Abstract
Human myelin basic protein (hMBP)-hydrolyzing activity was recently shown to be an intrinsic property of antibodies (Abs) from multiple sclerosis (MS) patients. Here, we present the first evidence demonstrating a significant diversity of different fractions of polyclonal IgGs (pIgGs) from MS patients in their affinity for hMBP and in the ability of pIgGs to hydrolyze hBMP at different optimal pHs (3–10.5). IgGs containing λ- and κ-types of light chains demonstrated comparable relative activities in the hydrolysis of hMBP. IgGs of IgG1–IgG4 sub-classes were analyzed for catalytic activity. IgGs of all four sub-classes were catalytically active, with their contribution to the total activity of Abzs in the hydrolysis of hMBP and its 19-mer oligopeptide increasing in the order: IgG1 (1.5–2.1%) < IgG2 (4.9–12.8%) < IgG3 (14.7–25.0%) < IgG4 (71–78%). Our findings suggest that the immune systems of individual MS patients generate a variety of anti-hMBP abzymes with different catalytic properties, which can attack hMBP of myelin-proteolipid shell of axons, playing an important role in MS pathogenesis.
Keywords: human blood, multiple sclerosis, catalytic IgG, hydrolysis of myelin basic protein
Introduction
Catalytically active artificial antibodies (Abs) or abzymes (Abzs) against transition chemical states of different reactions have been studied intensively (reviewed in [1]). The first example of a natural Abz was an IgG found in bronchial asthma patients, which hydrolyzes VIP [2]. During last two decades, it has become clear that auto-Abs from the sera of patients with different autoimmune (AI) diseases can possess enzymic activities and that their occurrence is a distinctive feature of AI diseases (reviewed in [3–6]). Natural Abzs hydrolyzing DNA, RNA, polysaccharides, oligopeptides and proteins are described from the sera of patients with several AI (SLE, Hashimoto’s thyroiditis, polyarthritis, multiple sclerosis [MS], asthma, rheumatoid arthritis, etc.) and viral diseases with a pronounced immune system disturbance (viral hepatitis and AIDS) [2–9]. Some healthy patients demonstrated Abzs with low proteolytic [2, 8] and polysaccharide-hydrolyzing activities [9], but healthy humans and patients with many diseases with insignificant autoimmune reactions usually lack Abzs or develop Abzs with very low catalytic activities, often on a borderline of the sensitivity of detection methods [3–6].
There are two general ways in which Abzs with different enzymic activities may appear in various AI diseases. First, similarly to artificial Abzs against analogs of transition states of catalytic reactions [1], naturally occurring Abzs may be Abs raised directly against the enzyme substrates acting as haptens and mimicking transition states of catalytic reactions [2–8]. On the other hand, anti-idiotypic Abs can be induced in AI diseases by a primary antigen and may show some of its features including the catalytic activity [3–6, 10, 11].
According to the current point of view, Abzs may play a significant role in broadening autoantibody properties, forming specific pathogenic patterns and clinical settings in different AI conditions [3–6]. Anti-VIP Abzs can have an important effect on pathogenesis due to a decrease in the concentration of VIP, which plays a major role in the asthma pathophysiology [12]. DNase Abzs from SLE, lymphoproliferative diseases [13] and MS [5] and DNA-hydrolyzing Bence–Jones proteins from multiple myeloma patients [14] are cytotoxic, cause nuclear DNA fragmentation and induce cell death by apoptosis. In addition, for 120 patients with Hashimoto thyroiditis, it was shown that relative activities of DNase Abzs correlate with a concentration of thyroid hormones and other biochemical and immunological indices of this pathology, and are related to the progressive deterioration of the clinical status of patients, including exacerbation of thyroid gland damage [15]. Proteolytic IgGs from patients with sepsis may participate in the control of disseminated microvascular thrombosis and play a role in recovery from the disease [16]. Obviously, the study of mechanisms of Abzs production and their biological role is very important for understanding the pathogenesis of AI diseases.
MS is a chronic demyelinating disease of the central nervous system. Its etiology remains unclear, and the most widely accepted theory of MS pathogenesis assigns the main role in the destruction of myelin to the inflammation related to AI reactions [17]. Evidence supports activated CD41 myelin-reactive T cells as major mediators of MS [17]. Several recent findings imply an important role of B cells and auto-Abs against myelin autoantigens in the pathogenesis of MS [17–19]. An important dual role of auto-Abs is suggested: they may be harmful in lesion formation but also potentially beneficial in repair [18]. Elevated Abs levels and oligoclonal IgG bands in the cerebrospinal fluid (CSF) as well as clonal B-cell accumulation in the CSF and lesions of MS patients are among the main lines of evidence [20]. High-affinity anti-DNA Abs have been recently identified as a major component of the intrathecal IgG in MS patient’s brains and CSF cells [21].
Recently, we have shown that homogeneous IgGs from the sera and CSF of MS patients are active in the hydrolysis of DNA, RNA and polysaccharides [22–24]. Whereas only 18 and 53% of MS patients contain increased concentrations of Abs to native and denatured DNA, respectively, as compared with healthy donors [6], DNase Abzs were found in approximately 80–90% of MS patients [22, 23]. Because DNase Abzs of MS patients are cytotoxic and induce apoptosis [5], they can play an important role in MS pathogenesis.
Immune-mediated demyelination initially manifests as a separation of myelin lamellae followed by the loss of myelin proteins and the eventual loss of myelin membranes. hMBP, one of the major structural proteins of myelin, is highly vulnerable to various proteases produced by diverse cell types [25]. Recently, we have proposed catalytic immune response against hMBP in MS patients, developed several rigid criteria and applied them to show that hMBP-hydrolyzing activity is indeed an intrinsic property of IgGs, IgMs and IgAs from the sera of patients with MS [26–28]. Later, hMBP-hydrolyzing activity of IgG from MS patients was confirmed and the specific sites of neural antigen cleaved by Abs were established [29]. Recognition and degradation of MBP peptides by serum auto-Abs was confirmed as a novel biomarker for MS [30].
Theoretically, a mammalian immune system can produce up to 106 variants of Abs against one antigen. An extreme diversity of RNase and DNase IgG and/or IgM Abzs from the sera of patients with MS and SLE and autoimmune prone MRL-lpr/lpr mice was observed [3–6, 23, 31–33]. It was shown that different patients may have a relatively small or an extremely large pool of polyclonal nuclease Abs containing different relative amounts of light chains of κ- and λ-types, demonstrating maximal activity at various optimal pHs, having a different net charge, activated or not by magnesium ions, and characterized by different substrate specificities [3–6]. At the same time, possible diversity of polyclonal Abs with proteolytic activity has not yet been analyzed. In addition, the data concerning possible catalytic activity of IgG1-IgG4 sub-classes are not available.
In this report, we use several different methods to provide the first evidence for significant diversity of polyclonal hMBP-hydrolyzing IgGs from MS patients.
