Abstract
Homing of endothelial progenitor cells (EPCs) is crucial for neoangiogenesis, which might be negatively affected by hypoxia. We investigated the influence of hypoxia on fibronectin binding integrins for migration and cell-matrix-adhesion. AMP-activated kinase (AMPK) and integrin-linked kinase (ILK) were examined as possible effectors of hypoxia.Human EPCs were expanded on fibronectin (FN) and integrin expression was profiled by flow cytometry. Cell-matrix-adhesion- and migration-assays on FN were performed to examine the influence of hypoxia and AMPK-activation. Regulation of AMPK and ILK was shown by Western blot analysis. We demonstrate the presence of integrin β1, β2 and α5 on EPCs. Adhesion to FN is reduced by blocking β1 and α5 (49% and 2% of control, P < 0.05) whereas α4-blockade has no effect. Corresponding effects were shown for migration. Hypoxia and AMPK-activation decrease adhesion on FN. Although total AMPK-expression remains unchanged, phospho-AMPK increases eightfold.The EPCs require α5 for adhesion on FN. Hypoxia and AMPK-activation decrease adhesion. As α5 is the major adhesive factor for EPCs on FN, this suggests a link between AMPK and α5-integrins. We found novel evidence for a connection between hypoxia, AMPK-activity and integrin activity. This might affect the fate of EPCs in ischaemic tissue.
Keywords: integrin, adhesion, hypoxia, EPC, AMPK
Introduction
The increasing evidence of postnatal neovascularization leads to new therapeutic concepts to restore function of damaged organs by infusion of ex vivo expanded circulating endothelial progenitor cells (EPC) [1]. Human EPCs are characterized by expression of endothelium specific proteins and by their ability to react similarly to endothelial cells [2]. They have been shown to home preferentially to areas of ischaemia and to increase vasculogenesis [3]. The cells exit the blood flow and migrate through the vessel wall by a complex series of adhesion processes to vascular endothelium and extracellular matrix [4].
Firm adhesion of EPCs to the vascular endothelium and the following invasion of the vessel wall is mediated by β2-integrins, while rolling and light adhesion are mainly mediated by selectins. The role of β1-integrins in this process remains unclear [4, 5].
Nevertheless, the ex vivo expansion of EPCs on fibronectin (FN) as described by Asahara et al. requires the presence of β1-integrins with the subunits α4 and α5 mediating adhesion to fibronectin [2]. Furthermore, expression of β1 as well as α4 and α5 integrin subunits has been demonstrated on RNA level in EPCs [4]. During the process of neovascularization EPCs specifically target hypoxic tissues. This is also known from leucocytes, which enter hypoxic areas as a result of inflammatory response and its consecutive high metabolism. The efficacy of EPCs to induce neovascularization is increased after hypoxic preconditioning. This might be due to superior homing capability after accumulation of β2-integrins on the cell surface [6].
The AMP-activated kinase (AMPK) plays a key role in energy metabolism of cells under hypoxic conditions. Its activation results in a metabolic switch from cellular energy storage to energy release under conditions of limited ATP-supply. Hypoxia as low as 0.3–5% oxygen does not decrease bioenergetics or induce cell death, but results in mitochondrial release of reactive oxygen species. This activates LKB1 and finally AMPK by phosphorylation at Thr172 of the α-subunit [7].
In this study, the role of FN-specific integrins for adhesion and migration of EPCs as well as the influence of hypoxia was examined. AMPK and ILK were studied as possible effectors of hypoxia. The findings of this study might clarify the behaviour of EPCs in ischaemic tissue.
Materials and methods
Cell culture
By using the identical protocol for in vitro expansion of EPCs as Assmus et al. [8], peripheral blood mononuclear cells (MNC) from healthy human volunteers were isolated by density gradient centrifugation (1.014g/ml, Ficoll, Biocoll; Biochrom AG, Berlin, Germany), followed by three washing steps with PBS. The cells were plated at 8Mio per 75 square centimetres of fibronectin coated cell culture flasks (10 μg/ml; Sigma-Aldrich Chemie GmbH, Munich, Germany) and maintained in high protein content endothelial basal medium (Cambrex) supplemented with hydrocortisone (1 μg/ml), bovine brain extract (12 μg/ml), gentamycin (50 μg/ml), epidermal growth factor (10 ηg/ml) and 20% foetal calve serum. After 3 days, non-viable cells were removed and fresh medium added for another day before experiments were started. EPCs were characterized by double staining with FITC-labelled lectin and incorporation of 1,1′-dioctadecyl-3,3′,3′-tetramethylindocarbocyanine-labelled acetyl-low-density lipoprotein (diI-Ac-LDL). Adherent cells were incubated with DiL-Ac-LDL (2.4 ng/ml) for 1 hr and stained using FITC-labelled U. europaeus agglutinin I (lectin, 10 ng/ml) for 3 hrs.
