Abstract
Insulin secretion from the pancreatic β cell is regulated principally by the ambient concentration of glucose. However, the molecular and cellular mechanisms underlying the stimulus – secretion coupling of glucose-stimulated insulin secretion (GSIS) remain only partially understood. Emerging evidence from multiple laboratories suggests key regulatory roles for GTP-binding proteins in the cascade of events leading to GSIS. This class of signalling proteins undergoes a series of requisite post-translational modifications (e.g. prenylation) at their C-terminal cysteines, which appear to be necessary for their targeting to respective membranous sites for optimal interaction with their respective effector proteins. This communication represents a perspective on potential regulatory roles for protein prenylation steps (i.e. protein farnesylation and protein geranylgeranylation) in GSIS from the islet β cell.Possible consequences of protein prenylation and potential mechanisms underlying glucose-induced regulation of prenylation, specifically in the context of GSIS, are also discussed.
Keywords: insulin secretion, islet β cell, G-proteins, protein prenylation, protein farnesylation, protein geranylgeranylation, Rac1, Cdc42
Insulin secretion – a simplified view
Insulin secretion from the pancreatic β cell is regulated principally by the ambient concentration of glucose. However, the molecular and cellular mechanisms underlying the stimulus – secretion coupling of glucose-stimulated insulin secretion (GSIS) remain only partially understood [1, 2]. In this context, it is widely accepted that GSIS is mediated largely via the generation of soluble second messengers, such as cyclic nucleotides, hydrolytic products of phospholipases A2, C and D [1, 2]. The principal signalling cascade has been shown to be initiated by the glucose-transporter protein (i.e. Glut-2)-mediated entry of glucose into the β cell followed by an increase in the intra-islet ATP/ADP ratio as a consequence of glucose metabolism. Such an increase in the ATP levels culminates in the closure of ATP-sensitive potassium channels localized on the plasma membrane resulting in membrane depolarization, and facilitation of the influx of extra-cellular calcium through the voltage-sensitive calcium channels also localized on the plasma membrane. A net increase in intracellular calcium that occurs via the translocation of extra-cellular calcium into the cytosolic compartment of the stimulated β cell in addition to the mobilization of intracellular calcium from the storage pools has been shown to be critical for the transport of insulin-laden secretory granules to the plasma membrane for fusion and release of insulin [1, 2].
Endogenous GTP and its binding proteins are important for GSIS
In addition to the regulation by adenine nucleotides of GSIS, earlier studies have examined possible contributory roles for guanine nucleotides (i.e. guanosine triphosphate [GTP]) in physiological insulin secretion [3]. For example, using selective inhibitors of GTP biosynthetic pathway (e.g. mycophenolic acid), a permissive role for GTP in GSIS was established [4, 5]. Although the precise molecular and cellular mechanisms underlying the roles of GTP in GSIS remain to be defined, available evidence indicates that it might involve activation of one (or more) GTP-binding proteins (G-proteins) endogenous to the islet β cell [3 and references therein]. Existing evidence clearly indicates localization of at least two major groups of G-proteins within the islet β cell. The first group consists of trimeric G-proteins composed of α (39–43kD), β (35–37 kD) and γ (5–10 kD) subunits. These are involved in the coupling of various G-protein-coupled receptors to their intracellular effector proteins, including adenylate cyclase, phosphodi-esterase and several forms of phospholipases. The second group of G-proteins is composed of low-molecular-mass G-proteins (20–25 kD), which are involved in sorting of proteins as well as trafficking of secretory vesicles. In support of the postulation that G-proteins, specifically the small G-proteins, are involved in GSIS is the well-established experimental support to suggest that the signalling steps involved in GSIS from the β cell involve well-regulated trafficking of insulin-laden secretory granules for their docking and fusion with the plasma membrane [3, 6–26].
Original observations from multiple laboratories, including our own, demonstrated critical involvement of small G-proteins, such as Rac1, Cdc42, Rap1 and ADP-ribosylation factor 6 (ARF6) in GSIS from normal rat islets, human islets and clonal β -cell preparations [3, 6–26]. Such conclusions were drawn primarily based on data from three mutually complementary experimental approaches. The first approach involved use of Clostridial toxins (e.g. toxin A or B), which monoglucosylate and inactivate specific G-proteins [7]. The second experimental manipulation involved molecular biological approaches, including expression of dominant negative mutants and/or selective knockdown (i.e. siRNA methodology) of candidate G-proteins [3, 8, 9, 11, 19, 23, 25]. The third approach involved the use of pharmacological inhibitors of G-protein activation to further decipher their regulatory roles in GSIS [3, 6, 12–14, 19].