Materials and methods
Chemicals and patients
hMBP was from the Department of Biotechnology, Research Center of Molecular Diagnostics and Therapy (Moscow), all other chemicals were from Sigma (St. Louis, MO, USA) or Pharmacia (GE Healthcare, Uppsala, Sweden). All protein-Sepharoses were obtained by immobilizing hMBP or monoclonal IgGs (Sigma) on BrCN-activated Sepharose according to the standard manufacturer’s protocol.
In our previous study, 35 patients with various differential MS diagnoses were used: 11 patients in remission, 5 patients at the evolutionary stage and 19 patients at the secondary chronic progressive stage of the disease were used [27]. In contrast to healthy donors, IgGs from approximately 89% of all investigated MS patients and IgMs from all patients with different diagnoses demonstrate detectable or significant activity in hydrolysis of hMBP. The relative level of Ab proteolytic activity statistically significantly increases with the exacerbation of the disease. In addition, it was shown that the relative level of DNase, RNase and amylase activities of Abs from patients with several AI diseases, including MS increases with exacerbation and decreases in remission [3–6]. Taking this into account, we have used the sera of 20 patients (16- to 55-year-old; 8 men and 12 women) with clinically defined MS according to the Poser criteria [34] in the present study to analyze a catalytic heterogeneity of hMBP-hydrolyzing IgGs. All 8 patients with the relapsing/remitting form of disease (RRMS) were in the relapse/exacerbation state at the time of blood collection. The remaining 12 patients had secondary progressive MS (SPMS) and four of these patients were in the relapse/exacerbation state at the time of blood collection. This special group of MS patients demonstrated EDSS from three to six; the average EDSS and expandability of disease were 4.5 6 0.3 and 3.2 6 1.2 years, respectively. None of these patients have received immunomodulatory therapy. During 8–12 months before collection of their blood prior to a visit to a neuropathologist or hospitalization, the patients have obtained only general restorative therapy. On the previous stages of MS or its exacerbation, only hormonal and restorative therapy was used.
The sera of 10 patients (18- to 49-year-old; 4 men and 6 women) diagnosed with schizophrenia according to the international standards were also used to study proteolytic Abzs. Igs from the sera of 10 healthy humans (18–65 year old; 5 men and 5 women) were used for control experiments.
The blood sampling protocol conformed to the local hospital human ethics committee guidelines.
Antibody purification and analysis
Twenty electrophoretically and immunologically homogeneous individual pIgG preparations from MS patients were obtained by sequential affinity chromatography of the serum proteins on protein A-Sepharose and FPLC gel filtration on a Superdex 200 HR 10/30 column [22–24, 26–28]. Using the same standard protocol, individual pIgG preparations were obtained from the sera of 10 healthy donors and 10 patients with schizophrenia.
IgGs were chromatographed on Sepharose bearing immobilized hMBP or monoclonal Abs (anti-IgG1, anti-IgG2, anti-IgG3, anti-IgG4, anti-κ-IgG-, or anti-λ-IgG). The column (1 ml) was equilibrated with 50 mM Tris–HCl (pH 7.5) containing 50 mM NaCl; the protein was applied and then the column was washed with the same buffer (hMBP-Sepharose) or with a buffer containing 0.5 M NaCl (anti-IgG-Sepharoses) to zero optical density. IgGs were eluted from hMBP-Sepharose with the same buffer containing different concentrations of NaCl (0.1–3 M) and then with 2–3 M MgCl2, whereas 0.1 M glycine-HCl (pH 2.6) was used for elution of Abs from the anti-IgG-Sepharoses. The column fractions were collected into cooled tubes containing 50 ml of 0.5 M Tris–HCl (pH 9.0), and each fraction was additionally neutralized with this buffer. Fractions after all chromatographies were dialyzed against 50 mM Tris–HCl (pH 7.5) containing 50 mM NaCl. SDS-PAGE analysis of Abs for homogeneity was performed in 5–16% gradient gels (0.1% SDS) as in [26–28]. The polypeptides were visualized by silver staining and by Western blotting onto a nitrocellulose membrane [22–24, 26–28].
ELISA of autoantibodies of different types
After chromatography of Abs on Sepharose bearing immobilized monoclonal mouse Abs (anti-IgG1, anti-IgG2, anti-IgG3, anti-IgG4, anti-κ-IgG or anti-λ-IgG), the IgGs were analyzed for isotype homogeneity by ELISA. Sodium carbonate buffer (50 ml, pH 9.6) containing 0.005 mg/ml of one of the tested IgGs was added to the ELISA strips and incubated overnight at 228C. The assembled strips were washed with TBS buffer containing 0.01% NaN3 and 0.05% Triton X-100 and twice with the same buffer without Triton X-100. The strips were blocked for 2 hrs at 37°C using TBS containing 0.2% bovine albumin, 0.01% NaN3 and washed 10 times with water and then with TBS containing 0.01% NaN3.
Each of the monoclonal mouse Abs (100 ml, 0.01 mg/ml; anti-IgG1, anti-IgG2, anti-IgG3, anti-IgG4; in some experiments. anti-κ-IgG, or anti-λ-IgG) in TBS containing 0.2% bovine albumin, 0.01% NaN3 and 0.05% Triton X-100 was added to the strips corresponding to human IgGs of different sub-classes and incubated for 2 hrs at 37°C. After washing of the strips with water (10 times) and TBS, 100 ml TBS containing 0.2% bovine albumin and 0.01% NaN3 were added, incubation 2 hrs at 37°C. The strips were washed 10 times with water and incubated with 100 ml TBS containing 1 mg/ml conjugate of polyclonal anti-mouse IgGs with horseradish peroxidase for 30 min. at 37°C and washed again 10 times with water. After addition of 50 ml citric-phosphate buffer containing 3,3′,5,5′-tetramethylbenzidine and H2O2, the strips were incubated for 15 min. at room temperature, and the reaction was stopped by addition of 50 ml of 50% H2SO4. The relative concentrations of Abs in samples analyzed were expressed as an optical density of the solution at 450 nm (units A450; average of three measurements). It was shown that each Ab preparation obtained using affinity chromatography contained IgGs of only one type.
Ab proteolytic activity assay
The reaction mixture (10–20 ml) for analysis of hMBP- or OP-19-hydrolyzing activity of IgGs, containing 50 mM Tris–HCl (pH 7.5), 0.19 mg/ml hMBP or 0.33 mM OP-19 and 10–100 mg/ml of IgGs, was incubated for 0.1–16 hrs at 37°C. OP-19 (R-LeuSerArgPheSerTrpGly-Ala-GluGlyGlnLysProGlyPheGlyTyrGlyGly) corresponding to one of four known IgG-dependent-specific cleavage sites of hMBP [29] and containing fluorescent residue 6-0-(carboxymethyl)fluorescein ethyl ester (R) on its N-terminus was used.