Additionally, flow cytometry showed expression of CD31, CD34 and CD18 as shown in supplementary figures (online supplement, Figs 1 and 2).
Flow cytometry
The 3 × 105 human EPCs, MNCs or cultivated monocytes were pre-incubated for 5min. with 5 μl Fc-block, washed in PBS containing 0.05% BSA and incubated for 30 min. at 4°C with 5 μl FITC-, PE- or APC-labelled antibodies (anti-β2, -β1, -α4, -α5, -CD14; BD Biosciences, Heidelberg, Germany) per 3 × 105 cells. Fluorochrome labelled isotype IgG1 served as control. 2 ml PBS was added and cells were centrifuged for 15 min. at 4°C with 800 ×g. Cells were then resuspended in PBS containing 0.05% BSA. Flow cytometry was performed before and after 4 days of cell-type specific culture.
Cell-matrix adhesion
The 96-well plates (Costar Corning, Amsterdam, The Netherlands) were coated overnight at 4°C with 10 μg/ml recombinant fibronectin (Sigma-Aldrich) in phosphate buffered saline (PBS). Wells were washed with PBS once and 50 μl adhesion buffer was added to prevent drying. Ex vivo expanded EPCs were stained with 5 μl CellTracker™ green (CMFDA; Molecular Probes/Invitrogen, Life Technologies, Darmstadt, Germany) per 3 ml culture medium for 5 min. and detached with trypsin. Trypsin was blocked, cells washed and resuspended in adhesion buffer (150 mM NaCl, 20 mM Hepes, 2 mM MgCl2, 0.05% fraction V bovine serum, 5% Glucose, pH 7.42). Experiments were performed as indicated in triplets with 105 cells per well. For inhibition experiments, EPCs were pre-incubated with antibodies for 15 min. at 4°C. Anti-α5 (CBL497, clone SAM-1), anti-β2 (CBL158, clone MEM-48), anti-β1 (CBL481, clone TDM29), anti-α4 (MAB1954Z, clone P4G9), anti-α5 (MAB1956Z, clone P1D6), stimulating anti-β1 (MAB1951Z, clone P4G11), blocking anti-β1 (MAB2253Z, clone 6S6) were obtained from Chemicon International. Goat IgG (R&D Systems, Minneapolis, MN, USA) served as control. Alternatively, experiments with cyclic RGD peptide (Sigma-Aldrich) at 50 μM were performed. Pre-incubation was performed similar to antibody treatment. After pre-incubation, the plate was centrifuged for 3 min. at 300 r.p.m. (RZF 17 g) to achieve simultaneous contact of the cells to the plate. After 20 min. of incubation at 37°C, non-adherent cells were removed and the plates were washed twice with adhesion buffer and fixed with 4% paraformaldehyde. Adherent cells were quantified by fluorescence microscopy (magnification 20×, Axiovert 100; Carl Zeiss, Oberkochen, Germany). Cells were counted in five randomly selected fields covering different areas of each well. Images were digitized and the software package ScionPro with custom macros was used for semi-automated counting. Data were normalized and presented as percentage of control.
Cell migration assay
The assays were performed as previously described [4]. Transwell membranes (8 μm; Costar) were coated with fibronectin (10 μg/ml; Sigma-Aldrich, Germany) overnight at 4°C. Ex vivo– expanded human EPCs were stained with 5 μl CellTracker™ green (5-chloromethylfluorescein diacetate, Molecular Probes, Invitrogen) per 3 ml culture medium for 5 min. at 37°C. EPCs were detached by trypsinization. Trypsin was neutralized and 1.2x105 EPCs were resuspended in 100 μl serum-free RPMI 1640 containing 0.05% BSA. Serum-free RPMI 1640 (600 μl) with 0.05% BSA containing 50 ng/ml of SDF-1α was placed in the lower chambers.