G-proteins undergo post-translational modifications
The majority of small G-proteins and the γ subunits of trimeric G-proteins undergo post-translational modification steps (e.g. prenylation) at their C-terminal cysteine residues (also referred to as the CAAX motif). Such modifications are felt to be responsible for targeting of the modified proteins to specific membranous compartments for optimal interaction with their effector proteins [27–31]. The farnesyl transferase (FTase) and the geranylgeranyl transferase (GGTase) catalyze the incorporation of either a 15-carbon (farnesyl moiety) or a 20-carbon (geranyl-geranyl moiety) derivative of mevalonic acid (MVA) into the C-terminal cysteine residues of the candidate proteins, respectively (Fig. 1). Collectively, the FTases and GGTases are referred to as protein prenyl transferases (PPTases). Examples of farnesylated proteins include Ras, nuclear lamin B, certain proteins involved in visual signal transduction and fungal mating factors. Small G-proteins, such as Cdc42, Rac and Rho as well as most γ subunits of trimeric G-proteins (other than γ subunit of transducin, which is farnesylated), represent some examples of geranylgeranylated proteins. In addition, the cysteine residues at the C-terminal CAAX motif of the prenylated proteins have been shown to undergo further post-translational modifications, including proteolysis and the carboxyl methylation (Fig. 1).
1.
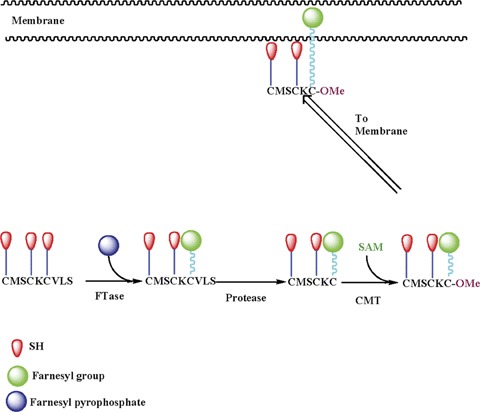
Schematic representation of post-translational modification of small G-proteins or the γ subunit of trimeric G-proteins. The first step of this reaction sequence is incorporation of either a 15 (farnesyl group)- or a 20 (geranylger-anyl group)-carbon derivative of MVA into the C-terminal cysteine via a thioether linkage. This reaction is catalyzed by either FTase or GGTase, respectively.Following this, the three amino acids after the prenylated cysteine are removed by a protease, thereby exposing the carboxylate anion.This site is then methylated by a carboxyl methyl transferase, which transfers a methyl group onto the carboxylate group.These enzymes use S-adenosyl methionine as the methyl donor.We have demonstrated that glucose promotes the carboxyl methylation of specific proteins in insulin-secreting cells (e.g. Cdc42, Rap1 and γ subunits of trimeric GTPases; see text for additional details).FTase: farnesyl transferase; CMT: carboxyl methyl transferase and SAM: S-adenosyl methionine.
Such modifications further increase the hydrophobicity of the modified protein, resulting in their targeting to the membrane fraction. Thus, the prenylation step represents the first committed reaction for G-protein activation mechanism [27–31].
To date, three distinct PPTases have been described in the literature. FTase and GGTase-I are also referred to as CAAX PPTases because they share the CAAX motif around the C-terminal cysteine region of their substrate proteins. GGTase-II (also referred to as Rab GGTase) prenylates the Rab subfamily of proteins at a different motif, and hence this group of PPTases is often referred to as non-CAAX PPTases. FTase and GGTase-I and -II are heterodimeric (i.e. consisting of β and β subunits) in nature. Interestingly, both FTase and GGTase-I share a common α subunit (48 kD) and different β subunits with apparent molecular weights of 46 and 43 kD, respectively, for FTase-β and GGTase-1β. The α subunit is the regulatory subunit, whereas the β subunit confers substrate specificity. The molecular sizes of GGTase-II α and β subunits are reported to be 60 and 38 kD, respectively [27–31].
In the following sections, I will review available evidence to support that protein prenylation is necessary for GSIS. For the sake of brevity, this section is divided into three parts. They are as follows: data accrued from studies involving generic inhibitors of protein prenylation; data accrued from studies involving use of site-specific inhibitors of protein prenylation and data accrued from studies involving over-expression of inactive mutants of PPTases.
