Abstract
We have shown that thymoquinone (TQ) is a potent antitumor agent in human colorectal cancer cells. In this study, we evaluated TQ's therapeutic potential in two different mice colon cancer models [1,2-dimethyl hydrazine (DMH) and xenografts]. We also examined TQ effects on the growth of C26 mouse colorectal carcinoma spheroids and assessed tumor invasion in vitro. Mice were treated with saline, TQ, DMH, or combinations once per week for 30 weeks and the multiplicity, size and distribution of aberrant crypt foci (ACF) and tumors were determined at weeks 10, 20 and 30. TQ injected intraperitoneally (i.p.) significantly reduced the numbers and sizes of ACF at week 10; ACF numbers were reduced by 86%. Tumor multiplicity was reduced at week 20 from 17.8 in the DMH group to 4.2 in mice injected with TQ. This suppression was observed at week 30 and was long-term; tumors did not re-grow even when TQ injection was discontinued for 10 weeks. In a xenograft model of HCT116 colon cancer cells, TQ significantly (P < 0.05) delayed the growth of the tumor cells. Using a matrigel artificial basement membrane invasion assay, we demonstrated that sub-cyto-toxic doses of TQ (40μM) decreased C26 cell invasion by 50% and suppressed growth in three-dimensional spheroids. Apoptotic signs seen morphologically were increased significantly in TQ-treated spheroids. TUNEL staining of xenografts and immunostaining for caspase 3 cleavage in DMH tumors confirmed increased apoptosis in mouse tumors in response to TQ. These data should encourage further in vivo testing and support the potential use of TQ as a therapeutic agent in human colorectal cancer.
Keywords: colon cancer; cancer therapy; 1,2-dimethyl hydrazine animal model; thymoquinone; invasion; xenograft
Introduction
Colorectal cancer is among the leading causes of cancer-related death and one of the most commonly diagnosed cancers [1]. The vast majority of deaths in colorectal cancer patients are caused by tumor metastases which are a consequence of a higher rate of migration. There is compelling evidence from epidemiological and experimental studies that highlight the importance of compounds derived from plants to reduce the risk of colon cancer [2] and inhibit the development and spread of tumors in experimental animals [3]. An ideal compound is one that possesses antitumor properties with minimal collateral defects, including toxicity.
Thymoquinone (TQ), the abundant component of black seed oil extract, is known to be the active principle responsible for many of the seed's antioxidant and anti-inflammatory effects [4]. Numerous studies have shown that the seeds and oil of this plant are characterized by a very low degree of toxicity [5]. Although TQ has been subjected to a range of investigations in animals, there have been no attempts to study its therapeutic effects in colon cancer. In animal models and systems other than the colon, TQ has been shown to have promising antitumor effects [6, 7]. It inhibited the incidence of fibrosarcoma tumors in mice induced with 20-methylcholanthrene [6] as well as forestomach tumors induced with benzo(a)pyrene [7]. TQ has been shown to attenuate ifosfamide-induced Fanconi syndrome in rats and to enhance its antitumor activity in mice [8]. TQ has been also shown to increase the antitumor effects of ifosamide [8]. Moreover, TQ was shown to reduce cisplatin-induced nephrotoxicity without affecting its antitumor activity [9].
We have recently shown that non-cytotoxic concentrations of TQ reduce the proliferation and induce apoptosis in human colon cancer cells via p53-dependent mechanisms [10]. The present studies were designed to extend our previous findings that showed growth inhibitory effects of TQ on HCT116 cells in vitro to an established mouse model of 1,2-dimethyl hydrazine (DMH)-induced colorectal carcinogenesis, which resembles histologically and pathologically the human disease, and to a mouse model of HCT116 xenografts. We also examined TQ effects on the growth of mouse colorectal carcinoma spheroids and on tumor cell invasion.
Methods
Cell culture and reagents
The mouse colorectal carcinoma cell line C26 was obtained from CLS (Heidelberg, Germany). This cell line was originally established from tumors induced in Balb/c mice by single rectal application of N-nitroso-N-methylurethane. The cells were cultivated in RPMI 1640 (PAA, Linz, Austria) supplemented with 10% fetal bovine serum (Biochrom, Berlin, Germany), antibiotics/antimycotics (PAA) under standard conditions at 37°C in humidified atmosphere containing 5% CO2. The adherent cells were detached from the culture flasks using trypsin/ethyl-enediaminetetraacetic acid (EDTA) (PAA, Linz, Austria). Cells were counted in the Coulter Counter ZII (Coulter Immunotech, Marseille, France). A purified preparation of TQ (>99% pure) was purchased from ICN Biochemicals (Irvine, CA).
Animal experimental design
Adult female Balb/c mice were bred in the animal care facility at the American University of Beirut. Mice were housed under optimum conditions of temperature set at 22 ± 2°C and light set at 12 hrs light–dark cycle. Mice were kept in plastic cages covered with sawdust and had unrestricted access to a commercial mouse diet (24% protein, 4.5% fat, 4% fiber) and water. Each mouse consumed an average of 6 ml of water daily. All animal studies were conducted using a protocol approved by the Institutional Animal Care and Use Committee of the American University of Beirut. The toxicity of TQ in mice was determined prior to the DMH animal experiment.
TQ toxicity in mice
Mice (9-week old) were divided into groups of 10 each and TQ toxicity was determined after daily intraperitoneal injections for 20 consecutive days. The doses of TQ were 5, 10, 20 or 30 mg/kgbw. TQ dilutions in isotonic saline (0.9%) were prepared by first dissolving TQ in ethanol and preparing subsequent dilutions in saline (as ethanol is toxic to animals). In all treatment groups (including the control), the animals received the same percentage of ethanol diluted in saline.
