Abstract
Neovascularization is an integral process of inflammatory reactions and subsequent repair cascades in tissue injury. Monocytes/macrophages play a key role in the inflammatory process including angiogenesis as well as the defence mechanisms by exerting microbicidal and immunomodulatory activity. Current studies have demonstrated that recruited monocytes/macrophages aid in regulating angiogenesis in ischemic tissue, tumours and chronic inflammation. In terms of neovascularization followed by tissue regeneration, monocytes/macrophages should be highly attractive for cell-based therapy compared to any other stem cells due to their considerable advantages: non-oncogenic, non-teratogenic, multiple secretary functions including pro-angiogenic and growth factors, straightforward cell harvesting procedure and non-existent ethical controversy. In addition to adult origins such as bone marrow or peripheral blood, umbilical cord blood (UCB) can be a potential source for autologous or allogeneic monocytes/macrophages. Especially, UCB monocytes should be considered as the first candidate owing to their feasibility, low immune rejection and multiple characteristic advantages such as their anti-inflammatory properties by virtue of their unique immune and inflammatory immaturity, and their pro-angiogenic ability. In this review, we present general characteristics and potential of monocytes/macrophages for cell-based therapy, especially focusing on neovascularization and UCB-derived monocytes.
Keywords: ischemia, inflammation, angiogenesis/arteriogenesis, monocyte/macrophage, umbilical cord blood, bone marrow, peripheral blood, transplantation, stem cells
Introduction
Monocytes, which are derived from monoblasts, haematopoietic stem cell precursors in the bone marrow (BM), circulate in the bloodstream before extravasating into tissues of the body. In the tissues, monocytes differentiate into various types of tissue resident macrophages depending on their anatomical locations, for example Langerhans cells in skin, Kupffer cells in liver, osteoclasts in bone, microglia in central nervous system, alveolar macrophages in lung and synovial type A cells in synovial joint [1–5] (Fig. 1). Monocytes/macrophages can perform phagocytosis by using mediators such as antibodies or complement components that coat the microbes or by binding to the pathogens directly via specific receptors that recognize them (endocytosis). Additionally, monocytes/macrophages are able to kill infected host cells, through an immune system response, termed antibody-mediated cellular cytotoxicity [4, 6]. Moreover, they are unique immunoregulatory cells able to both stimulate and suppress immune activities, including antigen presentation to T cells and controlled secretion of a wide range of cytokines and growth factors [1, 3, 7]. The bottom line in monocyte/macrophage function is that they play a major role in the inborn defence system by way of killing pathogens through phagocytosis and cellular cytotoxicity, and immunomodulation [1, 7].
Fig 1.
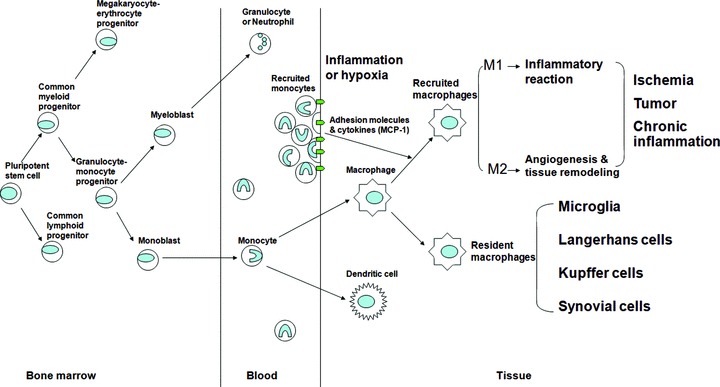
Schematic diagram depicting monocyte/macrophage ontogeny. The pluripotent stem cells differentiate into myeloid or lymphoid progenitors in BM. The granulocyte–monocyte progenitors are derived from the common myeloid progenitor cell before differentiating into myeloblast and monoblast. Monocytes are differentiated from monoblast and subsequently move from the BM into the blood. Blood monocytes differentiate into various types of resident macrophages depending on their anatomical locations after extravasating into tissues. On the other hand, during the early inflammatory process, recruitment and transendothelial migration of circulating monocytes is augmented by a series of adhesion and chemotactic materials, expressed by inflammatory cells. Recruited monocytes migrate along chemotactic and oxygen gradients between normal and injured tissues, and accumulate within inflammatory and hypoxic cores in ischemia, or solid tumours, or chronic inflammatory diseases before differentiation into recruited macrophages which have polarization, M1 or M2 subset.
Role of monocytes in neovascularization
Like neutrophils, one interesting function of monocytes/ macrophages is to promote angiogenesis related to inflammatory reactions [8–10]. Angiogenesis (or neovascularization) is a major element of inflammatory processes including subsequent repair cascades [4]. During the early inflammatory process, circulating blood monocytes extravasate into tissues [1]. Initially, neighbouring endothelial and inflammatory cells regulate this monocyte passage through vessel walls by releasing of a series of adhesion and chemotactic materials [1, 2, 11]. Along chemotactic and oxygen gradients between normal and injured tissues, extravasated monocytes move and gather into hypoxic and/or necrotic cores of diseased tissues before differentiation into tissue macrophages. The representative pathological tissues to which monocytes/macrophages are apt to accumulate are as follows: solid tumours, myocardial or cerebral infarction, synovial joints of chronic arthritis or atheromatous plaques, bacterial infection and healing wounds [1, 3, 11, 12] (Fig. 1).
After differentiation from monocytes, macrophages in tissue have been known to exist as polarized populations, M1 and M2 subsets [12–14]. Whereas M1 polarized macrophages are powerful inflammatory cells that produce pro-inflammatory cytokines and phagocytize pathogens, M2 macrophages modulate the inflammatory responses and help with angiogenesis and tissue repair [12–14]. Interestingly, in gene expression of macrophages, a combination of M1 and M2 subsets early in wound healing turns into dominantly M2 genes later [15]. During the early stage of the wound healing process, M1 macrophages lead to direct inflammatory reaction that cleans up the wound and debris of microbes and/or injured host tissues while tissue repair and angiogenesis are initiated by M2 macrophages at the same time. In the late stage when the cleansing by M1 macrophages is almost over, the prevailing M2 macrophages go on with their work, tissue regeneration including angiogenesis [15]. Accumulating evidence suggests that recruited monocytes/macrophages aid in modulating and regulating neovascularization in ischemic tissue, tumours and chronic inflammation such as arthritic joints and atherosclerosis.
Angiogenesis in ischemia
In recent years, the importance of circulating monocytes/ macrophages in neovascularization has been demonstrated in ischemic diseases [16–18]. Arteriogenesis, the structural growth of pre-established arteriolar webs into true effective collateral arteries, seems to be initiated by increased fluid shear stress which results from arterial obstruction within the developing collateral arteries and not induced by tissue hypoxia and ischemia [19, 20]. In contrast, angiogenesis, the formation of new capillaries from pre-existing blood vessels, is induced by hypoxia, and capillary density increases in sites of severe and acute ischemia [19, 21].