The hMBP cleavage products were analyzed by SDS-PAGE in 5–16% gradient gels with Coomassie R250 staining. The gels were imaged by scanning and quantified using GelPro v3.1 software. The OP-19 cleavage products were separated by TLC on Kieselgel F60 plates using the acetic acid–n-buthanol–H2O (1:4:5) system. The plates were dried and photographed. To quantify the intensities of the fluorescent spots after TLC, control OP-19 incubated without IgGs was used. Photographs of the plates were imaged by scanning and quantified using GelPro v3.1 software.
pH dependencies were analyzed using different buffers (50 mM): glycine-HCl (pH 2.6–3.5), MES-KOH (pH 5.4–6.6), MOPS-KOH (pH 6.6–7.6), Tris–HCl (pH 7.5–8.8) and glycine-KOH (pH 9.1–10.3).
Results
Recently, we presented evidence demonstrating that highly purified MS IgGs specifically catalyze hydrolysis of hMBP but not other proteins [26, 28]. In this work, electrophoretically and immunologically homogeneous pIgG was purified by sequential chromatography on Protein-G Sepharose under conditions that remove non-specifically bound proteins, followed by FPLC gel filtration in an acidic buffer destroying immune complexes [26–28]. The homogeneity of the 150 kD IgG was confirmed by SDS-PAGE with silver staining, which showed a single band under non-reducing conditions and two bands corresponding to the H and L chains after reduction. To exclude possible artefacts due to hypothetical traces of contaminating enzymes, the IgG was separated by SDS-PAGE and its hMBP-hydrolyzing activity was detected using an in-gel assay as in [26–28]. The activities were revealed only in the band corresponding to intact IgGs, and there were no other peaks of proteins or proteolytic activity. In addition, it was shown that, in contrast to canonical proteases, the preparation of pIgGs hydrolyzed specifically only hMBP but not many other tested proteins.
It was shown previously that anti-hMBP Abs were detectable in healthy donors, with their concentration in the 0.03–0.20 A450 units range, averaging 0.09 6 0.04 A450 units [26]. Relative indexes of anti-MBP Abs for 25 MS patients were between 0.67 and 0.98 A450 units, 0.8 6 0.1 A450 units on average. Thus, all MS patients analyzed by us previously demonstrated significantly higher levels of serum anti-MBP Abs than healthy individuals. IgGs from approximately 89% and IgMs from 100% of patients with different diagnoses demonstrate detectable or significant activity in hydrolysis of hMBP [26–28]. The relative level of Ab proteolytic activity statistically significant increase with the exacerbation of the disease. IgGs from healthy humans do not hydrolyze hMBP [26–29].
Here, we have used 10 new IgG preparations and confirmed that IgGs from that healthy humans do not possess MBP-hydrolyzing activity (Fig. 1). Specific IgG fractions from the sera of healthy donors and from MS patients with and without affinity for hBMP were obtained by affinity chromatography on hBMP-Sepharose (see below). The IgGs from MS patients having no affinity for hBMP-Sepharose and from the sera of healthy donors eluted by salt from hBMP-Sepharose (see below) also did not hydrolyze hBMP. In addition, it was shown that none of individual IgGs from 10 patients with schizophrenia hydrolyze hMBP (Fig. 1).
Fig 1.
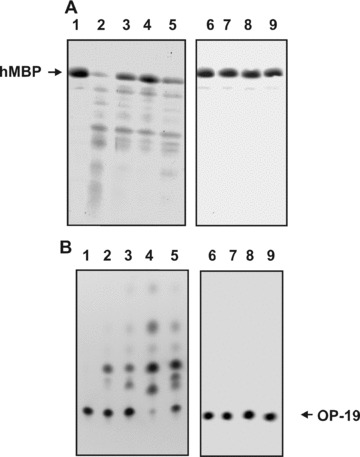
Examples of hMBP and OP-19 hydrolysis by non-fractionated pIgGs from different MS patients and control IgGs. The hydrolysis of 0.19 mg/ml hMBP (2.5 hrs) in the presence of pIgGs was followed by the decrease in the intensity of Coomassie-stained hMBP band after SDS-PAGE electrophoresis (A), and the hydrolysis of 0.33 mM OP-19 (2.0 hrs), by the decrease in the fluorescence of initial OP-19 spots after TLC (B). The difference in the intensities of these substrates incubated in the absence (lane 1) and in the presence of IgGs from four MS patients (lanes 2–5) was used for the calculations of their RAs. Several control IgGs were used: from a healthy donor (lane 6), a patient with schizophrenia (lane 7), IgGs from MS patients having no affinity for hMBP-Sepharose (lane 8) and IgGs from a healthy patient having affinity for hMPB-Sepharose and purified on this sorbent (lane 9). To quantitatively estimate the protease activity only experiments with the 15–40% conversation of substrate to its product of hydrolysis were used, for example, lane 4 (A) and lines 2 and 3 (B). In this experiment, 0.1 mg/ml pIgGs from eight different patients were used.
The efficiency of the hMBP cleavage was calculated from the decrease in the intensity of Coomassie-stained hMBP band after electrophoresis [26–28] and from hydrolysis of a specific 19-mer oligopeptide (OP-19) [29] following the decrease in fluorescence of the initial OP-19 sports after TLC; the difference in the intensities of these substrates incubated in the absence and in the presence of Abs was used for the calculations (Fig. 1). To quantitatively estimate the protease activity, we have found a low concentration for each IgG fraction corresponding to the reaction of the first order where the substrate is converted into products during hydrolysis within the linear regions of the time courses (15–40% of conversion).
It was shown that IgGs from all 20 MS patients demonstrate detectable level of MBP-hydrolyzing activity. Comparison of the relative activity of IgGs from 20 patients has shown that on average it increases on going from the secondary chronic progressive stage (8 patients, 16 6 8 nM MBP/1 hr /mg of IgGs) to the exacerbation stage of MS (12 patients; 39 6 11 nM MBP/1 hr /mg of IgGs). The differences between the IgG samples of these two groups according to Student’s t-test was statistically significant (P < 0.05). Ten IgG preparations from both groups (three from the secondary chronic progressive stage and seven from the exacerbation group) demonstrating different relative activities were used for a more detailed study of catalytic heterogeneity of Abzs.
pH dependencies of hMBP hydrolysis
Catalytic heterogeneity of polyclonal nuclease and polysaccharide-hydrolyzing Abzs from patients with different AI diseases, including MS patients was shown in many papers [23, 24, 31–33].
It is well known that canonical mammalian, bacterial and plant proteases, depending on their biological function, can have optimal pH values ranging from acidic (2.0) to neutral and alkaline (8–10) [35, 36]. Since the range of optimal pH of Abzs with proteolytic activity was not known, we have measured the relative activity of IgGs at pH from 2.6 to 10.5 and compared the results with the pH optima of canonical mammalian proteases. First, we have analyzed the pH dependencies of the initial rates of hMBP hydrolysis by five individual MS IgGs. The pH profile of each IgG was unique (Fig. 2). In contrast to all human proteases having one pronounced pH optimum, catalytic IgGs demonstrated high specific hBMP-hydrolyzing activity within a wide range of pH values (2.6–10). Interestingly, one of the pIgG preparations (number 1) had a single pronounced optimum of hMBP hydrolysis at pH 2.6; four preparations (numbers 2–5) demonstrated a notable pH optimum at pH from 4.2 to 5.4, whereas only three of them (numbers 2–4) have notable optima at pH from 8.2 to 9.8. The hydrolysis of the substrate proceeded with very different rates at pH values from 5.3 to 8.2 (Fig. 2). The above results clearly demonstrate that IgGs from individual MS patients can consist of different sets of catalytic IgG sub-fractions demonstrating quite distinct enzymic properties. At pH 2.6 IgGs are usually partially denatured, but, at the same time, the duration of the reaction allows them to hydrolyze hBMP with detectable or high efficiency (Fig. 2). Taking this into account, one cannot exclude that human immune system could in principle produce Abzs with a proteolytic activity similar to that of stomach acidic proteases.