DMH mouse experiment
Animals (30 mice per group) were treated once per week either with saline (group 0), TQ (group 6), DMH (group 1) or combinations (groups 2–5) and 10 animals were sacrificed at three time points during the experiment, at week 10 for counts of aberrant crypt foci (ACF) or at weeks 20 or 30 for the counts of adenoma and carcinoma, respectively (Table 1). DMH (20 mg/kgbw) was dissolved in isotonic saline and 0.2 ml was injected sub-cutaneously (s.c.) once per week on the dorsal back. TQ (5mg/kgbw, 0.2ml) was injected (i.p.) either at the start of DMH treatment (initiation, group 2) or at 10 weeks after DMH (post-initiation, groups 3–5). Groups 3 and 4 received 10 weeks of DMH injections followed by injection with TQ (5mg/kgbw, 0.2ml) for 10 or 20 weeks, respectively. To determine whether the therapeutic effects of TQ are long-lasting, a group of mice (group 5) was injected with TQ (5mg/kgbw, 0.2ml) at post-initiation for 10 weeks after which TQ treatment was discontinued and tumor multiplicity was determined at week 30.
1.
Treatment groups in the DMH animal experiment
| Groups | Treatment |
|---|---|
| 0 | Saline |
| 1 | DMH |
| 2 | TQ+DMH |
| 3 | DMH (10weeks)→TQ (10weeks) |
| 4 | DMH (10weeks)→TQ (20weeks) |
| 5 | DMH (10weeks)→TQ (10weeks)‡No treatment (10weeks) |
| 6 | TQ |
ACF multiplicity, size and distribution were determined after staining by Schiff's reagent as described previously [11]. Briefly, colons were removed, and after flushing with normal saline, opened longitudinally and visible tumors were examined either visually or using an optical microscope. Tumor locations (proximal, middle or distal colon) were recorded and tumors were rapidly excised and processed for immunohistochemistry.
Xenograft model
HCT116 colon cancer cells (kindly provided by Dr. Carlos Galmarini, Lyon, France) were harvested and resuspended in sterile physiologic NaCl solution. 5 × 106 cells in a volume of 0.2 ml were injected subcutaneously into the flank of 4–6 week old male Naval Medical Research Institute (NMRI) mice (Harlan Winkelmann GmbH, Germany) as described previously [12]. Animals were kept in a light- and temperature-controlled environment and provided with food and water ad libitum. Tumor size was determined daily by measurement with a caliper square and expressed relative to size at day 1 which was set at 1.0. Intraperitoneal treatment with TQ (20 mg/kg) or 10% methanol in physiologic saline three times per week was started when subcutaneous tumors reached a diameter of 7 mm. Animals were sacrificed by cervical dislocation and specimens of tumors were either fixed in 10% phosphate-buffered formalin or snap-frozen in liquid nitrogen. Ethical approval was obtained before the beginning of experiments.
Immunohistochemistry
Tumor tissues were placed in 10% neutral-buffered formalin, embedded in optimal cutting temperature (OCT) embedding compound (Leica Instruments, Nussloch, Germany). Samples were sectioned, stained with Hematoxylin and Eosin (H&E), and examined by light microscopy. Tissues were stained with Ki-67 antibody (mouse monoclonal antibody, clone MIB-1, M7240, dilution 1:50; DakoCytomation, Glostrup, Denmark) as a marker of cell proliferation and with an antibody specific for cleaved caspase 3 (polyclonal rabbit antibody, clone 9661, dilution 1:100, cell signaling technology, Danvers, MA, USA) as a marker of apoptosis. Immunohistochemical detection of Ki-67 was performed using the automated immunohistochemistry slide staining system by Ventana NexES (Ventana Medical System, Strasbourg, France) after antigen retrieval by microwave heating (450 W, 0.01 M EDTA, 20 min). No antigen retrieval was performed for cleaved caspase-3 immunohistochemistry. Positive immunohistochemical reactions were revealed using the iVIEWTM DAB Detection Kit (Ventana, Germany) or Vectastain ELITE ABC Kit (Vector Labarotories, Burlingame, CA, USA). All of the specimens were evaluated by a pathologist who was unaware of the treatment groups.
TUNEL staining of tissues
Apoptosis was studied using the method of TUNEL-assay after minor modifications. Deparaffinized and rehydrated 3μm tissue sections were treated with proteinase K solution (20 μg/ml) for 30min at 37°C. After rinsing twice with PBS containing glycine for 1 min the slides were incubated with 50 μl fresh prepared TUNEL reaction mixture in a humidified atmosphere in the dark (60 min, 37°C; Insitu cell death detection kit, Roche diagnostics, Mannheim, Germany). For fixation, phosphate-buffered saline (PBS)-rinsed slides were incubated with a solution of 4% paraformaldehyde containing 0.05% glutaraldehyde for 10 min. After repeated washing with PBS for 5 min, slides were incubated with 50 μl Converter-peroxidase (POD) (antifluorescein antiserum from sheep, conjugated with horseradish peroxidase; 30 min, 37°C, Roche). For color reaction, tissue was covered with 100 μl 3, 3′-diaminobenzidine tetrahydrochloride (DAB) substrate (5 min, Roche). Counterstaining with hematoxylin, dehydration and mounting followed.
The apoptotic index was determined by counting the number of TUNEL-labelled cells per 100 epithelial cancer cells in 10 fields of the most affected tumor areas with ×400 magnification.