Although arteriogenesis and angiogenesis both induce neovascularization through different mechanisms, monocytes/macrophages essentially contribute to both actions. In arteriogenesis, abrupt arterial flow obstruction resulting from an embolus or a progressive stenosis increases fluid shear stress in the arteriolar web, and subsequently adhesion molecules and chemokines such as endothelial adhesion molecule [22] and monocyte chemotactic protein-1 (MCP-1) [23] increase significantly. Blood monocytes are activated and are drawn to the collateral artery by MCP-1. Once there, they go through the vessel wall by way of binding to adhesion molecules and/or differentiate into tissue macrophages before producing plenty of growth factors and cytokines [4], which can promote endothelial and smooth muscle cell proliferation [16, 20].
Angiogenesis is a combination of more intricate processes, many of which are regulated by vascular endothelial growth factor (VEGF) and its receptors (VEGFR), which are known to initiate angiogenesis [16]. Recent data suggest, some subsets of angiopoietins (Ang-1 and 2) and their receptors (Tie) are critical to the secondary stages of the angiogenic process such as maturation, stabilization and remodelling of vessels [24]. Hypoxia and tissue necrosis significantly affect production of VEGF/VEGFR and angiopoietin/Tie receptors [25–28]. In turn, VEGF and angiopoietin induce the recruitment of endothelial progenitor cells and monocytes/macrophages [28, 29].
Recruited monocytes/macrophages promote angiogenesis by several potential mechanisms in each phase of the angiogenic process. First, macrophages degrade the extracellular matrix using matrix metalloproteinases (MMPs) (e.g. MMP-9) and proteolytic enzymes, leading to endothelial cell migration [30, 31]. Via a path through the extracellular matrix, growth factors and endothelial cells are mobilized from established vessels to form new capillaries [16].
Second, monocytes/macrophages release many pro-angiogenic cytokines such as interleukin (IL)-1β, basic fibroblast growth factor (bFGF), VEGF, IL-8, substance P, tumour necrosis factor (TNF)-α, transforming growth factor (TGF)-α and -β and prostaglandins [4, 30, 31], which act directly or indirectly on promoting endothelial cell proliferation, migration or tube formation [4, 16, 31]. Although monocytes/macrophages are also able to release angiostatic factors such as IL-12, IL-18 [31], thrombospodin 1, interferon-α and -γ[4], their production of inhibitory cytokines is regulated by pro-angiogenic factors. For example, IL-12 production is inhibited by increasing Ang-2 levels [28].
Third, monocytes/macrophages may differentiate into endothelial cells, which help directly in vessel wall production [30, 32, 33]. With specific pro-angiogenic factor stimulation, monocytes/macrophage progenitors can transdifferentiate into endothelial-like cells, which are directly incorporated into new blood vessels [16, 34–36].
Fourth, with exposure to VEGF or hypoxia, endothelial cells produce MCP-1 [37, 38] as well as VEGF [30] and angiopoietin [28], all of which activate and attract monocytes/macrophages [16]. In reverse, monocytes/macrophages not only up-regulate Tie-2 (angiopoietin receptor) [28], but also secrete MCP-1 and VEGF when they are activated by hypoxia, which in turn influences endothelial cell migration and proliferation [31], and even themselves by auto- and paracrine actions, and subsequently brings redoubling effect to the angiogenesis process [39].
Angiogenesis in tumours and chronic inflammation
For recent decades, there has been accumulating evidence that together with tumour cells themselves, monocytes/macrophages also play a major role in angiogenesis and progression of tumours [4] as neoplastic tissues exhibited neovascularization only with macrophages [40] and monocyte depleted animals showed a significant decrease in tumour angiogenesis [41]. The number of macrophages in tumour tissue is greater than in most normal tissues [42]. Augmented mobilization and differentiation from circulating monocytes more likely result in increased macrophages in tumour tissue, rather than simple proliferation of tissue macrophages [12, 43]. The pro-inflammatory cytokines which are induced by tumour cells and central hypoxia, attract monocytes/macrophages to sites of neoplastic necrosis and growth [44, 45].
Most of all, monocytes/macrophages appear to be recruited to promote neovascularization that is critical to tumour growth and progression. In most tumours, significantly more of the tumour-associated macrophages (TAMs) are of the M2 macrophage subpopulation, which potentiate angiogenesis, compared to the M1 subset which kills tumour cells [12, 14, 46]. M2 TAMs secrete plenty of pro-angiogenic factors, such as VEGF, TNF-α, IL-8, TGF-β and bFGF [3, 12–14, 31, 43, 47], and a broad range of proteolytic enzymes [47], which can break down the extracellular matrix and in turn, lead to endothelial cell migration for angiogenesis [30, 31, 43]. Of importance, significant correlations between TAM and vascular densities, have been observed in colon cancer [48], breast cancer [49] and pancreatic cancer [50], which suggest that TAMs potentiate tumour angiogenesis [43]. Besides, strong TAM recruitment is significantly related to poor prognosis in some tumour types [48, 49].
Angiogenesis also contributes to chronic inflammatory pathology. The chronic inflammatory status is maintained by virtue of new vessel formation, which continuously delivers inflammatory cells and supplies oxygen and nutrients to the area of inflammation [51]. Mechanisms and characteristics of neovascularization related to chronic inflammation are no different than angiogenesis induced by ischemia or tumour. Most cytokines and growth factors known to regulate angiogenesis can be produced by monocytes/macrophages [4]. During pannus formation in rheumatoid arthritis and atheromatous plaque formation in atherosclerosis, the proliferating inflamed tissue contains a number of inflammatory cells, especially monocytes/macrophages, newly forming vessels and derived inflammatory mediators [51]. The end result is that increased monocytes/macrophages can be observed at most inflammatory areas where angiogenesis is occurring in an abnormal environment, including pathological conditions such as ischemia, tumour and chronic inflammatory disease as well as wound healing [4, 51–53].
Monocytes versus stem cells for transplantation
A number of studies have been performed to explore the therapeutic potential of monocytes/macrophages for arteriogenesis and/or angiogenesis primarily in ischemic disease models [17, 54–57]. For arteriogenesis and angiogenesis, monocyte may be novel and fascinating as a target cell for cell-based therapy towards promotion of collateral vessel growth followed by tissue regeneration, which can attenuate local tissue ischemia and improve clinical outcome [58, 59]. Neovascularization from endogenous monocytes can be induced directly by either an infusion of MCP-1 [56] or granulocyte-macrophage colony stimulating factor (GM-CSF) [54], or indirectly through a rebound effect after administration of 5-fluorouracil [55]; all of these materials can promote homing to and accumulation around collateral arteries, or proliferation of endogenous monocytes.
