies have demonstrated that this factor has the ability to repair cartilage in vivo in various models of articular cartilage degradation (see [107] for review). When BMP-7 and BMP-2 are combined in a chondrocyte alginate culture, they work synergistically [122].
Next to the effect of one or two defined growth factors, a pool of various growth factors in PRP demonstrated the potency to increase collagen production in chondrocyte cultures [126]. The possibility to use autologous blood to make PRP is one of the prominent advantages of this approach and it is currently investigated in clinics for a large range of disorders to stimulate tissue repair.
Supportive effect of biomaterials
There is a tendency to move from ACI technologies to the use of CP using cell-seeded scaffolds, as they appear to be more ‘user-friendly’. However, the characterization and manufacturing of a 3D implant is more complex. Also, it is very likely that the mechanisms and timed events of tissue repair are quite different when compared to the use of cell suspensions.
There is a large variety of both natural and synthetic scaffolds available. The variety of polymers and fabrication techniques available continue to expand. Pore size, porosity, interconnectivity, bio-compatibility, shape specificity and degradation characteristics all influence the quality and/or amount of cartilage formed. Although many different scaffolds have been used for cartilage tissue engineering, not much is known about how matrices influence the quality of the matrix generated. Large differences in chondrogenic effects between matrices were described [127–131].
Hunziker and colleagues [132] reported that during normal development articular cartilage is physiologically reorganized by a process of tissue resorption and neoformation, rather than by internal remodelling [132], suggesting that formation of cartilage matrix in vitro before implantation might not be the best approach for long-term durability. Testing of this hypothesis in vivo is important because it has important consequences for the direction of future applications. Therefore, it might be better to implant ex vivo expanded cells (either with or without scaffold) and to deliver a soluble factor or a mixture of factors, intra-articularly thereby stimulating the cells to create a piece of hopefully anisotropic cartilage on the site where it is needed. The factors already identified in the above-mentioned in vitro research might prove very useful here. To locally deliver the growth factors, controlled release systems may be necessary. Carriers that release factors range from nanoparticles to complex three-dimensional scaffolds, membranes for tissue-guided regeneration, biomimetic surfaces and smart thermosensitive hydrogels. This is an important and growing area of research in regenerative medicine.
Examples of such a controlled release systems are emulsion-coated scaffolds that release TGF-β1. By varying the copolymer composition used as coating, the release rate of TGF-β could be precisely tailored from 12 to more than 50 days [133]. A poly lactic-co-glycolic acid (PGLA) microsphere system also seemed to be a functional device for the delivery of growth factors such as BMP-7 during the cultivation of articular chondrocytes [134]. Also systems have been designed for the controlled release of two factors [135, 136].
We have focused here on regulation of chondrogenic differentiation and matrix production. However, newer visions on cell therapy aim at cells secreting modulating factors to stimulate the intrinsic repair capacity of the cartilage or to reduce the local inflammatory reactions to enable tissue repair indirectly [56, 60]. Results in cardiovascular research showing paracrine effects of factors secreted by MSCs are promising [137].
Interference of inflammation with cartilage repair
In healthy joints, homeostasis exists. When this stable equilibrium of cartilage, bone, ligaments and synovium in a well-functioning biomechanical system is disturbed, a myriad of inflammatory factors and cellular responses come into play. The harmful effects on cartilage repair of biomechanical factors, such as malalignment lack of menisci and ligamentous insufficiency, have been recognized and correction of these conditions at the time of cartilage repair procedure is recommended. Much less attention has been paid to inflammation and its eventual harmful effects on the repair process. Inflammation in the medical jargon is often considered as the source of evil, leading to classical symptoms and signs of inflammation, rubor, tumour, dolour, calor and functio laesa. Inflammatory pain and autoimmune inflammation in the medical setting often require treatment with non-steroidal, steroidal and biological anti-inflammatory drugs. In contrast to this pragmatic medical view, inflammation can be more broadly considered as an essential phase in tissue repair and regeneration and as an integral part of almost any host response to any noxious physical or chemical stimulus. The avascularity of the cartilage and, therefore, its inability to mount an effective inflammatory response, is considered to be the main reason for the poor repair capacity of the cartilage. This is in some cartilage repair methods overcome by drilling, abrasion or lately in particular microfracturing of the sub-chondral plate. These techniques result in a fibrin-rich clot of platelets (which from their alpha granules release repair factors like vascular endothelial growth factor VEGF, TGF-β1, PDGF) and recruitment of autologous BMSC or progenitors to fill smaller < 2.5–3 cm2 cartilage defect. In a way, marrow stimulation is based on the provocation of inflammation, which leads to mobilization of endogenous bone marrow derived MSCs.
Inflammation is mediated mainly via pro-inflammatory cytokines, which may interfere with the chondrogenic and anabolic effects of growth factors used to stimulate chondrocytes and/or MSCs. Pro-inflammatory cytokines are considered to exert catabolic effects and to stimulate proteinases, which degrade extracellular cartilage matrix. Inflammation-induced functio laesa impairs cartilage tissue repair, maturation and vertical and lateral integration of the transplanted cells. Correspondingly, the most common complications of the procedure are symptomatic hypertrophy of the regenerated cartilage, disturbed fusion of the graft at the edges, partial graft failure as a result of too little cartilage production and delamination of already formed repair tissue. Several biomarkers have been described, which can be used to monitor inflammation and its consequences.
So far, ACI (ACI with a periosteal patch, ACI-P, or with a collagen cover, ACI-C) is perhaps optimally performed to repair critically located, relatively large >2 cm2 (1–15 cm2), focal cartilage defects caused by trauma in otherwise healthy cartilage in young athletes. Treatment of such defects might have prognostic value in the prevention of secondary osteoarthritis later in life. In young patients, both the systemic and local milieus are relatively healthy although the trauma and defect as well as implantation and implanted cells by themselves cause some inflammation that will affect repair [138]. Indeed, many of the clinical studies mentioned above reported that results were better in younger than older patients. High age and diabetes may compromise results of chondrocyte implantation and chondrogenesis also via increased production and accumulation of advanced glycation end-products (AGE). In immature cartilage under 20 years of age, the concentration of AGEs is low, but CML, CEL and pentosidine (different AGEs) increase 27-fold, 6-fold and 33-fold upon maturation, respectively [139]. This suggests that cartilage remodelling, via production of extracellular matrix, is much faster before the age of 20 years than later in life, when AGEs rapidly accumulate as a function of time. Acting through their receptor (RAGE), AGEs have immune-inflammation modulating properties but also impair expansion and trans-differentiation of MSCs to chondrocytes [140]. In the clinical setting, however, the effect of age per se on the outcome of various ACIs is not clear yet [141, 142].
In spite of the fact that pro-inflammatory cytokines are associated with enhanced remodelling, pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α) or interleukin-1β (IL-1β) have rarely been added to the chondrogenic inductive cell culture media, mainly because it is still a challenge to produce high-quality repair cartilage. Poor production of extracellular matrix and tissue destruction (catabolism) are locally present in patients even 1 year after ACI [143]. This local inflammation can be aggravated by the foreign body reaction caused by bioresorbable tissue engineering scaffolds [144] or by the iatrogenic inflammation eventually evoked by the vectors used for gene transfer to promote chondrogenesis. Pro-inflammatory cytokines are expected to be harmful for cartilage repair, therefore, in the clinical setting patients with cartilage damage due to arthritis are not considered to be eligible for (M)ACI although the progressive nature of the underlying disease is usually mentioned as the reason for a rather poor result in specific patients. To prove this paradigm, it would be necessary to show that, e.g. TNF antibodies or soluble TNF-receptors and IL-1 receptor antagonist exert synergistic effects together with the chondrogenic stimuli mentioned above. Attempts in this direction using in vitro co-culture systems suggest that the harmful effect paradigm holds [108, 145].
Pro-inflammatory cytokines and pro-apoptotic factors may be induced by local inflammation and also by the processing and storage of the cells and tissues prior to implantation [146]. It has been demonstrated that some chondrocytes used in chondrocyte transplantation undergo apoptosis [147], which may be due to a unique process known as chondroptosis. Therefore, the modulation of factors that interfere with this process may be of relevance to cartilage repair. Greater resistance to nitric oxide induced apoptosis in vitro was seen after transfection of an anti-apoptotic Bcl-2 gene [148] and after chondrocyte transplantation in vivo after transduction of an anti-apoptotic FNK factor [149]. Chondrocytes and MSCs require matrix interaction to avoid anoikis, which is the form of apoptosis developing when mesenchymal cells lose their contact with extracellular matrix. Improved attachment to extracellular matrix reduces chondrocyte apoptosis [150]. Apoptosis of MSCs undergoing chondrogenesis in 3D alginate scaffolds was diminished with low-intensity ultrasound, which seemed to up-regulate several anti-apoptotic genes [151]. Second and third generation ACI might help to avoid this form of chondrocyte apoptosis as the cells are seeded in or on a matrix.
TGF-β1 has recently been shown [152] also to protect osteoarthritic chondrocytes against apoptosis induced by a combination of TNF-α and mitogen-activated kinase phosphatase-1 inhibitor. Normal chondrocytes obtained from cadavers with no history of joint disease were not protected from apoptosis by TGF-β1 alone, but were protected from apoptosis if TGF-β1 treatment was done together with a phosphatase 2A inhibitor. These experiments show that TGF-β1 can be used to protect chondrocytes from apoptosis, but also that osteoarthritic chondrocytes behave differently from normal healthy chondrocytes [152].
