Alzheimer disease (AD) is characterized by the presence of two aberrant histopathological structures: the senile plaques and the neurofibrillary tangles. In the decade of the 1980s it was described that Aβ peptide is the major component of senile plaques [1]. Also, in the same decade, the pioneer works of Grundke-Iqbal et al. described the presence of tau [2], in hyperphosphorylated form [3], in the neurofibrillary tangles. Thus, two main features appear to be related with the tau pathology found in AD: tau phosphorylation and tau aggregation. Whereas the toxicity of tau aggregates is discussed at the present, there are some indications of a toxic behaviour for hyperphosphorylated tau (P-tau). In fact, it has been described that P-tau can sequester different microtubule-associated proteins, affecting the neuronal microtubule network [4], and, very recently, toxicity has been demonstrated in some animal models [5]. Also, the presence of P-tau has been correlated with cognitive impairment [6].
The level of P-tau is a consequence of the action of protein kinases, which favour tau phosphorylation, and of protein phosphatases, which decrease this post-translational modification. Tau kinases has been classified as proline-directed (PDPK) and non-proline-directed protein kinases (NPDPK) [7], one of the PDPK being glycogen synthase kinase 3 (GSK-3), the enzyme that could modify more sites in tau molecule [8]. Some of these sites are modified as well in mouse models overexpressing GSK3 [9].
On the other hand, tau phosphatases have also been described [10], the most significant one being protein phosphatase 2A (PP2A), a protein that accounts for more than 70% of the total phosphatase activity found in human brain [11].
One of the main objectives to understand tau pathology associated to AD is to identify the external signals that could favour the increase of tau phosphorylation. For instance, it was described that starvation may induce tau hyperphosphorylation in mouse brain [12]. Thus, alterations in glucose metabolism that induce hypothermia lead to tau hyperphosphorylation, mainly by inhibition of tau phosphatases [13]. More recently, it has also been reported that insulin dysfunction (a feature that has been related to AD [14]), may induce in vivo tau hyperphosphorylation [15].
Insulin dysfunction results in a decrease of both tau kinases and tau phosphatases (Fig. 1). In the case of tau phosphatase PP2A, composed of a catalytic subunit (PP2Ac) and two regulatory subunits, A and B, it was described that in peripheral tissues PP2Ac could be modified at tyrosine residues in response to insulin [16–18]. Tyrosine phosphorylation of PP2Ac mainly occurs at tyrosine 307, located at the C-terminus of the molecule, close to the carboxy-terminal end (residue 309), where a leucine residue, which could be modified by methylation, is present [19]. Thus, the analysis of tyrosine 307 phosphorylation could be an important task to understand tau phosphorylation in AD.
1.
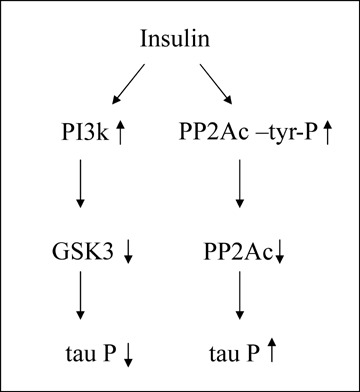
Insulin (or related compounds) signalling could affect, in two opposite ways, to tau phosphorylation. In one way (left), it will inhibit the main tau kinase (GSK3), decreasing the modification in tau molecule. On the other hand (right), it will decrease PP2A activity, increasing the level of tau phosphorylation. It seems that the overall effect, from these opposite effects, is an increase in tau phosphorylation.
In a work that is published in this issue of the Journal of Cellular and Molecular Medicine, Liu et al.[20] describe the presence of PP2Ac tyrosine phosphorylation in AD brains, a finding that could further explain tau hyperphosphorylation found in AD. For a future work it will be of interest to analyse if there is a decrease in this tyrosine phosphorylation process in response to external stimuli that could result in a dysfunction of insulin (or related factors) signalling pathways.