Cells were incubated at 37°C in 5% CO2 for 12 hrs. For inhibition experiments, EPCs were pre-incubated for 15 min. at 4°C with 5 μl of the indicated antibodies per 3.0 × 105 cells. The antibodies were free of sodium azide to reduce toxicity during the assay. Cells remaining on the upper surface of the filters were mechanically removed, and migrated cells at the lower surface were fixed with 4% formaldehyde and counted in five fields by using a fluorescence microscope (Axiovert 100; Carl Zeiss).
Hypoxic conditioning
According to the requirements of the experiment either adherent (Western blots) or resuspended cells (cell-matrix-adhesion, migration) were incubated in hypoxic environment. An electronically regulated incubator was used (Model C42; Labotect, Göttingen, Germany) to determine temperature and concentration of oxygen and carbondioxide during the experiments. Controls were preserved in regular incubators (37°C, 5% carbondioxide, oxygen as indicated, nitrogen ad 100%). The incubator was calibrated prior to each experiment.
Statistical analysis
Data were calculated using Microsoft™ Excel for Mac and statistical calculations were performed by Graphpad™ Prism (GraphPad Software Inc, La Jolla, CA, USA). Data are presented as mean ± S.D. Comparisons between groups were calculated using ANOVA with Bonferroni correction for multiple testing. For non-parametric distributions tests were used as appropriate.
Results
Integrin profile of EPCs
As revealed by flow cytometry, EPCs express β1 and α5 but not α4 (Fig. 1). In contrast, MNCs before and after non-cell-specific cultivation show unaltered expression of both α4 and α5. Both cell types were positive for β2 (see online supplement). Preselected cells expressing CD14 showed a similar expression of integrin subunits (Suppl. Fig. 6).
Fig 1.
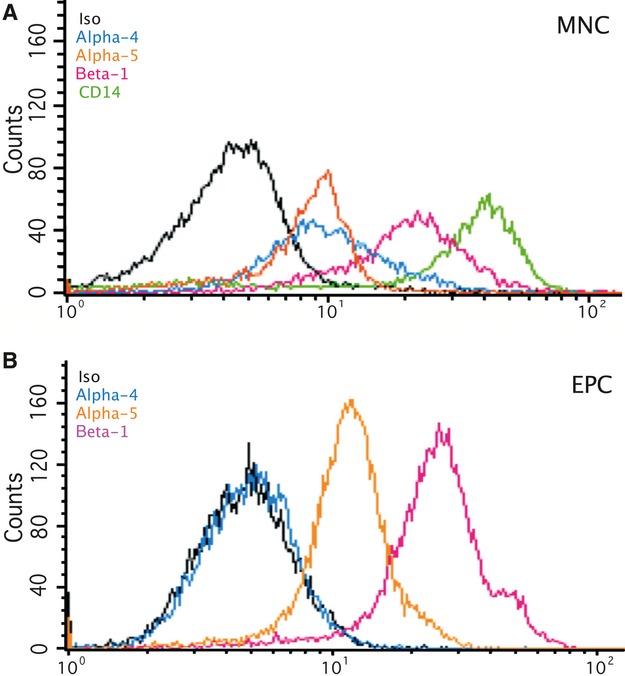
Human MNCs and in vitro expanded EPCs were stained with conjugated antibodies against β1, α5 and α4 integrins before integrin surface expression was analysed by flow cytometry. Significant lower levels of α4 (CD49d) were detected in EPCs. A representative FACS blot is shown.