In addition, neovascularization can be achieved by the transplantation of exogenous autologous or allogeneic monocytes with or without ex vivo engineering. By developing an effective method for isolation of monocytes from peripheral blood [60–63], an adequate autologous monocyte stock can be collected. Although peripheral blood yields a finite number of monocyte, an adequate monocyte stock can be accumulated for future usage through repeated harvesting of autologous monocytes from the patient’s peripheral blood by leucapharesis [60]. The advance of ex vivo tissue engineering leads to new strategies using monocytes as vehicles for therapeutic gene transfection, for example delivery of GM-CSF to promote neovascularization [17] as well as direct effectors for cell transplantation. This technical development from cell isolation to application should make monocytes more promising for regenerative medicine, especially in terms of augmentation of arteriogenesis and angiogenesis.
There may be several advantages of monocyte transplantation (Table 1) compared to stem cells. First, unlike progenitor/stem cells, they do not have the ability to self-renew and proliferate, thus decreasing the potential for tumorogenesis from the cell transplant. Second, they cannot differentiate into other cell lineages, which could exert undesired effects in specific tissues if they are transplanted into or migrate into them. Third, unlike embryonic and foetal tissues, they are free from ethical and moral issues for procurement and transplantation because they can be easily collected from perinatal cord blood and adult peripheral blood or BM. Fourth, monocyte transplantation can avoid an immune reaction such as graft-versus-host disease (GvHD), which often happens after allogenic leucocyte transfusion or BM transplantation in human leucocyte antigen (HLA) mis-matched host. GvHD is an immune reaction which results from activation of T cells in the graft (donor cells) after detecting host tissues (recipient cells) as antigenically different [64]. The activated donor T cells produce an abundance of cytotoxic and inflammatory cytokines and attack the host tissues. With respect to GvHD, regardless of HLA matching, transplantation of a monocyte fraction alone should be safe to a greater degree than that of mononuclear fraction of BM or GM-CSF mobilized peripheral blood, which is sure to include lymphocytes. Finally, monocytes/macrophages can secrete a number of cytokines, growth factors and trophic materials which may directly promote other tissue regeneration as well as angiogenesis [4]. For instance, VEGF has been known to be able to stimulate neurogenesis [65, 66].
Table 1.
Advantages versus disadvantages of monocytes/macrophages for cell transplantation
| Advantages | Disadvantages |
|---|---|
| No self-renewal | No cell replacement effect |
| No self-proliferation | Possibility to augment deleterious inflammatory reaction |
| No transdifferentiation | Possibility to promote tumour angiogenesis |
| No tumour induction | Additional isolation and expansion processes |
| No ethical and moral problem | Relative narrow applicable disorder criteria such as ischemic disease |
| Possible repeated autologous harvesting | |
| Secretory function of several cytokines and growth factors | |
| Possible tissue engineering as a vector for gene transfection |
Recently there has been accumulating evidence suggesting that monocytes can be a target cell for induced pluripotent stem (iPS) cell technology. This hypothesis is based on the fact that the monocyte itself is the progenitor of macrophages, dendritic cells, synovial cells, microglia, etc. Of particular interest are programmable cells of human monocyte origin (PCMO) with multipotent properties. PCMO which are dedifferentiated from CD14+ peripheral blood monocytes by a combination of M-CSF and IL-3 in a 6-day culture are responsive to inductive stimuli and consequently can re-transdifferentiate into hepatocyte-like cells [67, 68], pancreatic islet-like cells [67] and chondrocytes [69]. These transdifferentiated cells have shown a close resemblance to the original cells morphologically, serologically and functionally in vitro and in vivo[67–69]. Transplanted human monocyte-derived hepatocytes secreted albumin in severe combined immunodeficiency disease/non-obese diabetic mice [67]. Also, implanted islet-like cells released insulin according to glucose levels and normalized blood glucose in a diabetic mouse model [67]. Additionally, the chondrocytes derived from PCMO appeared to produce type II collagen based on messenger RNA expression [69]. Furthermore, transplantation of PCMO themselves improved impaired heart function from myocardial infarction (MI) in a mouse MI model [70]. In this study, the authors revealed that implanted PCMO induced angiogenesis in the infarction area, and PCMO from infracted donor expressed higher VEGF than PCMO from healthy donors and non-modulated monocytes. These findings suggest that PCMO may transdifferentiate into other phenotypes as well as hepatocytes and islet cells, including skin and neural cells. Thus, monocytes may be used as an autologous source of pluripotent stem cells for cell-based therapy, so-called patient specific iPS cells, in a variety of intractable diseases beyond ischemia, such as neurodegenerative disorders, diabetes, hepatic disease, etc.
However, like other cell-based therapies, clinical trials with monocytes for intractable ischemic diseases still need more careful pre-clinical investigations with regard to the issues of effectiveness or safety. Some studies have failed to show a significant effect of cell therapies with monocytes [71] or mononuclear cells [72–74] from BM in the ischemic heart disease animal models or real patients with MI. The mode of administration is also controversial. It is reasonable to expect that the cells could be even more effective if they were administered directly to the site of injury. Therefore, intramyocardial [71–73] or intracoronary [74] delivery of BM monocytes [71] or mononuclear cells [72–74] has been popular in the research field of ischemic heart disease. By contrast, more feasible routes such as intravenous injection might be considered because many adhesion molecules and chemoattractants are expressed at ischemic sites [22, 23] which could easily recruit intravenously administered monocytes into the damaged area. Moreover, we have demonstrated that intravenous injection of mononuclear human umbilical cord blood (hUCB) cells was at least similarly effective to intralesional implantation in short-term follow-up of neurological function and more effective than direct striatal implantation in producing long-term functional benefits to the stroke animal [75]. Recently, intraperitoneal injection of hUCB mononuclear cells has also shown a good effect in a neonatal rat model of hypoxic-ischemic brain damage [76]. Finally, the specific role and function of monocytes/macrophages in inflammation and immune modulation has still not been fully understood. Their deleterious potential in tumour vascularization, diabetic retinopathy, arthritic pannus and atherosclerotic plaque development still may need serious investigation [60].
Monocytes from umbilical cord blood
Monocytes constitute ∼5–10% of peripheral blood leucocytes in human beings [77, 78], whereas these cells comprise 2% of BM mononuclear cells [78]. By contrast, no difference in monocyte content between UCB and adult peripheral blood has been reported [79, 80]. Therefore, BM and UCB, as well as peripheral blood can be potential candidates as a source of autologous or allogeneic monocytes/macrophages. In fact, BM and UCB are the current gold standard sources of haematopoietic progenitor cells used to reconstitute blood lineages after myeloablative therapy in malignant and non-malignant blood disease. However, the potential of their stem cell population for cell-based therapy has also been demonstrated in other degenerative disorders, especially ischemic disease. There is accumulating evidence that delivery of BM- or UCB-derived stem or mononuclear cells to areas of ischemia by direct local transplantation or injection into blood, can improve the pathological lesion and functional impairment in pre-clinical [81–87] and clinical studies [88–92]. This improvement has been thought to result in part from paracrine effect with an increase in angiogenesis induced by stem cells in the ischemic and/or peri-ischemic core [82, 85, 93–95] even though the mechanisms to exert their angiogenic effects are not completely understood.
