Overview
Autoinflammatory bone diseases are a new branch of autoinflammatory diseases caused by seemingly unprovoked activation of the innate immune system leading to an osseous inflammatory process.1 The inflammatory bone lesions in these disorders are characterized by chronic inflammation that is typically culture negative with no demonstrable organism on histopathology.2–6 The most common autoinflammatory bone diseases in childhood include chronic non-bacterial osteomyelitis (CNO); synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome; Majeed syndrome; deficiency of interleukin-1 receptor antagonist (DIRA); and cherubism. In this chapter we will focus on chronic non-bacterial osteomyelitis (CNO) and summarize the distinct genetic autoinflammatory bone syndromes. Table 1.
Table 1.
Autoinflammatory Bone Diseases Summary Chart
| Autoinflammatory Bone Diseases Summary Chart | |||||
|---|---|---|---|---|---|
| CNO | Majeed Syndrome |
DIRA | Cherubism | Childhood SAPHO |
|
| CLINICAL MANIFESTATIONS | |||||
| Fever | Not typical | High fevers | Uncommon | No | Not typical |
| Common CNO Sites | Femur, tibia, pelvis, calcaneus, ankle, vertebrae, & clavicle | Similar to CNO | Long bones (especially proximal femur), vertebral bodies, ribs, & clavicle | Maxilla and mandible | Similar to CNO |
| Area of long Bone Affected | 75% around metaphyses | Metaphyses predominance | Metaphyses predominance | Long bones rarely affected | Similar to CNO |
| Extraosseous Manifestations | Skin, joints, gastrointestinal tract, and lungs | Congenital dyserythropoietic anemia, inflammatory dermatosis, growth failure, hepatomegaly, joint contractures | Generalized pustulosis, osteitis, periostitis, systemic organ involvement | Cervical lymphadenopathy | Palmoplantar pustulosis, severe acne, or psoriasis |
| Inflammatory markers | Normal to mildly elevated | Elevated | Elevated | Normal to mildly elevated | Normal to mildly elevated |
| GENETICS | |||||
| Inheritance | Unknown | Autosomal Recessive | Autosomal Recessive | Autosomal Dominant | Unknown |
| Gene Defect | Unknown | LPIN2 | IL1RN | SH3BP2 | Unknown |
| Protein Name | Unknown | Lipin2 | IL-1Ra | SH3BP2 | Unknown |
| Ethnicity | Worldwide Distribution | Arabic, Turkish | Puerto Rico, European, Lebanese | Worldwide Distribution | Likely similar to CNO |
Chronic Non-bacterial Osteomyelitis (CNO)
Introduction
In 1972, Gideon first described CNO as a subacute and chronic symmetrical osteomyelitis.7 In the past forty years, our understanding of CNO has become more sophisticated due to numerous breakthroughs. The recent renaissance advances? in imaging technology has led to the enhanced ability to diagnosis inflammatory bone lesions. Multiple breakthroughs in immunology have led to a more sophisticated appreciation of the function of the innate immune system. This understanding enabled the characterization of autoinflammatory diseases and therefore autoinflammatory bone diseases. There have been many discoveries of the genetic associations of autoinflammatory syndromes. Finally, use of TNFα antagonist and bisphosphonates enabled more effective treatments in non-steroidal anti-inflammatory drug (NSAID) resistant disease. These breakthroughs have enabled the rheumatology community to have a more advanced understanding of CNO.
Nomenclature and Disease Pattern
The terminology for CNO has changed multiple times in the past forty years. It was first called subacute and chronic symmetrical osteomyelitis.7 However, since that time it has most commonly been called chronic recurrent multifocal osteomyelitis (CRMO). The term CRMO was coined by Probst in 1978 and is characterized as a chronic inflammatory bone disorder that had multifocal bone lesions and had multiple recurrences.8 However, not all patients have multifocal bone lesions or numerous recurrences. Therefore, the term chronic nonbacterial osteomyelitis has been used as an umbrella term and is inclusive of all the varied presentations of this disease.2
There are three disease patterns for CNO: a course that resolves within six months, a persistent course, and a course characterized by multifocal bone lesions and multiple recurrences.2,9,10 The multifocal recurrent disease pattern is most consistent with CRMO and SAPHO (Synovitis, Acne, Pustulosis, Hyperostosis, and Osteitis) syndrome.11
Etiology and Pathogenesis of CNO
By definition, the bone lesions seen in CNO are culture negative and have no demonstrable organism on histopathology.2–6 Antibiotic therapy should not cause resolution of symptoms. In a small case series, azithromycin was shown to improve radiological and clinical signs and symptoms of CNO.12 This effect may be mediated through the anti-inflammatory properties of azithromycin instead of its antimicrobial properties. Although, there have been many proposed pathogens causing CNO, there have been no definitive evidence of that microbes trigger CNO. Propionibacterium acnes has been recently proposed as a cause for CNO. This pathogen has been cultured in adults with palmoplantar pustulosis, a common skin manifestation seen in SAPHO and CNO. Rarely was this bacteria cultured when the pustules of patients with palmoplantar pustulosis and SAPHO or CNO were analyzed for Propionibacterium acnes.13 In one series of adult SAPHO patients, P. acnes was cultured from the bone from the 7 of 15 of patients tested.13 However, in the vast majority of patients with SAPHO and CNO, cultures of the bone are negative or when positive present felt to be a contaminant.
The chronic inflammation seen with CNO appears to be due to activation in the innate immune system as typically seen in autoinflammatory diseases.1 This seemingly unprovoked activation may leads to an imbalance of pro- and anti-inflammatory cytokines and therefore disruption in immune homeostasis. Currently, the etiology and pathophysiology of CNO are not firmly established in non-syndromic forms of the disease. However, there have been several developments in the possible pathophysiology mechanisms and genetic associations for this disease.
There is evidence that links the interleukin-10 (IL-10) pathway to the development of CNO. Hofmann et al., reported that peripheral blood monocytes stimulated with the TLR-4 agonist LPS secreted significantly less IL-10 compared to healthy control monocytes.14 This occurred independently of IL-10 promoter polymorphisms as an association with CNO and the high IL-10 expressing −1082G/G alleles and GCC haplotype14 was found. Since LPS stimulated CNO monocytes have a decreased production of IL-10, this is the opposite of what would be expected with the high expressing allele suggesting other mechanisms are involved.