What then are the markers that might indicate that chondrogenic differentiation of cells and cartilage repair tissue is jeopardized? Probably they overlap to a large extent with the mediators and biomarkers used to assess osteoarthritis, which is often referred to as primarily a disease of the hyaline articular cartilage. These markers not only include pro-inflammatory cytokines, pro-teolytic enzymes (MMPs and ADAMTSs) and connective tissue degradation products, but also anabolic growth factors, pro-teinase inhibitors (TIMPs) and markers indicating synthesis of extracellular matrix components. This may be due to the fact that the intricate feedback systems always lead to tissue response upon damage as an attempt to reinstall the body's homeostasis. Some of these markers, often used in osteoarthritis research, but probably suitable and relevant for cartilage regeneration studies as well, are given in Table 1.
1.
Potentially suitable markers indicating that chondrogenic differentiation of cells or cartilage repair tissue is jeopardized
| Name | Abbreviation | Specification |
|---|---|---|
| Soluble and intracellular factors | ||
| Interleukin-1 | IL-1 | Pro-inflammatory molecules |
| Tumour necrosis factor-α | TNF-α | |
| Interleukin-6 | IL-6 | |
| Oncostatin M | ||
| High mobility group box-1 | HMGB-1 | |
| Bone morphogenetic proteins | BMP | Anabolic factors |
| Calcitonin | ||
| Fibroblast growth factor-2 | FGF-2 or bFGF | |
| Growth and differentiation factor-5 | GDF-5 | |
| Insulin-like growth factor-I (= somatomedin C) | IGF-I (SmC) | |
| Transforming growth factor-β | TGF-β | |
| Inducible nitrix oxide synthetase | iNOS | Enzyme producing nitric oxide |
| MAP kinases | MAPK | Intracellular signal transducing factors |
| Nuclear factor κB | NF-κB | |
| Prostaglandins | PGE2 etc. | Cause vasodilation and increase vascular permeability leading to swelling and sensitization and stimulation of the nociceptors |
| Caspases and various pro- and anti-apoptotic factors | Factors involved in the internal and external effector apoptosis pathways and its regulation | |
| Proteinases | ||
| Metalloproteinases able to degrade extracellular matrix | MMP-1, -2, -3, -9 and -13, MT1-MMP | Degrade collagen and/or gelatine, in particular in neutral pH, but can also degrade other components of the cartilage matrix |
| Tissue inhibitors of MMPs | TIMP-1 etc. | Inhibit, stabilize or target the action of the MMPs |
| Cathepsin K | Cat K | Degrades effectively collagen, in particular under acidic conditions |
| A disintegrin-, metalloproteinase- and trombospondin-containing molecules | ADAMTS-4 and -5 etc. | Degrade in particular the proteoglycans (aggrecans, the ground substance of the cartilage) in the cartilage extracellular matrix |
| Collagen and other structural components of the cartilage | ||
| Type II1, III, IX, XI collagens | Cartilage supporting fibres | |
| Cartilage oligomeric protein COMP and other collagen decorating molecules2 | Regulate the thickness of the collagen fibres and cross-link them to other molecules, store differentiation and growth factors | |
| Hyaluronan and hyaluronic acid binding protein HABP | Act as the core molecule in proteoglycans in the cartilage and form water binding hydrogel in the synovial fluid | |
| Aggrecans | Water binding molecules in the ground substance of the cartilage | |
| Degradation products | ||
| IICTP, IINTP, CTx-II | Degradation products of type II collagen | |
| Depolymerization products of hyaluronan | Depolymerization is also reflected in a diminished viscosity of the synovial fluid |
Production is measured by measuring the concentration of the amino- and carboxyterminal globular propeptides released from the procollagen molecules before they can organize into the near quarter-stagger.
In cartilage decorin/PGII, biglycan/PG I, fibromodulin, lumican, PRELP (Proline arginine-rich end leucine-rich repeat protein), mimecan (osteoglycin, osteoinductive factor) and chondroadherin.
Tracking of transplanted cells in vivo
To further explore the therapeutic potential of regenerative treatment protocols for cartilage repair, appropriate animal models are necessary that allow tracing the fate of naive or manipulated donor cells in the host. In the field of cartilage regeneration, mainly two cell labelling approaches have been used thus far.
(1) One possibility is to use ‘physicochemical labels’ such as certain dyes or magnetic nanoparticles. An additional advantage of magnetic nanoparticles is that they can potentially be used in clinical settings because they are non-toxic and detectable by high-resolution MRI. Magnetic nanoparticles have been used both in vivo and in vitro in different cell types relevant for cartilage repair, where they have been shown not to affect cell behaviour [153–155]. A drawback of magnetic particles for histological cell tracking is the fact that the specimens cannot be decalcified, because the decalcification process also removes the iron-containing particles. Another important disadvantage of cell labelling with particles or dyes is that the marker is diluted by cell divisions, and may be taken up by macrophages when transplanted cells are phagocytized [156]. For example, the membrane dye PKH26 was used to label chondrocytes in a goat study with ACI and the data indicated that the labelled, thus implanted, cells contributed to the repair tissue [157]. However, although some labelled cells were still visible after 2–3 months, most of the labelling was gone by this time-point. It is unclear whether this observation was due to loss of labelled cells or due to marker dilution by cell proliferation.
(2) Because of the shortcomings of particles or dyes, the most widely used approach to label cells is to introduce marker genes into the genome of the cells under investigation by transducing cells ex vivo with a marker gene, or by using cells from transgenic donor animals with a stable genetic marker. The advantage of a stable genetic marker is that it will also be found in the progeny of the transplanted cells. Therefore, use of cells from transgenic donor animals is probably the most robust way of exploring the long-term fate of transplanted cells. The most widely used genetic markers in regenerative treatment protocols for cartilage repair are Escherichia coliβ-galactosidase lacZ, and green fluorescent protein (GFP) from the jellyfish Aequorea Victoria. In a rabbit model of full thickness cartilage defects, muscle-derived cells and chondrocytes transduced to express lacZ were detected up to 4 weeks post-transplantation in the defects [158]. In GFP-transfected chondrocytes, using a retroviral vector, transgene expression and the number of implanted chondrocytes remained stable for at least 8 months in vitro and for 4 weeks in vivo[159]. In a goat model of osteoarthritis induced by ACL resection and total medial meniscectomy, MSC transduced to express GFP were found in the neomeniscal tissue, 6 weeks after intra-articular injection, but not at 20 weeks post-transplantation [160].
Although widely used, lacZ and GFP have several important disadvantages for histological cell tracking, especially in hard tissues. LacZ is heat labile, and requires pre-embedding staining [161]. Although GFP is an excellent marker for tracking of living cells [162], its fluorescent properties are greatly diminished during normal paraffin embedding, and sensitive detection of GFP in histological sections requires immunohistochemical detection methods [163]. Therefore, frozen sections are mostly used for histological analysis of GFP-labelled cells. However, preparation of frozen sections from hard tissues is technically extremely demanding. In addition, tissue autofluorescence is a major confounding factor in hard tissues. Recently, human placental alkaline phosphatase (hPLAP) has been described as highly suitable marker enzyme for studies involving genetically labelled cells in hard tissues [164]. This marker protein retains its enzymatic activity not only after paraffin embedding but also after a modified methylmethacrylate (MMA) embedding protocol [165], and can be detected by histochemistry and immunohistochemistry in a very sensitive fashion [164]. The availability of a genetic marker suitable for the detection of labelled cells in hard tissue sections may significantly advance the field of cell therapy for cartilage repair in the future.
Another important problem that has hampered the preclinical development of regenerative treatment protocols for cartilage repair is the issue of immune-mediated rejection of labelled cells in immunocompetent hosts. During recent years, it has become quite clear that membrane or even intracellular expression of any foreign protein, and, thus, of any marker protein, will elicit immune-mediated rejection of transplanted cells carrying the marker gene in the recipients [166–170]. Even though joints may be immune-privileged, immune-rejection might seriously interfere with the validity of animal models aimed at testing the usefulness of cell therapy, especially in long-term studies. Most investigators could not detect a large number of labelled cells in cartilage defects after a few months in immunocompetent hosts [157, 158, 160]. It is currently unknown whether this is due to loss of the marker gene, slow immune-mediated rejection of labelled cells, or replacement of transplanted cells by cells of host origin. Because inflammatory processes are crucial in the progression of osteoarthritis, immunocompetent animal models are highly desirable.
The problem of immune-mediated rejection of labelled cells can be circumvented by the use of genetic DNA markers such as the non-expressed TK-tsA cassette [171]. Also, cells from male donors can be tracked in female recipients by the presence of the Y chromosome. However, there is accumulating evidence that male cells can be rejected in immunocompetent females under certain circumstances. For example, skin grafts from male donors are rejected in female recipients, at least in certain mouse strains [172]. In any case, the detection of genetic DNA markers in histological sections requires in situhybridization or in situ PCR. Although methods for in situ hybridization in plastic-embedded soft tissues have been described [173], these methods are very difficult to apply to plastic sections of joints in a routine and reliable fashion. Recently, a novel in vivo technology for long-term studying of labelled cells in the complete absence of immune-mediated rejection in immunocompetent hosts was developed by inducing specific tolerance to the marker gene hPLAP by neonatal exposure of wild-type Fischer 344 rats to cells from hPLAP transgenic Fischer 344 rats of the same inbred strain [174]. This immunocompetent marker tolerant model is almost fully equivalent to the situation in human patients in whom autologous adult stem cells or autologous chondrocytes are used for cell therapy and holds great promise to provide answers to important questions in the further development of regenerative therapeutic protocols for cartilage repair: What is the long-term fate of transplanted chondrocytes? Do MSC engraft in cartilage defects? Do cells from different sources behave differently in terms of therapeutic potential for cartilage repair? Can scaffolds improve the engraftment of transplanted cells and the therapeutic outcome? How do inflammatory conditions in a traumatic or osteoarthritic joint influence engraftment, long-term survival and differentiation of transplanted cells?