Three-dimensional growth of tumor spheroids
Tumor cells were grown as ‘hanging drops’ (2000 cells per 20 μl RPMI 1640 per drop) for 4 days on the lid of the medium-filled culture dish [13]. The resulting tumor cell spheroids were culled from the lid and could be further cultivated under normal culture conditions in adherent culture dishes.
Cell invasion
Cellular invasion of tumor cells was evaluated in 24-well Transwell chambers (Costar, Bodenheim, Germany) as described previously [14]. The upper and lower culture compartments were separated by polycarbonate filters with 8 μm pore size. Prior to invasion assays, the polycarbonate filter was coated with 100 μg Matrigel matrix per cm2. Mouse colorectal cancer C26 cells (1.5 × 105/per well) were incubated on the reconstituted basement membrane for 24 hrs in RPMI 1640 culture medium. TQ was added at concentrations of 20, 40 and 60 mM. Cells passing the filters and attaching to the lower sites of matrigelcoated membranes were harvested using trypsin/EDTA. The percentage of migrating cells was calculated from controls grown under identical culture conditions for 24 hrs in 48 well plates. All quantifications were performed in triplicate.
Cell viability
Cells (105 cells/well) were plated in 96-well plates. At 24 rs of treatment with TQ, toxicity was studied using the CytoTox 96 non-radioactive cytotoxicity kit according to the manufacturer's suggestions (Promega Corp. Madison, WI). The CytoTox 96 assay quantitatively measures the lactate dehydrogenase (LDH), a stable cytosolic enzyme that is released upon cell lysis. Released LDH in culture supernatants is measured with a coupled enzymatic assay which results in the conversion of a tetrazolium salt into a red formazan product, the absorbance of which is recorded at 490 nm.
Statistical analysis
Students t-test was performed for comparing tumor cell invasion. anova followed by Dunnett test was done for comparing ACF, tumor size and number by SPSS Student Version 11.0 Software Package. Statistical significance was assigned if P < 0.05.
Results
Non-toxic doses of TQ reduce ACF and tumor multiplicity
To investigate TQ toxicity, different doses of TQ (5, 10, 20, 30 mg/kgbw) were injected i.p. for 20 consecutive days. TQ at doses of 5–20 mg/kgbw did not affect the body weight or result in animal death (data not shown), indicating that these doses are not toxic. Death was observed at 30 mg/kgbw (3 dead animals). For this reason, the lowest non-toxic dose of 5 mg/kg TQ was used for the tumor experiment. All animals survived during the entire course of the tumor experiment. At the beginning of the experiment the average body weight varied from 20 to 24 grams in each group with no significant difference between groups. At the end of the experiment before sacrificing the animals, the average weight varied from 24 to 30 grams. The carcinogen- and TQ-treated groups did not differ significantly in weight gain during the entire experimental period (P = 0.517, Fig. 1A).
1.

(A) The carcinogen- and TQ-treated groups did not differ significantly in weight gain during the entire experimental period (P= 0.517, one-way anova). (B) TQ-injected i.p. at initiation significantly (*P<0.01) reduced the number and sizes of ACF at week 10. (C) At week 10, ACF numbers in middle and distal colon were significantly (*P<0.01) higher in mice treated with 1,2-dimethyl hydrozine (DMH) only than those given DMH+TQ by i.p. injection. (D, E) TQ significantly (*P<0.01) decreased tumor multiplicity at weeks 20 and 30 if applied at initiation (group 2) or post-initiation (groups 3–5). Treatment groups 1–5 are described in Table 1. DMH (20 mg/kg) was injected sub-cutanenusly (s.c.). Saline- and TQ alone-treated mice did not develop ACF or tumors and are not represented in the graphs. At different time intervals, mice were sacrificed and colons were stained with Schiff's reagent for counting ACF or tumor numbers and for recording ACF sizes in mm2 using fluorescent microscopy. Statisitical analysis: anova followed by Dunnett test (*P<0.01). Means ± SD represent 20 measurements; level of significance was set at 0.05.
We then investigated the ability of the lowest nontoxic dose of 5 mg/kg bw of TQ to reduce ACF, the earliest identified putative premalignant precursors of both human and experimental colon cancer [15, 16]. The size and number of ACF in the colon of DMH-initiated mice was investigated. In this model the s.c. injection of the carcinogen DMH in the dorsal back of mice induces tumor development in the colon, specifically in the middle and distal parts.
As expected, no ACF or tumors were observed in animals given saline, the vehicle for DMH, or TQ alone (not shown). At 10 weeks of treatment with DMH only, all the animals developed ACF (Fig. 1B). These ACF were observed mainly in the middle and distal colon, with a greater distribution in the middle than in the distal colon (Fig. 1B). A significantly lower number of ACF, which were smaller in size, were found in mice injected with TQ at the initiation phase (Fig. 1B and C). The total number of ACF was reduced by 86% in mice injected with TQ. A further analysis of the size distribution of ACF in TQ-treated animals showed that the number of large foci (>0.2 mm2), which had accounted for almost 55% of the foci in DMH-treated mice, had fallen to around 6% after TQ treatment.