Data accrued from studies involving generic inhibitors of protein prenylation
Lovastatin (LOVA)
In 1993, Metz and coworkers addressed putative roles of protein isoprenylation through the use of LOVA, due to its ability to inhibit the synthesis of MVA from HMG CoA catalyzed by the enzyme HMG CoA-reductase [12]. In turn, MVA forms the precursor for the biosynthesis of isoprenoids, which are incorporated into respective proteins to complete the isoprenylation step. Data from these studies have suggested that pre-incubation of isolated normal rat islets or clonal β cells with LOVA, results in selective accumulation of non-prenylated proteins in the cytosolic fraction with a concomitant decrease in the abundance of these proteins in the membrane fraction [12]. These findings are compatible with the formulation that inhibition of protein prenylation leads to inhibition of targeting and/or translocation of candidate G-proteins to the membrane fraction (see above and Fig. 1). Under these conditions, LOVA markedly attenuated GSIS from normal rat islets [12]. Also in 1993, independent investigations by Li and coworkers have demonstrated significant inhibitory effects by LOVA on bombesin- and vasopressin-mediated insulin secretion in clonal β (HIT-T15) cells [13]. Based on these data, it was proposed that protein isoprenylation plays a critical regulatory role in insulin secretion from the β cell. Although the identity of the G-proteins affected by LOVA treatment has not been determined, indirect evidence tends to point out that Cdc42 might represent one such protein. For example, in HIT-T15 cells, LOVA has been shown to attenuate prenylation of Cdc42, thereby impeding its complexation with the guanosine diphosphate dissociation inhibitor (GDI; 32). Furthermore, in these studies, Ragazzi and coworkers [32] have demonstrated that pre-exposure of HIT-T15 cells to LOVA resulted in a significant increase in the relative abundance of low-molecular-mass G-proteins in the cytosolic fraction. Phase-partitioning analyses revealed that a large proportion of these G-proteins in the cytosolic fraction derived from LOVA-treated cells behaved as hydrophilic proteins. Based on these data, these investigators have concluded that, in LOVA-treated cells, the newly synthesized proteins were not prenylated optimally because of depletion of endogenous MVA pools, which are required for isoprenoid biosynthesis. Such an effect of LOVA was not seen with some other monomeric G-proteins (e.g. Rho or ADP-ribosylation factor [32]).
Further studies by Metz and coworkers questioned if the inhibitory effects of LOVA on GSIS are indeed mediated owing to inhibition of MVA biosynthesis, which is the precursor for isoprenoid moieties necessary for the isoprenylation step (see above). It was observed that pre-incubation of isolated islets with LOVA markedly reduced the ability of labelled acetate (a building block for cholesterol biosynthesis) into cholesterol, lanosterol, squalene and dolichols [12]. Furthermore, the LOVA-induced inhibition of GSIS was largely prevented by coprovision of exogenous MVA (either as a lactone or as a sodium salt). Likewise, studies by Li et al.[13] have demonstrated a marked protection by exogenous MVA against LOVA-induced inhibition of insulin secretion in HIT-T15 cells elicited by bombesin. It may also be mentioned that LOVA treatment also caused marked alterations in cellular morphology. For example, LOVA treatment of HIT-T15 cells caused rounding up of the cells; such effects could be prevented by exogenous MVA. These data suggested that inhibition of endogenous production of MVA (and thereby isoprenoids) following LOVA treatment impedes a signalling cascade necessary for exocytotic secretion of insulin. In conclusion, early 1990s have witnessed the first demonstration to suggest that protein prenylation may be necessary for GSIS. During the same time, two other generic inhibitors (e.g. limonene and perillic acid) of PPTases were also used to further support the hypothesis that protein prenylation regulates GSIS.
Limonene
d-Limonene is a monoterpene and is widely distributed in various foods and volatile oils, including citrus oils. Previous studies [33, 34] have demonstrated that limonene inhibits protein isoprenylation. Metz et al.[12] and Li et al.[13] investigated potential effects of limonene on insulin secretion. Li and coworkers observed that limonene significantly inhibited secret-agogue-induced insulin secretion in a dose-dependent manner in HIT-T15 cells. Interestingly, unlike LOVA, limonene inhibited nutrient – as well as forskolin-induced insulin secretion from HIT-T15 cells. More important, however, based on additional observations including its non-specific effects on protein content and resting calcium concentrations, Li and coworkers concluded that limonene is a nonspecific inhibitor of isoprenylation and that it exerts untoward cytotoxic effects on HIT-T15 cells [13]. Similar conclusions on potential cytotoxicity of limonene were also drawn from the studies of Metz and colleagues [12]. To the best of our knowledge, no followup studies have been carried out to further examine the use of limonene as an inhibitor of protein isoprenylation to study islet β-cell function. Crowell reviewed [35] the applicability and efficacy of dietary monoterpenes (e.g. limonene) as therapeutic tools for the prevention of cancer, primarily due to their ability to serve as inhibitors of protein prenylation (e.g. Ras superfamily of G-proteins) and thereby cell growth. This might explain some of the cytotoxic effects of limonene on the islet β cell.
Perillic acid (PA)
It is a major and biologically active metabolite of limonene and has been shown to inhibit prenylation of various low-molecular-mass G-proteins [34]. Treatment of isolated islets with PA markedly reduced insulin secretion induced by glucose or the amino acid metabolite α-oxo-4-methylpentanoic acid [12]. In addition to inhibiting incremental insulin release in response to stimulatory glucose concentrations, PA also reduced fractional insulin release in isolated rat islets. Therefore, PA appears to be a relatively more efficient inhibitor of insulin secretion with less cytotoxicity compared to limonene. Additional studies are needed however to further verify the specificity of this compound for its use to decipher regulatory roles for protein prenylation in GSIS.