Conclusion and future directions
In order to obtain more robust and more reproducible results in cartilage repair procedures, more basic information is needed on the processes of tissue formation and integration including the faith of the cells, cell viability and quality of the extracellular matrix. Also, variables that may affect the outcome of the clinical studies, such as patient characteristics and joint environment, should be taken into account. The role and use of factors that stimulate extracellular matrix formation (like growth factors, drugs but also biomechanical factors) as well as the vulnerability of the newly formed tissue to inflammation and degradation need more basic research.
Different applications will very likely have different optimal procedures. Cell type, culture parameters and scaffolds should be carefully selected and tested for each application. There are multiple potential sources for chondrogenic cells to engineer replacement tissue, none of which has yet been shown to be ideal. Articular cartilage has been extensively studied as a source for chondrogenic cells and autologous chondrocytes have been used in ACI procedures, but the results have shown limitations in expandability and ability to differentiate into hyaline cartilage. However, more recent modifications in growth conditions and the potential for using chondrocytes from alternative anatomic locations may overcome some of these limitations. Another promising source are the progenitor cell populations derived from bone marrow. Bone marrow stromal cells have the advantage of being very expandable but suffer from their propensity to form hyper-trophic chondrocytes, which invites replacement by bone. Other sources of chondrogenic progenitor cells also show promise, such as cells from perichondrium, synovium and adipose tissue, but these sources have not been studied extensively and some might bear the same risk of hypertrophy as BMSCs. The use of long-term cultures of these cells may overcome this limitation, but carries along with it the necessity to pre-form the cartilage in vitro prior to implantation, as opposed to seeding cells in vivo prior to differentiation.
One of the most challenging aspects in this field and the field of tissue engineering in general relates to the manufacturing processes. As was seen in the past with protein therapies, it may take quite some years to come to design and implement fully automated highly robust processes for the production of biological implants. Cell suspensions are still relatively ‘easy’, the manufacturing of 3D biological implants will require an additional level of monitoring involving sophisticated bioreactors and biosensors. These technology developments are required to make the production of these implants affordable. Quality controlled production processes are also a prerequisite if we want to gain more insight through clinical studies.
Stringent and challenging regulatory requirements have stimulated industry to find one stage surgical procedures that operate without cell culture. An example of such is the cartilage autograft implantation system (CAIS, dePuy) where cartilage is minced and mixed with a synthetic scaffold and fixed in the defect with resorbable staples. Animal experiments showed signs of hyaline cartilage formation [175] and clinical development is ongoing. Another development is the combination of commonly used bone marrow stimulation techniques with interactive biomaterials. Although initially the results of animal experiments appeared not very successful [176, 177], more promising results have been reported lately [178, 179].
The proof-of-the-principle study of ACI was first discovered in New York and verified in rabbits in 1989 [180]. Brittberg and colleagues [6] started with the first study in patients in the late 1980s and reported them in 1994. Since then, many patients have been treated with the ACI cell-based therapy. Although there are quite a few clinical studies at the moment, there is a need for more, well-designed clinical studies. The most used outcome parameters in cartilage repair studies are pain and function. Although from the patient's perspective these are the most important outcomes in the short term, there is a need for more objective and quantifiable outcome measures. These outcome measures should address structure/tissue-related aspects that are important for long-term outcome since studies have shown that there is no direct correlation between pain/function scores and radiographic scores [181]. Attention is now directed to expand imaging modalities such as MRI [182, 183], and to find biomarkers that predict progression or disease status. The search for the most optimal (panel of) bio-marker(s) is still in progress [184], and the incorporation of bio-markers in cartilage repair studies might be instrumental in helping to identify patients at risk, responders to treatment and uncover the mechanism(s) and processes taking place over time, after cartilage repair procedures.
However, these types of clinical studies are expensive and together with support from academic, national and other nonprofit resources such as the European Union, additional industrial support will be required to take this approach. In view of this, transparent regulatory requirements adapted to this field and the field of regenerative medicine in general are a prerequisite to achieve this goal.
We have started with investigating the possibility to repair traumatic defects in knee joint cartilage. However, the patient groups are often heterogeneous which complicates interpretations of the outcome of the studies. More information on the patient profile (including age, gender, weight, joint status, location of the defect, underlying cause) as well as the identification of responders to treatment, proper patient tailored rehabilitation and better information on the patients’ expectations will help improving treatment outcome. In addition, other groups of patients with cartilage lesions at other anatomic sites are waiting for repair too, such as patients with cartilage defects in ankle, hip and shoulder joints. Today, it is not known whether cartilage repair procedures can be used for these joints too and if or how the outcome will differ. Also patients with (early) osteoarthritis could in the future be eligible for cartilage repair treatment. This will be more complex because of the catabolic, inflammatory status of the joints and possibly the genetic basis of the disease. At the moment, we do not know enough about the vulnerability of the constructs when used in an inflamed joint.
Lastly, patients with non-joint cartilage defects such as nasal septum perforations, malformed auricle or defects in larynx or trachea after (repeated) airway stenosis or tumour resection are also potential patients for cartilage engineering [185]. If cartilage in the airway is to be repaired, cell injection is not an option. For these patients, we need to form a construct that has sufficient structure to keep the airway open from the moment it is implanted [186]. Because biomaterials in the airway almost always lead to inflammatory responses, the construct should be scaffold-free (at least upon implantation).
Finally, we have gained knowledge on cellular responses to stimuli as well as the experience with the clinical studies on cartilage repair that teach us more about the healing responses and what can be done in patients. The developments in the areas of bio-materials, cell culture, growth factors, imaging and the development of promising cell sources bodes well for the future for cartilage engineering. The challenge for the cell biologists is to develop the cell isolation and culture conditions necessary to expand and differentiate different cell sources efficiently and then, with the use of advances in the fields of biomaterials and bioreactors, to fabricate a provisional tissue or tissue intermediate into the appropriate size, shape and tissue organization. With the advent of advanced imaging capabilities, the challenge for the imaging engineers is to devise more and more effective means of assessing cartilage in vivo, so that all of these developing treatment modalities can be assessed accurately, and at different time-points. As these different technologies come together, the prospects of producing functional cartilage tissue for clinical applications with good long-term results become more and more likely. After the initial hype, it is time for hope. The knowledge gained in this area has raised many new questions on mechanisms, and there is a renewed and more realistic hope for improvements in technical and clinical outcomes in the years to come.
Acknowledgments
This review is an initiative of REMEDIC, a Network on Regenerative Medicine of the European Science Foundation. Sandra van den Bosch is acknowledged for her help with putting together the list of references. GJVMvO was supported by Erasmus MC, the Dutch Programme for Tissue Engineering, the SmartMix Programme of the Netherlands ministry of Economic Affairs and the Dutch Arthritis Association. YBJ was supported by Dutch Arthritis Association and Top Institute Pharma. RGE was supported by Deutsche Forschungsgemeinschaft and the Veterinary University Vienna. YTK was supported by the Sigrid JusÈlius Foundation, evo grants, Finska Läkaresällskapet, The Danish Council for Strategic Research, MATERA Bioactive Nanocomposite Constructs for Regeneration of Articular Cartilage, MNT ERA Net A New Generation of Titanium Biomaterials, EU cost 533. JED is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (R01 DE015322-01).
