Abstract
In the creation of stable tolerance to MHC-incompatible allografts, reducing the large mass of donor-reactive cells via apoptosis is often required. Apoptosis induction by immunotoxins targeting surface molecules specifically presented on donor-reactive cytopathic T effector (Teff) cells is a promising strategy. Traditionally, the toxin moieties are bacterial exotoxins or plant-derived ribosome-inactivating proteins (RIPs) with large molecular size and strong immunogenicity, hence causing the problems of tissue penetration, host immune reaction and quick clearance. We have identified a novel class of small molecule RIPs (<10 kD) from the seeds of the plant Luffa cylindrica. The smallest member of this family, Luffin P1, has a molecular weight of 5226.8 Da, yet possessing a highly potent inhibitory activity on cell-free protein synthesis with IC50 of 0.88 nM. We now report a recombinant hIL-2-Luffin P1 immunotoxin, which strongly inhibited T-cell proliferation in mixed lymphocyte reaction and ConA response with IC50 of 1.8–10 nM. In vivo, hIL-2-Luffin P1 significantly prolonged the survival of major MHC-mismatched skin and kidney allografts in animal models. Thus, we demonstrate for the first time the efficacy of the smallest immunotoxin that could be further combined with other pharmacological and immunological reagents for synergistic control of pathogenic lymphocytes in immune-mediated diseases.
Keywords: immunotoxin, mixed lymphocyte reaction, skin transplantation, renal transplantation, ribosome-inactivating proteins
Introduction
Before stem cell technology and tissue/organ bioengineering deliver their promise, organ transplantation is currently the only viable option to treat end-stage organ failure. In the past two decades, a large array of immunosuppressive agents has expanded the armamentarium used by transplant physicians and surgeons to prevent acute allograft rejection. However, long-term use of non-specific immunosuppressants has been associated with severe side effects. The goal for transplantation medicine is to achieve transplant tolerance, that is, specific and indefinite acceptance of grafts without ongoing immunosuppression.
In order to create an environment that facilitates tolerance induction, maximally minimizing the clone size of alloantigen-activated cytopathic Teff cells through apoptosis is necessary [1]. One strategy is to target immunotoxins against cell surface molecules, specifically presented on donor-reactive Teff cells for their demise. The concept of immunotoxin can be dated back 100 years ago, when Paul Ehrlich, the founder of modern chemotherapy and Nobel Prize winner postulated ‘magic bullets’ for use in the fight against human diseases [2]. This concept, inspiring generations of scientists to devise powerful molecular cancer therapeutics, was materialized by the use of monoclonal antibodies (Mabs) as vehicle, and bacteria- or plant-derived toxins as bullet [3]. Upon endocytosis, bacterial toxins, such as Pseudomonas exotoxin (PE) and diphtheria toxin (DT), are cleaved by cellular protease in the endosome and the resulting enzymatically active C-terminal fragments (37–38 kD) translocate to the cytosol where they ADP-ribosylate elongation factor 2 and inhibit protein synthesis. The plant toxins are usually ribosome-inactivating proteins (RIPs) that catalytically inactivate ribosomes by specifically modifying or cleaving high-molecular-weight rRNA [4], thus inhibiting protein synthesis and causing target cell apoptosis. RIPs recognize a highly conserved region in the large 28S rRNA and cleave a specific N-C glycosidic bond between an adenine and the nucleotide on the RNA, whereby the adenine residue is removed. For the most often used substrate – rat liver ribosome – this specific site is A4324 in 28S rRNA [5]. Besides the RNA N-glycosidase activity, RIPs also exhibit polynucleotide:adenosine glycosidase (PAG) activity, and presumed DNase-like and phosphatase activity [4]. Once inside the cytosol, 1-RIP molecule is capable of killing a cell, making immunotoxins some of the most potent killing agents.
Today, Mabs can be molecularly engineered to retain targeting capacity with its Fab portion or even in the form of single-chain Fv (ScFv). The reduced molecular size greatly enhanced the penetration of immunotoxins into deep tissue, such as the centre of the solid tumour. Humanization of murine Mabs or using complete human genes also significantly reduced the immunogenicity and host reaction towards the carrier moiety, hence increasing the in vivo half-life of the immunotoxins. However, the toxin moiety is still highly immunogenic because of their foreign origin and relatively large size (around 30 kD). It is therefore reasoned that if toxins of much smaller molecular size are used, they may confer the benefit of enhancing tissue penetration, as well as mitigating antigenic epitopes exposed to the host immune system.
In 1994, Gao et al. reported the discovery of a novel small RIP, Luffin-S, from the seeds of the plant Luffa cylindrica[6]. Since then, quite a number of small RIPs (<10 kD) have been discovered from various plants [7–9]. The smallest member of this new family, Luffin P1, has a molecular weigh of 5226.8 Da, yet possessing a highly potent inhibitory activity on protein synthesis in the cell-free rabbit reticulocyte lysate with IC50 of 0.88 nM [10, 11]. From biochemistry point of view, it is intriguing that such a 43-aa peptide could form into structures required for rRNA N-glycosidase activity. From application side, the engineering of Luffin P1 as a 43-aa peptide tag to immune molecules could provide opportunities to construct immunotoxins with improved pharmacological kinetics and tissue distribution, as well as reduced immunogenicity.