Fig 2.
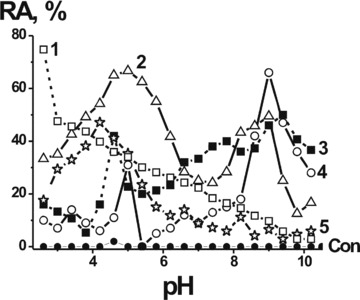
pH dependence of the relative hMBP-hydrolyzing activity (RA) of individual IgGs from the sera of five different MS patients (graphs 1–5). Hydrolysis of hMBP incubated alone was used as control (‘Con.’) The relative protease activity corresponding to a complete transition of 0.19 mg/ml hMBP to its shorter oligopeptides after 1.5 hrs in the presence of 0.1 mg/ml pIgGs was taken for 100%. The average error in the initial rate determination from two experiments did not exceed 7–10%. For other details see Materials and Methods.
Catalytic activity of IgGs of different sub-classes
As mentioned earlier, AI pIgGs can possess DNase, RNase, amylolytic and proteolytic activity [3–9]. However, at present nothing is known about possible catalytic activities of IgGs of different sub-classes. To analyze an ‘average’ situation concerning a possible catalytic heterogeneity of MBP-hydrolyzing IgGs, we have prepared a mixture of equal amounts of IgGs from the sera of 10 MS patients. We have separated mixture of IgGs to Ab sub-fractions of the first (IgG1), second (IgG2), third (IgG3) and fourth (IgG4) sub-classes as well as IgGs containing κ− and λ-type of light chains by affinity chromatography on the specific affinity adsorbents bearing immobilized monoclonal Abs to human IgGs of these types (Figs. 3 and 4). The purity of IgGs of all types was analyzed by ELISA; preparations of IgG1, IgG2, IgG3 and IgG4 were immunologically homogeneous and did not contain detectable amounts of IgGs of other sub-classes. Immunological homogeneity was also observed for IgGs containing κ− and λ-type of light chains.
Fig 3.
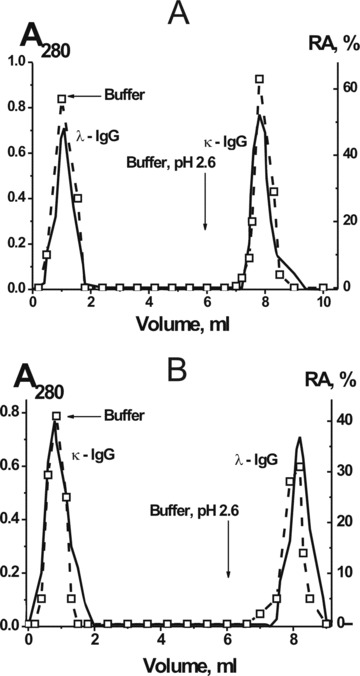
Affinity chromatography of the mixture of 10 pIgG preparations on anti-κ-Abs (A) and anti-λ-Abs (B) Sepharoses: (—), absorbance at 280 nm, (□) relative catalytic activity (RA). The complete transition of 0.19 mg/ml hMBP to its hydrolyzed forms after 1 hr of incubation in the presence of 0.1 mg/ml pIgGs was taken for 100%. The average error in the initial rate determination from two experiments in each case did not exceed 7–10%.
Fig 4.
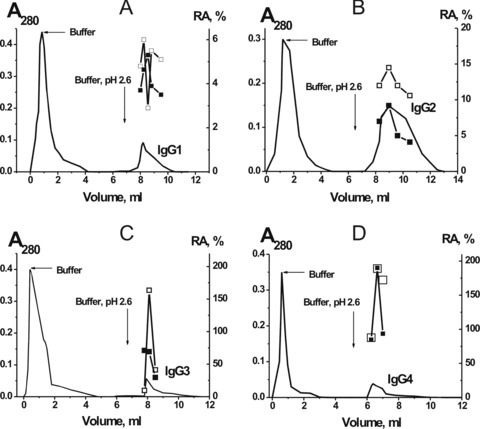
Affinity chromatography of pIgGs (mixture of 10 preparations) on anti-IgG1 (A), anti-IgG2 (B), anti-IgG3 (C) and anti-IgG4 (D) Sepharose: (—), absorbance at 280 nm, (□) and (▪), relative catalytic activities (RA) in the hydrolysis of hMBP and OP-19, respectively. Depending on the RA, the reaction mixtures were incubated for 0.3–16 hrs in the presence of 10–100 mg/ml IgGs and then the RAs were normalized to the standard conditions: the complete transition of 0.19 mg/ml hMBP 0.33 mM OP-19 to their hydrolyzed forms in the presence of 0.1 mg/ml pIgGs after 1 hr of incubation was taken for 100%. The average error in the initial rate determination from two experiments in each case did not exceed 7–10%.
The relative activities (RAs) of the fractions corresponding to the central parts of the peaks of different types of IgGs eluted with glycine buffer, pH 2.6, were measured in the hydrolysis of hMBP and OP-19 (Figs. 3 and 4) as in [26–29].
The profiles of the RAs in the hydrolysis of hMBP and OP-19 catalyzed by pIgGs of different sub-classes were to some extent similar (Fig. 4). All fractions of every peak corresponding to the specific type of IgGs were combined and their relative specific activity (RSA) in the hydrolysis of hBMP (0.19 mg/ml) and OP-19 (0.33 mM) at fixed concentrations of these substrates was measured (Table 1).
Table 1.