Manumycin A
Manumycin A, a natural substance isolated from Streptomyces parvulus, is a farnesyl pyrophosphate analog and acts as a potent selective and competitive inhibitor of FTase. In an attempt to examine putative regulatory roles for small G-proteins in cytokine-mediated metabolic dysfunction and demise of the islet β cell, we have reported the utility of manumycin A as a specific inhibitor of Ras function [36]. In followup studies, we were able to demonstrate that manumycin A selectively inhibits GSIS from clonal β (βTC3) cells [14]. These data provided the first evidence to suggest that the signalling mechanisms leading to GSIS might underlie activation of protein farnesylation, since manumycin A is an FTase inhibitor. This hypothesis was further confirmed via the use of more site-specific inhibitors of FTases (see below).
Taken together, the above findings favor the argument that protein prenylation plays a positive modulatory role in GSIS in a variety of insulin-secreting cells, including normal rat islets, HIT-T15 cells, INS-1 and β TC3 cells (Table 1). However, one of the potential caveats in the above studies, specifically the experiments involving the use of LOVA, is that it also inhibits both sterol and non-sterol limbs of the cholesterol biosynthetic pathway, affecting many compounds that could have specific functional roles in β-cell function [12]. In addition, LOVA inhibits the biosynthesis of both farnesyl and geranylgeranyl pyrophosphates and, thus, it is difficult to determine precisely which of the two signalling pathways (i.e. farnesylation and geranylgeranylation) is critical for GSIS. With these facts in mind, a series of investigations were undertaken to determine putative regulatory roles for each of these prenylation pathways in GSIS. Findings from these studies are reviewed later.
1.
Summary of experimental approaches/probes used to demonstrate that protein prenylation is necessary for GSIS
| Generic Inhibitors of protein prenylation |
|---|
| Lovastatin [12, 13] |
| Manumycin-A [14] |
| Perillic acid [12, 13] |
| Limonene [12, 13] |
| Site-specific inhibitors of protein prenylation |
| GGTI-2147 [3, 14, 19, 22, 37] |
| 3-allyl farnesol [14] |
| 3-vinyl farnesol [14] |
| 3-allyl geraniol [14] |
| 3-vinyl geraniol [14] |
| Molecular biological approach |
| Dominant negative mutant of the α subunit of FTase/GGTase [37] |
Data accrued from studies involving site-specific inhibitors of protein prenylation
3-Allyl and 3-vinyl farnesols and geranylgeraniols
In collaborative studies (with Dr. Richard Gibbs, Purdue University, IN) a series of site-specific pro-drug inhibitors of FTases (3-allyl or vinyl farnesols) and GGTases (3-allyl or vinyl geranylgeraniols) were developed [14]. The key issue of selectivity of allyl or vinyl farnesols or geranylgeraniols that we used in these studies was also addressed [14 and references therein]. For example, 3-allyl-farnesyl pyrophosphate, an active form of the prodrug 3-allyl farnesol, exhibits nearly 1600-fold selectivity for the inhibition of FTase versus GGTase I. In a similar manner, 3-vinyl-farnesyl pyrophosphate, the active form of 3-vinyl farnesol, is an effective tight-binding substrate for FTase, and exhibits no productive interaction with GGTase I (selectivity for FTase/GGTase I is nearly 600-fold). We then determined the effects of 3-allyl or vinyl farnesols (inhibitors of FTases) and 3-allyl or vinyl geranylgeraniols (inhibitors of GGTases) on GSIS from insulin-secreting β (βTC3) cells. We observed that each class of these inhibitors elicited significant inhibitory effects on GSIS. Moreover, in GTP-overlay assays, we observed a significant increase in the cytosolic accumulation of low-molecular-mass G-proteins in cells exposed to these inhibitors of FTases or GGTases. Based on these observations, it was concluded that both farnesylation as well as geranylation are essential for GSIS.
Geranylgeranyl transferase inhibitor-2147 (GGTI-2147)
In further support of our aforementioned observations using inhibitors of GGTases (i.e. allyl or vinyl geranylgeraniols), potential effects of yet another structurally dissimilar inhibitor of GGTase were investigated on GSIS from βTC3 cells. In these studies, GGTI-2147, a known inhibitor of GGTase I (with >60-fold in vivo selectivity for the inhibition of protein geranylgeranylation), was employed. Our findings indicated that GGTI-2147 elicits significant inhibitory effects on GSIS from β TC3 cells and INS 832/13 cells [14, 37]. Furthermore, GGTI-2147 markedly attenuated insulin secretion from β TC3 cells induced by succinic acid methyl ester, which is a mitochondrial fuel [22]. Additional studies revealed that GGTI-2147 significantly induced cytosolic accumulation of two small G-proteins, namely, Cdc42 and Rac1, under the conditions it inhibited GSIS from insulin-secreting cells [37]. Therefore, it is likely that GGTI-2147-mediated inhibition of post-translational preny-lation of candidate G-proteins (e.g. Cdc42 and Rac1) could, in turn, impede complexation of these G-proteins with their respective GDIs. In support of this formulation, Nevins and Thurmond [25] reported a significant degree of inhibition in the association between Cdc42 and its GDI, Caveolin-1 in MIN6 cells (see below for additional discussion). These findings implicate that Rac1 and Cdc42 could represent at least two target proteins whose geranylgeranylation is necessary for GSIS.