Recently, our group revealed that surgical intramyocardial multiple one-time injections of autologous BM mononuclear cells in patients with refractory angina showed a progressive improvement in angina classification as well as in reperfused myocardium area during 18 month follow-up [96]. It is of interest that a positive correlation was noted between monocyte concentration in the graft formulation and clinical improvement (Table 2). This suggests that monocytes should play a major role in functional recovery, probably through induction of myocardial angiogenesis.
Table 2.
Spearman’s correlation coefficients (rs) between monocytes of autologous BM mononuclear cells and improvement of angina symptoms for 18 months following transplantation into myocardium (from Hossne et al.[96])
| CCSAC | Monocytes | |||
|---|---|---|---|---|
| 3 months | rs | −0.759 | ||
| P-value | 0.048 | |||
| 6 months | rs | −0.759 | ||
| P-value | 0.048 | |||
| 12 months | rs | −0.759 | ||
| P-value | 0.048 | |||
| 18 months | rs | −0.759 | ||
| P-value | 0.048 | |||
CCSAC: Canadian Cardiovascular Society Angina Classification. months: months of follow-up.
If P < 0.05, there is a significant correlation between each subpopulation and improvement of CCSAC.
Meanwhile, monocytes in UCB essentially are unique compared to those originating in adult BM and peripheral blood [84]. Only adult monocytes are activated by hepatocyte growth factor, which is essential for normal monocyte functions such as antigen presentation [97]. The UCB monocytes express less HLA-DR than adult cells so their cytotoxic capacity is lower [98]. Furthermore, UCB monocytes do not differentiate into mature dendritic cells to the same extent as mature monocytes even with stimulation by IL-4 and GM-CSF [99]; dendritic cells play a major role in activation of naïve T cells. Secretory function is also different between UCB monocytes and adult blood monocytes. Less secretion of IL-1β and TNF-α, both of which stimulate inflammation as well as play a major role in immune reactions such as GvHD [100], from UCB monocytes after exposure to recombinant interferon-γ is most likely related to differences in the expression of monocytes antigens such as CD64, CD14, CD33 and CD45RO with adult blood monocytes [64]. CD14+ monocytes/macrophages in uterine decidual tissues and blood of normal pregnant women are predominantly of the M2 subset, which modulates maternal-foetal immune reaction and promotes tissue remodelling and angiogenesis to maintain successful pregnancy [101]. These findings suggest that most UCB monocytes may also become M2 polarized macrophages which are less inflammatory and more angiogenic because decidual macrophages probably are differentiated from UCB monocytes in part.
The immaturity of immune and inflammation stimulatory function in UCB monocytes may contribute to a lower incidence of immune rejection including GvHD and/or inhibition of deleterious inflammatory reaction after transplantation even though they are from an allogeneic source. Although, transplantation of monocytes from autologous BM or peripheral blood can avoid immune rejection and GvHD, BM harvesting itself needs additional time, cost and physical burden to the patients. Repeated monocyte harvesting and isolation from the patient’s own peripheral blood also necessitates extra time and cost. By contrast, in terms of target for cell-based therapy, one of the largest advantages of UCB monocytes compared to adult autologous monocytes is rapid availability. For example, in stroke patients, ‘proper timing’ is critical and it may not be feasible to recover, process and manipulate quality of monocyte cells, under current good manufacturing practices conditions from the patient within the therapeutic window. As far as feasibility and safety is concerned, UCB monocytes are the most promising allogenic cells that can be manipulated completely in advance without harm to donor or recipient before transplantation even if the cells are immunologically mismatched to those of the recipient. Table 3 shows the advantages of UCB monocytes for cell-based therapy.
Table 3.
Advantages of UCB monocytes/macrophages compared to adult origin for cell transplantation
| Immature immunoregulatory function |
| Immature inflammatory reaction |
| Possible anti-inflammatory reaction |
| More polarization to M2 macrophage subset that promotes tissue remodelling and angiogenesis |
| Low incidence of GvHD compared to adult origin BM and peripheral blood |
| Relatively easy procurement compared to BM |
| No burden to donors |
This superiority of UCB monocytes over BM and peripheral blood should lead to more brisk exploration of their promising role for cell-based therapy. Recently, our group showed that transplantation of hUCB cells from which the monocyte subpopulation (CD14+) was depleted failed to improve neurological outcome to the same extent as transplantation of the other UCB cells (T-cell depleted, B-cell depleted, CD133+ depleted and whole mononuclear fraction) in the middle cerebral artery occlusion rat model [102]. Further, removal of the CD14+ monocytes from UCB preparation resulted in a failure of the cells to reduce infarct size (Fig. 2). In regard to angiogenesis, these findings suggest that transplantation of monocyte-depleted hUCB could not induce angiogenesis properly, and in turn, could not rescue compromised cells surrounding the infarct core or improve neurological function. At least, the monocyte subpopulation of UCB should be critical to UCB-induced recovery following stroke.
Fig 2.
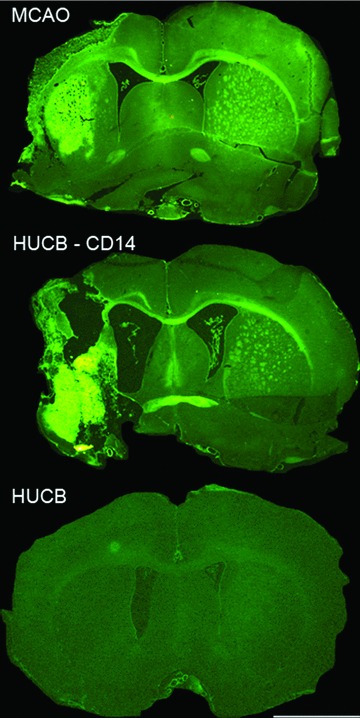
Neuroprotection of striatal and cortical degeneration after middle cerebral artery occlusion in a rat model of stroke is attenuated by removal of monocytes. There is extensive neurodegeneration in striatum and cortex after middle cerebral artery occlusion as determined with FluoroJade staining (top panel). Administering human UCB cells minimizes this damage (bottom panel) whereas removal of the CD14+ monocytes from the human UCB eliminates this neuroprotective effect. Scale bar = 2.0 mm. (From Womble et al.[102].)
In addition, we also demonstrated that locomotor dysfunction and memory were improved after intravenous transplantation of monocyte/macrophages from hUCB in a Sanfilippo syndrome type B (mucopolysaccharidosis type III B) mouse model [103]. In Sanfilippo syndrome type B, a deficiency of the α-N-acetylglucosaminidase (Naglu) enzyme leads to accumulation of heparan sulphate (HS), a glycosaminoglycan within cells and finally draws to progressive cerebral and systemic organ abnormalities. Although glycosaminoglycans are known as extracellular matrix molecules that have influence on the phagocytic ability of macrophages, the function of monocytes/ macrophages in a pathological HS-rich condition such as Sanfilippo syndrome is unclear. In this study, as well as neurological improvement, histopathological study showed that the number of microglia (macrophage in brain) increased in all hippocampal areas of Naglu mutant mice treated with monocytes and HS levels were reduced in the livers of treated mice. Furthermore, urinary distension, usually a significant problem in aged afflicted mice, was also improved in treated mice. These findings suggest that administration of hUCB monocytes/macrophages benefit mice modelling MPS III B, probably owing to the influence from transplanted cells on mechanisms of phagocytosis in the HS-rich environment of this disease [103].