The Km and kcat values characterizing interaction of initial pIgG and its sub-fractions separated by chromatography on different affinity adsorbents with hMBP and specific 19-mer oligopeptide
| IgG | Content, % | RSA (nM MBP/1 hr)/mg of IgGs | App. k cat, min−1 | Contribution to the total activity, % | RSA (mM OP-19/1 hr)/mg of IgGs | App. k cat, min−1 | Contribution to the total activity, % |
|---|---|---|---|---|---|---|---|
| IgG, non-fractionated | 100 | 43 ± 3.1 | 0.11 ± 0.01 | 100 | 0.9 ± 0.1 | 2.6 ± 0.5 | 100 |
| IgGs containing κ- and λ-types of light chains | |||||||
| λ-IgG | 45.2 (anti-λ) | 34.3 ± 3.0 | (8.8 ± 0.9)×10−2 | nd | 0.85 ± 0.1 | 2.1 ± 0.18 | nd |
| 48.2 (anti-κ) | 44.6 ± 4.0 | 0.11 ± 0.01 | nd | 1.1 ± 0.1 | 2.7 ± 0.24 | nd | |
| λ-IgG, average | 46.7 ± 2.0 | 39.5 ± 3.5 | 0.1 ± 0.01 | 50.3 ± 5 | 0.98 ± 0.1 | 2.4 ± 0.2 | 58.0 ± 5.0 |
| k-IgG | 54.8 (anti-λ) | 37.6 ± 3.1 | (9.9 ± 0.9)×10−2 | nd | 0.88 ± 0.09 | 2.2 ± 0.2 | nd |
| 51.8 (anti-κ) | 28.7 ± 2.7 | (7.5 ± 0.7)×10−2 | nd | 0.68 ± 0.07 | 1.7 ± 0.15 | nd | |
| κ-IgG, average | 53.3 ± 3.0 | 33.2 ± 3.5 | (8.7 ± 0.8)×10−2 | 49.7 ± 5 | 0.78 ± 0.08 | 2.0 ± 0.17 | 42.0 ± 5.0 |
| IgGs of different sub-classes | |||||||
| IgG1 | 22.0 ± 4.0 | 4.6 ± 0.4 | (1.2 ± 0.4)×10−2 | 1.5 ± 0.3 | 0.15 ± 0.02 | 0.36 ± 0.04 | 2.1 ± 0.3 |
| IgG2 | 36.0 ± 1.5 | 24.0 ± 1.5 | (6.3 ± 0.4)×10−2 | 12.8 ± 1.4 | 0.21 ± 0.02 | 0.52 ± 0.05 | 4.9 ± 0.5 |
| IgG3 | 12.3 ± 1.0 | 80.0 ± 3.0 | 0.21 ± 0.01 | 14.7 ± 2.0 | 1.9 ± 0.2 | 4.7 ± 0.5 | 15.0 ± 2.0 |
| IgG4 | 29.7 ± 3.0 | 159.0 ± 5.0 | 0.41 ± 0.01 | 71.0 ± 7.0 | 4.0 ± 0.3 | 10.1 ± 1.0 | 78.0 ± 8.0 |
For each fraction, a mean of two repeats is used. The apparent kcat values at fixed concentrations of hMBP (0.19 mg/ml) and OP-19 (0.33 mM) were calculated as kcat= V/[IgG]. Average values calculated from experiments with anti-κ-IgG and anti-λ-IgG Sepharoses. Contribution of IgGs of different types to the total activity of non-fractionated Abs was calculated taking into account the relative content of these IgGs within pIgGs and their RSAs in the hydrolysis of hMBP and OP-19.
IgGs containing κ− and λ-types of light chains were obtained in two ways: (i) by chromatography on anti-κ-IgG and (ii) on anti-λ-IgG Sepharose. Because the acidic treatment of IgGs leads to their partial inactivation and incomplete refolding after incubation of the purified IgGs in a neutral buffer, the RSAs of κ-IgGs and λ-IgGs eluted from the affinity sorbents by the acidic buffer were approximately 1.3-fold lower than those of corresponding IgG fractions of the flow-through peaks (Table 1). Since the purification of all types of IgGs (Figs. 3 and 4) was performed under the same standard conditions, it is reasonable to suggest that the activity of IgG1–IgG4 preparations eluted with the acidic buffer is also approximately 1.3-fold lower as compared with their activity before the affinity chromatography with an acidic treatment.
Interestingly, the RSA of IgGs of different sub-classes in the hydrolysis of hMBP increased in the order (expressed in nM hMBP/1 hr/mg of IgGs): IgG1 (4.6) < IgG2 (24.0) < IgG3 (80.0) < IgG4 (159.0) and at the same the order in the hydrolysis of OP-19 (expressed in mM OP-19/1 hr/mg of IgGs) was IgG1 (0.15) < IgG2 (0.21) < IgG3 (1.9) < IgG4 (4.0). To compare the hydrolysis of hMBP (0.19 mg/ml) and OP-19 (0.33 mM), the apparent kcat values of the hydrolysis of these substrates at their fixed concentrations were calculated as kcat= V/[IgG] (Table 1). On going from IgG1 with minimal to IgG4 with maximal RSA, the apparent kcat in the hydrolysis of hMBP and OP-19 increased approximately 34.6- and approximately 26.7-fold, respectively. A transition from hMBP (0.19 mg/ml = 10.2 mM) to OP-19 (0.33 mM) led to average increase in kcat approximately 21 ±-fold.
The relative content of λ-IgG (46.7%), κ-IgG (53.3%), IgG1(22%), IgG2 (36%), IgG3 (12.3%) and IgG4 (29.7%) within the mixture of pIgGs from 10 patients was estimated. Using these data and the RSAs of IgGs containing λ and κ-chains, the relative contribution of λ-IgGs and κ-IgGs into the total activity of pIgGs in the hydrolysis of hMBP and OP-19 was calculated to be 50.3–58.0% for λ-IgGs, and 42.0–49.7% for κ-IgGs (Table 1). The relative contribution of IgGs of different sub-classes to the total activity of pIgGs in the hydrolysis of hMBP and OP-19 was estimated in a similar way: IgG1 (1.5–2.1%) < IgG2 (4.9–12.8%) < IgG3 (14.7–25.0%) < IgG4 (71–78%). The contributions of λ-IgGs and κ-IgGs were comparable, whereas the contributions of IgG1 (approximately 34–54-fold), IgG2 (approximately 5.5–16-fold) and IgG3 (approximately 2.8–5.3-fold) in the hydrolysis of hMBP and OP-19 were significantly lower that that of IgG4, which showed the highest RSA. These data demonstrated for the first time that all types of human IgGs can possess catalytic activity, but differ in their contribution to the total activity of Abzs.
Chromatography of pIgGs on hMBP-Sepharose
It was shown previously that nuclease Abzs from the sera of AI patients and animals are very heterogeneous in their affinity for DNA and can be separated into many fractions by chromatography on DNA-cellulose [23, 31–33]. We have analyzed the affinity of MS pIgGs for hMBP by chromatography on hMBP-Sepharose (Fig. 5). The pIgG fractions of the first peak with no affinity for hMBP possessed no detectable proteolytic activity, whereas nine fractions of IgGs eluted from hMBP-Sepharose with different concentrations of NaCl (0.1–3.0 M) and MgCl2 (2–3 M) were active in the hydrolysis of hMBP. The distribution of the pIgG and its protease activity all over the profile of the affinity chromatography demonstrated a significant heterogeneity of MS IgGs in their affinity for hMBP.
Fig 5.