To control immune rejection while keeping the normal immune function intact, it is necessary to construct reagents specifically against donor antigen-activated Teff cells during tolerance induction. Upon activation, T cells rapidly express all three components of the high-affinity IL-2 receptor αβγ complex. Therefore, activated T cells have much higher affinity for IL-2 than resting naïve T cells. In this report, we successfully engineered an hIL-2-Luffin P1 immunotoxin and found that it exhibited potent inhibitory activity on T-cell proliferation in vitro and significantly prolonged the survival of MHC-mismatched skin and renal allografts in vivo.
Materials and methods
Expression of recombinant Luffin P1 and hIL-2-Luffin P1 in Escherichia coli
The cDNA of Luffin P1 (GenBank AF537345) was generated by primer annealing and extension with four synthetic oligos: P1 sense primer (75 bp), 5′-CCACGGACCGAGTATGAGGCGTGTCGAGTTCGATGCCAAGTGGCGGAGCATGGGGTGGAGCGGCAACGCAGGTGT-3′; P2 sense primer (75 bp, 5′ added phosphate group), 5′-CAACAGGTCTGTGAGAAGCGGCTGAGGGAGCGAGAGGGCCGGCGGGAGG GTGGCGGGCTACACCATCACCATCAC-3′; P3 antisense primer (76 bp, 5′ added phosphate group), 5′-ACCCTCCCGCCGGCCCTCTCGCTCCCTCAGCCGCTTCTCACA GACCTGTTGACACCTGCGTTGCCGCTCCACCCCA-3′; P4 antisense primer (58 bp), 5′-CAGGCTCGAGTTATAGCTCATCTTTACCGCCTCCGTGATGGTGATGGTGATGCCCG CC-3′. First, equal molar mixture of P1, P2, P3 and P4 was incubated at 70°C for 10 min and then annealed at 55°C for 15 min. T4 DNA ligase (TaKaRa, Japan) was added to join the nick between P1 and P2, and that between P3 and P4. Primer extension was performed with Taq enzyme to have the full-length Luffin-P1 gene. The hIL-2 gene was PCR-amplified from a PBMC cDNA library with primers hIL-2A 5′-TGATCCATGGCACCTACTTCAAGTTCTACAAAGAAAACACAGCTACAACTGG-3′ (53 bp) and hIL-2B 5′-CTCGACACGCCTCATACTCGGTCCG TGGAGATCCGCCCCCAGTCAGTGTTGAGATGAT-3′ (58 bp). The hIL-2B primer has a long overlapping sequence with the Luffin-P1 gene. Using gel-purified hIL-2 and Luffin P1 PCR products as template, together with hIL-2A and P4 antisense as primers, overlapping PCR was performed to amplify the full-length hIL-2-Luffin P1 gene. As a control, Luffin P1 was amplified with primers P4 antisense and P1.1 sense: 5′-TTAGCCATGGGGCCACGGACCGAGTATGAGGCGAGGTGT-3′. The P4 antisense primer encodes a His×6 tag to ease protein purification and a KDEL sequence for directing the toxin to the endoplasmic reticulum (ER), a necessary step for cytotoxicity [12]. Diagram of Luffin P1 and hIL-2-Luffin P1 gene synthesis is shown in Fig. 1.
Fig 1.
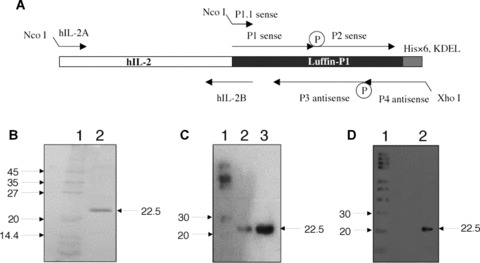
Expression of hIL-2-Luffin P1 immunotoxin. (A) Schematic illustration of gene synthesis for Luffin P1 and hIL-2-Luffin P1. Synthetic oligos P1, P2, P3 and P4 were annealed, ligated and extended to form the full-length Luffin P1 gene, which was further amplified with P1.1 and P4. The hIL-2 gene was amplified with primers hIL-2A and hIL-2B, and the resulting fragment was fused with Luffin P1 by overlapping PCR with hIL-2A and P4 primers. Both genes were cloned into the bacterial expression vector pET-20b(+) at NcoI and XhoI sites. P represents phosphate groups at the 5′ end of oligos required for the ligation reaction. The His×6 tag and KDEL sequence are indicated. (B) SDS-PAGE detection of hIL-2-Luffin P1 purified by His-Bind resin. A single 22.5 kD band of hIL-2-Luffin P1 in Lane 2 can be visualized. Molecular weight markers in Lane 1 were denoted in kD on the left. (C) Western blot detection of purified hIL-2-Luffin P1 (both Lane 2 and Lane 3 on different loading) with anti-His tag antibody. Positive bands at 22.5 kD were shown by arrow. Lane 1 is the molecular weight marker. A similar Western blot with anti-hIL-2 antibody was shown in (D).