References
- 1.Convery FR, Akeson WH, Keown GH. The repair of large osteochondral defects. An experimental study in horses. Clin Orthop Relat Res. 1972;82:253–62. [PubMed] [Google Scholar]
- 2.Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460–6. [PubMed] [Google Scholar]
- 3.Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532–53. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Verwoerd-Verhoef HL, Ten Koppel PG, Van Osch GJ, Meeuwis CA, Verwoerd CD. Wound healing of cartilage structures in the head and neck region. Int J Pediatr Otorhinolaryngol. 1998;43:241–51. doi: 10.1016/s0165-5876(98)00003-2. [DOI] [PubMed] [Google Scholar]
- 5.Bos PK, Van Osch GJ, Frenz DA, Verhaar JA, Verwoerd-Verhoef HL. Growth factor expression in cartilage wound healing: temporal and spatial immunolocalization in a rabbit auricular cartilage wound model. Osteoarthritis Cartilage. 2001;9:382–9. doi: 10.1053/joca.2000.0399. [DOI] [PubMed] [Google Scholar]
- 6.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 7.Brittberg M, Peterson L, Sjogren-Jansson E, Tallheden T, Lindahl A. Articular cartilage engineering with autologous chondrocyte transplantation. A review of recent developments. J Bone Joint Surg Am. 2003;85-A:109–15. doi: 10.2106/00004623-200300003-00017. [DOI] [PubMed] [Google Scholar]
- 8.Minas T, Chiu R. Autologous chondrocyte implantation. Am J Knee Surg. 2000;13:41–50. [PubMed] [Google Scholar]
- 9.Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, Skinner JA, Pringle J. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223–30. doi: 10.1302/0301-620x.85b2.13543. [DOI] [PubMed] [Google Scholar]
- 10.Gillogly SD. Treatment of large full-thickness chondral defects of the knee with autologous chondrocyte implantation. Arthroscopy. 2003;19:147–53. doi: 10.1016/j.arthro.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 11.Lohmander LS. Tissue engineering of cartilage: do we need it, can we do it, is it good and can we prove it? Novartis Found Symp. 2003;249:2–10. [PubMed] [Google Scholar]
- 12.Behrens P, Bitter T, Kurz B, Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI) – 5-year follow-up. Knee. 2006;13:194–202. doi: 10.1016/j.knee.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Zheng M, Willer C, Krilak L, Yates P, Xu J, Wood D, Shimmen A. Matrix-induced autologous chondrocyte implantation (MACI): biological and histological assessment. Tissue Eng. 2007;13:737–46. doi: 10.1089/ten.2006.0246. [DOI] [PubMed] [Google Scholar]
- 14.Marcacci M, Zaffagnini S, Kon E, Visani A, Iacono F, Loreti I. Arthroscopic autologous chondrocyte transplantation: technical note. Knee Surg Sports Traumatol Arthrosc. 2002;10:154–9. doi: 10.1007/s00167-001-0275-6. [DOI] [PubMed] [Google Scholar]
- 15.Marcacci M, Kon E, Zaffagnini S, Filardo G, Delcogliano M, Neri M, Iacono F, Hollander A. Arthroscopic second generation autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. 2007;15:610–9. doi: 10.1007/s00167-006-0265-9. [DOI] [PubMed] [Google Scholar]
- 16.Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85-A:185–92. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Dozin B, Malpeli M, Cancedda R, Bruzzi P, Calcagno S, Molfetta L, Priano F, Kon E, Marcacci M. Comparative evaluation of autologous chondrocyte implantation and mosaicplasty: a multicentered randomized clinical trial. Clin J Sport Med. 2005;15:220–6. doi: 10.1097/01.jsm.0000171882.66432.80. [DOI] [PubMed] [Google Scholar]
- 18.Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105–12. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 19.Saris D, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, Vandekerckhove B, Almqvist KF, Claes T, Handelberg F, Lagae K, Van Der Bauwhede J, Vandenneucker H, Yang KG, Jelic M, Verdonk R, Veulemans N, Bellemans J, Luyten FP. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235–46. doi: 10.1177/0363546507311095. [DOI] [PubMed] [Google Scholar]
- 20.Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, Bentley G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640–5. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 21.Gooding CR, Bartlett W, Bentley G, Skinner J, Carrington R, Flanagan A. A Prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: periosteum covered versustype I/II collagen covered. Knee. 2006;13:203–10. doi: 10.1016/j.knee.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Wasiak J, Clar C, Villanueva E. Autologous cartilage implantation for full thickness articulair cartilage defects of the knee. Cochrane Database Rev. 2006;3:CD003323. doi: 10.1002/14651858.CD003323.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Vavken P, Gruber M, Dorotka R. Tissue engineering in orthopaedic surgery – clinical effectiveness and cost effectiveness of autologous chondrocyte transplantation. Z Orthop Unfall. 2008;146:26–30. doi: 10.1055/s-2007-989435. [DOI] [PubMed] [Google Scholar]
- 24.Dell’Accio F, De Bari C, Luyten FP. Molecular markers predictive of the capacity of expanded human articular chondro-cytes to form stable cartilage in vivo. Arthritis Rheum. 2001;44:1608–19. doi: 10.1002/1529-0131(200107)44:7<1608::AID-ART284>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 25.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–24. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 26.Von Der Mark K, Gauss V, Von Der Mark H, Muller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267:531–2. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda T, Kamekura S, Mabuchi A, Kou I, Seki S, Takato T, Nakamura K, Kawaguchi H, Ikegawa S, Chung UI. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. 2004;50:3561–73. doi: 10.1002/art.20611. [DOI] [PubMed] [Google Scholar]
- 28.Kafienah W, Jakob M, Demarteau O, Frazer A, Barker MD, Martin I, Hollander AP. Three-dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue Eng. 2002;8:817–26. doi: 10.1089/10763270260424178. [DOI] [PubMed] [Google Scholar]
- 29.Van Osch GJ, Marijnissen W, Van Der Veen S, Verwoerd-Verhoef H. The potency of culture-expanded nasal septum chondrocytes for tissue engineering of cartilage. Am J Rhinol. 2001;15:187–92. doi: 10.2500/105065801779954166. [DOI] [PubMed] [Google Scholar]
- 30.Van Osch GJ, Van Der Veen S, Verwoerd-Verhoef H. In vitro redifferentiation of culture-expanded rabbit and human auriculair chondrocytes for cartilage reconstruction. Plast Reconstr Surg. 2001;107:433–40. doi: 10.1097/00006534-200102000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Henderson JH, Welter JF, Mansour JM, Niyibizi C, Caplan AI, Dennis JE. Cartilage tissue engineering for laryngotra-cheal reconstruction: comparison of chondrocytes from three anatomic locations in the rabbit. Tissue Eng. 2007;13:843–53. doi: 10.1089/ten.2006.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naumann A, Dennis JE, Awadallah A, Carrino DA, Mansour JM, Kastenbauer E, Caplan AI. Immunochemical and mechanical characterization of cartilage subtypes in rabbit. J Histochem Cytochem. 2002;50:1049–58. doi: 10.1177/002215540205000807. [DOI] [PubMed] [Google Scholar]
- 33.Isogai N, Kusuhara H, Ikada Y, Ohtani H, Jacquet R, Hillyer J, Lowder E, Landis WJ. Comparison of different chondrocytes for use in tissue engineering of cartilage model structures. Tissue Eng. 2006;12:691–703. doi: 10.1089/ten.2006.12.691. [DOI] [PubMed] [Google Scholar]
- 34.Chung C, Erickson IE, Mauck RL, Burdick JA. Differential behavior of auricular and articular chondrocytes in hyaluronic acid hydrogels. Tissue Eng Part A. 2008;14:1121–31. doi: 10.1089/ten.tea.2007.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panossian A, Ashiku S, Kirchhoff CH, Randolph MA, Yaremchuk MJ. Effects of cell concentration and growth period on articular and ear chondrocyte transplants for tissue engineering. Plast Reconstr Surg. 2001;108:392–402. doi: 10.1097/00006534-200108000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Tay AG, Farhadi J, Suetterlin R, Pierer G, Heberer M, Martin I. Cell yield, proliferation, and postexpansion differentiation capacity of human ear, nasal, and rib chondrocytes. Tissue Eng. 2004;10:762–70. doi: 10.1089/1076327041348572. [DOI] [PubMed] [Google Scholar]
- 37.Yokoyama A, Muneta T, Nimura A, Koga H, Mochizuki T, Hata Y, Sekiya I. FGF2 and dexamethasone increase the production of hyaluronan in two-dimensional culture of elastic cartilage-derived cells: in vitro analyses and in vivo cartilage formation. Cell Tissue Res. 2007;329:469–78. doi: 10.1007/s00441-007-0438-y. [DOI] [PubMed] [Google Scholar]
- 38.Van Osch GJ, Mandl EW, Jahr H, Koevoet W, Nolst-Trenite G, Verhaar JA. Considerations on the use of ear chondro-cytes as donor chondrocytes for cartilage tissue engineering. Biorheology. 2004;41:411–21. [PubMed] [Google Scholar]
- 39.Klein TJ, Schumacher BL, Schmidt TA, Li KW, Voegtline MS, Masuda K, Thonar EJ, Sah RL. Tissue engineering of stratified articular cartilage from chondrocyte sub-populations. Osteoarthritis Cartilage. 2003;11:595–602. doi: 10.1016/s1063-4584(03)00090-6. [DOI] [PubMed] [Google Scholar]