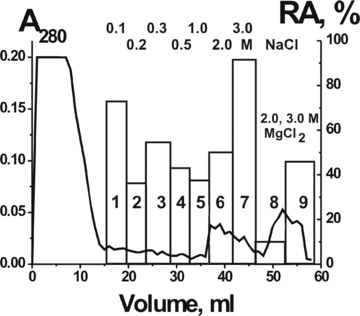
Affinity chromatography of the mixture of 10 preparations pIgGs on hMBP-Sepharose: (—), absorbance at 280 nm, the bars indicate the relative catalytic activity (RA) in the hydrolysis of hMBP. The samples were incubated 0.3–1.0 hrs and the complete transition of 0.19 mg/ml hMBP to its hydrolyzed forms in the presence of 0.01 mg/ml pIgGs after 1 hr of incubation was taken for 100%. The average error in the initial rate determination from two experiments in each case did not exceed 7–10%.
We have estimated the apparent Km and Vmax (kcat) values for the hydrolysis of both substrates by IgGs corresponding to the fractions 1, 5 and 9 with the weakest, medium and strongest binding to the affinity sorbent (Fig. 6). The initial rate data obtained at increasing hMBP or OP-19 concentration (e.g.Fig. 6) were consistent with the Michaelis–Menten kinetics. The approximate affinity of pIgG fractions for hMBP (in terms of Km values) increased gradually with the increase in eluting salt concentration; for pIgGs eluted with 3 M MgCl2 (Fig. 6B, fraction 9), it was approximately 5-fold higher than for the first pIgG fraction and approximately 1.5-fold higher than for the fifth fraction eluted with 1 M NaCl (Table 2). The approximate affinity of all analyzed fractions for OP-19 was approximately 100–200-fold lower than for hMBP, and the affinity of the ninth fraction for OP-19 was 10- and 4-fold higher than that of the first and fifth IgG fractions, respectively (Fig. 6C and D; Table 2). The data in Table 2 are indicative of catalytic and affinity heterogeneity of non-fractionated pIgG.
Fig 6.
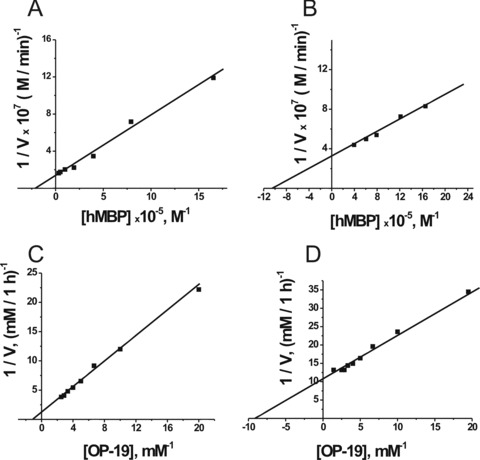
Determination of the Km and Vmax values for hMBP (A and B) and OP-19 (C and D) using a Lineweaver–Burk plot. The reactions were performed as described in Materials and Methods using 5 mg/ml (A and B) and 16 mg/ml pIgGs (C and D) of IgG fractions after chromatography on hMBP-Sepharose: fraction 1 (A and C) and fraction 9 (B and D). The average error in the initial rate determination at each substrate concentration from two independent experiments did not exceed 7–10%.
Table 2.
The Km and kcat values characterizing interaction of pIgG sub-fractions separated by chromatography on hMBP–Sepharose with hMBP and specific 19-mer oligopeptide
| Fraction number* | Condition of elution | Protease activity, hMBP | Protease activity, OP-19 | ||
|---|---|---|---|---|---|
| K m, μM | k cat, min−1 | K m, mM | k cat, min−1 | ||
| 1 | 0.1 M NaCl | 5.0 6 1.3 | 2.3 6 0.3 | 1.0 6 0.2 | 156.0 6 30.0 |
| 5 | 1.0 M NaCl | 1.5 6 0.2 | 1.0 6 0.18 | 0.25 6 0.05 | 33.0 6 5.0 |
| 9 | 3 M MgCl2 | 0.9 6 0.015 | 0.98 6 0.15 | 0.1 6 0.018 | 14.3 6 2.5 |
Numbers of fractions corresponds to Fig. 5. For each fraction, a mean of three repeats is used. The kcat values were calculated as kcat=Vmax/[IgG].
Discussion
Here, we analyzed possible proteolytic diversity of hMBP-hydrolyzing pIgGs of MS patients. It was shown that MS pIgGs hydrolyzing, in contrast to canonical proteases, only hMBP could be separated by chromatography on hMBP-Sepharose into many fractions with different affinity for hMBP. The approximate affinity of the separated IgG fractions (in terms of Km values) for hMBP and OP-19 increased with the increase in NaCl and MgCl2 concentrations and for the first Ab fraction eluted with 0.1 M NaCl it was approximately 6–10-fold lower than for the ninth fraction eluted with 3 M MgCl2 (Table 2). In addition, the affinity of all pIgG fractions for intact hMBP was approximately 100–200-fold higher than for its specific 19-mer peptide fragment (OP-19) (Table 2).
The affinity of MS pIgGs for hMBP (Km= 0.9–5.0 mM, Table 2) corresponds to the typical affinity (Km= 0.038–7.3 [2, 37, 38]) of Abzs for different protein antigens. The apparent Km values (0.1–1.0 mM, Table 2) for OP-19 short peptide were comparable with those for short peptides in reactions catalyzed by IgGs from patients with rheumatoid arthritis (0.39–0.53 mM [8]) and by different light chains with proteolytic activity (0.015–0.29 mM [39]), but lower than the values shown by recombinant light chains (4.8 mM [40]).
The catalysis mediated by artificial Abzs is usually characterized by relatively low reaction rates: kcat values are 102–106-fold lower than for canonical enzymes [1]. The known kcat values for natural Abzs from AI patients vary in the range of 0.001–40 min−1[2–9, 23–24, 31–33, 38–41]. The apparent kcat values for hMBP (1–2.3 min−1) and OP-19 (14.3–156 min−1) in the reactions catalyzed by IgG sub-fractions separated by affinity chromatography (Table 2) are comparable with or even higher than those for known Abzs characterized by the highest kcat values. It should be mentioned that currently there are no methods that could efficiently separate Abzs from catalytically inactive Abs against the same protein. Since the specific activities were calculated using the total concentrations of pIgGs, and affinity chromatography on Sepharose bearing immobilized hBMP or Abs against IgGs of different sub-classes leads only to partial enrichment of individual fractions with protease Abzs, the specific hMBP-hydrolyzing activities of the individual monoclonal sub-fractions in a polyclonal IgGs pool may be significantly higher than those of the non-fractionated or partially fractionated pIgGs.
Taken together, our previous findings show that MS pIgGs contain mainly Abzs of two types: serine and metal-dependent proteases [26, 28]. Here, we have shown that MS pIgGs contain many sub-fractions with different affinity for hMBP and its oligopeptide. In addition, IgGs from individual MS patients are characterized by individual pH dependencies (Fig. 1).
In this paper we show for the first time, using MS IgGs with hMBP-hydrolyzing activity as an example, that Abs with proteolytic activity similar to nuclease Abzs ([3–6, 31–32] can contain κ- and λ-types of light chains, and the relative contributions of κ- and λ-IgGs to the total activity of Abzs is comparable (Table 1).