Both Luffin P1 and hIL-2-Luffin P1 were cloned into the prokaryotic expression plasmid pET-20b(+) (Novagen, USA) at NcoI (CCATGG) and XhoI (CTCGAG) sites (underlined in primers) and were confirmed by sequencing. The pET-20b(+) vector carries an N-terminal pelB signal sequence for periplasmic localization of secreted products. Recombinant plasmids, pET-Luffin P1 and pET-hIL-2-Luffin P1, were transformed into host cell Origami (DE3) (Novagen, USA) carrying the chromatin T7 RNA polymerase gene. After induction with 0.4 mM IPTG (Sigma, USA) at 30°C, hIL-2-Luffin P1 was detected by SDS-PAGE and Western blot with antibodies against either His tag or hIL-2 (Santa Cruz, USA). Purification of His-tagged Luffin P1 and hIL-2-Luffin P1 fusion protein from bacterial lysates was carried out with His-Bind resin (Novagen, USA), according to the instructions of the manufacturer.
Lymphocyte proliferation in MLR or in response to ConA
Mixed lymphocyte reaction (MLR) was set up as previously described [13]. Briefly, splenocytes from C57BL/6 (H-2b) and BALB/c (H-2d) mice were isolated, and 1×106 cells from each strain were mixed in individual U-bottom 96 wells and cultured with different doses of Luffin P1 or hIL-2-Luffin P1 at 37°C for 5 days. Alternatively, isolated splenocytes from BALB/c mice were adjusted to 1 x 107 cells/ml in RPMI-1640 medium with 10% FBS. A total of 1 x 106 cells were seeded in individual U-bottom 96 wells and stimulated with 10 μg/ml of ConA (Sigma, USA) in the presence of different doses of Luffin P1 or hIL-2-Luffin P1 at 37°C for 3 days. 3H-TdR (0.5 μCi/well) was added during the last 16 hrs of culture, and counts per minute (CPM) were measured by a liquid scintillation counter (Beckman, USA). The experiments were repeated for three times. Percentage of inhibition on lymphocyte proliferation was calculated by the following formula: Inhibition (%) =[(Positive control CPM − Negative control CPM) − (Sample CPM − Negative control CPM)]/(Positive control CPM − Negative control CPM) × 100%.
Mouse skin transplantation
Skin grafts of 1.5–2.0 cm2 in size were harvested from C57BL/6 mice. Graft beds were prepared by excising 1.5–2.0 cm2 skin from the lateral dorsal thoracic wall of BALB/c recipients. The grafted skins were covered with Vaseline gauze and an aseptic adhesive bandage for 7 days. Five groups of BALB/c recipients with C57BL/6 skins were injected via tail vein with HBSS (group I), Luffin P1 (group II, 70 μg/kg) or hIL-2-Luffin P1 (group III, 2.25 μg/kg; group IV, 22.5 μg/kg and group V, 225 μg/kg), starting on the day of transplantation for every 2 days till rejection occurred. Grafts were examined daily beginning at day 7 post-transplantation and were considered rejected when approximately 80% or more of the graft tissue was encrusted, hardened and destroyed as assessed by visual examination.
Rat renal transplantation
Male Wistar rats and SD rats about 250–300 g in body weight were served as donors and recipients, respectively. As described [14], the left kidney of donor rat was surgically removed and grafted into recipient’s abdomen cavity, with artery anastomosis between recipient abdominal aorta and donor left renal artery, and vein anastomosis between recipient caval vein and donor left renal vein. Three groups of SD recipients with Wistar kidneys were injected via tail vein with HBSS (group I), Luffin P1 (group II, 70 μg/kg) or hIL-2-Luffin P1 (group III, 22.5 μg/kg), starting on the day of transplantation for every 2 days till rejection occurred. The day of anuria was considered as the rejection day.
Histology and immunohistochemistry
Multiple skin and renal sections were fixed in 10% buffered formalin, embedded in paraffin and stained with hematoxylin and eosin to evaluate histological changes.
Statistical analysis
Probit regression analysis was used to analyse the IC50 of inhibition on lymphocyte proliferation. One-way anova was used to analyse the mean survival time. The Kaplan–Meier survival plots were obtained with StatView software. P-value <0.05 was considered statistically significant.
Results
Expression of immunotoxin hIL-2-Luffin P1
The Luffin P1 gene (GenBank AF537345) encodes a short 43-aa peptide [10, 11] (GSPRTEYEACRVRCQVAEHGVERQRRCQQVCEKRLREREGRRE) that is similar to the 6.5 kD arginine/glutamate-rich polypeptide isolated from the seeds of sponge gourd (L. cylindrica) [8]. Luffin P1 (5.2 kD) is the smallest peptide so far reported that possesses rRNA N-glycosidase activity and strongly inhibits protein translation in cell-free system [10, 11]. Its short sequence enabled us to chemically synthesize the gene by primer annealing and extension, followed by overlapping PCR to obtain the hIL-2-Luffin P1 fusion gene as depicted in Fig. 1A. We then cloned the fusion gene into pET-20b(+) and used E. coli to express this immunotoxin, as prokaryotic protein translation machinery should be insensitive to RIP inhibition. After induction with IPTG, 1.5 mg hIL-2-Luffin P1 protein could be purified with His-Bind resin from the lysate of bacteria grown in 1 litre of fermentation culture. SDS-PAGE analysis showed the purity of hIL-2-Luffin P1 that has a molecular weight of 22.5 kD (Fig. 1B), which is also confirmed by Western blot, using either anti-His tag or anti-IL-2 antibody (Fig. 1C and D). In parallel, His-tagged Luffin P1 was similarly purified using the same procedure (data not shown).