- 40.Kim TK, Sharma B, Williams CG, Ruffner MA, Malik A, McFarland EG, Elisseeff JH. Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthritis Cartilage. 2003;11:653–64. doi: 10.1016/s1063-4584(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 41.Skoog T, Ohlsen L, Sohn SA. Perichondrial potential for cartilagenous regeneration. Scand J Plast Reconstr Surg. 1972;6:123–5. doi: 10.3109/02844317209036711. [DOI] [PubMed] [Google Scholar]
- 42.Homminga G, Bulstra S, Bouwmeester P, Van Der Linden A. Perichondral grafting for cartilage lesions of the knee. J Bone Joint Surg Br. 1990;72:1003–7. doi: 10.1302/0301-620X.72B6.2246280. [DOI] [PubMed] [Google Scholar]
- 43.Bouwmeester P, Kuijer R, Homminga G, Bulstra S, Geesink R. A retrospective analysis of two independent prospective cartilage repair studies: antogenous perichondral grafting versus subchondral drilling 10 years post-surgery. J Orthop Res. 2002;20:267–73. doi: 10.1016/S0736-0266(01)00099-7. [DOI] [PubMed] [Google Scholar]
- 44.Van Osch GJ, Van Der Veen SW, Burger EH, Verwoerd-Verhoef HL. Chondrogenic potential of in vitro multiplied rabbit perichondrium cells cultured in alginate beads in defined medium. Tissue Eng. 2000;6:321–30. doi: 10.1089/107632700418047. [DOI] [PubMed] [Google Scholar]
- 45.Togo T, Utani A, Naitoh M, Ohta M, Tsuji Y, Morikawa N, Nakamura M, Suzuki S. Identification of cartilage progenitor cells in the adult ear perichondrium: utilization for cartilage reconstruction. Lab Invest. 2006;86:445–57. doi: 10.1038/labinvest.3700409. [DOI] [PubMed] [Google Scholar]
- 46.Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745–57. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–92. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Solchaga LA, Yoo JU, Lundberg M, Dennis JE, Huibregtse BA, Goldberg VM, Caplan AI. Hyaluronan-based polymers in the treatment of osteochondral defects. J Orthop Res. 2000;18:773–80. doi: 10.1002/jor.1100180515. [DOI] [PubMed] [Google Scholar]
- 49.Solchaga LA, Gao J, Dennis JE, Awadallah A, Lundberg M, Caplan AI, Goldberg VM. Treatment of osteochondral defects with autologous bone marrow in a hyaluronan-based delivery vehicle. Tissue Eng. 2002;8:333–47. doi: 10.1089/107632702753725085. [DOI] [PubMed] [Google Scholar]
- 50.Zhou XZ, Leung VY, Dong QR, Cheung KM, Chan D, Lu WW. Mesenchymal stem cell-based repair of articular cartilage with polyglycolic acid-hydroxyapatite biphasic scaffold. Int J Artif Organs. 2008;31:480–9. doi: 10.1177/039139880803100603. [DOI] [PubMed] [Google Scholar]
- 51.Guo X, Zheng Q, Yang S, Shao Z, Yuan Q, Pan Z, Tang S, Liu K, Quan D. Repair of full-thickness articular cartilage defects by cultured mesenchymal stem cells transfected with the transforming growth factor beta1 gene. Biomed Mater. 2006;1:206–15. doi: 10.1088/1748-6041/1/4/006. [DOI] [PubMed] [Google Scholar]
- 52.Park MS, Kim SS, Cho SW, Choi CY, Kim BS. Enhancement of the osteogenic efficacy of osteoblast transplantation by the sustained delivery of basic fibroblast growth factor. J Biomed Mater Res. 2006;79:353–9. doi: 10.1002/jbm.b.30549. [DOI] [PubMed] [Google Scholar]
- 53.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, M Y. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 54.Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004;13:595–600. doi: 10.3727/000000004783983747. [DOI] [PubMed] [Google Scholar]
- 55.Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted autologous mesenchymal stem cells. Pain Physician. 2008;11:343–53. [PubMed] [Google Scholar]
- 56.Dennis JE, Carbillet JP, Caplan Al, Charbord P. The STRO-1 + marrow cell population is multipotential. Cells Tissues Organs. 2002;170:73–82. doi: 10.1159/000046182. [DOI] [PubMed] [Google Scholar]
- 57.Farrell E, Van Der Jagt OP, Koevoet W, Kops N, Van Manen CJ, Hellingman CA, Jahr H, O’Brien FJ, Verhaar JA, Weinans H, Van Osch GJ. Chondrogenic priming of human bone marrow stromal cells: a better route to bone repair? Tissue Eng Part A. doi: 10.1089/ten.tec.2008.0297. doi: DOI: 10.1089/ten.tea.2008.0297. [DOI] [PubMed] [Google Scholar]
- 58.Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–66. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 59.Liu K, Zhou GD, Liu W, Zhang WJ, Cui L, Liu X, Liu TY, Cao Y. The dependence of in vivo stable ectopic chondrogenesis by human mesenchymal stem cells on chondrogenic differentiation in vitro. Biomaterials. 2008;29:2183–92. doi: 10.1016/j.biomaterials.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 60.Caplan AL, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 61.Hoogduijn MJ, Crop MJ, Peeters AM, Van Osch GJ, Balk AH, Ijzermans JN, Weimar W, Baan CC. Human heart spleen, and perirenal fat-derived mesenchymal stem cells have immunomodulatory capacities. Stem Cells Dev. 2007;16:597–604. doi: 10.1089/scd.2006.0110. [DOI] [PubMed] [Google Scholar]
- 62.Chen L, Tredget E, Wu P, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endolithial lineage cells and enhance wound healing. PLoS ONE. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 64.Rich JT, Rosova I, Nolta JA, Myckatyn TM, Sandell LJ, McAlinden A. Upregulation of Runx2 and Osterix during in vitrochondrogenesis of human adipose-derived stromal cells. Biochem Biophys Res Commun. 2008;372:230–5. doi: 10.1016/j.bbrc.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hennig T, Lorenz H, Thiel A, Goetzke K, Dickhut A, Geiger F, Richter W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682–91. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 66.De Bari C, Dell’Accio F, Luyten FP. Failure of in vitro differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum. 2004;50:142–50. doi: 10.1002/art.11450. [DOI] [PubMed] [Google Scholar]
- 67.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–9. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 68.Shirasawa S, Sekiya I, Sakaguchi Y, Yagishita K, Ichinose S, Muneta T. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J Cell Biochem. 2006;97:84–97. doi: 10.1002/jcb.20546. [DOI] [PubMed] [Google Scholar]
- 69.Jones EA, English A, Henshaw K, Kinsey SE, Markham AF, Emery P, McGonagle D. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum. 2004;50:817–27. doi: 10.1002/art.20203. [DOI] [PubMed] [Google Scholar]
- 70.Koyama E, Shibukawa Y, Nagayama M, Sugito H, Young B, Yuasa T, Okabe T, Ochiai T, Kamiya N, Rountree RB, Kingsley DM, Iwamoto M, Enomoto-Iwamoto M, Pacifici M. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol. 2008;316:62–73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J, Lee Y, Kim H, Hwang KJ, Kwon HC, Kim SK, Cho DJ, Kang SG, You J. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. 2007;40:75–90. doi: 10.1111/j.1365-2184.2007.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C, Greschat S, Knipper A, Bender J, Degistirici O, Gao J, Caplan AI, Colletti EJ, Almeida-Porada G, Muller HW, Zanjani E, Wernet P. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–35. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mos L, Cooper GA, Serben K, Cameron M, Koop BF. Effects of diesel on survival, growth, and gene expression in rainbow trout (Oncorhynchus mykiss) fry. Environ Sci Technol. 2008;42:2656–62. doi: 10.1021/es702215c. [DOI] [PubMed] [Google Scholar]
- 74.Vats A, Bielby RC, Tolley N, Dickinson SC, Boccaccini AR, Hollander AP, Bishop AE, Polak JM. Chondrogenic differentiation of human embryonic stem cells: the effect of the micro-environment. Tissue Eng. 2006;12:1687–97. doi: 10.1089/ten.2006.12.1687. [DOI] [PubMed] [Google Scholar]
- 75.Koay EJ, Athanasiou KA. Hypoxic chondrogenic differentiation of human embryonic stem cells enhances cartilage protein synthesis and biomechanical functionality. Osteoarthritis Cartilage. 2008;16:1450–6. doi: 10.1016/j.joca.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 76.Jukes JM, Both SK, Leusink A, Sterk LM, Van Blitterswijk CA, De Boer J. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:6840–5. doi: 10.1073/pnas.0711662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hegert C, Kramer J, Hargus G, Muller J, Guan K, Wobus AM, Muller PK, Rohwedel J. Differentiation plasticity of chondrocytes derived from mouse embryonic stem cells. J Cell Sci. 2002;115:4617–28. doi: 10.1242/jcs.00171. [DOI] [PubMed] [Google Scholar]
- 78.Hwang N, Varghese S, Elisseeff J. Derivation of chondrogenically-committed cells from human embryonic cells for cartilage tisssue regeneration. PLoS ONE. 2008;3:e2498. doi: 10.1371/journal.pone.0002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 80.Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nature Biotechnol. 2007;25:1177–81. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- 81.Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 82.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 83.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 84.Yaeger PC, Masi TL, De Ortiz JL, Binette F, Tubo R, McPherson JM. Synergistic action of transforming growth factor-beta and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Exp Cell Res. 1997;237:318–25. doi: 10.1006/excr.1997.3781. [DOI] [PubMed] [Google Scholar]