Next, these investigators demonstrated that the decrease in IL-10 secretion from LPS-stimulated CNO monocytes is associated with attenuated extracellular-signal regulated kinase (ERK)1/2 activity.15 This decrement in ERK1/2 signaling then results in reduced levels of the transcription factor, Sp-1, a transcription factor that drives IL-10 gene expression in monocytes.15,16 They showed that Sp1 recruitment to the IL-10.636 Sp1 element is reduced in LPS-stimulated CNO monocytes. In addition, they found that histone H3 serine-10 phosphorylation (HS3S10p), an activating marker, is decreased around the IL-10-636 element of the IL-10 promoter.15,16 The attenuation of Sp1 and reduced histone H3 serine-10 phosphorylation suggest that epigenetic factors play a role in the decreased gene expression of IL-10 seen in CNO.15,16 A unified hypothesis has been proposed to explain these separate pathophysiology mechanisms in CNO. The investigators concluded that impaired MAPK signaling, decreased H3S10 phosphorylation, and attenuated Sp1 recruitment to the IL10 promoter results in impaired gene expression of IL-10 with subsequent disruption of the pro and anti-inflammatory cytokine balance. This disruption in immune homeostasis might explain part of the clinical presentation of CNO.16
There is increasing evidence for the theory that CNO is genetically driven. The two strongest pieces of evidence are that two similar diseases with autoinflammatory bone lesions are genetically driven and animal models with genetic defects have similar defects. Majeed syndrome and deficiency of the interleukin-1 receptor antagonist (DIRA) are genetically linked diseases with features that include autoinflammatory bone lesions. Majeed syndrome is caused by mutations in LPIN2 and DIRA with mutations in IL1RN.17–19
There have been many animal models with genetic defects leading to autoinflammatory bone lesions. Currently, there are reports of autoinflammatory bone lesions seen in mice, lemurs, and dogs. Two mice models have developed CNO - the cmo mice and the Lupo mice.20,21 Both of these murine models have a mutation in pstpip2 and present with similar clinical features as seen in humans. The cmo mice generally develop a clinically severe presentation.20 Pstpip2 acts in the cytosol as an F-actin associated phosphoprotein, interacts with PEST-type protein tyrosine phosphatases (PTP PEST) and is involved in cytoskeletal organization.22,23 Murine pstpip2 is similar to human PSTPIP1 and PSTPIP2. PSTPIP1 regulates the NLRP3 inflammasome through binding to pyrin and is the genetic defect seen in the autosomal dominant autoinflammatory syndrome pyogenic arthritis pyoderma gangrenosum acne (PAPA) syndrome.24
There is a possible genetic association locus at chromosome 18q21.3–18q22 and CRMO.25 This genetic association is not yet linked to the pathophysiological development of CNO. There have been cases of families with multiple affected members with CNO and cases with first- and second-degree relatives with inflammatory bowel disease, psoriasis, and other chronic inflammatory conditions. This provides additional evidence of a genetic association.2,5,6 Genetic mutations in PSTPIP1, PSTPIP2, CARD15/NOD2, and IL1RN have been examined in small series and do not appear to be the causative genetic feature in CNO.2,26,27
Epidemiology
CNO is primarily a disease of childhood. It has many similarities to SAPHO syndrome, which is a disorder primarily seen in adults. The incidence and prevalence of CNO is unknown. Although, a diversity of ethnicities and populations throughout the world is affected by CNO, the majority of reports are from Scandinavia, Europe, Australia and North America. There is a female predominance and the mean age of disease onset is around 10 years-old.2,5,6
Clinical Presentation
CNO is a diagnosis of exclusion and is established by the clinical presentation, imaging studies, and a culture negative bone biopsy. Pain with or without swelling at the site of the bony lesion is the typical presenting symptoms. Bone lesions tend to cluster around the metaphysis, can occur at atypical locations for bacterial osteomyelitis such as the clavicle, and when multifocal, often have a symmetric distribution.3,28 Seventy-five percent of bone lesions are perimetaphyseal.29 Appendicular and axial skeletal lesions are also seen. The most common CNO sites are the femur, tibia, pelvis, calcaneus, ankle, vertebrae, and clavicle.2,3,5,6 CNO is the most common disease etiology to affect the medial third of the clavicle in all age groups.28 A unifocal pattern of disease occurs in 10–20% of patients.5,6 CNO is a systemic disease that can affect skin, joints, gastrointestinal tract, and lungs. Patients with CNO frequently have other coexisting chronic inflammatory diseases.2,5,6,27 In one study, 20–50% of patients had or developed another autoimmune/inflammatory disease.2,5 The most frequent associated autoimmune and/or inflammatory diseases included arthritis, psoriasis, inflammatory bowel disease, vasculitis, myositis/fasciitis and parotitis. Typically, these patients have more bony lesions than patients without a comorbid inflammatory disease.5 CNO patients tend to have normal, mild or moderately elevated inflammatory laboratory changes. Markers of inflammation including ESR, CRP, WBC and platelet count can be moderately increased or completely normal.2–6,30 Inflammatory markers are typically higher in patients with comorbid autoimmune diseases.5
Imaging Studies
Radiographs, technetium bone scans, and/or MRI are generally used to detect the lesions and screen for multifocality.28,31,32 Computerized tomography (CT) can detect lesions but exposes children to significant levels of radiation so is generally not recommended. Typical radiographic findings include a lytic lesion at or around the metaphysis that progress to sclerosis or hyperostosis.28 Recently whole-body MRI imaging has been studied as a radiation-free method of imaging.31,33 Whole-body MRI imaging is a useful method to detect asymptomatic lesions without radiation exposure. The lesions are best seen on short tau inversion recovery sequences (STIR) and can be utilized to identify subclinical spinal involvement, synovitis of adjacent joints and sacroiliitis. Whole-body MRI tends to poorly detect lesions in the small joints of the hands and feet, ribs, sternoclavicular and costovertebral joint junctions. There has never been a study to compare bone scintigraphy and whole-body MRI. Whole-body MRI should be considered in working up indeterminate cases of CNO and as an imaging technique used to monitor disease activity and response to therapy.
Histopathology
A bone biopsy is often needed to confirm a diagnosis of CNO.2,4 This is especially necessary in isolated bone lesions since bone malignancies can mimic CNO. In children who present with multifocal disease for many months in duration, the need for a confirmatory bone biopsy is debatable.4,30 The presence of clavicular involvement with palmar-plantar pustulosis or psoriasis vulgaris is very strong evidence of CNO. However, caution should be used in the absence of strong supporting evidence of CNO as serious disorders such as intraosseous lymphoma and other forms of neoplasia can mimic CNO.34 A classification criteria and clinical algorithm has been proposed to aid the clinician in the diagnosis of CNO.2,4
CNO is characterized by subacute or chronic inflammation, with a lymphocytic or mixed inflammatory infiltrate, and often marrow fibrosis.3,9 By definition, the biopsy is culture negative and has no demonstrable organism on histopathology.