hIL-2-Luffin P1 strongly suppressed ConA-induced T-cell proliferation
Upon polyclonal activation with mitogen ConA, T cells rapidly up-regulate all three subunits of the IL-2 receptor. We tested whether hIL-2-mediated binding of the immunotoxin hIL-2-Luffin P1 to mouse IL-2R complex could induce cytotoxicity. Indeed, hIL-2-Luffin P1 effectively suppressed ConA-stimulated lymphocyte proliferation in a dose-dependent manner. At the concentration of 10−6 M, hIL-2-Luffin P1 inhibited more than 91% of proliferation, compared with untreated control. Inhibition of proliferation was due to apoptosis but not anergy, as assayed by Annexin V staining (data not shown). The IC50 of hIL-2-Luffin P1 was calculated to be around 10−8 M, whereas Luffin P1 showed no suppression across the dose range of 10−11–10−6 M (Fig. 2A). This indicates that the specific interaction between cytokine receptor and the cytokine moiety of immunotoxin is necessary for mediating the cytotoxicity of target cells.
Fig 2.
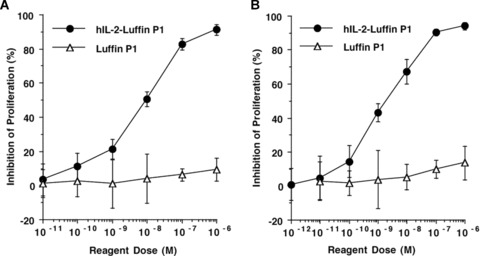
Inhibition of T-cell proliferation by immunotoxin hIL-2-Luffin P1. Increasing doses of hIL-2-Luffin P1 or Luffin P1 were added to BALB/c splenocytes stimulated with ConA (A), or to the mixed lymphocyte reaction using BALB/c and C57BL/6 splenocytes (B). CPM values from triplicate wells were averaged and inhibition percentages (±s.d.) were calculated based on the formula described in Materials and Methods section. Data were from one of three repeated experiments.
hIL-2-Luffin P1 potently inhibited T-cell proliferation in MLR
To determine whether hIL-2-Luffin P1 could also suppress T-cell proliferation induced by MHC-mismatched alloantigens, different doses of hIL-2-Luffin P1 were added into the MLR culture. Results showed that proliferation of alloreactive T cells was inhibited by hIL-2-Luffin P1 in a dose-dependent manner (Fig. 2B). At the concentration of 10−6 M, hIL-2-Luffin P1 inhibited more than 94% of proliferation, compared with untreated control. The IC50 of hIL-2-Luffin P1 was calculated to be around 1.8 x 10−9 M, whereas Luffin P1 only showed mild inhibition on lymphocyte proliferation at 10−6 M (Fig. 2B).
hIL-2-Luffin P1 prolonged the survival of mouse skin allografts
In an animal model of allorejection, wherein BALB/c mice were transplanted with MHC-mismatched skin grafts from C57BL/6 mice, the effects of recombinant immunotoxin hIL-2-Luffin P1 on graft survival were compared with those of HBSS or Luffin P1 given to control groups. HBSS-treated BALB/c mice promptly rejected C57BL/6 skin grafts with a mean survival time (MST) of 9.1 ± 1.2 days. There was no obvious difference in the survival time when Luffin P1 was administrated at 70 μg/kg (MST 9.9 ± 1.5 days, P= 0.14). In contrast, hIL-2-Luffin P1 already significantly prolonged the survival time of skin allografts at 2.25 μg/kg (MST 14.4 ± 1.6 days, P < 0.001). Increasing doses of the immunotoxin to 22.5 and 225 μg/kg further prolonged the survival time of skin allografts, with MST of 15.8 ± 1.7 (P < 0.001) and 18.2 ± 2.5 (P < 0.001) days, respectively (Fig. 3A and Table 1). These doses were chosen based on the molecular weights of Luffin P1 (7.0 kD with His tag and KDEL tag) and hIL-2-Luffin P1 (22.5 kD with tags). Thus, the dose of Luffin P1 at the molar basis is equivalent to the highest dose of hIL-2-Luffin P1. Yet, Luffin P1 is ineffective, whereas hIL-2-Luffin P1 is at least more than 100-fold effective in prolonging the survival of skin allografts. At day 10 post-transplantation, histological examination showed that there was much less lymphocyte infiltration in skin grafts of hIL-2-Luffin P1-treated group as compared with that of control group at the same time point. Moreover, the structures of skin grafts, that is, epidermis and dermis, were kept much better in hIL-2-Luffin P1-treated group than those in control groups (Fig. 3B).
Fig 3.