As shown in Figures 1D and 1E, the tumor multiplicity (average number of tumors per tumor bearing mouse) in the DMH only group (group 1) at weeks 20 and 30 was 17.8 and 15.6, respectively. TQ injected i.p. concomitantly with carcinogen treatment (group 2) significantly inhibited tumor development decreasing tumor multiplicity to 4.3. Interestingly, 10-or 20-week administration of TQ by i.p injection at post-initiation and after the 10th DMH dose (groups 3 and 4) significantly decreased tumor multiplicity (Fig. 1D E). The suppression of tumor development was long-term since tumors did not re-grow even when TQ injection was discontinued for 10 weeks (group 5). Selective histologic studies in the DMH group have shown that most of the intestinal tumors were poorly-differentiated adenocarcinomas with extensive growth lymphangiosis carcinomatosa and partly of signet ring cell type (Fig. 2E). Furthermore, large adenomas with mostly high grade intraepithelial neoplasia were observed in the DMH group (Fig. 2C). In contrast, mice of the TQ treated group (group 2) showed adenocarcinomas of early tumor stage which were histologically well differentiated. Here, single adenomas with low grade intraepithelial neoplasia occurred (Fig. 2D and F). Normal mucosa was observed in the control (group 0) and the TQ-alone treated mice (group 6) (Fig. 2A and B).
2.
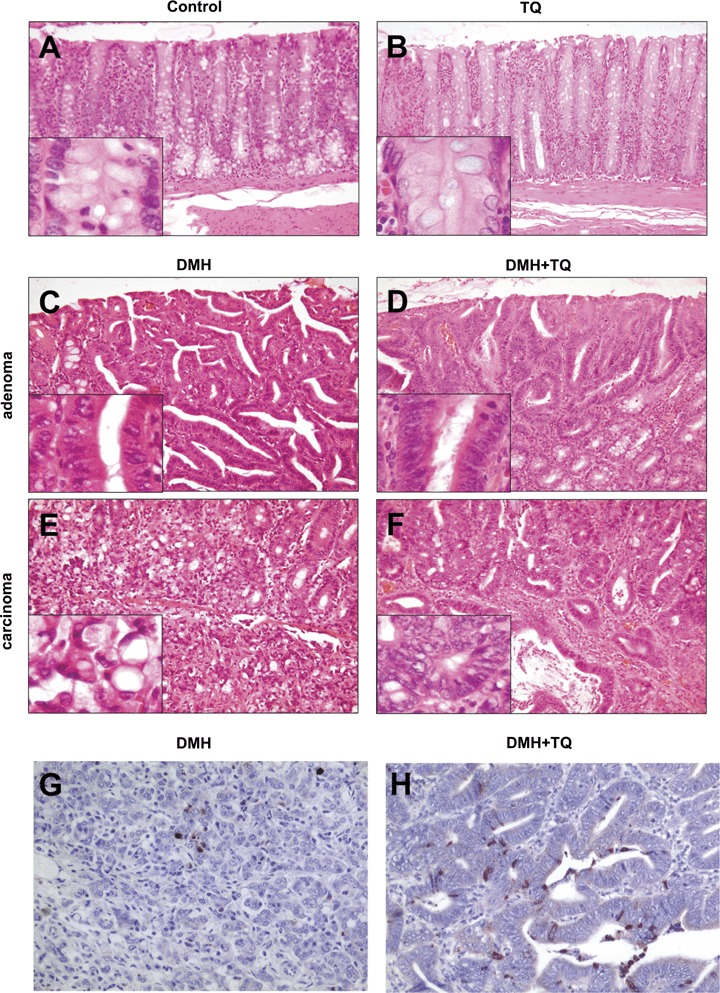
Haematoxyline and Eosin (H&E) and cleaved caspase-3 staining of tissues of the DMH mouse experiment: (A) Untreated and (B) TQ alone-treated mice showed normal colon mucosa. In the DMH-treated group adenomas with high-grade intraepithelial neoplasia (C) and poorly differentiated carcinoma (E), partly of signet cell ring type (E, magnification), were observed. Mice given DMH+TQ developed adenomas with low-grade intraepithelial neoplasia (D) and demonstrated well differentiated carcinomas of early tumor stage (F). (G and H) Immunohistochemical detection of cleaved caspase-3 disclosed few positive cells and apoptotic bodies in the carcinoma of untreated animals (group 1), whereas tumors of TQ-treated animals (group 3) had considerably higher proportion of cleaved caspase-3 expressing cells (Zeiss Axioscope 50, camera:Nikon Coolpix 990; x100, x200, x400).
Staining of the DMH and DMH+TQ (group 3) tissues with an anticleaved caspase-3 antibody showed that the tumor growth inhibition by TQ was due to the induction of apoptosis (Fig. 2G,H). Immunohistochemical detection disclosed few positive cells and apoptotic bodies in the carcinomas of DMH-treated mice compared to considerably higher proportion of cleaved caspase-3 expressing cells in the tumors of TQ-treated mice (Fig. 2G and H).
TQ inhibits growth and induces apoptosis in HCT116 xenografts
Mice were treated for 21 days with intraperitoneal injections of 20 mg/kg TQ or vehicle. Tumor size was set at 1.0 at day 1 of treatment and growth is expressed relative to day 1. At the end of the treatment period, xenografts of HCT116 cells reached a relative size of 2.8 mm2 in the control group, while tumor size in the TQ group was 2.0 (P < 0.05) (Fig. 3).
3.
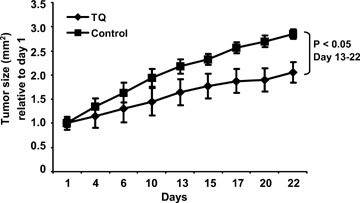
Relative growth of HCT116 xenografts in male NMRI mice during 21 days of i.p. treatment (3x/week) with either 20 mg/kg TQ or 10% methanol in physiologic saline. Mean tumor size was set at 1.0 at day 1 and growth is expressed relative to day 1. P<0.05 between TQ and vehicle treated animals.