Treatment
NSAIDs are the gold standard initial therapy for CNO.2,26,27,35 However, there is a discrepancy in the literature regarding the efficacy of NSAIDs in the treatment of CNO. In 2 published studies from Germany, Beck et al. demonstrated a complete response to naproxen in 43% of the patients26 whereas Girschick et al. noted that while 100% of patients with a non-relapsing course responded to naproxen only 42% of patients with a relapsing course responded.9 Indomethacin might be more effective than naproxen but is associated with more side effects.36 Case studies and small case series have been published addressing the treatment of CNO with various medications including corticosteroids and DMARDs (MTX and sulfasalazine).37 Due to small numbers there is no conclusion about the efficacy of these agents.
TNFα appears to be an important cytokine in CNO. This cytokine plays an important role in the activation of osteoclasts in CNO.2,14 There have been several case reports, small retrospective series, and a few larger series on TNFα antagonist treatment in CNO.5,38,39 In a larger series involving 11 patients on TNFα antagonists, 10 of the 11 responded to the therapy and there was a 46% remission rate.5 Smaller case series have also showed a favorable response to TNFα antagonists in the treatment of CNO. Eleftheriou et al. evaluated 3 pediatric patients treated TNFα blocking agents with CRMO or SAPHO and all 3 showed a clinical improvement.38 However, 1 patient stopped therapy prematurely due to an invasive fungal infection. Catalano-Pons et al. published the results from a French dataset cohort of 40 pediatric cases of which 2 had used TNFα antagonists although treatment response was not thoroughly detailed.6 Other biologic medications have been used in case reports that include INF-α, INF-γ, and anakinra. The case report on anakinra initially showed a response but the response was not sustained.38
Pamidronate have also been recently used for the treatment of CNO.40–44 This therapy is hypothesized to treat CNO by inactivating osteoclasts, decreasing pain, and possibly through an anti-inflammatory mechanism. In one study, all 9 patients treated responded to pamidronate.43 The clinical response typically occurred in the first 3 days. Four patients experienced a recurrence in their CNO 12–18 months after their first pamidronate course. All 4 patients responded to a repeat course of pamidronate. In another study evaluating response to pamidronate, 4 of 5 patients showed clinical improvement.41 In a third study, 6 of the 7 patients improved with pamidronate. However, synovial joint disease was unresponsive to the therapy.42 Pamidronate therapy may be particular beneficial in spinal lesions and vertebral fractures by improving vertebral shape and a decreasing the kyphotic angle.42,44 Hospach et al. reported on patients with axial disease, and found that all 7 had an improvement in spinal lesions after pamidronate therapy.44 Urinary N-telopeptise/urine creatinine (uNTX/uCr), a marker of collagen-I breakdown, has been proposed as a marker for disease flare after bisphosphonate therapy.43 This marker has used to monitor disease with accelerated bone turnover. When used in cases with CNO, no clinically evident relapses occurred while uNTX/uCr was suppressed. Concerns have been raised about using bisphosphonates in the pediatric population since long-term safety data is limited. The most common adverse events are minor flu-like symptoms for a day after the infusion. Currently, osteonecrosis of the jaw is a possible but is an unreported side effect of pamidronate use in children with CNO. This adverse event primarily occurs in elderly patients with myeloma. Due to this potential side effect, it is recommended to have pediatric patients have a dental screening and wisdom teeth extraction prior to pamidronate therapy whenever possible and postpone elective dental procedures for at least 6 months following therapy.43
No study has compared the efficacy of bisphosphonates to TNFα antagonists. There have been two larger observational studies that had treated patients with either bisphosphonates or TNFα antagonists (3 and 2 in one cohort and 3 and 1 in the other cohort) but no data was included on the efficacy of these therapies.2,6 Figure 1.
Figure 1.
Prognosis
In North America, CNO continues to be difficult to treat with high rates of treatment nonresponders. In a study, only 43% of patients were in remission 22 months after diagnosis and only 13% were in remission off therapy.5 In general, European cohorts appear to have more favorable outcomes.2,3 Functional and cosmetic consequences of hyperostosis are frequently seen.2 Pathologic fractures are common and most frequently occur in the vertebrae.2,3 Scoliosis, bony overgrowth, and generalized growth failure are possible long term morbidities associated with CNO.2,5,6 Table 2.
Table 2.
| Common Morbidities Associated with CNO |
|---|
| Pathologic Fractures |
| Scoliosis |
| Bony Overgrowth/ Hyperostosis |
| Generalized Growth Failure |
Conclusion
CNO is a diagnosis of exclusion that is established by the clinical presentation, imaging studies, and a culture negative bone biopsy. Recently, there have been several advancements in the understanding the etiology and genetic links for CNO. Treatment remains challenging. TNFα antagonist and bisphosphonates appear to be possible second line agents for CNO. There are high rates of pathologic fractures and morbidity when untreated.
Distinct Genetic Autoinflammatory Bone Syndromes
Majeed Syndrome
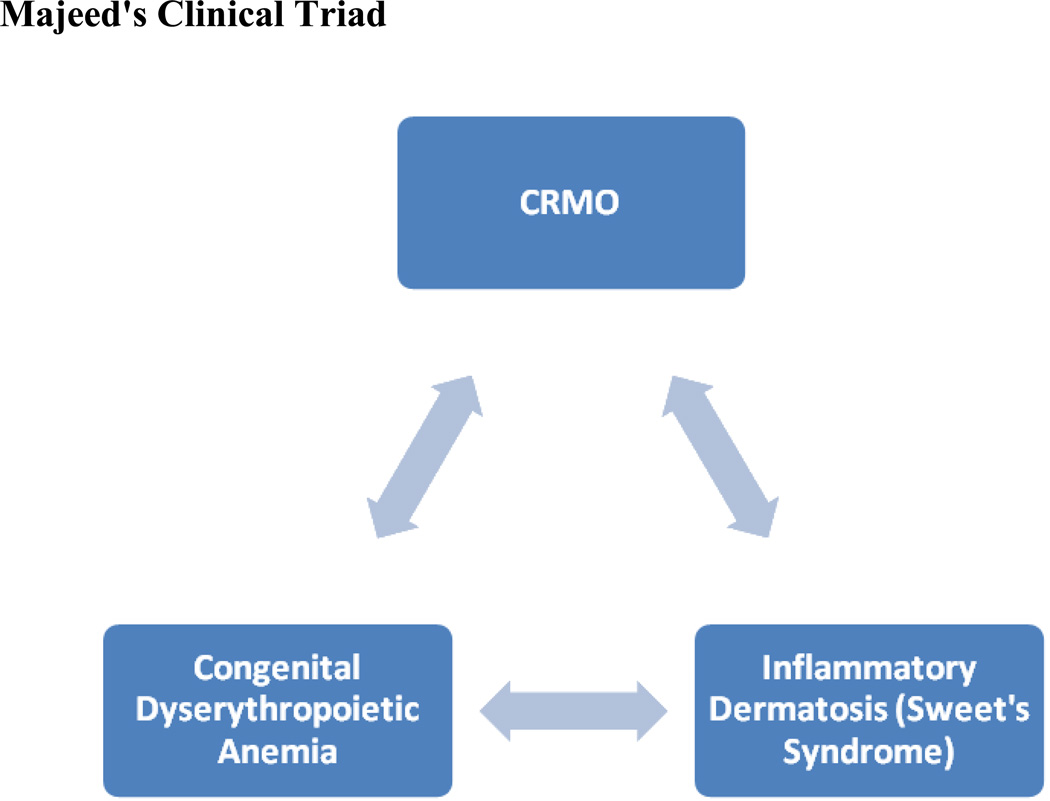
Majeed Syndrome is a rare autosomal recessive disorder first described by Majeed in 1989 that is characterized by a clinical triad of features: CRMO, congenital dyserythropoietic anemia, and inflammatory dermatosis.45,46 The disease course of Majeed syndrome differs from CNO in that it is more severe, has an earlier age of onset, and is associated with congenital dyserythropoietic anemia (CDA) and inflammatory dermatosis.45,46 Figure 2.