Functionality of expressed integrins
The EPCs were pretreated with integrin specific antibodies and data were normalized for control. As expected, there is no significant loss of adhesion to fibronectin due to blockade of β2 (81.8 ± 9.3% of control). Inhibition of β1 leads to partial loss of adhesion and inhibition of α5 to a complete ablation of adhesive capacity to fibronectin (49.4 ± 15.8% and 2.1 ± 1.3% respectively). The use of different clones of antibodies leads to the same results (clone TDM29 and clone 6S6 for β1, clone SAM-1 and clone P1D6 for α5), although application of a stimulating antibody targeting β1 leads to a slight albeit statistically not significant increase of adhesion (111.2 ± 18.7%). α4-blockade does not alter adhesion (106 ± 26%). In consistency with the expression profile seen in flow cytometry, only inhibition of the expressed fibronectin-specific integrins β1 and α5 leads to a significant loss of adhesion in this model. The effect of α5-blockade on EPCs was much higher than blockade of β1. In contrast, blockade of each of the integrin subunits α4, α5 and β1 showed to diminish adhesion to fibronectin in peripheral blood MNC. Using a cyclic RGD peptide, adhesion to fibronectin decreased by 50% in endothelial progenitor cells (see online supplement, Figs 4 and 5).
To determine the role of the expressed integrins in the dynamic process of migration, EPCs migrated in a fibronectin coated modified Boyden chamber along a SDF-α-gradient. Migration is markedly reduced when β1 or α5 are blocked (45.4 ± 22.1% and 57.5 ± 9.1% of control, respectively). Blockade of α4 showed no effect on migration capacity (98.4 ± 16.9% of control, Fig. 2).
Fig 2.
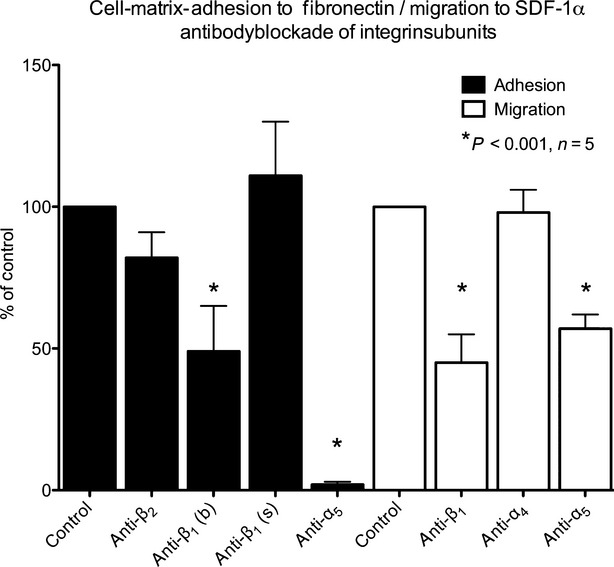
Human EPCs were pre-incubated with the indicated blocking antibodies. While blocking anti-β1 (CD29b) and anti-α5 (CD49e) decreased adhesion to fibronectin, inhibition of α4 (CD49d) and β2 (CD18) had no significant effect. A stimulating β1 antibody (CD29s) moderately increased adhesion (black bars). White bars show VEGF stimulated EPC-migration on fibronectin. The results parallel adhesion experiments (n = 5, data presented as percent of control, mean±S.D., *P < 0.05 versus control).
Adhesion of EPCs under hypoxic conditions
We further investigated the effects of hypoxia on integrin activity. Ex vivo expanded human EPCs were subjected to hypoxia. The static adhesion assay was performed as described above. With increasing duration of hypoxia, EPCs are less able to adhere to fibronectin in this experiment. Statistical significance is reached at 1.5 hrs of 1% oxygen (Fig. 3). After 2 hrs, adhesion capacity is reduced to 55.1 ± 22.3% of control and reaches a minimum of 39.9 ± 22.5% of control after 4hrs. Further increase of time under hypoxia up to 12 hrs had no additional effect. The cells show no signs of necrosis or apoptosis as determined by Roche® Cell death detection kit (see online supplement, Fig. 3).
Fig 3.