The macroscopically observed growth delay was not due to reduced proliferation of tumor cells as evidenced by MIB1 staining (Fig. 4). Staining showed that the proliferation index of tumors of untreated controls (87.9%) was similar to that of the TQ-treated xenografts (86.7%) (Fig. 4C and D). On the other hand, TQ treatment lead to increased TUNEL positivity (Fig. 4E and F), indicating that, in this model as well, the diminished tumor size in TQ-treated xenografts was mainly due to the induction of cell death. In the TQ-treated group, median apoptotic index estimated by TUNEL was 3.8% as compared to 2.4% in vehicle controls.
4.
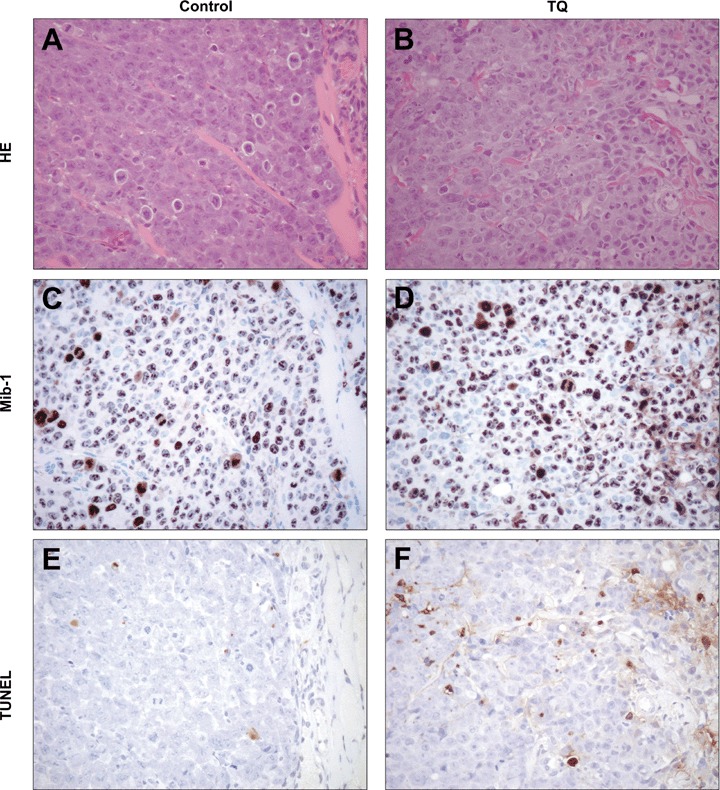
Histology of the HCT116 xenograft tumors upon staining with H&E, Ki-67 and TUNEL: (A, B) Hematoxylin and Eosin staining of xenograft tumors. The proliferation index determined by Ki-67 staining showed no significant difference between the TQ-treated xenografts (D) and the untreated control (C). An increased apoptotic index measured by TUNEL was observed in the TQ-treated xenograft (F) as compared to the untreated control (E). (Zeiss Axioscope 50, camera:Nikon Coolpix 990; x200).
Inhibition of cell invasion potential of C26 cells by TQ
To quantify cell invasion, we tested the effect of TQ on the invasion of C26 colon carcinoma cells. C26 cells were suspended in conditioned medium, added to the upper components of the matrigel invasion chamber supplemented with various concentrations of TQ (0, 20, 40, 60 μM), and incubated for 24 hours at 37°C and 5% CO2. In normal culture conditions, 1.15% of C26 cells passed the Matrigelcoated filters. As shown in Figure 5A, the total number of cells that invaded to the underside of the filters was significantly decreased by TQ treatment (IC50= 40 μM). Whereas the addition of 20 μM TQ showed only a minor effect on the invasive potential (8% inhibition) of the cell line, treatment with 40 μM TQ resulted in a significantly reduced invasion of these cells which was not further decreased by higher concentrations. Since C26 cell viability did not significantly decrease at 24 hours after TQ treatment (Fig. 5B), the inhibitory effect of TQ may occur as a result of reduced cell-invasive capacity rather than a reduction in cell number.
5.
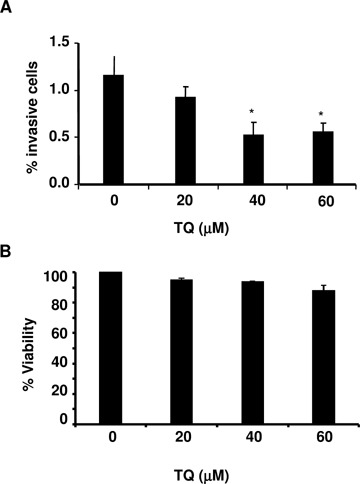
(A) The effect of TQ treatment on cellular invasion and (B) viability of C26 murine colon carcinoma cells. For invasion studies, C26 cells (1.5 × 105) were incubated on reconstituted basement membranes and cellular invasion was evaluated in 24-well Transwell chambers after 24 hrs of TQ treatment. For viability studies, C26 cells (1 × 105) were cultured in 96-well plates and cell viability was evaluated after 24 hrs of TQ treatment using the CytoTox 96 non-radioactive cytotoxicity kit (Promega Corp. Madison, WI). In both experiments TQ was added every 24 hrs. Results from Student's t-test are given on top of bars. *P values of <0.01 between TQ treatment versus control were considered to be significant.