It was very interesting whether pIgGs of different sub-classes may function as Abzs. It was surprising that MS IgGs of all four sub-classes efficiently hydrolyzed hMBP, but their contribution to the total activity of Abzs increased in the order: IgG1 (1.5–2.1%) < IgG2 (4.9–12.8%) < IgG3 (14.7–25.0%) < IgG4 (71–78%). Thus, hMBP-hydrolyzing IgGs of MS patients are very catalytically heterogeneous; IgG-abzymes of different sub-classes can contain κ- and λ-types of light chains, catalyze the hydrolysis of hMBP as serine-like or metalloproteases, demonstrate different affinity for hMBP and different pH optima.
Interestingly, structural heterogeneity was observed earlier for monoclonal Abs after immunization of mice with p-nitrobenzyl phosphonate hapten; approximately 10 different catalytic clones were usually obtained from a single fusion of lymphocytes taken from normal mice, whereas several hundred different catalytic clones were obtained in SJL or MRL/lpr mice [42]. Several different types of haptens were used to mimic the transition state of the substrate in the ester/amide hydrolysis [43]. Autoimmune mice results in a dramatically higher incidence of various Abzs with a higher activity than in conventionally used normal mouse strains.
In MS, the protease activity of anti-hMBP Abzs can attack hMBP of the myelin-proteolipid shell of axons. An established MS therapeutic Copaxone appears to be a specific hMBP-hydrolyzing Abzs inhibitor [29]. Consequently, the Abzs may play an important role in MS pathogenesis.
High-affinity anti-DNA Abs have been recently identified as a major component of the intrathecal IgG in brain and CSF cells of
MS patients [21]. In addition, it was shown that DNase Abzs from MS patients are cytotoxic and induce apoptotic cell death [5]. Moreover, MS Abs possess a very high polysaccharide-hydrolyzing activity [24]. Taking these data in account, it is reasonably to propose that polysaccharide-hydrolyzing, DNase and especially hMBP-hydrolyzing Abzs may cooperatively promote important neuropathologic mechanisms in this chronic inflammatory disorder and MS pathogenesis development.
Acknowledgments
This research was made possible in part by grants from the Presidium of the Russian Academy of Sciences (Molecular and Cellular Biology Program, 10.5; Fundamental Sciences to Medicine, 11.8), Russian Foundation for Basic Research (04-04-48211), RFBR-BFBR (04-04-81017) and funds from the Siberian Division of the Russian Academy of Sciences.
References
- 1.Keinan EE. Catalytic antibodies. Weinheim: Wiley-VCH Verlag GmbH and Co. KgaA; 2005. pp. 1–586. [Google Scholar]
- 2.Paul S, Volle DJ, Beach CM, et al. Catalytic hydrolysis of vasoactive intestinal peptide by human autoantibody. Science. 1989;244:1158–62. doi: 10.1126/science.2727702. [DOI] [PubMed] [Google Scholar]
- 3.Nevinsky GA, Buneva VN. Human catalytic RNA- and DNA-hydrolyzing antibodies. J Immunol Methods. 2002;269:235–45. doi: 10.1016/s0022-1759(02)00234-x. [DOI] [PubMed] [Google Scholar]
- 4.Nevinsky GA, Favorova OO, Buneva VN. Natural catalytic antibodies – new characters in the protein repertoire. In: Golemis E, editor. Protein-protein interactions: a molecular cloning manual. New York: Cold Spring Harbor: Cold Spring Harbor Lab. Press; 2002. pp. 523–34. [Google Scholar]
- 5.Nevinsky GA, Buneva VN. Catalytic antibodies in healthy humans and patients with autoimmune and viral pathologies. J Cell Mol Med. 2003;7:265–76. doi: 10.1111/j.1582-4934.2003.tb00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nevinsky GA, Buneva VN. Natural catalytic antibodies-abzymes. In: Keinan E, editor. Catalytic antibodies. Weinheim: Wiley-VCH Verlag GmbH and Co. KgaA; 2005. pp. 503–67. [Google Scholar]
- 7.Shuster AM, Gololobov GV, Kvashuk OA, et al. DNA hydrolyzing autoantibodies. Science. 1992;256:665–7. doi: 10.1126/science.1585181. [DOI] [PubMed] [Google Scholar]
- 8.Kalaga R, Li L, O’Dell JR, et al. Unexpected presence of polyreactive catalytic antibodies in IgG from unimmunized donors and decreased levels in rheumatoid arthritis. J Immunol. 1995;155:2695–702. [PubMed] [Google Scholar]
- 9.Savel’ev AN, Eneyskaya EV, Shabalin KA, et al. Autoantibodies with amylolytic activity. Prot Pept Lett. 1999;6:179–84. [Google Scholar]
- 10.Izadyar LA, Friboulet MH, Remy A, et al. Monoclonal anti-idiotypic antibodies as functional internal images of enzyme active sites: production of a catalytic antibody with a cholinesterase activity. Proc Natl Acad Sci USA. 1993;90:8876–80. doi: 10.1073/pnas.90.19.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolesnikov AV, Kozyr AV, Alexandrova ES, et al. Enzyme mimicry by the antiidiotypic antibody approach. Proc Natl Acad Sci USA. 2000;97:13526–31. doi: 10.1073/pnas.200360497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul S. Mechanism and functional role of antibody catalysis. Appl Biochem Biotechnol. 1998;75:13–23. doi: 10.1007/BF02787705. [DOI] [PubMed] [Google Scholar]
- 13.Kozyr AV, Kolesnikov A, Aleksandrova ES, et al. Novel functional activities of anti-DNA autoantibodies from sera of patients with lymphoproliferative and autoimmune diseases. Appl Biochem Biotechnol. 1998;75:45–61. doi: 10.1007/BF02787708. [DOI] [PubMed] [Google Scholar]
- 14.Sinohara H, Matsuura K. Does catalytic activity of Bens-Jones proteins contribute to the pathogenesis of multiple myeloma. Appl Biochem Biotechnol. 2000;83:85–94. doi: 10.1385/abab:83:1-3:85. [DOI] [PubMed] [Google Scholar]
- 15.Nevinsky GA, Breusov AA, Baranovskii AG, et al. Effect of different drugs on the level of DNA-hydrolyzing polyclonal IgG antibodies in sera of patients with Hashimoto’s thyroiditis and nontoxic nodal goiter. Med Sci Monit. 2001;7:201–11. [PubMed] [Google Scholar]
- 16.Lacroix-Desmazes S, Bayry J, Kaveri SV, et al. High levels of catalytic antibodies correlate with favorable outcome in sepsis. Proc Natl Acad Sci USA. 2005;102:4109–13. doi: 10.1073/pnas.0500586102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connor KC, Bar-Or A, Hafler DA. Neuroimmunology of multiple sclerosis. J Clin Immunol. 2001;21:81–92. doi: 10.1023/a:1011064007686. [DOI] [PubMed] [Google Scholar]