In conclusion, it is clear from the above discussion that activation of FTase and GGTase are essential for GSIS in a variety of insulin-secreting cells. It is also likely that Rac1 and Cdc42 represent at least two of the geranylgeranylated proteins whose activation may be necessary for GSIS. The precise identity of the farnesylated protein(s) involved in the signalling cascade leading to GSIS still remains to be determined. We speculate that they might include small G-proteins, such as Ras and the β subunit of trimeric G-proteins.
Data accrued from studies involving over-expression of inactive mutants of PPTases
As discussed earlier, three distinct PPTases have been described in the literature.
FTase and GGTase-I are also referred to as CAAX -PPTases because they share the CAAX motif around the C-terminal cysteine region of their substrate proteins. GGTase-II (also referred to as Rab GGTase) prenylates the Rab subfamily of proteins at a different motif, and hence this group of PPTases is often referred to as non-CAAX-PPTases. Interestingly, both FTase and GGTase-I share a common α subunit (48 kD) and different β subunits with apparent molecular weights of 46 and 43 kD, respectively, for FTase-β and GGTase-1β . The α subunit is the regulatory subunit, whereas the β subunit confers substrate specificity. The molecular sizes of GGTase-II α and β subunits are reported to be 60 and 38 kD, respectively.
More recently, to further strengthen the pharmacologic data (Table 1) on essential roles of PPTase activation in GSIS, we undertook a molecular biological approach in which we over-expressed a dominant negative mutant of the β subunit of FTase/GGTase in INS 832/13 cells and studied GSIS in control cells or in cells in which PPTase function is compromised via the transfection approach [37]. We noticed a significant reduction in GSIS in cells over-expressing the dominant negative mutant compared to the cells expressing the vector alone. We were also able to demonstrate a marked reduction in the ability of glucose to promote translocation of Rac1 to the membrane fraction in cells over-expressing the dominant negative mutant; we and others have demonstrated this to be a requisite for GSIS [37]. Together, these data afford further support to the data accrued from the pharmacological experiments and confirm our original hypothesis that protein prenylation is necessary for GSIS (Table 1).
What are the functional consequences of prenylation in the islet β cell?
Several earlier studies in multiple cell types, including the islet β cell, have suggested that inhibition of prenylation leads to cytosolic accumulation of G-proteins, which may, in part, be due to decreased hydrophobicity of the unmodified proteins. Compatible with these data is our formulation that prenylation promotes targeting of candidate proteins to relevant membranes for optimal interaction with their effector proteins. Along these lines, we reported potential regulation of phospholipase C function by Cdc42 [6]. More recent studies from Thurmond's laboratory have identified Pak1 as one of the effector proteins for Cdc42 [38]. It is also likely that prenylation of proteins promotes interaction with their regulatory proteins, including GDIs. In this context, we have reported localization of GDI in the islet β cell [23]. Using multiple experimental approaches, including coimmuno-precipitation, Triton X-114 phase partitioning assay, sucrose density gradient centrifugation etc., we reported that Rac1 and GDI remain complexed in insulin-secreting cells [23]. Although not demonstrated by direct experimental evidence, it is likely that prenylation of specific G-proteins (e.g. Rac1 and Cdc42) is necessary for its interaction with their respective GDIs optimally. Along these lines, recent studies by Nevins and Thurmond have demonstrated a significant decrease in the interaction of Cdc42 with caveolin-1, its GDI, after inhibition of protein prenylation with GGTI-2147 in insulin-secreting MIN6 cells. Based on these observations, it was concluded that caveolin-1 interacted more efficiently with the prenylated form of Cdc42 [25]. In addition, it has also been proposed that post-translational prenylation and the carboxyl methylation of the γ subunits of trimeric G-proteins are tightly regulated in the pancreatic β cell by extra-cellular glucose concentrations [26]. It is suggested that such modifications at the C-terminal cysteines of the γ subunits promotes their interaction with the β subunit [26 and references therein]. Lastly, it is also likely that inhibition of protein prenylation of specific G-proteins (e.g. Rac1) might prevent their degradation, resulting in increased abundance of these proteins in the cytosolic fraction [37 and references therein]. Additional studies are needed to conclusively demonstrate the functional consequences of PPTase activation on G-protein function, including an increase in their trafficking to the relevant membranous sites for effector regulation and/or degradation.
How are PPTases regulated by glucose in the islet?
One such regulatory mechanism might include acute regulation of PPTase function via post-translational modification (i.e. phosphorylation–dephosphorylation). Available evidence suggests that the α subunit of FTase/GGTase undergoes phosphorylation which, in turn, results in the functional activation of the enzyme. Indeed, emerging evidence from Goalstone's laboratory demonstrated that insulin stimulates the phosphorylation and activation of the α subunit of PPTases, thereby providing increased amounts of prenylated p21Ras (farnesylated) and Rho A (geranylgeranylated) necessary for its mitogenic effects [39–42]. They also reported that substitution of ala-nine for two serine residues (i.e. S60A and S62A) of the β subunit of FTase/GGTase creates a dominant negative mutant for both PPTases. Finally, these investigators also demonstrated that over-expression of the dominant negative FTase/GGTase α mutant markedly attenuated the ability of insulin to increase the activities of FTase/GGTase and the abundance of prenylated p21Ras and Rho A [39–42].