- 85.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 86.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchy-mal progenitor cells. Exp Cell Res. 1998;238:265–72. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 87.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–28. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 88.Mehlhorn AT, Niemeyer P, Kaschte K, Muller L, Finkenzeller G, Hartl D, Sudkamp NP, Schmal H. Differential effects of BMP-2 and TGF-beta1 on chondrogenic differentiation of adipose derived stem cells. Cell Prolif. 2007;40:809–23. doi: 10.1111/j.1365-2184.2007.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hanada K, Solchaga LA, Caplan AI, Hering TM, Goldberg VM, Yoo JU, Johnstone B. BMP-2 induction and TGF-beta 1 modulation of rat periosteal cell chondrogenesis. J Cell Biochem. 2001;81:284–94. doi: 10.1002/1097-4644(20010501)81:2<284::aid-jcb1043>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 90.Luyten FP, Yu YM, Yanagishita M, Vukicevic S, Hammonds RG, Reddi AH. Natural bovine osteogenin and recombi-nant human bone morphogenetic protein-2B are equipotent in the maintenance of proteoglycans in bovine articular cartilage explant cultures. J Biol Chem. 1992;267:3691–5. [PubMed] [Google Scholar]
- 91.Ito T, Sawada R, Fujiwara Y, Tsuchiya T. FGF-2 increases osteogenic and chondrogenic differentiation potentials of human mesenchymal stem cells by inactivation of TGF-beta signaling. Cytotechnology. 2008;56:1–7. doi: 10.1007/s10616-007-9092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu D, Gechtman Z, Hughes A, Collins A, Dodds R, Cui X, Jolliffe L, Higgins L, Murphy A, Farrell F. Potential involvement of BMP receptor type IB activation in a synergistic effect of chondrogenic promotion between rhTGFbeta3 and rhGDF5 or rhBMP7 in human mesenchymal stem cells. Growth Factors. 2006;24:268–78. doi: 10.1080/08977190601075865. [DOI] [PubMed] [Google Scholar]
- 93.Longobardi L, O’Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, Horton WA, Moses HL, Spagnoli A. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J Bone Miner Res. 2006;21:626–36. doi: 10.1359/jbmr.051213. [DOI] [PubMed] [Google Scholar]
- 94.Chen WH, Lo WC, Lee JJ, Su CH, Lin CT, Liu HY, Lin TW, Lin WC, Huang TY, Deng WP. Tissue-engineered intervertebral disc and chondrogenesis using human nucleus pulposus regulated through TGF-beta1 in platelet-rich plasma. J Cell Physiol. 2006;209:744–54. doi: 10.1002/jcp.20765. [DOI] [PubMed] [Google Scholar]
- 95.Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84:276–88. doi: 10.1302/0301-620x.84b2.11167. [DOI] [PubMed] [Google Scholar]
- 96.Sellers RS, Zhang R, Glasson SS, Kim HD, Peluso D, D’Augusta DA, Beckwith K, Morris EA. Repair of articular cartilage defects one year after treatment with recombinant human bone morphogenetic protein-2 (rhBMP-2) J Bone Joint Surg Am. 2000;82:151–60. doi: 10.2106/00004623-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 97.Louwerse RT, Heyligers IC, Klein-Nulend J, Sugihara S, Van Kampen GP, Semeins CM, Goei SW, De Koning MH, Wuisman PI, Burger EH. Use of recombinant human osteogenic protein-1 for the repair of sub-chondral defects in articular cartilage in goats. J Biomed Mater Res. 2000;49:506–16. doi: 10.1002/(sici)1097-4636(20000315)49:4<506::aid-jbm9>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 98.Harrison ET, Jr, Luyten FP, Reddi AH. Transforming growth factor-beta: its effect on phenotype reexpression by dedifferentiated chondrocytes in the presence and absence of osteogenin. In Vitro Cell Dev Biol. 1992;28A:445–8. doi: 10.1007/BF02634049. [DOI] [PubMed] [Google Scholar]
- 99.Barbero A, Ploegert S, Heberer M, Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003;48:1315–25. doi: 10.1002/art.10950. [DOI] [PubMed] [Google Scholar]
- 100.Jakob M, Demarteau O, Schafer D, Hintermann B, Dick W, Heberer M, Martin I. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81:368–77. doi: 10.1002/1097-4644(20010501)81:2<368::aid-jcb1051>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 101.Mandl EW, Jahr H, Koevoet JL, Van Leeuwen JP, Weinans H, Verhaar JA, Van Osch GJ. Fibroblast growth factor-2 in serum-free medium is a potent mitogen and reduces dedifferentiation of human ear chondrocytes in monolayer culture. Matrix Biol. 2004;23:231–41. doi: 10.1016/j.matbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 102.Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203:398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 103.Luyten FP, Hascall VC, Nissley SP, Morales TI, Reddi AH. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988;267:416–25. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- 104.Jenniskens YM, Koevoet W, De Bart AC, Weinans H, Jahr H, Verhaar JA, DeGroot J, Van Osch GJ. Biochemical and functional modulation of the cartilage collagen network by IGF1, TGFbeta2 and FGF2. Osteoarthritis Cartilage. 2006;14:1136–46. doi: 10.1016/j.joca.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 105.Hui W, Rowan AD, Cawston T. Modulation of the expression of matrix metalloproteinase and tissue inhibitors of metalloproteinases by TGF-beta1 and IGF-1 in primary human articular and bovine nasal chondrocytes stimulated with TNF-alpha. Cytokine. 2001;16:31–5. doi: 10.1006/cyto.2001.0950. [DOI] [PubMed] [Google Scholar]
- 106.Hui W, Rowan AD, Cawston T. Insulin-like growth factor 1 blocks collagen release and down regulates matrix metalloproteinase-1, -3, -8, and -13 mRNA expression in bovine nasal cartilage stimulated with oncostatin M in combination with interleukin 1alpha. Ann Rheum Dis. 2001;60:254–61. doi: 10.1136/ard.60.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chubinskaya S, Hurtig M, Rueger DC. OP-1/BMP-7 in cartilage repair. Int Orthop. 2007;31:773–81. doi: 10.1007/s00264-007-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nixon AJ, Haupt JL, Frisbie DD, Morisset SS, McIlwraith CW, Robbins PD, Evans CH, Ghivizzani S. Genemediated restoration of cartilage matrix by combination insulin-like growth factor-I/interleukin-1 receptor antagonist therapy. Gene Ther. 2005;12:177–86. doi: 10.1038/sj.gt.3302396. [DOI] [PubMed] [Google Scholar]
- 109.Im HJ, Muddasani P, Natarajan V, Schmid TM, Block JA, Davis F, Van Wijnen AJ, Loeser RF. Basic fibroblast growth factor stimulates matrix metallo-proteinase-13 viathe molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes. J Biol Chem. 2007;282:11110–21. doi: 10.1074/jbc.M609040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaul G, Cucchiarini M, Arntzen D, Zurakowski D, Menger MD, Kohn D, Trippel SB, Madry H. Local stimulation of articular cartilage repair by transplantation of encapsulated chondrocytes overex-pressing human fibroblast growth factor 2 (FGF-2) in vivo. J Gene Med. 2006;8:100–11. doi: 10.1002/jgm.819. [DOI] [PubMed] [Google Scholar]
- 111.Ishii I, Mizuta H, Sei A, Hirose J, Kudo S, Hiraki Y. Healing of full-thickness defects of the articular cartilage in rabbits using fibroblast growth factor-2 and a fibrin sealant. J Bone Joint Surg Br. 2007;89:693–700. doi: 10.1302/0301-620X.89B5.18450. [DOI] [PubMed] [Google Scholar]
- 112.Mandl EW, Van Der Veen SW, Verhaar JA, Van Osch GJ. Serum-free medium supplemented with high-concentration FGF2 for cell expansion culture of human ear chondrocytes promotes redifferentiation capacity. Tissue Eng. 2002;8:573–80. doi: 10.1089/107632702760240490. [DOI] [PubMed] [Google Scholar]
- 113.Mandl EW, Van Der Veen SW, Verhaar JA, Van Osch GJ. Multiplication of human chondrocytes with low seeding densities accelerates cell yield without losing redifferentiation capacity. Tissue Eng. 2004;10:109–18. doi: 10.1089/107632704322791754. [DOI] [PubMed] [Google Scholar]
- 114.Sawaji Y, Hynes J, Vincent T, Saklatvala J. Fibroblast growth factor 2 inhibits induction of aggrecanase activity in human articular cartilage. Arthritis Rheum. 2008;58:3498–509. doi: 10.1002/art.24025. [DOI] [PubMed] [Google Scholar]
- 115.Moore EE, Bendele AM, Thompson DL, Littau A, Waggie KS, Reardon B, Ellsworth JL. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis Cartilage. 2005;13:623–31. doi: 10.1016/j.joca.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 116.Ellsworth JL, Berry J, Bukowski T, Claus J, Feldhaus A, Holderman S, Holdren MS, Lum KD, Moore EE, Raymond F, Ren H, Shea P, Sprecher C, Storey H, Thompson DL, Waggie K, Yao L, Fernandes RJ, Eyre DR, Hughes SD. Fibroblast growth factor-18 is a trophic factor for mature chondrocytes and their progenitors. Osteoarthritis Cartilage. 2002;10:308–20. doi: 10.1053/joca.2002.0514. [DOI] [PubMed] [Google Scholar]
- 117.Bastiaansen-Jenniskens YM, Koevoet W, De Bart AC, De Bart AC, Zuurmond AM, Bank RA, Verhaar JA, Degroot J, Van Osch GJ. TGFbeta affects collagen cross-linking independent of chondrocyte phenotype but strongly depending on physical environment. Tissue Eng Part A. 2008;14:1059–66. doi: 10.1089/ten.tea.2007.0345. [DOI] [PubMed] [Google Scholar]
- 118.Redini F, Mauviel A, Pronost S, Loyau G, Pujol JP. Transforming growth factor beta exerts opposite effects from interleukin-1 beta on cultured rabbit articular chondrocytes through reduction of interleukin-1 receptor expression. Arthritis Rheum. 1993;36:44–50. doi: 10.1002/art.1780360108. [DOI] [PubMed] [Google Scholar]