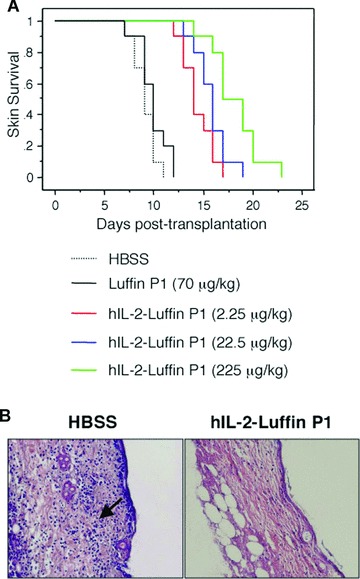
Prolongation of skin allograft survival by hIL-2-Luffin P1. (A) C57BL/6 skin grafts were transplanted onto five groups (n= 10) of BALB/c recipients, which were then injected with HBSS as control, or with different doses of hIL-2-Luffin P1 or Luffin P1 every 2 days till rejection occurred. Accumulative survival curves were plotted. Kaplan–Meier survival analysis indicated significant prolongation of survival in all three hIL-2-Luffin P1 groups (P < 0.001 versus control), but not in Luffin P1 group (P > 0.14 versus control). (B) Histological comparison of skin grafts from recipients treated with HBSS (left panel) or hIL-2-Luffin P1 (right panel) at the 10-day post-transplantation time point. In the HBSS group, there was obvious necrosis of epidermal cells, oedema, fibrinoid degeneration, necrosis and lysis of dermis, lymphocyte infiltration and inflammatory reaction (shown by the arrow), which was not seen in the hIL-2-Luffin P1 group (haematoxylin and eosin χ200).
Table 1.
Survival of C57BL/6 skin grafts in BALB/c mice
| Groups (n= 10) | Treatment | Dose (μg/kg) | MST 6 s.d. | P-value (versus Group I) |
|---|---|---|---|---|
| I | HBSS | 9.1 ± 1.2 | ||
| II | Luffin P1 | 70 | 9.9 ± 1.5 | >0.14 |
| III | hIL-2-Luffin P1 | 2.25 | 14.4 ± 1.6 | <0.001 |
| IV | hIL-2-Luffin P1 | 22.5 | 15.8 ± 1.7 | <0.001 |
| V | hIL-2-Luffin P1 | 225 | 18.2 ± 2.5 | <0.001 |
hIL-2-Luffin P1 prolonged the survival of rat renal allografts
To further confirm the beneficial effect of immunotoxin hIL-2-Luffin P1 on allograft survival, we used a rat renal transplantation model, wherein Wistar rats were served as kidney donors and SD rats were used as recipients. The results showed that administration of hIL-2-Luffin P1 could significantly prolong the MST of renal allografts to 13.9 ± 2.1 days, compared with animals treated with HBSS (MST 8.6 ± 1.5 days, P < 0.001) or Luffin P1 (MST 9.3 ± 1.2 days, P < 0.001). There was no significant difference between HBSS group and Luffin P1 group in MST (Fig. 4A and Table 2). Histological examination showed that the structures of kidney allografts were destroyed and there was obvious lymphocyte infiltration in both HBSS group and Luffin P1 group. Whereas at the same time point, kidney structures were kept much better and there were much fewer infiltrated cells in hIL-2-Luffin P1-treated group (Fig. 4B). In line with this, the percentages of CD3+, CD4+ and CD8+ T cells were significantly reduced in the PBL of recipient rats 1 week post-transplantation, following hIL-2-Luffin P1 treatment (Table 3).
Fig 4.
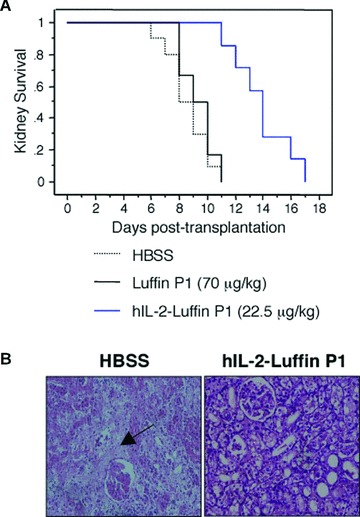
Prolongation of kidney allograft survival by hIL-2-Luffin P1. (A) Kidneys from male donor Wistar rats were transplanted into three groups (n= 6–10) of SD recipient rats, which were then injected with HBSS as control, or with hIL-2-Luffin P1 or Luffin P1 every 2 days till rejection occurred. Accumulative survival curves were plotted. Kaplan–Meier survival analysis indicated significant prolongation of survival in hIL-2-Luffin P1 groups (P= 0.001 versus control). (B) Histological comparison of sections of kidney grafts from recipients treated with HBSS (left panel) or hIL-2-Luffin P1 (right panel) at the 8-day post-transplantation time point. The structures of the allografts were almost completely destroyed with extensive necrosis (shown by the arrow) in the control group, whereas there were only some inflammatory cells in glomerular and interstitial tissues, with low degree of degeneration in epithelial cells of proximal and distal convoluted tubules in hIL-2-Luffin P1 group (haematoxylin and eosin ×200).
Table 2.
Survival of Wistar rat kidneys in SD rat recipients
| Groups | Treatment | Dose (μg/kg) | MST 6 s.d. | P-value (versus Group I) |
|---|---|---|---|---|
| I (n= 10) | HBSS | 8.6 ± 1.5 | ||
| II (n= 6) | Luffin P1 | 70 | 9.3 ± 1.2 | >0.05 |
| III (n= 7) | hIL-2-Luffin P1 | 22.5 | 13.9 ± 2.1 | <0.001 |
Table 3.