Therefore, it is likely that glucose promotes the phosphorylation of the FTase/GGTase α subunit in insulin-secreting cells, thereby facilitating PPTase activation and prenylation of candidate G-proteins (e.g. Rac1/Cdc42). This, in turn, leads to association of prenylated G-proteins, such as Rac1, with their regulatory protein GDI. On receipt of appropriate signals, the Rac1/GDI complex then moves to the membrane, where the GDI dissociates from the complex, enabling Rac1 to attain its GTP-bound active conformation for regulation of its effector proteins [43]. We have recently performed localization of GDI in insulin-secreting cells and have provided experimental evidence to suggest key regulatory roles for this protein in GSIS [23]. Taken together, and at least based on the transfection experiments involving a mutant lacking serine phosphorylation sites (see above and Table 1), these results have led us to conclude that glucose-mediated effects on insulin secretion might require phosphorylation of serine-60 and -62 residues of the PPTase α subunits. Studies are in progress to precisely determine the effects of glucose on PPTase phosphorylation and functional activation in the events necessary for GSIS.
Conclusions and future directions
Together, based on the available evidence discussed in this review, I propose that isoprenylation of specific proteins plays a significant regulatory role in GSIS. It is also apparent from the above discussion that still a substantial amount of work is needed, especially in the area of possible identity of these prenylated proteins as well as the PPTases. Furthermore, data are lacking in the area of potential mechanisms underlying glucose-induced activation of the islet endogenous PPTases. Initial progress along these lines was somewhat slow, principally because of the unavailability of specific inhibitors of each of these enzymes, and now that more specific inhibitors are becoming available, it is likely that we will see considerable amount of data emerging in this area in the near future. As indicated earlier, even though protein prenylation is not acutely regulable, it seems to dictate the subsequent modification steps (e.g. carboxyl methylation) that are acutely regulated and could determine the functional status of a given G-protein (Fig. 1). Lastly, potential abnormalities in the ability of glucose to finely regulate the functional activation of this signalling cascade could contribute to the impaired insulin secretion in islets derived from models of type 2 diabetes. This remains to be verified and represents an active area of investigation in our laboratory.
Acknowledgments
I thank the Medical Research Service of the Department of VA, the National Institutes of Health (RO1 DK 56605 and RO1 DK 74921), the American Diabetes Association (RA-45-ADA) and the Juvenile Diabetes Research Foundation (JDRF 1-2006-4) for supporting the research described in this review article. I also thank my collaborators at the University of Wisconsin School of Medicine and William S. Middleton Memorial VA Medical Center in Madison and at Wayne State University and John D. Dingell VA Medical Center in Detroit for their contributions to the research described in this review.
References
- 1.Prentki M, Matschinsky FM. Calcium, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987;67:1185–248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- 2.Newgard CB, McGarry JD. Metabolic coupling factors in pancreatic β-cell signal transduction. Annu Rev Biochem. 1995;64:689–719. doi: 10.1146/annurev.bi.64.070195.003353. [DOI] [PubMed] [Google Scholar]
- 3.Kowluru A. Regulatory roles for small G proteins in the pancreatic beta-cell: lessons from models of impaired insulin secretion. Am J Physiol Endocrinol Metab. 2003;285:E669–84. doi: 10.1152/ajpendo.00196.2003. [DOI] [PubMed] [Google Scholar]
- 4.Metz SA, Rabaglia ME, Pintar TJ. Selective inhibitors of GTP synthesis impede exocytotic insulin release from intact rat islets. J Biol Chem. 1992;267:12517–27. [PubMed] [Google Scholar]