Meanwhile, evidence is accumulating that suggests hUCB mononuclear cells containing monocytes may be a good candidate for cell-based therapies for stroke and ischemic heart diseases including acute MI. Of interest, recently Pimentel-Coelho et al.[76] demonstrated that intraperitoneal transplantation of hUCB mononuclear cells, 3 hrs after the hypoxic-ischemic insult, improved developmental sensorimotor reflexes, in the first week after the injury in a neonatal rat model of hypoxic-ischemic brain damage. They also observed a decrease in the number of dying neurons in the striatum as well as a decline in the activated microglia in the cerebral cortex of treated animals compared to the control group. With respect to the field of cardiovascular diseases, a number of studies revealed that intravenous [104] or intramyocardial [105, 106] injection of hUCB mononuclear cells after acute MI increased microvessels and decreased collagen deposition in the acute MI rodent models. The grafted human cells still survived in the animals’ myocardium. Besides, at 4–6 weeks after transplantation, improvement of left ventricular wall function was observed [105, 106].
In contrast to their original role in inflammation, the use of UCB monocytes may prevent probable harmful effects or exert anti-inflammatory effects. There has been accumulating evidence that UCB mononuclear cells provide anti-inflammatory effects in several disease conditions. Previously, we demonstrated that the transplantation of hUCB mononuclear cells significantly decreased the number of CD45+/CD11b+ (microglia/macrophage) and CD45+/B220+ (B cell) cells in the brain of rats with middle cerebral artery occlusion [107]. In addition, UCB transplantation decreased the pro-inflammatory cytokines such as TNF-α and IL-1β[107]. Recently, we also revealed that transplantation of hUCB mononuclear cells via intravenous injection in older rats can significantly reduce the number of activated microglia, and increase neurogenesis [108]. Chronic microgliosis reflects chronic inflammatory reactions in brain tissue, and is involved in structural damage to neurons in ischemic injury as well as in other neurodegenerative diseases such as Parkinson’s and Alzheimer’s disease [109]. Therefore, this finding suggests that UCB mononuclear cells can ameliorate the hostile environment of the aged hippocampus by way of an anti-inflammatory reaction, and subsequently regenerate potential of the aged neural stem/progenitor cells. On the basis of above studies, a monocyte fraction which comprises a considerable proportion of UCB mononuclear cells, could have the potential, anti-inflammatory effects which may be partially responsible for the functional improvements seen in animal models of injury, including stroke.
Conclusions
Monocytes/macrophages may provide a promising alternative to stem cell transplantation for therapeutic purposes with the ability to promote arteriogenesis and angiogenesis in varied ischemic diseases. Potential hazardous side effects from transplantation with monocytes/macrophages can be prevented by careful evaluation to check whether the proposed recipient has a pre-existing malignancy, uncontrolled diabetes, arthritis, atherosclerosis or not. If during pre-treatment evaluation, it is confirmed that the patients have a pre-existing disease which may be exacerbated by monocyte transplantation, they would be excluded before cell transplantation. This is unlikely because to the best of our knowledge, monocyte-related complications such as pre-existing tumour or chronic inflammatory disease progression, have not been reported after BM or UCB transplantation in patients with haematological malignant or non-malignant diseases even though monocytes comprise a considerable portion of BM and UCB. These UCB monocytes should be the first candidate out of several due to their outstanding feasibility, safety and multiple functions such as anti-inflammatory reaction as well as angiogenesis.
Acknowledgments
P.R.S. and D.-H.P. equally wrote the paper. N.K.-N., E.C., N.A.H. Jr, E.B. and A.E.W. critically edited the paper. P.R.S. has overall responsibility for its contents. A.E.W. is a consultant for and P.R.S. is cofounder and Chairman of Saneron CCEL Therapeutics, Inc. (SCTI, Tampa, FL, USA). SCTI is a University of South Florida start-up company, which is developing UCB-derived treatments for neurodegenerative and cardiovascular disorders. E.C. is a chairman of Cryopraxis, which is developing UCB-derived treatments.
References
- 1.Bosco MC, Puppo M, Blengio F, et al. Monocytes and dendritic cells in a hypoxic environment: spotlights on chemotaxis and migration. Immunobiology. 2008;213:733–49. doi: 10.1016/j.imbio.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 2.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4:432–44. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- 3.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–34. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 4.Sunderkotter C, Steinbrink K, Goebeler M, et al. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–22. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 5.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 6.Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79:319–26. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paulnock DM, Demick KP, Coller SP. Analysis of interferon-gamma-dependent and -independent pathways of macrophage activation. J Leukoc Biol. 2000;67:677–82. doi: 10.1002/jlb.67.5.677. [DOI] [PubMed] [Google Scholar]
- 8.Hoefer IE, Grundmann S, Van Royen N, et al. Leukocyte subpopulations and arteriogenesis: specific role of monocytes, lymphocytes and granulocytes. Atherosclerosis. 2005;181:285–93. doi: 10.1016/j.atherosclerosis.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 9.Schruefer R, Lutze N, Schymeinsky J, et al. Human neutrophils promote angiogenesis by a paracrine feedforward mechanism involving endothelial interleukin-8. Am J Physiol Heart Circ Physiol. 2005;288:H1186–92. doi: 10.1152/ajpheart.00237.2004. [DOI] [PubMed] [Google Scholar]
- 10.Kusumanto YH, Dam WA, Hospers GA, et al. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283–7. doi: 10.1023/B:AGEN.0000029415.62384.ba. [DOI] [PubMed] [Google Scholar]
- 11.Baggiolini M, Loetscher P. Chemokines in inflammation and immunity. Immunol Today. 2000;21:418–20. doi: 10.1016/s0167-5699(00)01672-8. [DOI] [PubMed] [Google Scholar]