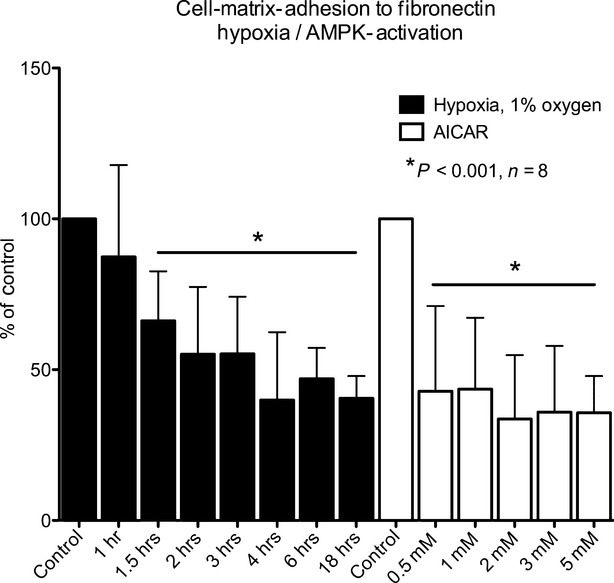
Human EPCs were either submitted to hypoxia (black) or stimulated with AICAR (white). Integrin activity was tested by adhesion to fibronectin. Increase of AMPK-activity by both hypoxia and AICAR-stimulation decreases integrin activity (n = 8, data presented as percent of control, mean ± S.D., *P < 0.05 versus control).
As the onset of this effect is too early to be caused by altered protein translation, we focused on protein interactions. AMP-dependent kinase is a sensor molecule for intracellular energy content expressed as ADP/ATP and AMP/ATP ratio. It is stimulated by hypoxia and the highly specific activator aminoimidazole carboxamide ribonucleotide (AICAR). In vitro expanded EPCs were treated with increasing doses of AICAR for 4hrs and then tested for their adhesion capacity to fibronectin. Even low doses of AICAR result in diminished integrin activity beginning with 42.8 ± 23.7% at 0.5 mM AICAR to 35.7 ± 12.2% at 5 mM AICAR (Fig. 3).
Activation of AMP in EPCs under hypoxic conditions
Activation of AMPK by phosphorylation at Thr172 has been shown in other cell types to activate AMPK during hypoxia. In EPCs the level of phosphorylation increased eightfold, whereas total expression remained unchanged (Fig. 4). As demonstrated by GSK3-assay, ILK-activity was not altered by hypoxia (Fig. 4).
Fig 4.
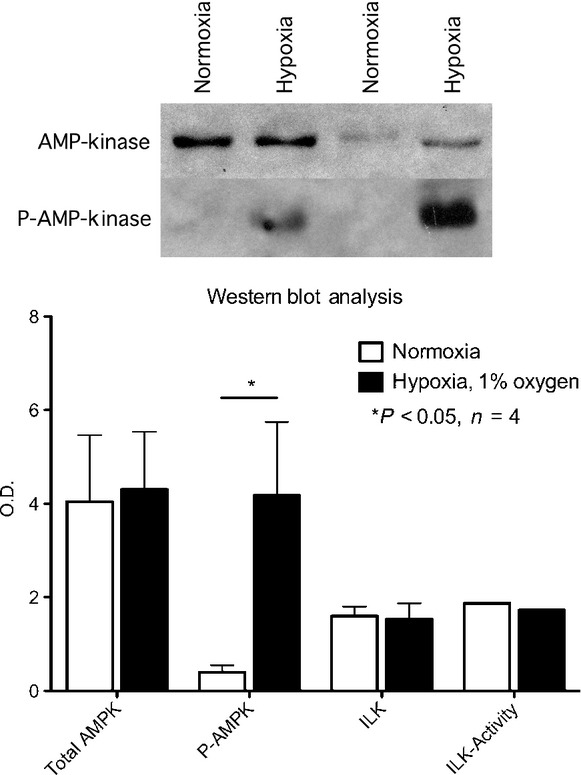
Western blot analyses showed unaltered total level of AMPK but an increase of phosphorylation at an activating phosphorylation site. ILK-activity as shown by GSK3-assay remained unchanged.
Discussion
In this study we were able to demonstrate the expression of fibronectin-specific integrins on EPCs and the markedly decreased expression of α4 compared to MNCs. We further showed the functional relevance of this finding for adhesion and migration on FN. Both hypoxic and pharmacological AMPK-activation lead to diminished activity of these integrins. We finally demonstrated the phosphorylation and therefore activation of AMPK during hypoxia. Our results add to present knowledge about behaviour of EPCs under hypoxic conditions as found in targeted tissues for cell based therapy.