Figure 2.
Majeed's Clinical Triad
The disease onset of Majeed syndrome is in infancy. This disease is associated with high course in fevers, severe pain, chronic anemia, and soft tissue swelling that typically affects large joints. Frequent exacerbations, as often as 3 to 4 per month, each lasting a few days with short remissions, characterize disease Majeed syndrome.46,47 The distribution of the CRMO is similar to CNO in that it also tends to affect the metaphyses of long bones.46,48 The inflammatory neutrophilic dermatosis, Sweet’s syndrome, is the associated skin manifestation.45–47 The CDA seen with Majeed syndrome is variable in its presentation and severity and different from other known forms of CDA. This microcytic anemia typically appears by 9 months of age. Children with Majeed syndrome often have multiple complications and poor outcomes that include growth retardation, failure to thrive, hepatomegaly, joint contractures and muscle atrophy.45–48 Neonatal cholestatic jaundice and mild neutropenia were seen in one patient.48
LPIN2 is the mutation responsible for Majeed syndrome and encodes LIPIN2.48,49 LIPIN2 is part of the LIPIN family. This protein family plays a role in glycerolipid biosynthesis by acting as phosphatidate phosphatase (PAP).50,51 Homozygous mutations in LPIN2 have been found in 4 families with four unique mutations: a missense mutation (S734L), a frame shift mutation (T180fs), a splice site mutation (R776Sfs), 2-base pair deletion in LPIN2 (c.1312_1313delCT; p.Leu438fs+16X).52 LPIN2 is expressed in multiple different organs: liver, lung, kidney, placenta, spleen, thymus, lymph node, prostate, testes, small intestine, and colon.49 Mutations in LPIN1 in mice cause lipodystrophy, fatty liver, hypertriglyceridemia, glucose intolerance, peripheral neuropathy, and atherosclerosis. The LPIN2 mutation in humans does not appear to produce lipid abnormalities.48 Recently, it has been suggested that the phenotypic picture seen with Majeed syndrome results from a loss of PAP activity in LIPIN2.50 In an in vitro model, the mutation of serine to leucine at amino acid 734 (S734L) is required for appropriate PAP function. This mutation appears to impact the PAP activity without affecting the other lipid and metabolic functions of lipin2. It is still unclear how dysfunction of LIPIN2 produces all of the phenotypic features of Majeed syndrome however; Valdearcos et al. recently demonstrated that LPIN2 dampens the pro-inflammatory signaling that is produced by macrophages when exposed to excessive saturated fatty acids.53
Majeed syndrome can be difficult to treat. Typically, NSAIDs and oral corticosteroids have been used with variable success.47 Recently, two brothers failed treatment with a TNF inhibitor but treatment with IL-1 beta blockade resulted in clinical, laboratory, and radiologic improvement.52 This supports the hypothesis that Majeed syndrome is an autoinflammatory syndrome.
Deficiency of the Interleukin-1 Receptor Antagonist
Deficiency of the Interleukin-1 Receptor Antagonist (DIRA) is a newly recognized autosomal recessive autoinflammatory disorder.54–57 Prose et al. described a child with what was likely DIRA in 1994 and it was recognized as a distinct syndrome in 2009.57–59 DIRA is due to a mutation in the ILIRN, is potentially life threatening and can mimic neonatal sepsis.56,57 A high index of suspicion is critical to prevent multisystem organ damage and death.
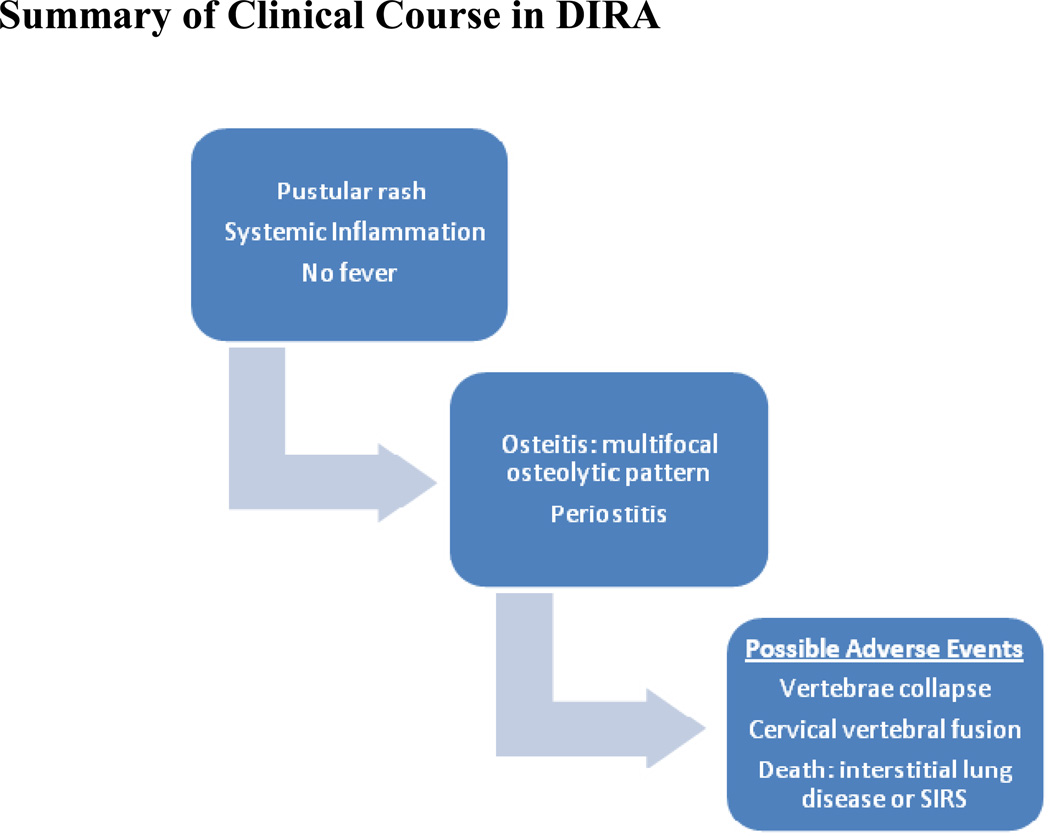
The characteristic clinical presentation includes generalized pustulosis, osteitis, periostitis, and systemic inflammation.47,54–57,59 Within the first few weeks after birth, a pustular rash and systemic signs of inflammation develop.54,56,57,59 Even though systemic inflammatory markers are markedly elevated, fever is usually absent.54,56,57,59 Weeks after the rash presents, osteitis is detected.54,56,57 The osteitis seen in DIRA is severe with extensive bone involvement, a multifocal osteolytic pattern of disease, and marked periostitis.54,56,57,59 These bony lesions typically affect long bones, vertebral bodies, and have a predilection for the proximal femur. The bone biopsy in DIRA is characterized by a purulent osteomyelitis that is culture negative with fibrosis and sclerosis.57 Three radiographic characteristic findings are typically seen that include widening of the anterior rib ends, multifocal osteolytic lesions, and periosteal elevation of the long bones.54,56,57,59 Other less common findings include heterotopic ossification of the proximal femurs, widening of the clavicles, metaphyseal osteolytic areas of long bones, and osteolytic skull lesions.57 As with CNO vertebral involvement and morbidities are common. Collapse of the vertebrae due to osteolytic lesions can occur and cause cervical vertebral fusion.54,57 Figure 3.