- 12.Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 13.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Sica A, Schioppa T, Mantovani A, et al. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–27. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Deonarine K, Panelli MC, Stashower ME, et al. Gene expression profiling of cutaneous wound healing. J Transl Med. 2007;5:11. doi: 10.1186/1479-5876-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shireman PK. The chemokine system in arteriogenesis and hind limb ischemia. J Vasc Surg. 2007;45:A48–56. doi: 10.1016/j.jvs.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herold J, Pipp F, Fernandez B, et al. Transplantation of monocytes: a novel strategy for in vivo augmentation of collateral vessel growth. Hum Gene Ther. 2004;15:1–12. doi: 10.1089/10430340460732517. [DOI] [PubMed] [Google Scholar]
- 18.Capoccia BJ, Gregory AD, Link DC. Recruitment of the inflammatory subset of monocytes to sites of ischemia induces angiogenesis in a monocyte chemoattractant protein-1-dependent fashion. J Leukoc Biol. 2008;84:760–8. doi: 10.1189/jlb.1107756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito WD, Arras M, Scholz D, et al. Angiogenesis but not collateral growth is associated with ischemia after femoral artery occlusion. Am J Physiol. 1997;273:H1255–65. doi: 10.1152/ajpheart.1997.273.3.H1255. [DOI] [PubMed] [Google Scholar]
- 20.Heil M, Eitenmuller I, Schmitz-Rixen T, et al. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scholz D, Ziegelhoeffer T, Helisch A, et al. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–87. doi: 10.1006/jmcc.2002.2013. [DOI] [PubMed] [Google Scholar]
- 22.Nagel T, Resnick N, Atkinson WJ, et al. Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J Clin Invest. 1994;94:885–91. doi: 10.1172/JCI117410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eischen A, Vincent F, Bergerat JP, et al. Long term cultures of human monocytes in vitro. Impact of GM-CSF on survival and differentiation. J Immunol Methods. 1991;143:209–21. doi: 10.1016/0022-1759(91)90046-i. [DOI] [PubMed] [Google Scholar]
- 24.Thurston G. Role of Angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis. Cell Tissue Res. 2003;314:61–8. doi: 10.1007/s00441-003-0749-6. [DOI] [PubMed] [Google Scholar]
- 25.Milkiewicz M, Ispanovic E, Doyle JL, et al. Regulators of angiogenesis and strategies for their therapeutic manipulation. Int J Biochem Cell Biol. 2006;38:333–57. doi: 10.1016/j.biocel.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Zhang RL, Zhang ZG, Chopp M. Neurogenesis in the adult ischemic brain: generation, migration, survival, and restorative therapy. Neuroscientist. 2005;11:408–16. doi: 10.1177/1073858405278865. [DOI] [PubMed] [Google Scholar]
- 27.Beck H, Acker T, Wiessner C, et al. Expression of angiopoietin-1, angiopoietin-2, and tie receptors after middle cerebral artery occlusion in the rat. Am J Pathol. 2000;157:1473–83. doi: 10.1016/S0002-9440(10)64786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murdoch C, Tazzyman S, Webster S, et al. Expression of Tie-2 by human monocytes and their responses to angiopoietin-2. J Immunol. 2007;178:7405–11. doi: 10.4049/jimmunol.178.11.7405. [DOI] [PubMed] [Google Scholar]
- 29.Tammela T, Enholm B, Alitalo K, et al. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–63. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Moldovan L, Moldovan NI. Role of monocytes and macrophages in angiogenesis. Exs. 2005:127–46. doi: 10.1007/3-7643-7311-3_9. [DOI] [PubMed] [Google Scholar]
- 31.Dirkx AE, Oude Egbrink MG, Wagstaff J, et al. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80:1183–96. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 32.Moldovan NI, Goldschmidt-Clermont PJ, Parker-Thornburg J, et al. Contribution of monocytes/macrophages to compensatory neovascularization: the drilling of metalloelastase-positive tunnels in ischemic myocardium. Circ Res. 2000;87:378–84. doi: 10.1161/01.res.87.5.378. [DOI] [PubMed] [Google Scholar]
- 33.Anghelina M, Moldovan L, Zabuawala T, et al. A subpopulation of peritoneal macrophages form capillarylike lumens and branching patterns in vitro. J Cell Mol Med. 2006;10:708–15. doi: 10.1111/j.1582-4934.2006.tb00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmeisser A, Graffy C, Daniel WG, et al. Phenotypic overlap between monocytes and vascular endothelial cells. Adv Exp Med Biol. 2003;522:59–74. doi: 10.1007/978-1-4615-0169-5_7. [DOI] [PubMed] [Google Scholar]
- 35.Schmeisser A, Strasser RH. Phenotypic overlap between hematopoietic cells with suggested angioblastic potential and vascular endothelial cells. J Hematother Stem Cell Res. 2002;11:69–79. doi: 10.1089/152581602753448540. [DOI] [PubMed] [Google Scholar]
- 36.Hoenig MR, Bianchi C, Sellke FW. Hypoxia inducible factor-1 alpha, endothelial progenitor cells, monocytes, cardiovascular risk, wound healing, cobalt and hydralazine: a unifying hypothesis. Curr Drug Targets. 2008;9:422–35. doi: 10.2174/138945008784221215. [DOI] [PubMed] [Google Scholar]
- 37.Marumo T, Schini-Kerth VB, Busse R. Vascular endothelial growth factor activates nuclear factor-kappaB and induces monocyte chemoattractant protein-1 in bovine retinal endothelial cells. Diabetes. 1999;48:1131–7. doi: 10.2337/diabetes.48.5.1131. [DOI] [PubMed] [Google Scholar]
- 38.Lakshminarayanan V, Lewallen M, Frangogiannis NG, et al. Reactive oxygen intermediates induce monocyte chemotactic protein-1 in vascular endothelium after brief ischemia. Am J Pathol. 2001;159:1301–11. doi: 10.1016/S0002-9440(10)62517-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 40.Mostafa LK, Jones DB, Wright DH. Mechanism of the induction of angiogenesis by human neoplastic lymphoid tissue: studies on the chorioallantoic membrane (CAM) of the chick embryo. J Pathol. 1980;132:191–205. doi: 10.1002/path.1711320302. [DOI] [PubMed] [Google Scholar]
- 41.Evans R. Effect of X-irradiation on host-cell infiltration and growth of a murine fibrosarcoma. Br J Cancer. 1977;35:557–66. doi: 10.1038/bjc.1977.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gouon-Evans V, Lin EY, Pollard JW. Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 2002;4:155–64. doi: 10.1186/bcr441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmid MC, Varner JA. Myeloid cell trafficking and tumor angiogenesis. Cancer Lett. 2007;250:1–8. doi: 10.1016/j.canlet.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pugh-Humphreys RG. Macrophage-neoplastic cell interactions: implications for neoplastic cell growth. FEMS Microbiol Immunol. 1992;5:289–308. doi: 10.1111/j.1574-6968.1992.tb05914.x. [DOI] [PubMed] [Google Scholar]
- 45.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sironi M, Martinez FO, D’Ambrosio D, et al. Differential regulation of chemokine production by Fcgamma receptor engagement in human monocytes: association of CCL1 with a distinct form of M2 monocyte activation (M2b, Type 2) J Leukoc Biol. 2006;80:342–9. doi: 10.1189/jlb.1005586. [DOI] [PubMed] [Google Scholar]
- 47.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA. 2006;103:12493–8. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oosterling SJ, Van Der Bij GJ, Meijer GA, et al. Macrophages direct tumour histology and clinical outcome in a colon cancer model. J Pathol. 2005;207:147–55. doi: 10.1002/path.1830. [DOI] [PubMed] [Google Scholar]