In this study we were able to verify the expression of α5- and β1-integrins by flow cytometry. Although MNCs also expressed α4-integrin, this could not be seen after endothelium specific culture of the cells. Despite the previously described heterogeneity of ex vivo expanded early EPCs [9], our finding represents a common denominator of these subpopulations similar to their endothelial surface markers. Chavakis et al. compared the mRNA expression of EPCs to HUVECs by using a microarray. In that investigation, both β2 and CD11b/c were significantly increased in EPCs compared to HUVECs on mRNA level, whereas β1 and α5 were slightly decreased without statistical significance. α4-integrin was slightly increased without reaching statistical significance.
We perceived a high expression of β1 and α5, whereas α4 was markedly decreased during expansion. Both α4β1 and α5β1 integrins have a high affinity to fibronectin [10], which is an important regulator of various cellular processes including survival, differentiation, growth and migration. It is deposited actively in the ECM by cells and circulates freely in the plasma. Recent data suggested an important role in flow-induced vascular remodelling by influencing the invasion of leucocytes and the proliferation of vascular smooth muscle cells [11]. Most importantly, it is used as immobilized form for the in vitro expansion of EPCs [8]. Therefore, we investigated the functional relevance of the altered integrin expression. Our findings emphasize the importance of α5β1 integrins for EPCs to adhere on the provided fibronectin matrix during in vitro expansion. Interestingly, inhibition of α5 shows a markedly pronounced effect on the cells compared to blockade of β1. Furthermore, the possibility of an insufficient affinity of the antibody was addressed by comparing different clones of blocking antibodies without any effect on this finding. Hence, one can speculate about the signalling properties of integrins and the effects on the signalling pathways of such blockage. These pathways have been subject to investigation in case of β1, but data on α5 remain scarce [12, 13]. The used cell culture protocol has been shown to produce subpopulations of EPCs probably because of the short time for differentiation. The phenotypic characterization with both functional assays (diLDL-uptake) and flowcytometry for endothelial surface markers showed a high content of endothelial type cells (see online supplement) in concordance with previous literature [9]. The integrin profiles were found irrespective of this heterogeneity, but further analysis of subpopulations may help our understanding of cell based tissue regeneration.
Migration comprises a series of complex actions of the cell. It depends on a coordinated sequence of adhesion and release of molecules on the cell surface as well as the cell moves along a chemotactic gradient. This process, in which integrins and selectins are important effectors, is only incompletely understood [14]. In our experiments with EPCs we demonstrated the functional relevance of β1-integrins for migration on a fibronectin matrix. The inhibition of both β1 and α5 but not α4 resulted in decreased adhesion and migration capacity of EPCs. This finding matches the expression profile.
Akita and coworkers found an increased efficacy of EPCs regarding vasculogenesis after hypoxic preconditioning. This was mainly due to an accumulation of β2-integrins [6]. Kong et al. investigated the effects of hypoxia on the integrin expression of leucocytes. They reported an up-regulation of β2 but not β1 under hypoxic conditions. Additionally they found an increased β2-integrin dependent increase of adhesion to endothelial cells after hypoxia [15]. Taking into account the importance of β2-integrins for the homing of EPCs [4], this mechanism explains the effect of hypoxic preconditioning of EPCs. The effect of hypoxia on β1-integrins remained unclear. In this study, we subjected EPCs to hypoxic conditions and demonstrated a decreased adhesion of EPCs on fibronectin. As the adhesion to fibronectin of these cells was strictly dependent from α5β1 integrins, we deducted an influence of hypoxia on expression or function of these integrins. Consistent with the aforementioned literature, no increase of β1 or α5 could be detected after hypoxia (Suppl. Fig. 7A–C).
The rapid onset of the effect leads to the hypothesis of protein modifications due to hypoxia. Hypoxia activates AMP-dependent kinase (AMPK) in EPCs, which is a sensor molecule for metabolic stress and energy level [7]. Recent data suggest a role of mitochondrial ROS-release during hypoxia as the main activator of AMPK in this setting [7]. Hypoxia leads to phosphorylation of AMPKα at Thr172 and enhances its enzymatic activity. This influences an extensive number of pathways [16–19]. Aminoimidazole carboxamide ribonucleotide (AICAR) is a specific activator of AMPK. In our experiments we demonstrated the same dose-dependent reduction of adhesion by AICAR as was seen in hypoxia. As described before, we observed phosphorylation of AMPK on Thr172 during hypoxia. Taking into account these findings, we conclude that upon activation AMPK affects cell-matrix adhesion directly or indirectly by modification of either α5 or β1.