Effects of TQ treatment in a three-dimensional culture system
Tumor spheroids of C26 cells were treated with TQ at 40mM, the concentration which represents the IC50 of cell invasivenenss. The growth pattern and morphological changes were examined by phase contrast microscopy (Fig. 6A and B) or on H&E-stained paraffin-sections (Figs. 6C–F). TQ significantly changed the growth pattern in the three-dimensional culture system. Spheroids of untreated C26 colon carcinoma cells show a dense growth pattern and signs of high proliferation, enlargement and multiplication of the nucleoli as well as numerous atypical mitoses (Figs. 6 A, C and E). By contrast, treated C26 spheroids showed a loss of intercellular adhesion resulting in a sponge-like growth pattern (Fig. 6B). Furthermore, increased apoptotic features were observed after treatment of C26 spheroids with TQ including homogenous eosinophil cytoplasma, blebs of the cell membrane, condensation of chromatin as well as fragmentation and loss of nuclei (Figs. 6D and F).
6.

The effect of TQ treatment on the growth pattern of C26 murine colon carcinoma cell spheroids. Spheroids of untreated C26 colon carcinoma cells show a dense growth pattern (A, C) and signs of high proliferation such as cytoplasmatic basophilia, hyperchromasia of the nucleus, enlargement and multiplication of the nucleoli as well as numerous atypical mitosis (E). In contrast, after treatment of C26 spheroids by TQ increased apoptotic features can be observed: loss of intercellular contact, homogenous eosinophil cytoplasma, blebs of the cell membrane, condensation of chromatin as well as fragmentation and loss of the nucleus (B, D, F). (a = apoptosis, b = blebs, f = fragmented nuclei, m = mitosis, n = nucleoli; * loss of intercellular contact; microscope: Nikon Eclypse TE300, camera:Nikon Coolpix 990; x200, x1000).
Discussion
Many contemporary medical drugs are derived from herbs and about one-quarter of prescription drugs contain at least one active ingredient derived from plant material [17]. Herbal therapies, including the black seed (Nigella sativa) from which TQ is extracted, have been used by diverse human cultures around the world for centuries. TQ has shown antitumor promotion activities in rats or mice treated with chemical carcinogens [6, 7]. Although TQ has been subjected to a range of pharmacological investigations in animals, there have been no attempts to study its effects in the colon. Recent investigations in our laboratories have shown that TQ exerts antiproliferative and pro-apoptotic effects via p53-dependent mechanisms in HCT116 human colon cancer cells [10]. The results of the present study clearly indicate that TQ inhibits tumor cell invasion in vitro (IC50= 40 μM) as well as ACF formation and tumor development in DMH-induced mouse colon carcinogenesis and in a xenograft model of human HCT-116 colon cancer cells. Here we show that TQ demonstrates potent and long-term therapeutic effects at the post-initiation phase of colon carcinogenesis and results in a pronounced inhibition of tumor growth of three-dimensional spheroids. TQ has been shown to upregulate p53 and p21WAF1 protein expression in human colon cancer cells and to induce apoptosis by increasing bax/bcl-2 protein expression levels [10]. Although it is not known whether TQ induces epigenetic changes, the long-term therapeutic effects of TQ resemble epigenetic interference with the p53 and p21WAF1 pathways as observed by HDAC inhibitors which are known to be long-lasting under physiologic conditions [18]. Epigenetic changes modify the long-term phenotypic behaviour of tumor cells regarding their differentiation potential [19].
The exact mechanism(s) by which TQ exerts its antitumor activity in vivo is still not known, since no data is available on the molecular mechanisms of TQ effects. Tumor growth inhibition by TQ in the DMH and xenograft mouse models may be attributed to its potent antiinflammatory and antioxidant effects, in addition to the induction of apoptosis as indicated by TUNEL and caspase-3 cleavage. Oxygen radicals, especially nitric oxide, cause p53 gene mutations, chromosomal change and activation of signal transduction pathways involved in cell growth [20]. TQ has been shown to inhibit superoxide anions [21, 22] and nitric oxide production [23, 24] involved in tumorigenesis. The antioxidant and pro-apoptotic effects of TQ are involved in reducing tumor size, although the antioxidant effect may be the more important contributing factor in group 2 and the pro-apoptotic effects may be the predominant effect in the established tumors. In groups 3 and 4, the antioxidant effect of TQ may contribute to a diminished ‘de novo’ formation of tumors.
This is the first report that demonstrates the in vivo therapeutic activities of TQ in colon cancer models. In previous work, black seed oil has been shown to suppress the development of ACF in DMH-induced rat colon carcinogenesis particularly in the postinitiation stage and this inhibition was associated with the suppression of cell proliferation in the colonic mucosa [25]. Considering that TQ is the most abundant component of black seed oil, the inhibition of ACF development in the latter study may be attributed to the activity of TQ in the oil. We confirmed these findings in the DMH mouse model as the administration of TQ reduced the mean number and size of ACF. While DMH treatment alone caused the development of many, highly neoplastic, large and poorly differentiated adenocarcinomas, mice treated with DMH+TQ developed significantly less, small and low-grade neoplastic adenomas and well-differentiated carcinomas of early tumor stage. At week 20 we found 76% inhibition (P<0.01) of tumor formation associated with TQ injected i.p..
To corroborate our previous findings on the growth inhibiting and pro-apoptotic effects of TQ in human HCT116 cells and to use a second mouse model, we investigated the therapeutic potential of TQ in subcutaneous flank model in NMRI mice. Although the pharmacologic properties of TQ are not well known and still need to be optimized, we observed a significant growth delay of TQ-treated xenografts. This also demonstrates that TQ is a very well-tolerated drug in mice. We show that TQ administered for 20 consecutive days did not induce death in mice or affect their mean body weight, which is a very sensitive parameter for toxicity in rodents. This is in agreement with numerous studies which have demonstrated the absence of toxicity by TQ when administered to animals [26–28]. In fact TQ treatment has been found to alleviate the toxicity of several anticancer compounds by reducing their toxicity to the heart, kidney, liver and nervous system [9, 29–31]. The macroscopically observed growth delay in HCT116 mouse xenografts was not due to the reduced proliferation of tumor cells but rather to drug-induced apoptosis as evidenced by enhanced TUNEL positivity in xenografts treated with TQ. Our results on anticleaved caspase-3 antibody staining in DMH induced tumors confirmed that one major effect of TQ in vivo is through an increased apoptosis. Therefore, TQ is a drug that induces apoptosis both in cultured colorectal cancer cells [10] as well as in colon cancer cells implanted in mice.