References
- 1.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–9. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–9. [PubMed] [Google Scholar]
- 3.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–7. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci USA. 1997;94:298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinhilb ML, Dias-Santagata D, Fulga TA, Felch DL, Feany MB. Tau phosphorylation sites work in concert to promote neurotoxicity in vivo. Mol Biol Cell. 2007;18:5060–8. doi: 10.1091/mbc.E07-04-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura T, Yamashita S, Fukuda T, Park JM, Murayama M, Mizoroki T, Yoshiike Y, Sahara N, Takashima A. Hyperphosphorylated tau in parahippocampal cortex impairs place learning in aged mice expressing wild-type human tau. EMBO J. 2007;26:5143–52. doi: 10.1038/sj.emboj.7601917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Titani K, Ihara Y. Proline-directed and non-proline-directed phosphorylation of PHF-tau. J Biol Chem. 1995;270:823–9. doi: 10.1074/jbc.270.2.823. [DOI] [PubMed] [Google Scholar]
- 8.Avila J, Lucas JJ, Perez M, Hernandez F. Role of tau protein in both physiological and pathological conditions. Physiol Rev. 2004;84:361–84. doi: 10.1152/physrev.00024.2003. [DOI] [PubMed] [Google Scholar]
- 9.Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegen-eration in GSK-3beta conditional transgenic mice. EMBO J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem. 1993;61:921–7. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci. 2005;22:1942–50. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- 12.Yanagisawa M, Planel E, Ishiguro K, Fujita SC. Starvation induces tau hyperphosphorylation in mouse brain: implications for Alzheimer's disease. FEBS Lett. 1999;461:329–33. doi: 10.1016/s0014-5793(99)01480-5. [DOI] [PubMed] [Google Scholar]
- 13.Planel E, Miyasaka T, Launey T, Chui DH, Tanemura K, Sato S, Murayama O, Ishiguro K, Tatebayashi Y, Takashima A. Alterations in glucose metabolism induce hypothermia leading to tau hyper-phosphorylation through differential inhibition of kinase and phosphatase activities: implications for Alzheimer's disease. J Neurosci. 2004;24:2401–11. doi: 10.1523/JNEUROSCI.5561-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao D, Lu H, Lewis TL, Li L. Intake of Sucrose-sweetened Water Induces Insulin Resistance and Exacerbates Memory Deficits and Amyloidosis in a Transgenic Mouse Model of Alzheimer Disease. J Biol Chem. 2007;282:36275–82. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- 15.Planel E, Richter KE, Nolan CE, Finley JE, Liu L, Wen Y, Krishnamurthy P, Herman M, Wang L, Schachter JB, Nelson RB, Lau LF, Duff KE. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J Neurosci. 2007;27:3090–7. doi: 10.1523/JNEUROSCI.4854-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srinivasan M, Begum N. Regulation of protein phosphatase 1 and 2A activities by insulin during myogenesis in rat skeletal muscle cells in culture. J Biol Chem. 1994;269:12514–20. [PubMed] [Google Scholar]
- 17.Begum N, Ragolia L. cAMP counter-regulates insulin-mediated protein phosphatase-2A inactivation in rat skeletal muscle cells. J Biol Chem. 1996;271:31166–71. doi: 10.1074/jbc.271.49.31166. [DOI] [PubMed] [Google Scholar]
- 18.Begum N, Ragolia L. Role of janus kinase-2 in insulin-mediated phosphorylation and inactivation of protein phosphatase-2A and its impact on upstream insulin signalling components. Biochem J. 1999;344:895–901. [PMC free article] [PubMed] [Google Scholar]
- 19.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–39. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu R, Zhou X, Tanila2 H, Bjorkdahl C, Wang J-Z, Guan Z-Z, Cao Y, Gustafsson J-A, Winblad B, Pei J-J. Phosphorylated PP2A (tyrosine 307) is associated with Alzheimer neurofibrillary pathology. J Cell Mol Med. 2007;12:241–5. doi: 10.1111/j.1582-4934.2008.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