Several phosphorylation sites on the cytosolic domains are believed to control conformation and alignment of the branches of α- and β-subunit causes altered adhesion [10]. Neither the exact impact of phosphorylation on these sites nor the phosphorylating enzymes have been sufficiently examined in this context. Our study contributes to knowledge of inside-out signalling of integrins. We have previously reported the down-regulation of the active conformation of β1 in response to ex vivo deletion of ILK in endothelial cells. The mechanism led to apoptosis of the cells and was independent of akt [20]. Our experiments show neither decreased ILK expression in response to hypoxia nor decreased ILK-activity in contrast to previous reports [21] (Fig. 4).
Increasing intensity of both hypoxia and AICAR leads to marked reduction of adhesion. But this effect did not abolish the adhesion capacity. Therefore, one might speculate whether inactivation of β1 or α5 might be the possible mechanism of hypoxia and whether activation of AMPK is causative for this.
As endothelial nitric oxide synthetase is activated by AMPK [22, 23], we carried out adhesion experiments using nitric oxide donators and found no impact on adhesion. Therefore, not only eNOS but also most heme-group dependent enzymes appeared unlikely targets.
In summary, we demonstrated the expression of α5β1-integrin on ex vivo expanded EPCs in contrast to α4β1 and α5β1 on MNCs. We were able to demonstrate the importance of α5 for adhesion to fibronectin as well as the influence of hypoxia on functional capacity of α5β1-integrin. We then confirmed the activation of AMPK by phosphorylation of AMPKα-Thr172 in response to hypoxia in EPCs. Finally, data could be presented implicating a causative relation of AMPK and function of α5β1-integrin. As previously described, α5β1-integrins might not be important for the homing of EPCs, as their adhesion to endothelium is not affected. But binding of fibronectin to α5β1-integrins might play a role in cell growth and differentiation of EPCs during tissue repair. The influence of hypoxia on α5β1-integrins and its downstream targets LKB1, mTOR, eNOS, KLF and many more remains subject of further investigation.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Characterization of EPCs (A–D) Representative fluorescence microscopic images showing DiI-Ac-LDL uptake and Ulex europaeus-lectin binding of adherent cells as well as typical spindle-shaped endothelial cell-like morphology after 4 days of cell culture under endothelial selection pressure (100× magnification). A: green – Ulex europaeus-lectin, B: red – DiI-Ac-LDL, C: DAPI counterstaining, D: fusion image of A, B and C.
Figure S2 Characterization of EPCs (A–C) Representative flowcytometry analysis showing iso-IgG1 (A), co-staining of CD31-FITC (B&D) and CD34-APC (B&D) (B) and co-staining of CD31-FITC and CD18-APC (B&D) (C). As described previously, EPCs express highly CD31, CD34 and CD18 (beta2-integrin subunit) after in vitro expansion.
Figure S3 Cell death and apoptosis. Two experiments were performed to detect cell death during hypoxia at 1% oxygen for 8 hrs. The assay was performed following vendor instructions (Roche, Roche Diagnostics Deutschland GmbH, Mannheim, Germany).
Figure S4 Cell-matrix-adhesion to fibronectin. Peripheral blood mononuclear cells (MNC) and in vitro expanded endothelial progenitor cells (EPC) from the same individuals were compared. Integrin subunits were blocked selectively by antibodies by pre-incubation. Although adhesion is blocked by antibodies targeting α5 and β1, only MNCs are blocked by the anti-α4-antibody.
Figure S5 Cell-matrix-adhesion to fibronectin. EPCs were pre-incubated with 50 μM of cyclic RGD-peptide (Sigma-Aldrich), an established inhibitor of αVβ3, αVβ5, α4β1 and α5β1. EPCs show a decreased adhesion capacity to the fibronectin matrix, when RGD-recognizing integrins are blocked.
Figure S6 Flow cytometry for integrin subunits. Peripheral blood mononuclear cells were isolated by density gradient. The cells were enriched by CD14 labelling by using magnetic beads. After 4 days of endothelial specific culture, flow cytometry was performed (see methods). CD14-enriched in vitro expanded cells demonstrated the same integrin profile as endothelial progenitor cells obtained from the Asahara protocol (Fig. 1).