- 119.Grimaud E, Heymann D, Redini F. Recent advances in TGF-beta effects on chondrocyte metabolism. Potential therapeutic roles of TGF-beta in cartilage disorders. Cytokine Growth Factor Rev. 2002;13:241–57. doi: 10.1016/s1359-6101(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 120.Grunder T, Gaissmaier C, Fritz J, Stoop R, Hortschansky P, Mollenhauer J, Aicher WK. Bone morphogenetic protein (BMP)-2 enhances the expression of type II collagen and aggrecan in chondrocytes embedded in alginate beads. Osteoarthritis Cartilage. 2004;12:559–67. doi: 10.1016/j.joca.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 121.Stove J, Schneider-Wald B, Scharf HP, Schwarz ML. Bone morphogenetic protein 7 (bmp-7) stimulates proteoglycan synthesis in human osteoarthritic chondrocytes in vitro. Biomed Pharmacother. 2006;60:639–43. doi: 10.1016/j.biopha.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 122.Hicks DL, Sage AB, Shelton E, Schumacher BL, Sah RL, Watson D. Effect of bone morphogenetic proteins 2 and 7 on septal chondrocytes in alginate. Otolaryngol Head Neck Surg. 2007;136:373–9. doi: 10.1016/j.otohns.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 123.Chubinskaya S, Segalite D, Pikovsky D, Hakimiyan AA, Rueger DC. Effects induced by BMPS in cultures of human articular chondrocytes: comparative studies. Growth Factors. 2008;26:275–83. doi: 10.1080/08977190802291733. [DOI] [PubMed] [Google Scholar]
- 124.Mailhot G, Yang M, Mason-Savas A, Mackay CA, Leav I, Odgren PR. BMP-5 expression increases during chondrocyte differentiation in vivo and in vitro and promotes proliferation and cartilage matrix synthesis in primary chondrocyte cultures. J Cell Physiol. 2008;214:56–64. doi: 10.1002/jcp.21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Blaney Davidson E, Vitters E, Van Lent P, Van De Loo F, Van Den Berg W, Van Der Kraan P. Elevated extracellulairmatrix production and degradation upon bone morphogenetic protein -2 (BMP-2) stimulation point toward a role for (BMP-2) in cartilagerepair and remodeling. Arthritis Res Ther. 2007;9:R102. doi: 10.1186/ar2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Akeda K, An HS, Okuma M, Attawia M, Miyamoto K, Thonar EJ, Lenz ME, Sah RL, Masuda K. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14:1272–80. doi: 10.1016/j.joca.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 127.Marijnissen WJ, Van Osch GJ, Aigner J, Verwoerd-Verhoef HL, Verhaar JA. Tissue-engineered cartilage using serially passaged articular chondrocytes. Chondrocytes in alginate, combined in vivo with a synthetic (E210) or biologic biodegradable carrier (DBM) Biomaterials. 2000;21:571–80. doi: 10.1016/s0142-9612(99)00218-5. [DOI] [PubMed] [Google Scholar]
- 128.Marijnissen WJ, Van Osch GJ, Aigner J, Van Der Veen SW, Hollander AP, Verwoerd-Verhoef HL, Verhaar JA. Alginate as a chondrocyte-delivery substance in combination with a non-woven scaffold for cartilage tissue engineering. Biomaterials. 2002;23:1511–7. doi: 10.1016/s0142-9612(01)00281-2. [DOI] [PubMed] [Google Scholar]
- 129.Miot S, Woodfield T, Daniels AU, Suetterlin R, Peterschmitt I, Heberer M, Van Blitterswijk CA, Riesle J, Martin I. Effects of scaffold composition and architecture on human nasal chondrocyte redifferentiation and cartilaginous matrix deposition. Biomaterials. 2005;26:2479–89. doi: 10.1016/j.biomaterials.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 130.Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152–63. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 131.Oliveira JT, Crawford A, Mundy JM, Moreira AR, Gomes ME, Hatton PV, Reis RL. A cartilage tissue engineering approach combining starch-polycaprolactone fibre mesh scaffolds with bovine articular chondrocytes. J Mater Sci Mater Med. 2007;18:295–302. doi: 10.1007/s10856-006-0692-7. [DOI] [PubMed] [Google Scholar]
- 132.Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis Cartilage. 2007;15:403–13. doi: 10.1016/j.joca.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 133.Sohier J, Hamann D, Koenders M, Cucchiarini M, Madry H, Van Blitterswijk C, De Groot K, Bezemer JM. Tailored release of TGF-beta1 from porous scaffolds for cartilage tissue engineering. Int J Pharm. 2007;332:80–9. doi: 10.1016/j.ijpharm.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 134.Gavenis K, Klee D, Pereira-Paz RM, Von Walter M, Mollenhauer J, Schneider U, Schmidt-Rohlfing B. BMP-7 loaded microspheres as a new delivery system for the cultivation of human chondrocytes in a collagen type-I gel. J Biomed Mat Res. 2007;82:275–83. doi: 10.1002/jbm.b.30731. [DOI] [PubMed] [Google Scholar]
- 135.Sohier J, Vlugt TJ, Cabrol N, Van Blitterswijk C, De Groot K, Bezemer JM. Dual release of proteins from porous polymeric scaffolds. J Control Release. 2006;111:95–106. doi: 10.1016/j.jconrel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 136.Ginty PJ, Barry JJ, White LJ, Howdle SM, Shakesheff KM. Controlling protein release from scaffolds using polymer blends and composites. Eur J Pharm Biopharm. 2008;68:82–9. doi: 10.1016/j.ejpb.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 137.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–9. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 138.Saris DB, Dhert WJ, Verbout AJ. Joint homeostasis. The discrepancy between old and fresh defects in cartilage repair. J Bone Joint Surg Br. 2003;85:1067–76. doi: 10.1302/0301-620x.85b7.13745. [DOI] [PubMed] [Google Scholar]
- 139.Verzijl N, DeGroot J, Bank RA, Bayliss MT, Bijlsma JW, Lafeber FP, Maroudas A, TeKoppele JM. Age-related accumulation of the advanced glycation endproduct pentosidine in human articular cartilage aggrecan: the use of pentosidine levels as a quantitative measure of protein turnover. Matrix Biol. 2001;20:409–17. doi: 10.1016/s0945-053x(01)00158-5. [DOI] [PubMed] [Google Scholar]
- 140.Kume S, Kato S, Yamagishi S, Inagaki Y, Ueda S, Arima N, Okawa T, Kojiro M, Nagata K. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J Bone Miner Res. 2005;20:1647–58. doi: 10.1359/JBMR.050514. [DOI] [PubMed] [Google Scholar]
- 141.Kreuz PC, Erggelet C, Steinwachs MR, Krause SJ, Lahm A, Niemeyer P, Ghanem N, Uhl M, Sudkamp N. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006;22:1180–6. doi: 10.1016/j.arthro.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 142.Rosenberger RE, Gomoll AH, Bryant T, Minas T. Repair of large chondral defects of the knee with autologous chondrocyte implantation in patients 45 years or older. Am J Sports Med. 2008;36:2336–44. doi: 10.1177/0363546508322888. [DOI] [PubMed] [Google Scholar]
- 143.Vasara AI, Konttinen YT, Peterson L, Lindahl A, Kiviranta I. Persisting high levels of synovial fluid markers after cartilage repair: a pilot study. Clin Orthop Relat Res. 2009;467:267–72. doi: 10.1007/s11999-008-0434-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rotter N, Ung F, Roy AK, Vacanti M, Eavey RD, Vacanti CA, Bonassar LJ. Role for interleukin 1alpha in the inhibition of chondrogenesis in autologous implants using polyglycolic acid-polylactic acid scaffolds. Tissue Eng. 2005;11:192–200. doi: 10.1089/ten.2005.11.192. [DOI] [PubMed] [Google Scholar]
- 145.Haupt JL, Frisbie DD, McIlwraith CW, Robbins PD, Ghivizzani S, Evans CH, Nixon AJ. Dual transduction of insulin-like growth factor-I and interleukin-1 receptor antagonist protein controls cartilage degradation in an osteoarthritic culture model. J Orthop Res. 2005;23:118–26. doi: 10.1016/j.orthres.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 146.Robertson CM, Allen RT, Pennock AT, Bugbee WD, Amiel D. Upregulation of apoptotic and matrix-related gene expression during fresh osteochondral allograft storage. Clin Orthop Relat Res. 2006;442:260–6. doi: 10.1097/01.blo.0000187058.42820.39. [DOI] [PubMed] [Google Scholar]
- 147.Lee JH, Prakash KV, Pengatteeri YH, Park SE, Koh HS, Han CW. Chondrocyte apoptosis in the regenerated articular cartilage after allogenic chondrocyte transplantation in the rabbit knee. J Bone Joint Surg Br. 2007;89:977–83. doi: 10.1302/0301-620X.89B7.18983. [DOI] [PubMed] [Google Scholar]
- 148.Surendran S, Kim SH, Jee BK, Ahn SH, Gopinathan P, Han CW. Anti-apoptotic Bcl-2 gene transfection of human articular chondrocytes protects against nitric oxide-induced apoptosis. J Bone Joint Surg Br. 2006;88:1660–5. doi: 10.1302/0301-620X.88B12.17717. [DOI] [PubMed] [Google Scholar]
- 149.Nakachi N, Asoh S, Watanabe N, Mori T, Matsushita T, Takai S, Ohta S. Transduction of anti-cell death protein FNK suppresses graft degeneration after autologous cylindrical osteochondral transplantation. J Histochem Cytochem. 2009;57:197–206. doi: 10.1369/jhc.2008.952754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cao L, Lee V, Adams ME, Kiani C, Zhang Y, Hu W, Yang BB. Beta-integrin-collagen interaction reduces chondrocyte apoptosis. Matrix Biol. 1999;18:343–55. doi: 10.1016/s0945-053x(99)00027-x. [DOI] [PubMed] [Google Scholar]
- 151.Lee HJ, Choi BH, Min BH, Park SR. Low-intensity ultrasound inhibits apoptosis and enhances viability of human mesenchymal stem cells in three-dimensional alginate culture during chondrogenic differentiation. Tissue Eng. 2007;13:1049–57. doi: 10.1089/ten.2006.0346. [DOI] [PubMed] [Google Scholar]