- 49.Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:177–89. doi: 10.1023/a:1020304003704. [DOI] [PubMed] [Google Scholar]
- 50.Esposito I, Menicagli M, Funel N, et al. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J Clin Pathol. 2004;57:630–6. doi: 10.1136/jcp.2003.014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jackson JR, Seed MP, Kircher CH, et al. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11:457–65. [PubMed] [Google Scholar]
- 52.Wagner EM, Sanchez J, McClintock JY, et al. Inflammation and ischemia-induced lung angiogenesis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L351–7. doi: 10.1152/ajplung.00369.2007. [DOI] [PubMed] [Google Scholar]
- 53.Hunt TK, Knighton DR, Thakral KK, et al. Studies on inflammation and wound healing: angiogenesis and collagen synthesis stimulated in vivo by resident and activated wound macrophages. Surgery. 1984;96:48–54. [PubMed] [Google Scholar]
- 54.Buschmann IR, Hoefer IE, Van Royen N, et al. GM-CSF: a strong arteriogenic factor acting by amplification of monocyte function. Atherosclerosis. 2001;159:343–56. doi: 10.1016/s0021-9150(01)00637-2. [DOI] [PubMed] [Google Scholar]
- 55.Heil M, Ziegelhoeffer T, Pipp F, et al. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol. 2002;283:H2411–9. doi: 10.1152/ajpheart.01098.2001. [DOI] [PubMed] [Google Scholar]
- 56.Ito WD, Arras M, Winkler B, et al. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res. 1997;80:829–37. doi: 10.1161/01.res.80.6.829. [DOI] [PubMed] [Google Scholar]
- 57.Hirose N, Maeda H, Yamamoto M, et al. The local injection of peritoneal macrophages induces neovascularization in rat ischemic hind limb muscles. Cell Transplant. 2008;17:211–22. doi: 10.3727/000000008783906919. [DOI] [PubMed] [Google Scholar]
- 58.Sasayama S, Fujita M. Recent insights into coronary collateral circulation. Circulation. 1992;85:1197–204. doi: 10.1161/01.cir.85.3.1197. [DOI] [PubMed] [Google Scholar]
- 59.Krupinski J, Kaluza J, Kumar P, et al. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–8. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 60.Herold J, Tillmanns H, Xing Z, et al. Isolation and transduction of monocytes: promising vehicles for therapeutic arteriogenesis. Langenbecks Arch Surg. 2006;391:72–82. doi: 10.1007/s00423-006-0033-9. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez-Barderas M, Gallego-Delgado J, Mas S, et al. Isolation of circulating human monocytes with high purity for proteomic analysis. Proteomics. 2004;4:432–7. doi: 10.1002/pmic.200300629. [DOI] [PubMed] [Google Scholar]
- 62.De Almeida MC, Silva AC, Barral A, et al. A simple method for human peripheral blood monocyte isolation. Mem Inst Oswaldo Cruz. 2000;95:221–3. doi: 10.1590/s0074-02762000000200014. [DOI] [PubMed] [Google Scholar]
- 63.Repnik U, Knezevic M, Jeras M. Simple and cost-effective isolation of monocytes from buffy coats. J Immunol Methods. 2003;278:283–92. doi: 10.1016/s0022-1759(03)00231-x. [DOI] [PubMed] [Google Scholar]
- 64.Brichard B, Varis I, Latinne D, et al. Intracellular cytokine profile of cord and adult blood monocytes. Bone Marrow Transplant. 2001;27:1081–6. doi: 10.1038/sj.bmt.1703037. [DOI] [PubMed] [Google Scholar]
- 65.Jin K, Zhu Y, Sun Y, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–50. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teng H, Zhang ZG, Wang L, et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28:764–71. doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruhnke M, Ungefroren H, Nussler A, et al. Differentiation of in vitro-modified human peripheral blood monocytes into hepatocyte-like and pancreatic islet-like cells. Gastroenterology. 2005;128:1774–86. doi: 10.1053/j.gastro.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 68.Ruhnke M, Nussler AK, Ungefroren H, et al. Human monocyte-derived neohepatocytes: a promising alternative to primary human hepatocytes for autologous cell therapy. Transplantation. 2005;79:1097–103. doi: 10.1097/01.tp.0000157362.91322.82. [DOI] [PubMed] [Google Scholar]
- 69.Pufe T, Petersen W, Fandrich F, et al. Programmable cells of monocytic origin (PCMO): a source of peripheral blood stem cells that generate collagen type II-producing chondrocytes. J Orthop Res. 2008;26:304–13. doi: 10.1002/jor.20516. [DOI] [PubMed] [Google Scholar]
- 70.Dresske B, El Mokhtari NE, Ungefroren H, et al. Multipotent cells of monocytic origin improve damaged heart function. Am J Transplant. 2006;6:947–58. doi: 10.1111/j.1600-6143.2006.01289.x. [DOI] [PubMed] [Google Scholar]
- 71.Wisenberg G, Lekx K, Zabel P, et al. Cell tracking and therapy evaluation of bone marrow monocytes and stromal cells using SPECT and CMR in a canine model of myocardial infarction. J Cardiovasc Magn Reson. 2009;11:11. doi: 10.1186/1532-429X-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nasseri BA, Kukucka M, Dandel M, et al. Intramyocardial delivery of bone marrow mononuclear cells and mechanical assist device implantation in patients with end-stage cardiomyopathy. Cell Transplant. 2007;16:941–9. doi: 10.3727/096368907783338235. [DOI] [PubMed] [Google Scholar]
- 73.Moelker AD, Baks T, Van Den Bos EJ, et al. Reduction in infarct size, but no functional improvement after bone marrow cell administration in a porcine model of reperfused myocardial infarction. Eur Heart J. 2006;27:3057–64. doi: 10.1093/eurheartj/ehl401. [DOI] [PubMed] [Google Scholar]
- 74.Lunde K, Solheim S, Forfang K, et al. Anterior myocardial infarction with acute percutaneous coronary intervention and intracoronary injection of autologous mononuclear bone marrow cells: safety, clinical outcome, and serial changes in left ventricular function during 12-months’ follow-up. J Am Coll Cardiol. 2008;51:674–6. doi: 10.1016/j.jacc.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 75.Willing AE, Lixian J, Milliken M, et al. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosci Res. 2003;73:296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- 76.Pimentel-Coelho PM, Magalhães ES, Lopes LM, et al. Human cord blood transplantation in a neonatal rat model of hypoxic-ischemic brain damage: functional outcome related to neuroprotection in the striatum. Stem Cells Dev. 2009 doi: 10.1089/scd.2009.0049. Doi: 10.1089/scd.2009.0049. [DOI] [PubMed] [Google Scholar]
- 77.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 78.Mielcarek M, Martin PJ, Torok-Storb B. Suppression of alloantigen-induced T-cell proliferation by CD14+ cells derived from granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells. Blood. 1997;89:1629–34. [PubMed] [Google Scholar]