Figure 3.
Summary of Clinical Course in DIRA
DIRA is due to a mutation in the ILIRN. There are presently 6 known mutations in the ILIRN.54,56,57,59 The most common mutation is E77X. Other mutations seen are N52KfsX25, Q54X, D72_176del, T47Tfs, 175-kb deletion on chromosome 2q13. All but 1 child had homozygous mutations in the gene.54,57,59 One child was a compound heterozygote (E77X and T47TfsX4).60 The 75 kb chromosomal deletion on chromosome 2q13 includes ILIRN gene and 5 other IL1 family members.57,59 The genetic understanding of this disease has enabled improved outcomes due to treatment of IL-1 blocking agents such as anakinra.47,55,61 Prior to the use of anakinra there was a 33% mortality rate.47 These children died of systemic inflammatory response syndrome (SIRS) early in life (n=2) or pulmonary interstitial disease in childhood (n=1). Long-term outcome data of children treated with anakinra is unknown.
Cherubism
Cherubism is an autosomal dominant autoinflammatory bone disorder affecting the maxilla and mandible.62–65 The bony changes of the jaw give these children’s faces a chubby cheeked appearance; hence the disorder was named after their likeness to cherubs depicted in Renaissance art. Children present in childhood (2–7years) with a large multilocular, cystic lesion of the mandible or less commonly the maxilla.62–65 Similar multilocular cysts can be found in Noonan syndrome and is considered part of the Noonan spectrum.66
Cherubism is an autoinflammatory disorder that is driven by two mechanisms - macrophage activity leading to high levels of TNF-α with subsequent inflammation, and osteoclast activation causing excessive bone resorption.66 In 2001, three heterozygous missense mutations in the SH3 binding protein 2 (SH3BP2) were identified: primarily affecting amino acid 415, 418, and 420.66 Since that time, three additional mutations were identified: amino acid 420, 418, and 419.66 SH3BP2 is an adapter protein involved in innate and adaptive immune system signaling through interacting and forming complexes with binding and scaffolding proteins.66 In various immune cells especially osteoclasts, SH3BP2 can cause phosphorylation and therefore affect signal pathways.66 Mutations in this regulator cause uncontrolled bone resorption of the jaw. This genetic locus is commonly deleted in bladder cancer and contained within the locus for Wolf-Hirschhorn syndrome: a syndrome characterized by craniofacial malformations, intellectual disability, muscle hypotonia, and heart defects.66
Cherubism continues to be difficult to treat. Recently, 2 patients have been unsuccessfully treated with adalimumab.67 There has been another recent case-report of a patient receiving adalimumab and oral bisphosphonate with no clinical improvement. However, this child was only treated with these therapies for a short period of time.68
Synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome in childhood
SAPHO syndrome is an autoinflammatory disease that affects both skin and bones which has been diagnosed primarily in adults.69,70 The bone manifestations seen in SAPHO are generally described as CRMO. Palmoplantar pustulosis, severe acne, or psoriasis are the typical skin manifestations and have a neutrophilic predominance.69–71 Recently, SAPHO has also been described in childhood. It is unclear if it is a distinct entity or is a more severe form of CRMO. The etiology for SAPHO remains unknown. Recent studies suggest the polymorphonuclear cell dysfunction could be a possible cause of SAPHO. LPIN2, PSTPIP2, and NOD2 mutations do not appear to be associated with SAPHO.72 Recently a child and mother were reported who both had a clinical presentation of SAPHO and had abnormal PMN intracellular production of reactive oxygen species.73 It has been proposed that P. acnes might stimulate IL-8 and IL-18 release by PMNs leading to the pathogenesis of SAPHO.74
First line treatment of childhood SAPHO is typically NSAIDs.71 Other agents that have been used are methotrexate, oral corticosteroids, colchicine, and sulfasalazine. Ben Abdelghani et al. studied 6 adults treated with TNF-α antagonist for SAPHO syndrome and 66.6% had a beneficial? response.75 Bisphosphonates have also been used. In a recent case series, all 7 patients had a marked clinical improvement with pamidronate.76 In this study, some children were classified as SAPHO without classic skin manifestations and may have better been classified as CRMO.
Juvenile Mandibular Chronic Osteomyelitis
Diffuse sclerosing osteomyelitis is a sclerosing osteomyelitis of the jaw first described by Carl Garré in 1893.77 Recently, the name juvenile mandibular chronic osteomyelitis (JMCO) has been used to describe this disease in children.77 The mean age of presentation of JMCO is 13 years.77 JMCO is characterized by a mixed osteolytic and sclerotic process that can cause mandibular nerve canal enlargement and adjacent soft tissue involvement.77 Typically unilateral mandible involvement is seen.77 Similar findings can occur in patients with jaw involvement in typical CRMO. It is unclear if these are the same disease entity, i.e. JMCO is CNO of the mandible, or they are distinct clinical entities.
Conclusions
CNO is an autoinflammatory bone disease that is culture negative and has no demonstrable organism on histopathology. It is a systemic disease that affects many organ systems in addition to bones and has high rates of comorbid autoinflammatory/autoimmune diseases. This disease can also be part of a larger genetic syndrome such as seen with DIRA and Majeed syndrome. Recently, TNF-α antagonists and pamidronate has shown great promise in treating CNO in NSAID resistant disease. Early control is essential to decrease morbidities such as pathologic fractures and scoliosis.