- 152.Lires-Dean M, Carames B, Cillero-Pastor B, Galdo F, Lopez-Armada MJ, Blanco FJ. Anti-apoptotic effect of transforming growth factor-beta1 on human articular chondrocytes: role of protein phosphatase 2A. Osteoarthritis Cartilage. 2008;16:1370–8. doi: 10.1016/j.joca.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 153.Farrell E, Wielopolski P, Pavljasevic P, Van Tiel S, Jahr H, Verhaar J, Weinans H, Krestin G, O’Brien FJ, Van Osch GJ, Bernsen M. Effects of iron oxide incorporation for long term cell tracking on MSC differentiation in vitro and in vivo. Biochem Biophys Res Commun. 2008;369:1076–81. doi: 10.1016/j.bbrc.2008.02.159. [DOI] [PubMed] [Google Scholar]
- 154.Farrell E, Wielopolski P, Pavljasevic P, Kops N, Weinans H, Bernsen MR, Van Osch GJ. Cell labelling with superpara-magnetic iron oxide has no effect on chondrocyte behaviour. Osteoarthritis Cartilage. doi: 10.1016/j.joca.2008.11.016. doi: DOI: 10.1016/j.joca.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 155.Jing XH, Yang L, Duan XJ, Xie B, Chen W, Li Z, Tan HB. In vivo MR imaging tracking of magnetic iron oxide nanoparticle labeled, engineered, autologous bone marrow mesenchymal stem cells following intra-articular injection. Joint Bone Spine. 2008;75:432–8. doi: 10.1016/j.jbspin.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 156.Brazelton TR, Blau HM. Optimizing techniques for tracking transplanted stem cells in vivo. Stem Cells. 2005;23:1251–65. doi: 10.1634/stemcells.2005-0149. [DOI] [PubMed] [Google Scholar]
- 157.Dell’Accio F, Vanlauwe J, Bellemans J, Neys J, De Bari C, Luyten FP. Expanded phenotypically stable chondrocytes persist in the repair tissue and contribute to cartilage matrix formation and structural integration in a goat model of autologous chondrocyte implantation. J Orthop Res. 2003;21:123–31. doi: 10.1016/S0736-0266(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 158.Adachi N, Sato K, Usas A, Fu FH, Ochi M, Han CW, Niyibizi C, Huard J. Muscle derived, cell based ex vivo gene therapy for treatment of full thickness articular cartilage defects. J Rheumatol. 2002;29:1920–30. [PubMed] [Google Scholar]
- 159.Hirschmann F, Verhoeyen E, Wirth D, Bauwens S, Hauser H, Rudert M. Vital marking of articular chondrocytes by retroviral infection using green fluorescence protein. Osteoarthritis Cartilage. 2002;10:109–18. doi: 10.1053/joca.2001.0486. [DOI] [PubMed] [Google Scholar]
- 160.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–74. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 161.Gossler A. Gene and enhancer trap screens in ES cell chimeras. In: Joyner CJ, editor. Gene targeting: a practical approach. New York, NY: Oxford University Press; 1993. pp. 187–227. [Google Scholar]
- 162.Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien RY. Understanding, improving and using green fluorescent proteins. Trends Biochem Sci. 1995;20:448–55. doi: 10.1016/s0968-0004(00)89099-4. [DOI] [PubMed] [Google Scholar]
- 163.Zagzag D, Miller DC, Chiriboga L, Yee H, Newcomb EW. Green fluorescent protein immunohistochemistry as a novel experimental tool for the detection of glioma cell invasion in vivo. Brain Pathol. 2003;13:34–7. doi: 10.1111/j.1750-3639.2003.tb00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Unger NJ, Odorfer KI, Weber K, Sandgren EP, Erben RG. Utility of human placental alkaline phosphatase as a genetic marker for cell tracking in bone and cartilage. Histochem Cell Biol. 2007;127:669–74. doi: 10.1007/s00418-007-0286-6. [DOI] [PubMed] [Google Scholar]
- 165.Erben RG. Embedding of bone samples in methylmethacrylate: an improved method suitable for bone histomorphom-etry, histochemistry, and immunohisto-chemistry. J Histochem Cytochem. 1997;45:307–13. doi: 10.1177/002215549704500215. [DOI] [PubMed] [Google Scholar]
- 166.Sarukhan A, Soudais C, Danos O, Jooss K. Factors influencing cross-presentation of non-self antigens expressed from recombinant adeno-associated virus vectors. J Gene Med. 2001;3:260–70. doi: 10.1002/jgm.175. [DOI] [PubMed] [Google Scholar]
- 167.Gross DA, Leboeuf M, Gjata B, Danos O, Davoust J. CD4+CD25+ regulatory T cells inhibit immune-mediated transgene rejection. Blood. 2003;102:4326–8. doi: 10.1182/blood-2003-05-1454. [DOI] [PubMed] [Google Scholar]
- 168.Andersson G, Illigens BM, Johnson KW, Calderhead D, LeGuern C, Benichou G, White-Scharf ME, Down JD. Nonmyeloablative conditioning is sufficient to allow engraftment of EGFP-expressing bone marrow and subsequent acceptance of EGFP-transgenic skin grafts in mice. Blood. 2003;101:4305–12. doi: 10.1182/blood-2002-06-1649. [DOI] [PubMed] [Google Scholar]
- 169.Heim DA, Hanazono Y, Giri N, Wu T, Childs R, Sellers SE, Muul L, Agrlcola BA, Metzger ME, Donahue RE, Tlsdale JF, Dunbar CE. Introduction of a xenogeneic gene via hematopoietic stem cells leads to specific tolerance in a rhesus monkey model. Mol Ther. 2000;1:533–44. doi: 10.1006/mthe.2000.0072. [DOI] [PubMed] [Google Scholar]
- 170.Rosenzweig M, Connole M, Glickman R, Yue SP, Noren B, DeMaria M, Johnson RP. Induction of cytotoxic T lymphocyte and antibody responses to enhanced green fluorescent protein following transplantation of transduced CD34(+) hematopoietic cells. Blood. 2001;97:1951–9. doi: 10.1182/blood.v97.7.1951. [DOI] [PubMed] [Google Scholar]
- 171.Kaab G, Brandl G, Marx A, Wekerle H, Bradl M. The myelin basic protein-specific T cell repertoire in (transgenic) Lewis rat/SCID mouse chimeras: preferential V beta 8.2 T cell receptor usage depends on an intact Lewis thymic microenvironment. Eur J Immunol. 1996;26:981–8. doi: 10.1002/eji.1830260504. [DOI] [PubMed] [Google Scholar]
- 172.Obhrai JS, Oberbarnscheidt M, Zhang N, Mueller DL, Shlomchik WD, Lakkis FG, Shlomchik MJ, Kaplan DH. Langerhans cells are not required for efficient skin graft rejection. J Invest Dermatol. 2008;128:1950–5. doi: 10.1038/jid.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mueller M, Wacker K, Hickey WF, Ringelstein EB, Kiefer R. Co-localization of multiple antigens and specific DNA. A novel method using methyl methacrylate-embedded semithin serial sections and catalyzed reporter deposition. Am J Pathol. 2000;157:1829–38. doi: 10.1016/S0002-9440(10)64822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Odorfer Kl, Unger NJ, Weber K, Sandgren EP, Erben RG. Marker tolerant, immuno-competent animals as a new tool for regenerative medicine and long-term cell tracking. BMC Biotechnol. 2007;7:30. doi: 10.1186/1472-6750-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lu Y, Dhanaraj S, Wang Z, Bradley DM, Bowman SM, Cole BJ, Binette F. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24:1261–70. doi: 10.1002/jor.20135. [DOI] [PubMed] [Google Scholar]
- 176.Dorotka R, Windberger U, Macfelda K, Bindreiter U, Toma C, Nehrer S. Repair of articular cartilage defects treated by microfracture and a three-dimensional collagen matrix. Biomaterials. 2005;26:3617–29. doi: 10.1016/j.biomaterials.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 177.Breinan HA, Martin SD, Hsu HP, Spector M. Healing of canine articular cartilage defects treated with microfracture, a type-II collagen matrix, or cultured autologous chondrocytes. J Orthop Res. 2000;18:781–9. doi: 10.1002/jor.1100180516. [DOI] [PubMed] [Google Scholar]
- 178.Chevrier A, Hoemann CD, Sun J, Buschmann MD. Chitosan-glycerol phosphate/blood implants increase cell recruitment, transient vascularization and subchondral bone remodeling in drilled cartilage defects. Osteoarthritis Cartilage. 2007;15:316–27. doi: 10.1016/j.joca.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 179.Zhang X, Zheng Z, Liu P, Ma Y, Lin L, Lang N, Fu X, Zhang J, Ma K, Chen P, Zhou C, Ao Y. The synergistic effects of microfracture, perforated decalcified cortical bone matrix and adenovirus-bone morphogenetic protein-4 in cartilage defect repair. Biomaterials. 2008;29:4616–29. doi: 10.1016/j.biomaterials.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 180.Grande DA, Pitman MI, Peterson L, Menche D, Klein M. The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation. J Orthop Res. 1989;7:208–18. doi: 10.1002/jor.1100070208. [DOI] [PubMed] [Google Scholar]
- 181.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30:914–8. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 182.Gray ML. Toward imaging biomarkers for glycosaminoglycans. J Bone Joint Surg Am. 2009;91:44–9. doi: 10.2106/JBJS.H.01498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Eckstein F, Mosher T, Hunter D. Imaging of knee osteoarthritis: data beyond the beauty. Curr Opin Rheumatol. 2007;19:435–43. doi: 10.1097/BOR.0b013e328248b4be. [DOI] [PubMed] [Google Scholar]
- 184.Bauer DC, Hunter DJ, Abramson SB, Attur M, Corr M, Felson D, Heinegård D, Jordan JM, Kepler TB, Lane NE, Saxne T, Tyree B, Kraus VB Osteoarthritis Biomarkers Network. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. 2006;14:723–7. doi: 10.1016/j.joca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 185.Weidenbecher M, Henderson JH, Tucker HM, Baskin JZ, Awadallah A, Dennis JE. Hyaluronan-based scaffolds to tissue-engineer cartilage implants for laryngotracheal reconstruction. Laryngoscope. 2007;117:1745–9. doi: 10.1097/MLG.0b013e31811434ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Weidenbecher M, Tucker HM, Awadallah A, Dennis JE. Fabrication of a neotrachea using engineered cartilage. Laryngoscope. 2008;118:593–8. doi: 10.1097/MLG.0b013e318161f9f8. [DOI] [PMC free article] [PubMed] [Google Scholar]