- 79.Sorg RV, Andres S, Kogler G, et al. Phenotypic and functional comparison of monocytes from cord blood and granulocyte colony-stimulating factor-mobilized apheresis products. Exp Hematol. 2001;29:1289–94. doi: 10.1016/s0301-472x(01)00735-4. [DOI] [PubMed] [Google Scholar]
- 80.Mills KC, Gross TG, Varney ML, et al. Immunologic phenotype and function in human bone marrow, blood stem cells and umbilical cord blood. Bone Marrow Transplant. 1996;18:53–61. [PubMed] [Google Scholar]
- 81.Henning RJ, Burgos JD, Vasko M, et al. Human cord blood cells and myocardial infarction: effect of dose and route of administration on infarct size. Cell Transplant. 2007;16:907–17. doi: 10.3727/096368907783338299. [DOI] [PubMed] [Google Scholar]
- 82.Chen J, Zhang ZG, Li Y, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–9. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 83.Willing AE, Lixian J, Milliken M, et al. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosci Res. 2003;73:296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- 84.Newcomb JD, Sanberg PR, Klasko SK, et al. Umbilical cord blood research: current and future perspectives. Cell Transplant. 2007;16:151–8. [PMC free article] [PubMed] [Google Scholar]
- 85.Chang YC, Shyu WC, Lin SZ, et al. Regenerative therapy for stroke. Cell Transplant. 2007;16:171–81. [PubMed] [Google Scholar]
- 86.Kinnaird T, Stabile E, Burnett MS, et al. Bone-marrow-derived cells for enhancing collateral development: mechanisms, animal data, and initial clinical experiences. Circ Res. 2004;95:354–63. doi: 10.1161/01.RES.0000137878.26174.66. [DOI] [PubMed] [Google Scholar]
- 87.Norol F, Merlet P, Isnard R, et al. Influence of mobilized stem cells on myocardial infarct repair in a nonhuman primate model. Blood. 2003;102:4361–8. doi: 10.1182/blood-2003-03-0685. [DOI] [PubMed] [Google Scholar]
- 88.Beeres SL, Bax JJ, Kaandorp TA, et al. Usefulness of intramyocardial injection of autologous bone marrow-derived mononuclear cells in patients with severe angina pectoris and stress-induced myocardial ischemia. Am J Cardiol. 2006;97:1326–31. doi: 10.1016/j.amjcard.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 89.Tse HF, Thambar S, Kwong YL, et al. Comparative evaluation of long-term clinical efficacy with catheter-based percutaneous intramyocardial autologous bone marrow cell implantation versus laser myocardial revascularization in patients with severe coronary artery disease. Am Heart J. 2007;154(982):e1–6. doi: 10.1016/j.ahj.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 90.Briguori C, Reimers B, Sarais C, et al. Direct intramyocardial percutaneous delivery of autologous bone marrow in patients with refractory myocardial angina. Am Heart J. 2006;151:674–80. doi: 10.1016/j.ahj.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 91.Losordo DW, Schatz RA, White CJ, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–72. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 92.Van Ramshorst J, Bax JJ, Beeres SL, et al. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. Jama. 2009;301:1997–2004. doi: 10.1001/jama.2009.685. [DOI] [PubMed] [Google Scholar]
- 93.Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–8. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Laflamme MA, Zbinden S, Epstein SE, et al. Cell-based therapy for myocardial ischemia and infarction: pathophysiological mechanisms. Annu Rev Pathol. 2007;2:307–39. doi: 10.1146/annurev.pathol.2.010506.092038. [DOI] [PubMed] [Google Scholar]
- 95.Tse HF, Siu CW, Zhu SG, et al. Paracrine effects of direct intramyocardial implantation of bone marrow derived cells to enhance neovascularization in chronic ischaemic myocardium. Eur J Heart Fail. 2007;9:747–53. doi: 10.1016/j.ejheart.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 96.Hossne NA, Jr, Invitti AL, Buffolo E, et al. Intramyocardial injection of a specific formulation of autologous bone marrow stem cells as a sole therapy in patients with refratory angina, without left ventricular dysfunction: 18 month follow-up. Cell Transplant. 2009;18:1299–310. doi: 10.3727/096368909X484671. [DOI] [PubMed] [Google Scholar]
- 97.Jiang Q, Azuma E, Hirayama M, et al. Functional immaturity of cord blood monocytes as detected by impaired response to hepatocyte growth factor. Pediatr Int. 2001;43:334–9. doi: 10.1046/j.1442-200x.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- 98.Theilgaard-Monch K, Raaschou-Jensen K, Palm H, et al. Flow cytometric assessment of lymphocyte subsets, lymphoid progenitors, and hematopoietic stem cells in allogeneic stem cell grafts. Bone Marrow Transplant. 2001;28:1073–82. doi: 10.1038/sj.bmt.1703270. [DOI] [PubMed] [Google Scholar]
- 99.Liu E, Tu W, Law HK, et al. Decreased yield, phenotypic expression and function of immature monocyte-derived dendritic cells in cord blood. Br J Haematol. 2001;113:240–6. doi: 10.1046/j.1365-2141.2001.02720.x. [DOI] [PubMed] [Google Scholar]
- 100.Hill GR, Krenger W, Ferrara JL. The role of cytokines in acute graft-versus-host disease. Cytokines Cell Mol Ther. 1997;3:257–66. [PubMed] [Google Scholar]
- 101.Gustafsson C, Mjosberg J, Matussek A, et al. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS ONE. 2008;3:e2078. doi: 10.1371/journal.pone.0002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Womble TA, Green S, Sanberg PR, et al. CD14+ human umbilical cord blood cells are essential for neurological recovery following MCAO. Cell Transplant. 2008;17:485–6. [Google Scholar]
- 103.Garbuzova-Davis S, Xie Y, Danias P, et al. Transplantation of cord blood monocyte/ macrophage cells to treat Sanfilippo type B. Cell Transplant. 2008;17:466–7. [Google Scholar]
- 104.Ma N, Stamm C, Kaminski A, et al. Human cord blood cells induce angiogenesis following myocardial infarction in NOD/scid-mice. Cardiovasc Res. 2005;66:45–54. doi: 10.1016/j.cardiores.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 105.Hu CH, Wu GF, Wang XQ, et al. Transplanted human umbilical cord blood mononuclear cells improve left ventricular function through angiogenesis in myocardial infarction. Chin Med J (Engl) 2006;119:1499–506. [PubMed] [Google Scholar]
- 106.Cortes-Morichetti M, Frati G, Schussler O, et al. Association between a cell-seeded collagen matrix and cellular cardiomyoplasty for myocardial support and regeneration. Tissue Eng. 2007;13:2681–7. doi: 10.1089/ten.2006.0447. [DOI] [PubMed] [Google Scholar]
- 107.Vendrame M, Gemma C, De Mesquita D, et al. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev. 2005;14:595–604. doi: 10.1089/scd.2005.14.595. [DOI] [PubMed] [Google Scholar]
- 108.Bachstetter AD, Pabon MM, Cole MJ, et al. Peripheral injection of human umbilical cord blood stimulates neurogenesis in the aged rat brain. BMC Neurosci. 2008;9:22. doi: 10.1186/1471-2202-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–81. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]