Key Points.
Chronic non-bacterial osteomyelitis (CNO); synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome; Majeed syndrome; deficiency of interleukin-1 receptor antagonist (DIRA); cherubism; and Juvenile Mandibular Chronic Osteomyelitis (JMCO) are autoinflammatory bone diseases.
Autoinflammatory bone diseases are innate immune system activation disorders.
The bone inflammation in these syndromes is characterized by a subacute or chronic inflammation that is culture negative and has no demonstrable organism on histopathology.
Anti-inflammatory medications are typically used as first line therapies in CNO. Recently bisphosphonates and TNFα antagonists have been used for second line therapies to prevent pathologic fractures, pain, and disease relapse.
Majeed syndrome, deficiency of interleukin-1 receptor antagonist (DIRA), and cherubism are distinct genetic autoinflammatory bone syndromes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Masters SL, Simon A, Aksentijevich I, et al. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*) Annu Rev Immunol. 2009;27:621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jansson A, Renner ED, Ramer J, et al. Classification of non-bacterial osteitis: retrospective study of clinical, immunological and genetic aspects in 89 patients. Rheumatology (Oxford) 2007;46:154–160. doi: 10.1093/rheumatology/kel190. [DOI] [PubMed] [Google Scholar]
- 3.Girschick HJ, Zimmer C, Klaus G, et al. Chronic recurrent multifocal Osteomyelitis: what is it and how should it be treated? Nat Clin Pract Rheumatol. 2007;3:733–738. doi: 10.1038/ncprheum0653. [DOI] [PubMed] [Google Scholar]
- 4.Jansson AF, Müller TH, Gliere L, et al. Clinical score for nonbacterial osteitis in children and adults. Arthritis Rheum. 2009;60:1152–1159. doi: 10.1002/art.24402. [DOI] [PubMed] [Google Scholar]
- 5.Borzutzky A, Stern S, Reiff A, et al. Pediatric chronic nonbacterial osteomyelitis. Pediatrics. 2012;130:e1190–e1197. doi: 10.1542/peds.2011-3788. [DOI] [PubMed] [Google Scholar]
- 6.Catalano-Pons C, Comte A, Wipff J, et al. Clinical outcome in children with chronic recurrent multifocal osteomyelitis. Rheumatology (Oxford) 2008;47:1397–1399. doi: 10.1093/rheumatology/ken249. [DOI] [PubMed] [Google Scholar]
- 7.Giedion A, Holthusen W, Masel LF, et al. Subacute and chronic “symmetrical“ osteomyelitis. Ann. Radiol (Paris) 1972;15:329–342. [PubMed] [Google Scholar]
- 8.Probst FP, Bjorksten B, Gustavson KH. Radiological aspect of chronic recurrent multifocal osteomyelitis. Ann Radiol (Paris) 1978;21:115–125. [PubMed] [Google Scholar]
- 9.Girschick HJ, Raab P, Surbaum S, et al. Chronic non-bacterial osteomyelitis in children. Ann Rheum Dis. 2005;64:279–285. doi: 10.1136/ard.2004.023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gikas PD, Islam L, Aston W, et al. Nonbacterial osteitis: a clinical, histopathological, and imaging study with a proposal for protocol-based management of patients with this diagnosis. J Orthop Sci. 2009;14:505–516. doi: 10.1007/s00776-009-1381-4. [DOI] [PubMed] [Google Scholar]
- 11.Beretta-Piccoli BC, Sauvain MJ, Gal I, et al. Synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome in childhood: a report of ten cases and review of the literature. Eur J Pediatr. 2000;159:594–601. doi: 10.1007/s004310000500. [DOI] [PubMed] [Google Scholar]
- 12.Schilling F, Wagner AD. Azithromycin: an anti-inflammatory effect in chronic recurrent multifocal osteomyelitis? A preliminary report. Z Rheumatol. 2000;59:352–353. doi: 10.1007/s003930070059. [DOI] [PubMed] [Google Scholar]
- 13.Edlund E, Johnsson U, Lidgren L, et al. Palmoplantar pustulosis and sternocostoclavicular arthro-osteitis. Ann. Rheum. Dis. 1988;47:809–815. doi: 10.1136/ard.47.10.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann SR, Schwarz T, Möller JC, et al. Chronic non-bacterial osteomyelitis is associated with impaired Sp1 signaling, reduced IL10 promoter phosphorylation, and reduced myeloid IL-10 expression. Clin Immunol. 2011;141:317–327. doi: 10.1016/j.clim.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann SR, Morbach H, Schwarz T, et al. Attenuated TLR4/MAPK signaling in monocytes from patients with CRMO results in impaired IL-10 expression. Clin Immunol. 2012;145:69–76. doi: 10.1016/j.clim.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann SR, Roesen-Wolff A, Hahn G, et al. Update: Cytokine Dysregulation in Chronic Nonbacterial Osteomyelitis (CNO) Int J Rheumatol. 2012;2012:310206. doi: 10.1155/2012/310206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson PJ, Chen S, Tayeh MK, et al. Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anemia (Majeed syndrome) J Med Genet. 2005;42:551–557. doi: 10.1136/jmg.2005.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aksentijevich I, Masters SL, Ferguson PJ, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438–2444. doi: 10.1056/NEJMoa0809568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferguson PJ, Bing X, Vasef MA, et al. A missense mutation in pstpip2 is associated with the murine autoinflammatory disorder chronic multifocal osteomyelitis. Bone. 2006;38:41–47. doi: 10.1016/j.bone.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grosse J, Chitu V, Marquardt A, et al. Mutation of mouse Mayp/Pstpip2 causes a macrophage autoinflammatory disease. Blood. 2006;107:3350–3358. doi: 10.1182/blood-2005-09-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeung YG, Soldera S, Stanley ER. A novel macrophage actin-associated protein (MAYP) is tyrosine-phosphorylated following colony stimulating factor-1 stimulation. J Biol Chem. 1998;273:30638–30642. doi: 10.1074/jbc.273.46.30638. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Dowbenko D, Lasky LA. PSTPIP 2, a second tyrosine phosphorylated, cytoskeletal-associated protein that binds a PEST-type protein-tyrosine phosphatase. J Biol Chem. 1998;273:30487–30496. doi: 10.1074/jbc.273.46.30487. [DOI] [PubMed] [Google Scholar]