T-cell subsets in PBL of SD rats 1 week post-transplantation
Discussion
Immunotoxins are proteins that contain a toxin along with an antibody or growth factor that binds specifically to target cells. The majority of toxin moieties are bacterial exotoxins or plant-derived RIPs that enzymatically inhibit protein synthesis, leading to cell apoptosis. For immunotoxin to work, it must bind to and be internalized by the target cells, and the toxin must translocate to the cytosol. Once in the cytosol, one molecule is capable of killing a cell. However, most toxins (or toxin fragments) currently used are relatively large molecules (molecular weigh ranging from 25–38 kD). These protein toxins are highly immunogenic to the host immune system for their foreign origin, which decreases immunotoxin in vivo half-life and limits their repeated use due to neutralizing antibodies. In addition, the large molecular size also poses difficulty in translocation into the cytosol, thus hampering the efficacy of immunotoxins.
In this study, we genetically fused the genes encoding human IL-2 and a novel small RIP, Luffin P1, to make a recombinant immunotoxin hIL-2-Luffin P1. Luffin P1 (43 aa) is the smallest ribosome-inactivating peptide so far reported that has potent N-glycosidase activity to inhibit protein translation (IC50 of 0.88 nM) [10, 11]. We intend to use the smallest RIP to overcome some of the above-mentioned difficulties associated with the application of immunotoxins. It is conceivable however, such a 43-aa peptide, even smaller than insulin (51 aa), may have very strict structure–function restriction that any addition of sequences at its ends may disrupt its enzymatic activity. To our surprise, hIL-2-Luffin P1 is highly potent in inhibiting in vitro T-cell proliferation stimulated by ConA and alloantigens, with IC50 reaching 1.8–10 nM, placing it among the strongest immunotoxins reported. It is noteworthy that the hIL-2 gene we used was of wild-type sequence, not encoding a mutated antagonist for competitive binding to IL-2R. Therefore, the cytotoxicity of hIL-2-Luffin P1 was achieved by overcoming the proliferation stimulatory activity of the cytokine moiety. Obviously, the fusion of hIL-2 to Luffin P1 at N-terminus did not affect Luffin P1 activity. The addition of His×6 tag and KDEL sequence at its C-terminus was not harmful either. In fact, although we did not have a KDEL-less construct as a control, we believe adding the four amino acids to the very end of the immunotoxin would help to boost its cytotoxicity.
As one of the cellular mechanisms for protein compartmentalization, C-terminal KDEL sequence is an ER retention signal. It was first noticed by Munro and Pelham that soluble ER chaperones encode a conserved KDEL sequence at their C-termini [15]. Deletion of KDEL sequence in proteins otherwise permanently residing in ER rendered them secretary. Conversely, addition of KDEL sequence to otherwise secretary proteins prevented their secretion and made them accumulated in ER [15]. Interestingly, the PE toxin has a motif REDLK at its C-terminus, very similar to the KDEL sequence. Mutation of this motif neither affected PE binding to target cells nor its internalization by endocytosis, but the mutant toxin could not translocate into the cytosol, hence is non-toxic. Replacement of the mutated motif with the KDEL sequence restored its full cytotoxicity [16]. It was later reported by many studies that recombinant immunotoxins with KDEL sequence added at the C-terminus could increase cytotoxicity by more than 100-fold [12, 17, 18]. These data indicate that KDEL improves immunotoxin cytotoxicity by increasing binding to a sorting receptor, which transports the toxin from the transreticular Golgi apparatus to the ER, where it translocates to the cytosol and inhibits protein synthesis.
With this molecular design and bolstered by its potent in vitro cytotoxicity on activated T cells up-regulating IL-2R, we tested hIL-2-Luffin P1 in vivo in allogenic skin and kidney transplantation models. Compared to control animals injected with HBSS or Luffin P1, the immunotoxin hIL-2-Luffin P1 significantly prolonged the survival of allografts (Figs 3 and 4), with no apparent histological abnormalities found in liver, lung, heart and kidney tissues during the experimental period (data not shown). Thus, hIL-2-Luffin P1 is a powerful immunotoxin that exhibits no obvious side effects.
Nevertheless, although prolongation of allograft survival by hIL-2-Luffin P1 was statistically significant, even after repeated injections for every 2 days, rejection still occurred in all the animals having received immunotoxin treatment. We think several issues need to be considered for future improvement. First, the molecular weight of hIL-2-Luffin P1 is only 22.5 kD, much below the 50–60 kD molecular cut-off value of the kidney. Thus, the majority of injected hIL-2-Luffin P1 protein could be excreted into the urine. The half-life of cytokines in circulation could be dramatically increased from minutes to more than 2 weeks by fusing cytokines with the immunoglobulin Fc domain, such as in the cases for IL-2/Fc and IL-15/Fc [19]. Thus, an improved version of hIL-2/Fc-Luffin P1 may exhibit more potent effects because of longer half-life in circulation. Nonetheless, such complicated fusion proteins with multiple pairs of disulfide bonds may not be correctly folded and secreted in E. coli systems. It should also not to be expressed in mammalian cells as the protein may be retained in the ER and the producing cells may be sensitive to cytotoxicity due to any leakage in translocation. A likely candidate for expressing hIL-2/Fc-Luffin P1 might be a plant cell-based expression system [20], which needs to be worked out in future. An alternative way is to administer the agent via different routes, such as subcutaneous or intramuscular. As compared to intravenous, the half-life of short-lived agents can be significantly increased via these routes. It would be interesting in future studies to compare side by side the pharmacokinetics of hIL-2-Luffin P1 and hIL-2/Fc-Luffin P1 administered via different routes, and to determine if in vivo activity of Luffin P1 immunotoxin can be improved when its half-life in circulation is prolonged.