References
- 1.Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–32. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Aicher A, Brenner W, Zuhayra M, et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107:2134–9. doi: 10.1161/01.CIR.0000062649.63838.C9. [DOI] [PubMed] [Google Scholar]
- 4.Chavakis E, Aicher A, Heeschen C, et al. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavakis E, Urbich C, Dimmeler S. Homing and engraftment of progenitor cells: a prerequisite for cell therapy. J Mol Cell Cardiol. 2008;45:514–22. doi: 10.1016/j.yjmcc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Akita T, Murohara T, Ikeda H, et al. Hypoxic preconditioning augments efficacy of human endothelial progenitor cells for therapeutic neovascularization. Lab Invest. 2003;83:65–73. doi: 10.1097/01.lab.0000050761.67879.e4. [DOI] [PubMed] [Google Scholar]
- 7.Emerling BM, Weinberg F, Snyder C, et al. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic Biol Med. 2009;46:1386–91. doi: 10.1016/j.freeradbiomed.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assmus B, Schachinger V, Teupe C, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–17. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 9.Schachinger V, Assmus B, Britten MB, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44:1690–9. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 11.Chiang HY, Korshunov VA, Serour A, et al. Fibronectin is an important regulator of flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol. 2009;29:1074–9. doi: 10.1161/ATVBAHA.108.181081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okamura M, Yamaji S, Nagashima Y, et al. Prognostic value of integrin beta1-ILK-pAkt signaling pathway in non-small cell lung cancer. Hum Pathol. 2007;38:1081–91. doi: 10.1016/j.humpath.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Wederell ED, de Iongh RU. Extracellular matrix and integrin signaling in lens development and cataract. Semin Cell Dev Biol. 2006;17:759–76. doi: 10.1016/j.semcdb.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Muller WA. Leukocyte-endothelial cell interactions in the inflammatory response. Lab Invest. 2002;82:521–33. doi: 10.1038/labinvest.3780446. [DOI] [PubMed] [Google Scholar]
- 15.Kong T, Eltzschig HK, Karhausen J, et al. Leukocyte adhesion during hypoxia is mediated by HIF-1-dependent induction of beta2 integrin gene expression. Proc Natl Acad Sci USA. 2004;101:10440–5. doi: 10.1073/pnas.0401339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinberg GR, Watt MJ, Febbraio MA. Cytokine Regulation of AMPK signalling. Front Biosci. 2009;14:1902–16. doi: 10.2741/3350. [DOI] [PubMed] [Google Scholar]
- 17.Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 2008;32:S7–12. doi: 10.1038/ijo.2008.116. [DOI] [PubMed] [Google Scholar]
- 18.Williams T, Brenman JE. LKB1 and AMPK in cell polarity and division. Trends Cell Biol. 2008;18:193–8. doi: 10.1016/j.tcb.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Brenman JE. AMPK/LKB1 signaling in epithelial cell polarity and cell division. Cell Cycle. 2007;6:2755–9. doi: 10.4161/cc.6.22.4927. [DOI] [PubMed] [Google Scholar]
- 20.Friedrich EB, Liu E, Sinha S, et al. Integrin-linked kinase regulates endothelial cell survival and vascular development. Mol Cell Biol. 2004;24:8134–44. doi: 10.1128/MCB.24.18.8134-8144.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SP, Youn SW, Cho HJ, et al. Integrin-linked kinase, a hypoxia-responsive molecule, controls postnatal vasculogenesis by recruitment of endothelial progenitor cells to ischemic tissue. Circulation. 2006;114:150–9. doi: 10.1161/CIRCULATIONAHA.105.595918. [DOI] [PubMed] [Google Scholar]
- 22.Murphy BA, Fakira KA, Song Z, et al. AMP-activated protein kinase (AMPK) and nitric oxide (NO) regulate the glucose sensitivity of ventromedial hypothalamic (VMH) glucose-inhibited (GI) neurons. Am J Physiol Cell Physiol. 2009;297:C750–8. doi: 10.1152/ajpcell.00127.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz E, Schuhmacher S, Munzel T. When metabolism rules perfusion: AMPK-mediated endothelial nitric oxide synthase activation. Circ Res. 2009;104:422–4. doi: 10.1161/CIRCRESAHA.109.194274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