In our experimental settings, TQ also exhibited 50% inhibition of C26 tumor cell invasion at concentrations that have almost no cytotoxic effects. Tumor invasion is the hallmark of malignant phenotype and is considered one of the most important factors determining the prognosis of several cancers, including colorectal cancer. Considering the complex processes on the invasion fronts, it is recommended to apply three-dimensional tumor cell models (i.e spheroids in vitro) thus getting closer to the in vivo situation (DMH and xenograft model) than do single cell cultures in the monolayer [13]. Sutherland et al. were the first to demonstrate that strong similarities exist between spheroids and solid tumors regarding morphology and functional characteristics [32]. Using three-dimensional cultures, it is possible to investigate in vitro drug-cell interactions, particularly the influence of drugs on many different processes such as apoptosis, angiogenesis, intravasation or extravasation [33, 34]. We have demonstrated that TQ treatment not only inhibited tumor cell invasion and proliferation in C26 tumor spheroids but also reduced the growth of colon cancer cells in two different in vivo models.
In conclusion, the findings herein represent evidence that TQ, the bioactive component of black seed, has antiinvasive activities in C26 colorectal cancer cells, in addition to a therapeutic role against DMH-induced colon cancer when administered at the initiation or post-initiation phases. This, coupled with the apoptotic effects of TQ in human colorectal cancer cell cultures and xenografts indicates that this relatively non-toxic and inexpensive compound merits further clinical investigation, especially when considering that there are only a few effective and nontoxic anticancer agents available for clinical use.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft, Germany: DFG 477/6–1 and 477/7–1 and the Lebanese National Council for Scientific Research.
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–43. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Lipkin M, Reddy B, Newmark H, Lamprecht SA. Dietary factors in human colorectal cancer. Annu Rev Nutr. 1999;19:545–86. doi: 10.1146/annurev.nutr.19.1.545. [DOI] [PubMed] [Google Scholar]
- 3.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 4.Worthen D, Ghosheh O, Crooks P. The in vitro antitumor activity of some crude and purified components of black seed, Nigella sativa L. Anticancer Res. 1998;18:1527–32. [PubMed] [Google Scholar]
- 5.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17:299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 6.Badary OA, Gamal El-Din AM. Inhibitory effects of thymoquinone against 20 methylcholanthrene-induced fibrosarcoma tumorigenesis. Cancer Detect Prev. 2001;25:362–8. [PubMed] [Google Scholar]
- 7.Badary OA, Al-Shabanah OA, Nagi MN, Al-Rikabi AC, Elmazar MM. Inhibition of benzo(a)pyrene-induced forestomach carcinogenesis in rats by thymoquinone. Eur J Cancer Prev. 1999;8:435–40. doi: 10.1097/00008469-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Badary OA. Thymoquinone attenuates ifosfamide-induced Fanconi syndrome in rats and enhances its antitumor activity in mice. J Ethnopharmacol. 1999;67:135–42. doi: 10.1016/s0378-8741(98)00242-6. [DOI] [PubMed] [Google Scholar]
- 9.Badary OA, Nagi MN, Al-Shabanah OA, Al-Sawaf HA, Al-Sohaibani MO, Al-Bekairi AM. Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Can J Physiol Pharmacol. 1997;75:1356–61. [PubMed] [Google Scholar]
- 10.Gali-Muhtasib H, Diab-Assaf M, Boltze C, Al-Hmaira J, Hartig R, Roessner A, Schneider-Stock R. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int J Oncol. 2004;25:857–66. [PubMed] [Google Scholar]
- 11.Gali-Muhtasib HU, Younis I, Karchesy J, El-Sabban M. Plant tannins inhibit the induction of aberrant crypt foci and colonic tumors by 1,2-dimethyl hydrazine in mice. Nutr Cancer. 2001;39:108–16. doi: 10.1207/S15327914nc391_15. [DOI] [PubMed] [Google Scholar]
- 12.Ocker M, Neureiter D, Leuders M, Zopf S, Ganslmayer M, Hahn EG, Herold C, Schuppan D. Variants of bcl-2 specific siRNA for silencing antiapoptotic bcl-2 in pancreatic cancer. Gut. 2005;54:1298–308. doi: 10.1136/gut.2004.056192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krueger S, Kalinski T, Wolf H, Kellner U, Roessner A. Interactions between human colon carcinoma cells, fibroblasts and monocytic cells in coculture-regulation of cathepsin B expression and invasiveness. Cancer Lett. 2005;223:313–22. doi: 10.1016/j.canlet.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 14.Krueger S, Kellner U, Buehling F, Roessner A. Cathepsin L antisense oligonucleotides in a human osteosarcoma cell line: effects on the invasive phenotype. Cancer Gene Ther. 2001;8:522–8. doi: 10.1038/sj.cgt.7700341. [DOI] [PubMed] [Google Scholar]