- 5.Metz SA, Meredith M, Rabaglia ME, Kowluru A. Small elevations of glucose concentration redirect and amplify the synthesis of guanosine 5(-triphosphate in rat islets. J Clin Invest. 1993;92:872–82. doi: 10.1172/JCI116662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowluru A, Seavey SE, Li G, Sorenson RL, Weinhaus AJ, Nesher R, Rabaglia ME, Vadakekalam J, Metz SA. Glucose-and GTP-dependent stimulation of the carboxylmethylation of Cdc42 in rodent and human pancreatic islets and pure β -cells: evidence for an essential role for GTP-binding proteins in nutrient-induced insulin secretion. J Clin Invest. 1996;98:540–55. doi: 10.1172/JCI118822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kowluru A, Li G, Rabaglia ME, Segu VB, Hofmann F, Aktories K, Metz SA. Evidence for differential roles of the Rho subfamily of GTP-binding proteins in glucose-and calcium-induced insulin secretion from pancreatic β-cells. Biochem Pharmacol. 1997;54:1097–108. doi: 10.1016/s0006-2952(97)00314-6. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Luo R, Kowluru A, Li G. Novel regulation by Rac1 of glucose- and forskolin-induced insulin secretion in INS-1 beta-cells. Am J Physiol Endocrinol Metab. 2004;286:E818–27. doi: 10.1152/ajpendo.00307.2003. [DOI] [PubMed] [Google Scholar]
- 9.Nevins AK, Thurmond DC. A direct interaction between Cdc42 and vesicle-associated membrane protein 2 regulates SNARE-dependent insulin exocytosis. J Biol Chem. 2004;280:1944–52. doi: 10.1074/jbc.M409528200. [DOI] [PubMed] [Google Scholar]
- 10.Nevins AK, Thurmond DC. Glucose regulates the cortical actin network through modulation of Cdc42 cycling to stimulate insulin secretion. Am J Physiol Cell Physiol. 2003;285:C698–710. doi: 10.1152/ajpcell.00093.2003. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence JT, Birnbaum MJ. ADP-ribosylation factor 6 regulates insulin secretion through plasma membrane phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA. 2003;100:13320–5. doi: 10.1073/pnas.2232129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metz SA, Rabaglia ME, Stock JB, Kowluru A. Modulation of insulin secretion from normal rat islets by inhibitors of the posttranslational modifications of the GTP-binding proteins. Biochem J. 1993;295:31–40. doi: 10.1042/bj2950031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li G, Regazzi R, Roche E, Wollheim CB. Blockade of mevalonate production by lovastatin attenuates bombesin and vasopressin potentiation of nutrient-induced insulin secretion from HIT-T15 cells. Biochem J. 1993;289:379–85. doi: 10.1042/bj2890379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin R, Chen HQ, Tannous M, Gibbs R, Kowluru A. Inhibition of glucose-and calcium-induced insulin secretion from βTC3 cells by novel inhibitors of iso-prenylation. J Pharmacol Exp Ther. 2002;303:82–8. doi: 10.1124/jpet.102.036160. [DOI] [PubMed] [Google Scholar]
- 15.Daniel S, Noda M, Cerione RA, Sharp GW. A link between Cdc42 and syntaxin is involved in mastoparan-stimulated insulin release. Biochemistry. 2002;41:9663–71. doi: 10.1021/bi025604p. [DOI] [PubMed] [Google Scholar]
- 16.Kowluru A, Seavey SE, Rhodes CJ, Metz SA. A novel regulatory mechanism for trimeric GTP-binding proteins in the membrane and secretory granule fractions of human and rodent beta cells. Biochem J. 1996;313:97–107. doi: 10.1042/bj3130097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowluru A, Rabaglia ME, Muse KE, Metz SA. Subcellular localization and characterization of gua-nine nucleotide binding proteins in normal rat and human pancreatic islets and transformed β-cells. Biochim Biophys Acta. 1994;1222:348–59. doi: 10.1016/0167-4889(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 18.Kowluru A, Metz SA. Stimulation by PGE2 of a high-affinity GTPase in the secretory granules of normal rat and human pancreatic islets. Biochem J. 1994;297:399–406. doi: 10.1042/bj2970399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amin RH, Chen H-Q, Veluthakal R, Silver RB, Li J, Li G, Kowluru A. Mastoparan-induced insulin secretion from insulin-secreting β TC3 and INS-1 cells: evidence for its regulation by Rho subfamily of G-proteins. Endocrinology. 2003;144:4508–18. doi: 10.1210/en.2003-0106. [DOI] [PubMed] [Google Scholar]
- 20.Straub SG, James RF, Dunne MJ, Sharp GW. Glucose augmentation of mastoparan-stimulated insulin secretion in rat and human pancreatic islets. Diabetes. 1998;47:1053–7. doi: 10.2337/diabetes.47.7.1053. [DOI] [PubMed] [Google Scholar]
- 21.Kowluru A, Metz SA. Subcellular distribution and posttranslational modifications of GTP-binding proteins in insulin-secreting cells. Method Neurosci. 1996;29:298–318. [Google Scholar]
- 22.Kowluru A, Chen HQ, Tannous M. Novel roles for the rho subfamily of GTP-binding proteins in succinate induced insulin secretion from betaTC3 cells: further evidence in support of the succinate mechanism of insulin release. Endocr Res. 2003;29:363–76. doi: 10.1081/erc-120025043. [DOI] [PubMed] [Google Scholar]