- 24.Shoham NG, Centola M, Mansfield E, et al. Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc Natl Acad Sci USA. 2003;100:13501–13506. doi: 10.1073/pnas.2135380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golla A, Jansson A, Ramser J, et al. Chronic recurrent multifocal osteomyelitis (CRMO): evidence for a susceptibility gene located on chromosome 18q21.3–18q22. Eur J Hum. Genet. 2002;10:217–221. doi: 10.1038/sj.ejhg.5200789. [DOI] [PubMed] [Google Scholar]
- 26.Beck C, Morbach H, Beer M, et al. Chronic nonbacterial Osteomyelitis in childhood: prospective follow-up during the first year of anti-inflammatory treatment. Arthritis Res Ther. 2010;12:R74. doi: 10.1186/ar2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morbach H, Dick A, Beck C, et al. Association of chronic non-bacterial Osteomyelitis with Crohn’s disease but not with CARD15 gene variants. Rheumatol Int. 2010;30:617–621. doi: 10.1007/s00296-009-1029-x. [DOI] [PubMed] [Google Scholar]
- 28.Khanna G, Sato TS, Ferguson P. Imaging of chronic recurrent multifocal Osteomyelitis. Radiographics. 2009;29:1159–1177. doi: 10.1148/rg.294085244. [DOI] [PubMed] [Google Scholar]
- 29.Mandell GA, Contreras SJ, Conard K, et al. Bone scintigraphy in the detection of chronic recurrent multifocal osteomyelitis. J Nucl Med. 1998;39:1778–1783. [PubMed] [Google Scholar]
- 30.Wipff J, Adamsbaum C, Kahan A, et al. Chronic recurrent multifocal osteomyelitis. Joint Bone Spine. 2011;78:555–560. doi: 10.1016/j.jbspin.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Fritz J, Tzaribatchev N, Claussen CD, et al. Chronic recurrent multifocal Osteomyelitis: comparison of whole-body MR imaging with radiography and correlation with clinical and laboratory data. Radiology. 2009;252:842–851. doi: 10.1148/radiol.2523081335. [DOI] [PubMed] [Google Scholar]
- 32.Jurik AG, Egund N. MRI in chronic recurrent multifocal Osteomyelitis. Skeletal Radiol. 1997;26:230–238. doi: 10.1007/s002560050227. [DOI] [PubMed] [Google Scholar]
- 33.Guérin-Pfyffer S, Guillaume-Czitrom S, Tammam S, et al. Evaluation of chronic recurrent multifocal osteitis in children by whole-body magnetic resonance imaging. Joint Bone Spine. 2012;79:616–620. doi: 10.1016/j.jbspin.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Sato TS, Ferguson PJ, Khanna G. Primary multifocal osseous lymphoma in a child. Pediatr Radiol. 2008;38:1338–1341. doi: 10.1007/s00247-008-0964-0. [DOI] [PubMed] [Google Scholar]
- 35.Ferguson PJ, El-Shanti HI. Autoinflammatory bone disorders. Curr Opin Rheumatol. 2007;19:492–498. doi: 10.1097/BOR.0b013e32825f5492. [DOI] [PubMed] [Google Scholar]
- 36.Abril JC, Ramirez A. Successful treatment of chronic recurrent multifocal osteomyelitis with indomethacin: a preliminary report of five cases. J Pediatr Orthop. 2007;27:587–591. doi: 10.1097/BPO.0b013e318070cbd3. [DOI] [PubMed] [Google Scholar]
- 37.Twilt M, Laxer RM. Clinical care of children with sterile bone inflammation. Curr Opin Rheumatol. 2011;23:424–431. doi: 10.1097/BOR.0b013e328349c363. [DOI] [PubMed] [Google Scholar]
- 38.Eleftheriou D, Gerschman T, Sebire N, et al. Biologic therapy in refractory chronic non-bacterial osteomyleitis in childhood. Rheumatology (Oxford) 2010;49:1505–1512. doi: 10.1093/rheumatology/keq122. [DOI] [PubMed] [Google Scholar]
- 39.Deutschmann A, Mache CJ, Bodo K, et al. Successful treatment of chronic recurrent multifocal Osteomyelitis with tumor necrosis factor-alpha blockage. Pediatrics. 2005;116:1231–1233. doi: 10.1542/peds.2004-2206. [DOI] [PubMed] [Google Scholar]
- 40.Marangoni RG, Halpern AS. Chronic recurrent multifocal osteomyelitis primarily affecting the spine treated with anti-TNF therapy. Spine (Phila Pa 1976) 2010;35:E253–E256. doi: 10.1097/BRS.0b013e3181c09601. [DOI] [PubMed] [Google Scholar]
- 41.Simm PJ, Allen RC, Zacharin MR. Bisphosphonate treatment in chronic recurrent multifocal osteomyelitis. J Pediatr. 2008;152:571–575. doi: 10.1016/j.jpeds.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 42.Gleeson H, Wiltshire E, Briody J, et al. Childhood chronic recurrent multifocal osteomyelitis: pamidronate therapy decreases pain and improves vertebral shape. J Rheumatol. 2008;35:707–712. [PubMed] [Google Scholar]
- 43.Miettunen PM, Wei X, Kaura D, et al. Dramatic pain relief and resolution of bone inflammation following pamidronate in 9 pediatric patients with persistent chronic recurrent multifocal osteomyelitis (CRMO) Pediatr Rheumatol Online J. 2009;7:2. doi: 10.1186/1546-0096-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hospach T, Langendoerfer M, von Kalle T, et al. Spinal involvement in chronic recurrent multifocal osteomyelitis (CRMO) in childhood and effect of pamidronate. Eur J Pediatr. 2010;169:1105–1111. doi: 10.1007/s00431-010-1188-5. [DOI] [PubMed] [Google Scholar]
- 45.Majeed HA, Kalaawi M, Mohanty D, et al. Congenital dyserythropoietic anemia and chronic recurrent multifocal osteomyelitis in three related children and the association with Sweet syndrome in two siblings. J Pediatr. 1989;115:730–734. doi: 10.1016/s0022-3476(89)80650-x. [DOI] [PubMed] [Google Scholar]
- 46.Majeed HA, Al-Tarawna M, El-Shanti H, et al. The syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia. Report of a new family and a review. Eur J Pediatr. 2001;160:705–710. doi: 10.1007/s004310100799. [DOI] [PubMed] [Google Scholar]
- 47.Ferguson PJ, Sandu M. Current understanding of the pathogenesis and management of chronic recurrent multifocal osteomyelitis. Curr Rheumatol Rep. 2012;14:130–141. doi: 10.1007/s11926-012-0239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Mosawi ZS, Al-Saad KK, Ijadi-Maghsoodi R, et al. A splice site mutation confirms the role of LPIN2 in Majeed syndrome. Arthritis Rheum. 2007;56:960–964. doi: 10.1002/art.22431. [DOI] [PubMed] [Google Scholar]
- 49.Ferguson PJ, Chen S, Tayeh MK, et al. Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome) J Med Genet. 2005;42:551–557. doi: 10.1136/jmg.2005.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donkor J, Zhang P, Wong S, et al. A conserved serine residue is required for the phosphatidate phosphatase activity but not the transcriptional coactivator functions of lipin-1 and lipin-2. J Biol Chem. 2009;23(284):29968–29978. doi: 10.1074/jbc.M109.023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donkor J, Sariahmetoglu M, Dewald J, et al. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J Biol Chem. 2007;282:3450–3457. doi: 10.1074/jbc.M610745200. [DOI] [PubMed] [Google Scholar]