- 15.Bird RP, Good CK. The significance of aberrant crypt foci in understanding the pathogenesis of colon cancer. Toxicol Lett. 2000;112–113:395–402. doi: 10.1016/s0378-4274(99)00261-1. [DOI] [PubMed] [Google Scholar]
- 16.Takayama T, Katsuki S, Takahashi Y, Ohi M, Nojiri S, Sakamaki S, Kato J, Kogawa K, Miyake H, Niiysu Y. Aberrant crypt foci of the colon as precursors of adenoma and cancer. N Engl J Med. 1998;339:1277–84. doi: 10.1056/NEJM199810293391803. [DOI] [PubMed] [Google Scholar]
- 17.Bent S, Ko R. Commonly used herbal medicines in the United States: a review. Am J Med. 2004;116:478–85. doi: 10.1016/j.amjmed.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 18.Kelly WK, Richon VM, O'Connor O, Curley T, MacGregor-Curtelli B, Tong W, Mark K, Lawrence S, Stacie R, Eddie R, Marija D, Carlos C, Judy H, Paul A, Howard S. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res. 2003;9:3578–88. [PubMed] [Google Scholar]
- 19.Neureiter D, Zopf S, Leu T, Dietze O, Herold C, Ocker M. Apoptosis, proliferation and differentiation patterns are influenced by Zebularine and SAHA in pancreatic cancer models. Scand J Gastroenterol. 2007;42:103–16. doi: 10.1080/00365520600874198. [DOI] [PubMed] [Google Scholar]
- 20.Cerutti PA. Oxy-radicals and cancer. Lancet. 1994;344:862–3. doi: 10.1016/s0140-6736(94)92832-0. [DOI] [PubMed] [Google Scholar]
- 21.Mansour MA, Nagi MN, El-Khatib AS, Al-Bekairi AM. Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT-diaphorase in different tissues of mice: a possible mechanism of action. Cell Biochem Funct. 2002;20:143–51. doi: 10.1002/cbf.968. [DOI] [PubMed] [Google Scholar]
- 22.Badary OA, Taha RA, Gamal el-Din AM, Abdel-Wahab MH. Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol. 2003;26:87–98. doi: 10.1081/dct-120020404. [DOI] [PubMed] [Google Scholar]
- 23.El-Mahmoudy A, Shimizu Y, Shiina T, Matsuyama H, El-Sayed M, Takewaki T. Successful abrogation by thymoquinone against induction of diabetes Mellitus with streptozotocin via nitric oxide inhibitory mechanism. Int Immunopharmacol. 2005;5:195–07. doi: 10.1016/j.intimp.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 24.El-Mahmoudy A, Matsuyama H, Borgan MA, Shimizu Y, El-Sayed MG, Minamoto N, Takewati T. Thymoquinone suppresses expression of inducible nitric oxide synthase in rat macrophages. Int Immunopharmacol. 2002;2:1603–11. doi: 10.1016/s1567-5769(02)00139-x. [DOI] [PubMed] [Google Scholar]
- 25.Salim EI, Fukushima S. Chemopreventive potential of volatile oil from black cumin (Nigella sativa L.) seeds against rat colon carcinogenesis. Nutr Cancer. 2003;45:195–202. doi: 10.1207/S15327914NC4502_09. [DOI] [PubMed] [Google Scholar]
- 26.Kirui PK, Cameron J, Benghuzzi HA, Tucci M, Patel R, Adah F, Russel G. Effects of sustained delivery of thymoqiunone on bone healing of male rats. Biomed Sci Instrum. 2004;40:111–6. [PubMed] [Google Scholar]
- 27.Hosseinzadeh H, Parvardeh S. Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine. 2004;11:56–64. doi: 10.1078/0944-7113-00376. [DOI] [PubMed] [Google Scholar]
- 28.Badary O, Al-Shabanah O, Nagi M, Al-Bekairi A, Elmazar M. Acute and subchronic toxicity of thymoquinone in mice. Drug Dev Res. 1998;44:56–61. [Google Scholar]
- 29.Al-Shabanah OA, Badary OA, Nagi MN, Al-Gharably NM, Al-Rikabi AC, Al-Bekairi AM. Thymoquinone protects against doxorubicin-induced cardiotoxicity without compromising its antitumor activity. J Exp Clin Cancer Res. 1998;17:193–8. [PubMed] [Google Scholar]
- 30.Mansour MA, Ginawi OT, El-Hadiyah T, El-Khatib AS, Al-Shabanah OA, Al-Sawaf HA. Effects of volatile oil constituents of Nigella sativa on carbon tetrachloride-induced hepatotoxicity in mice: evidence for antioxidant effects of thymoquinone. Res Commun Mol Pathol Pharmacol. 2001;110:239–51. [PubMed] [Google Scholar]
- 31.Mansour MA. Protective effects of thymoquinone and desferrioxamine against hepatotoxicity of carbon tetrachloride in mice. Life Sci. 2000;66:2583–91. doi: 10.1016/s0024-3205(00)00592-0. [DOI] [PubMed] [Google Scholar]
- 32.Sutherland RM, Durand RE. Growth and cellular characteristics of multicell spheroids. Recent Results Cancer Res. 1984;95:24–49. doi: 10.1007/978-3-642-82340-4_2. [DOI] [PubMed] [Google Scholar]
- 33.Santini MT, Rainaldi G, Indovina PL. Apoptosis, cell adhesion and the extracellular matrix in the three-dimensional growth of multicellular tumor spheroids. Crit Rev Oncol Hematol. 2000;36:75–87. doi: 10.1016/s1040-8428(00)00078-0. [DOI] [PubMed] [Google Scholar]
- 34.Mueller-Klieser W. Tumor biology and experimental therapeutics. Crit Rev Oncol Hematol. 2000;36:123–39. doi: 10.1016/s1040-8428(00)00082-2. [DOI] [PubMed] [Google Scholar]