- 23.Kowluru A, Veluthakal R. Rho GDP-dissociation inhibitor (GDI) plays a negative modulatory role in glucose-stimulated insulin secretion. Diabetes. 2005;54:3523–9. doi: 10.2337/diabetes.54.12.3523. [DOI] [PubMed] [Google Scholar]
- 24.Kowluru A, Robertson RP, Metz SA. GTP-binding proteins in the regulation of pancreatic β-cell function. In: LeRoith D, Taylor SI, Olefsky JM, editors. Diabetes mellitus: a fundamental and clinical text. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 78–94. [Google Scholar]
- 25.Nevins AK, Thurmond DC. Caveolin-1 functions as a novel Cdc42 guanine nucleotide dissociation inhibitor in pancreatic β-cells. J Biol Chem. 2006;281:18961–72. doi: 10.1074/jbc.M603604200. [DOI] [PubMed] [Google Scholar]
- 26.Kowluru A, Li G, Metz SA. Glucose activates the carboxylmethylation of subunits of trimeric GTP-binding proteins in pancreatic β-cells. J Clin Invest. 1997;100:1596–610. doi: 10.1172/JCI119684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 28.Casey PJ, Seabra MC. Protein prenyltransferases. J Biol Chem. 1996;271:5289–92. doi: 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- 29.Maurer-Stroh S, Washietl S, Eisenhaber F. Protein prenyltransferases. Genome Biol. 2003;4:212. doi: 10.1186/gb-2003-4-4-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seabra MC, Reiss Y, Casey PJ, Brown MS, Goldstein JL. Protein farnesyltransferase and geranylgeranyl transferase share a common alpha subunit. Cell. 1991;65:429–34. doi: 10.1016/0092-8674(91)90460-g. [DOI] [PubMed] [Google Scholar]
- 31.Fu HW, Casey PJ. Enzymology and biology of CaaX protein prenylation. Rec Prog Horm Res. 1999;54:315–42. [PubMed] [Google Scholar]
- 32.Regazzi R, Kikuchi A, Taka Y, Wollheim CB. The small GTP-binding proteins in the cytosol of insulin-secreting cells are complexed to GDP dissociation inhibitor proteins. J Biol Chem. 1992;267:17512–9. [PubMed] [Google Scholar]
- 33.Hohl RJ, Lewis K. Differential effects of monoter-penes and lovastatin on RAS processing. J Biol Chem. 1995;270:17508–12. doi: 10.1074/jbc.270.29.17508. [DOI] [PubMed] [Google Scholar]
- 34.Crowell PL, Chang RR, Ren ZB, Elson CE, Gould MN. Selective inhibition of isoprenylation of 21-26 kDa proteins by the anticarcinogen d-limonene and its metabolites. J Biol Chem. 1991;266:17679–85. [PubMed] [Google Scholar]
- 35.Crowell PL. Monoterpenes in breast cancer chemo-prevention. Breast Cancer Res Treat. 1997;46:191–7. doi: 10.1023/a:1005939806591. [DOI] [PubMed] [Google Scholar]
- 36.Tannous M, Amin R, Popoff MR, Fiorentini C, Kowluru A. Positive modulation by Ras of interleukin-1β-mediated nitric oxide generation in insulin-secreting clonal beta (HIT-T15) cells. Biochem Pharmacol. 2001;62:1459–68. doi: 10.1016/s0006-2952(01)00818-8. [DOI] [PubMed] [Google Scholar]
- 37.Veluthakal R, Kaur H, Goalstone M, Kowluru A. Dominant-negative alpha-subunit of farnesyl-and geranyltransferase inhibits glucose-stimulated, but not KCl-stimulated insulin secretion in INS 832/13 cells. Diabetes. 2007;56:204–10. doi: 10.2337/db06-0668. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Oh E, Thurmond DC. Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J Biol Chem. 2007;282:9536–46. doi: 10.1074/jbc.M610553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solomon CS, Leitner JW, Goalstone ML. Dominant negative α subunit of farnesyl- and geranylgeranyl transferase-I inhibits insulin-induced differentiation of 3T3-L1 pre-adipocytes. Int J Obes Relat Metab Disord. 2003;27:40–7. doi: 10.1038/sj.ijo.0802189. [DOI] [PubMed] [Google Scholar]
- 40.Solomon CS, Goalstone ML. Dominant negative subunit of FTase inhibits effects of insulin and IGF in MCF-7 cells. Biochem Biophys Res Commun. 2002;291:458–65. doi: 10.1006/bbrc.2002.6471. [DOI] [PubMed] [Google Scholar]
- 41.Goalstone ML, Draznin B. Effect of insulin on farne-syltransferase gene transcription and mRNA stability. Biochem Biophys Res Commun. 1999;254:243–7. doi: 10.1006/bbrc.1998.9922. [DOI] [PubMed] [Google Scholar]
- 42.Goalstone ML, Draznin B. Effect of insulin on farne-syltransferase activity in 3T3-L1 adipocytes. J Biol Chem. 1996;271:27585–9. doi: 10.1074/jbc.271.44.27585. [DOI] [PubMed] [Google Scholar]
- 43.Mcdonald P, Veluthakal R, Kaur H, Kowluru A. Biologically active lipids promote trafficking and membrane association of Rac1 in insulin-secreting INS 832/13 cells. Am J Physiol Cell Physiol. 2007;292:C1216–20. doi: 10.1152/ajpcell.00467.2006. [DOI] [PubMed] [Google Scholar]