Secondly, although we aim to use IL-2 to specifically target the immunotoxin to IL-2R-positive-activated donor-reactive Teff cells, hIL-2-Luffin P1 may also kill Foxp3+ regulatory T (Treg) cells. Treg cells are absolutely required for induction of tolerance to transplants. They highly express CD25, the α-subunit of the IL-2R complex, and use IL-2 for maintaining their survival and phenotype. In this sense, hIL-2-Luffin P1 may offer limited benefit as it could harm the regulatory arm of tolerance. On the other hand, previous studies suggested that Treg cells are relatively resistant to apoptosis induced by a lytic form of hIL-2/Fc [19]. The differential killing effect of hIL-2-Luffin P1 on Teff versus Treg cells awaits future careful analysis by using Luffin P1 immunotoxin, containing a mutant hIL-2 antagonist that can bind to its receptor but fail to signal. This would help accurately interpret the data by abolishing the growth stimulatory activity of wild-type IL-2 on T-cell subsets. In similar designs to extend the application, Luffin P1 could be fused with mutant IL-12 to dampen Th1 response, and with mutant IL-21 or IL-23 to inhibit Th17 cell expansion [21, 22]. It might also be beneficial to fuse Luffin P1 with pro-inflammatory cytokines and chemokines, as illustrated by diphtheria toxin-based cytokine-toxins, to control early inflammation [23–25]. Whether hIL-2-Luffin P1 and these reagents can synergize with each other and with other pharmacological and immunological reagents to achieve long-term engraftment and even tolerance needs to be further tested.
Regardless of the final entity of the constructs, our study is the first report of a novel recombinant immunotoxin using the smallest RIP as the toxin moiety. Although its mechanism of action and effects on various cell types in the immune system await further detailed analysis, its potent in vitro and in vivo activities provided proof of principle that minimizing toxin structure without compromising function is possible. Currently, FDA has approved DAB(389)IL-2 (denileukin diftitox, ONTAK), which contains human interleukin-2 and truncated diphtheria toxin, for treating cutaneous T-cell lymphoma [26]. Another cytokine-fusion immunotoxin IL13-PE38QQR (cintredekin besudotox), composed of human IL-13 and a truncated form of Pseudomonas exotoxin A, has completed clinical trials and is being evaluated for its efficacy for treating malignant glioma [27]. However, application of these immunotoxins has been associated with some side effects, such as myalgia, oedema, hypocalcaemia, low albuminemia, hypofibrinogenaemia and hyperpotassaemia. One of the reasons responsible for these side effects could be due to the large molecular weight and strong immunogenicity of the toxin moiety. Applying bacterial toxins in previously vaccinated hosts may also limit their repeated use and aggravate these symptoms. The refinement of existing immunotoxins with novel small ribosome-inactivating peptides from plants might decrease immunogenicity and improve pharmacokinetics and ensure better drug delivery. A side-by-side comparison of different generations of immunotoxins with large or small toxin molecules in terms of circulation half-life and eliciting neutralizing antibodies will be necessary. If the advantages of using small RIPs are confirmed, these second-generation immunotoxins will find broadened use in treating transplant rejection, autoimmune diseases, as well as lymphocyte-derived tumours.
In conclusion, immunotoxin hIL-2-Luffin P1 strongly inhibited lymphocyte activation in vitro and significantly prolonged the mean survival time of skin and kidney allografts, demonstrating promising clinical potential. On the other hand, as IL-2 receptors are highly expressed on some T- and B-cell tumours, hIL-2-Luffin P1 may also be used for the management of certain lymphocytic caners.
Acknowledgments
This work was supported in part by grants from National Science Foundation of China (30672174 to J.W., 30771933 to R.W.), National ‘863’ Project (2006AA02A121 to J.W.), State Key Laboratory Funding 2009 (SKLZZ200808 to J.W.) and Juvenile Diabetes Research Foundation (1-2007-551 to W.G.).