- 18.Archelos JJ, Storch MK, Hartung HP. The role of B cells and autoantibodies in multiple sclerosis. Ann Neurol. 2000;47:694–706. [PubMed] [Google Scholar]
- 19.Hemmer B, Archelos JJ, Hartung HP. New concepts in the immunopathogenesis of multiple sclerosis. Nat Rev Neurosci. 2002;3:291–301. doi: 10.1038/nrn784. [DOI] [PubMed] [Google Scholar]
- 20.Cross AH, Trotter JL, Lyons J. B cells and antibodies in CNS demyelinating disease. J Neuroimmunol. 2001;112:1–14. doi: 10.1016/s0165-5728(00)00409-4. [DOI] [PubMed] [Google Scholar]
- 21.Williamson RA, Burgoon MP, Owens GP, et al. Anti-DNA antibodies are a major component of the intrathecal B cell response in multiple sclerosis. Proc Natl Acad Sci USA. 2001;98:1793–8. doi: 10.1073/pnas.031567598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baranovskii AG, Kanyshkova TG, Mogelnitskii AS, et al. Polyclonal antibodies from blood and cerebrospinal fluid of patients with multiple sclerosis effectively hydrolyze DNA and RNA. Biochemistry. 1998;63:1239–48. [PubMed] [Google Scholar]
- 23.Baranovskii AG, Ershova NA, Buneva VN, et al. Catalytic heterogeneity of polyclonal DNA-hydrolyzing antibodies from the sera of patients with multiple sclerosis. Immunol Lett. 2001;76:163–7. doi: 10.1016/s0165-2478(01)00185-7. [DOI] [PubMed] [Google Scholar]
- 24.Savel’iev AN, Ivanen DR, Kulminskaya AA, et al. Amylolytic activity of IgM and IgG antibodies from patients with multiple sclerosis. Immunol Lett. 2003;86:291–7. doi: 10.1016/s0165-2478(03)00042-7. [DOI] [PubMed] [Google Scholar]
- 25.Vanguri P, Lee E, Henkart P, et al. Hydrolysis of myelin basic protein in myelin membranes by granzymes of large granular lymphocytes. J Immunol. 1993;150:2431–9. [PubMed] [Google Scholar]
- 26.Polosukhina DI, Kanyshkova TG, Doronin BM, et al. Hydrolysis of myelin basic protein by polyclonal catalytic IgGs from the sera of patients with multiple sclerosis. J Cell Mol Med. 2004;8:359–68. doi: 10.1111/j.1582-4934.2004.tb00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polosukhina DI, Buneva VN, Doronin BM, et al. Hydrolysis of myelin basic protein by IgM and IgA antibodies from the sera of patients with multiple sclerosis. Med Sci Monit. 2005;11:BR266–72. [PubMed] [Google Scholar]
- 28.Polosukhina DI, Buneva VN, Doronin BM, et al. Metal-dependent hydrolysis of myelin basic protein by IgGs from the sera of patients with multiple sclerosis. Immunol Lett. 2006;103:75–81. doi: 10.1016/j.imlet.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Ponomarenko NA, Durova OM, Vorobiev II, et al. Autoantibodies to myelin basic protein catalyze site-specific degradation of their antigen. Proc Natl Acad Sci USA. 2006;103:281–6. doi: 10.1073/pnas.0509849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belogurov AA, Kurkova IN, Friboulet A, et al. Recognition and degradation of myelin basic protein peptides by serum autoantibodies: novel biomarker for multiple sclerosis. J Immunol. 2008;180:1258–67. doi: 10.4049/jimmunol.180.2.1258. [DOI] [PubMed] [Google Scholar]
- 31.Andrievskaya OA, Buneva VN, Naumov VA, et al. Catalytic heterogeneity of polyclonal RNA-hydrolyzing IgM from sera of patients with lupus erythematosus. Med Sci Monit. 2000;6:460–70. [PubMed] [Google Scholar]
- 32.Andrievskaya OA, Buneva VN, Baranovskii AG, et al. Catalytic diversity of polyclonal RNA-hydrolyzing IgG antibodies from the sera of patients with systemic lupus erythematosus. Immunol Lett. 2002;81:191–8. doi: 10.1016/s0165-2478(02)00006-8. [DOI] [PubMed] [Google Scholar]
- 33.Kuznetsova I, Orlovskaya IA, Buneva VN, et al. Activation of DNA-hydrolyzing antibodies from the sera of autoimmune-prone MRL-lpr/lpr mice by different metal ions. Biochim Biophys Acta. 2007;1774:884–96. doi: 10.1016/j.bbapap.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Poser CM. The diagnosis of multiple sclerosis. New York: Thieme-Stratton; 1984. pp. 3–13. [Google Scholar]
- 35.Horl WH, Wanner C, Schollmer P. Proteinases in catabolism and malnutrition. JPEN J Parenter Enteral Nutr. 1987;11:98S–103S. doi: 10.1177/014860718701100515. [DOI] [PubMed] [Google Scholar]
- 36.Rao MB, Tanksale AM, Ghatge MS, et al. Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev. 1998;62:597–635. doi: 10.1128/mmbr.62.3.597-635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odintsova ES, Buneva VN, Nevinsky GA. Casein-hydrolyzing activity of sIgA antibodies from human milk. J Mol Recognit. 2005;18:413–21. doi: 10.1002/jmr.743. [DOI] [PubMed] [Google Scholar]
- 38.Tyutyulkova S, Gao QS, Thompson A, et al. Efficient vasoactive intestinal polypeptide hydrolyzing autoantibody light chains selected by phage display. Biochim Biophys Acta. 1996;1316:217–23. doi: 10.1016/0925-4439(96)00028-2. [DOI] [PubMed] [Google Scholar]
- 39.Paul S, Li L, Kalaga R, et al. Natural catalytic antibodies: peptide-hydrolyzing activities of Bence Jones proteins and V fragment. J Biol Chem. 1995;270:15257–61. doi: 10.1074/jbc.270.25.15257. [DOI] [PubMed] [Google Scholar]
- 40.Sun M, Paul S. Altered cleavage site preference of a proteolytic antibody light chain induced by denaturation. FEBS Lett. 1997;407:289–90. doi: 10.1016/s0014-5793(97)00355-4. [DOI] [PubMed] [Google Scholar]
- 41.Gololobov GV, Chernova EA, Schourov DV, et al. Cleavage of supercoiled plasmid DNA by autoantibody Fab fragment: application of the flow linear dichroism technique. Proc Natl Acad Sci USA. 1995;92:254–7. doi: 10.1073/pnas.92.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tawfik DS, Chap R, Green BS, et al. Unexpectedly high occurrence of catalytic antibodies in MRL/lpr and SJL mice immunized with a transition-state analog: is there a linkage to autoimmunity? Proc Natl Acad Sci USA. 1995;92:2145–9. doi: 10.1073/pnas.92.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishi Y. Evolution of catalytic antibody repertoire in autoimmune mice. J Immunol Methods. 2002;269:213–33. doi: 10.1016/s0022-1759(02)00233-8. [DOI] [PubMed] [Google Scholar]