- 52.Herlin T, Fiirgaard B, Bjerre M, et al. Efficacy of anti-IL-1 treatment in Majeed syndrome. Ann Rheum Dis. 2013;72:410–413. doi: 10.1136/annrheumdis-2012-201818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valdearcos M, Esquinas E, Meana C, et al. Lipin-2 reduces proinflammatory signaling induced by saturated fatty acids in macrophages. J Biol Chem. 2012;287:10894–10904. doi: 10.1074/jbc.M112.342915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jesus AA, Osman M, Silva CA, et al. A novel mutation of IL1RN in the deficiency of interleukin-1 receptor antagonist syndrome: description of two unrelated cases from Brazil. Arthritis Rheum. 2011;63:4007–4017. doi: 10.1002/art.30588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Altiok E, Aksoy F, Perk Y, et al. A novel mutation in the interleukin-1 receptor antagonist associated with intrauterine disease onset. Clin Immunol. 2012;145:77–81. doi: 10.1016/j.clim.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schnellbacher C, Ciocca G, Menendez R, et al. Deficiency of Interleukin-1 Receptor Antagonist Responsive to Anakinra. Pediatr Dermatol. 2012 doi: 10.1111/j.1525-1470.2012.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aksentijevich I, Masters SL, Ferguson PJ, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prose NS, Fahrner LJ, Miller CR, et al. Pustular psoriasis with chronic recurrent multifocal osteomyelitis and spontaneous fractures. J Am Acad Dermatol. 1994;31:376–379. doi: 10.1016/s0190-9622(94)70176-8. [DOI] [PubMed] [Google Scholar]
- 59.Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438–2444. doi: 10.1056/NEJMoa0809568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stenerson M, Dufendach K, Aksentijevich I, et al. The first reported case of compound heterozygous IL1RN mutations causing deficiency of the interleukin-1 receptor antagonist. Arthritis Rheum. 2011;63:4018–4022. doi: 10.1002/art.30565. [DOI] [PubMed] [Google Scholar]
- 61.Ter Haar N, Lachmann H, Ozen S, et al. Treatment of autoinflammatory diseases: results from the Eurofever Registry and a literature review. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2011-201268. [DOI] [PubMed] [Google Scholar]
- 62.Ueki Y, Tiziani V, Santanna C, et al. Mutations in the gene encoding c-Abl-binding protein SH3BP2 cause cherubism. Nat Genet. 2001;28:125–126. doi: 10.1038/88832. [DOI] [PubMed] [Google Scholar]
- 63.Tiziani V, Reichenberger E, Buzzo CL, et al. The gene for cherubism maps to chromosome 4p16. Am J Hum Genet. 1999;65:158–166. doi: 10.1086/302456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones WA, Gerrie J, Pritchard J. Cherubism: familial fibrous dysplasia of the jaws. J Bone Joint Surg Br. 1950;32-B:334–347. doi: 10.1302/0301-620X.32B3.334. [DOI] [PubMed] [Google Scholar]
- 65.Mangion J, Rahman N, Edkins S, et al. The gene for cherubism maps to chromosome 4p16.3. Am J Hum Genet. 1999;65:151–157. doi: 10.1086/302454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reichenberger EJ, Levine MA, Olsen BR, et al. The role of SH3BP2 in the pathophysiology of cherubism. Orphanet J Rare Dis. 2012;7:S5. doi: 10.1186/1750-1172-7-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hero M, Suomalainen A, Hagström J, et al. Anti-tumor necrosis factor treatment in cherubism--clinical, radiological and histological findings in two children. Bone. 2013;52:347–353. doi: 10.1016/j.bone.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Pagnini I, Simonini G, Mortilla M, et al. Ineffectiveness of tumor necrosis factor-alpha inhibition in association with bisphosphonates for the treatment of cherubism. Clin Exp Rheumatol. 2011;29:147. [PubMed] [Google Scholar]
- 69.Chamot AM, Benhamou CL, Kahn MF, et al. Acne-pustulosis-hyperostosis-osteitis syndrome: results of a national survey. 85 cases. Rev Rhum Mal Osteoartic. 1987;54:187–196. [PubMed] [Google Scholar]
- 70.Benhamou CL, Chamot AM, Kahn MF. Synovitis-acne-pustulosis hyperostosis-osteomyelitis syndrome (SAPHO): a new syndrome among the spondyloarthropathies? Clin. Exp. Rheumatol. 1988;6:109–112. [PubMed] [Google Scholar]
- 71.Beretta-Piccoli BC, Sauvain MJ, Gal I, et al. Synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome in childhood: a report of ten cases and review of the literature. Eur J Pediatr. 2000;159:594–601. doi: 10.1007/s004310000500. [DOI] [PubMed] [Google Scholar]
- 72.Hurtado-Nedelec M, Chollet-Martin S, Chapeton D, et al. Genetic susceptibility factors in a cohort of 38 patients with SAPHO syndrome: a study of PSTPIP2, NOD2, and LPIN2 genes. J Rheumatol. 2010;37:401–409. doi: 10.3899/jrheum.090456. [DOI] [PubMed] [Google Scholar]
- 73.Ferguson PJ, Lokuta MA, El-Shanti HI, et al. Neutrophil dysfunction in a family with a SAPHO syndrome-like phenotype. Arthritis Rheum. 2008;58:3264–3269. doi: 10.1002/art.23942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hurtado-Nedelec M, Chollet-Martin S, Nicaise-Roland P, et al. Characterization of the immune response in the synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome. Rheumatology (Oxford) 2008;47:1160–1167. doi: 10.1093/rheumatology/ken185. [DOI] [PubMed] [Google Scholar]
- 75.Ben Abdelghani K, Dran DG, Gottenberg JE, et al. Tumor necrosis factor-alpha blockers in SAPHO syndrome. J Rheumatol. 2010;37:1699–1704. doi: 10.3899/jrheum.091086. [DOI] [PubMed] [Google Scholar]
- 76.Kerrison C, Davidson JE, Cleary AG, et al. Pamidronate in the treatment of childhood SAPHO syndrome. Rheumatology (Oxford) 2004;43:1246–1251. doi: 10.1093/rheumatology/keh295. [DOI] [PubMed] [Google Scholar]
- 77.Kadom N, Egloff A, Obeid G, et al. Juvenile mandibular chronic osteomyelitis: multimodality imaging findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:e38–e43. doi: 10.1016/j.tripleo.2010.10.027. [DOI] [PubMed] [Google Scholar]