References
- 1.Wells AD, Li XC, Li Y, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–7. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 2.Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–80. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 3.Kreitman RJ. Immunotoxins for targeted cancer therapy. Aaps J. 2006;8:E532–51. doi: 10.1208/aapsj080363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peumans WJ, Hao Q, Van Damme EJ. Ribosome-inactivating proteins from plants: more than RNA N-glycosidases? FASEB J. 2001;15:1493–506. doi: 10.1096/fj.00-0751rev. [DOI] [PubMed] [Google Scholar]
- 5.Endo Y, Mitsui K, Motizuki M, et al. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem. 1987;262:5908–12. [PubMed] [Google Scholar]
- 6.Gao W, Ling J, Zhong X, et al. Luffin-S – a small novel ribosome-inactivating protein from Luffa cylindrica. Characterization and mechanism studies. FEBS Lett. 1994;347:257–60. doi: 10.1016/0014-5793(94)00554-0. [DOI] [PubMed] [Google Scholar]
- 7.Ishihara H, Sasagawa T, Sakai R, et al. Isolation and molecular characterization of four arginine/glutamate rich polypeptides from the seeds of sponge gourd (Luffa cylindrica. Biosci Biotechnol Biochem. 1997;61:168–70. doi: 10.1271/bbb.61.168. [DOI] [PubMed] [Google Scholar]
- 8.Kimura M, Park SS, Sakai R, et al. Primary structure of 6.5k-arginine/glutamate-rich polypeptide from the seeds of sponge gourd (Luffa cylindrica. Biosci Biotechnol Biochem. 1997;61:984–8. doi: 10.1271/bbb.61.984. [DOI] [PubMed] [Google Scholar]
- 9.Parkash A, Ng TB, Tso WW. Isolation and characterization of luffacylin, a ribosome inactivating peptide with anti-fungal activity from sponge gourd (Luffa cylindrica) seeds. Peptides. 2002;23:1019–24. doi: 10.1016/s0196-9781(02)00045-1. [DOI] [PubMed] [Google Scholar]
- 10.Li F, Xia HC, Yang XX, et al. Purification and partial characterization of luffin P1, a peptide with translational inhibitory activity and trypsin inhibitory activity, from seeds of Luffa cylindrica. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2003;35:847–52. [PubMed] [Google Scholar]
- 11.Li F, Yang XX, Xia HC, et al. Purification and characterization of Luffin P1, a ribosome-inactivating peptide from the seeds of Luffa cylindrica. Peptides. 2003;24:799–805. doi: 10.1016/s0196-9781(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 12.Kreitman RJ, Pastan I. Importance of the glutamate residue of KDEL in increasing the cytotoxicity of Pseudomonas exotoxin derivatives and for increased binding to the KDEL receptor. Biochem J. 1995;307:29–37. doi: 10.1042/bj3070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Barisoni D, Armato U. Donor’s and recipient’s antigen presenting cells co-operatively enhance the allo-reactive proliferation of peripheral blood lymphocytes. Life Sci. 1994;54:1009–17. doi: 10.1016/0024-3205(94)00503-6. [DOI] [PubMed] [Google Scholar]
- 14.Schumacher M, Van Vliet BN, Ferrari P. Kidney transplantation in rats: an appraisal of surgical techniques and outcome. Microsurgery. 2003;23:387–94. doi: 10.1002/micr.10139. [DOI] [PubMed] [Google Scholar]
- 15.Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhary VK, Jinno Y, Fitzgerald D, et al. Pseudomonas exotoxin contains a specific sequence at the carboxyl terminus that is required for cytotoxicity. Proc Natl Acad Sci U S A. 1990;87:308–12. doi: 10.1073/pnas.87.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitcher C, Roberts L, Fawell S, et al. Generation of a potent chimeric toxin by replacement of domain III of Pseudomonas exotoxin with ricin A chain KDEL. Bioconjug Chem. 1995;6:624–9. doi: 10.1021/bc00035a018. [DOI] [PubMed] [Google Scholar]
- 18.Seetharam S, Chaudhary VK, FitzGerald D, et al. Increased cytotoxic activity of Pseudomonas exotoxin and two chimeric toxins ending in KDEL. J Biol Chem. 1991;266:17376–81. [PubMed] [Google Scholar]
- 19.Zheng XX, Sanchez-Fueyo A, Sho M, et al. Favorably tipping the balance between cytopathic and regulatory T cells to create transplantation tolerance. Immunity. 2003;19:503–14. doi: 10.1016/s1074-7613(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 20.Decker EL, Reski R. Moss bioreactors producing improved biopharmaceuticals. Curr Opin Biotechnol. 2007;18:393–8. doi: 10.1016/j.copbio.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 23.Jia J, Li H, Tai S, et al. Construction and preliminary investigation of a plasmid containing a novel immunotoxin DT390-IL-18 gene for the prevention of murine experimental autoimmune encephalomyelitis. DNA Cell Biol. 2008;27:279–85. doi: 10.1089/dna.2007.0642. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Li H, Jia Y, et al. In vivo administration of plasmid DNA encoding recombinant immunotoxin DT390-IP-10 attenuates experimental autoimmune encephalomyelitis. J Autoimmun. 2007;28:30–40. doi: 10.1016/j.jaut.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Jia Y, Li H, Chen W, et al. Prevention of murine experimental autoimmune encephalomyelitis by in vivo expression of a novel recombinant immunotoxin DT390-RANTES. Gene Ther. 2006;13:1351–9. doi: 10.1038/sj.gt.3302799. [DOI] [PubMed] [Google Scholar]
- 26.Foss FM. Interleukin-2 fusion toxin: targeted therapy for cutaneous T cell lymphoma. Ann N Y Acad Sci. 2001;941:166–76. doi: 10.1111/j.1749-6632.2001.tb03720.x. [DOI] [PubMed] [Google Scholar]
- 27.Mut M, Sherman JH, Shaffrey ME, et al. Cintredekin besudotox in treatment of malignant glioma. Expert Opin Biol Ther. 2008;8:805–12. doi: 10.1517/14712598.8.6.805. [DOI] [PubMed] [Google Scholar]


