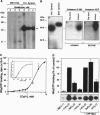
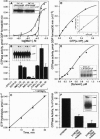
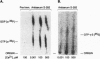
Abstract
Exocytotic membrane fusion and secretion are promoted by the concerted action of GTP and Ca2+, although the precise site(s) of action in the process are not presently known. However, the calcium-dependent membrane fusion reaction driven by synexin (annexin VII) is an in vitro model for this process, which we have now found to be further activated by GTP. The mechanism of fusion activation depends on the unique ability of synexin to bind and hydrolyze GTP in a calcium-dependent manner, both in vitro and in vivo in streptolysin O-permeabilized chromaffin cells. The required [Ca2+] for GTP binding by synexin is in the range of 50-200 microM, which is known to occur at exocytotic sites in chromaffin cells, neurons, and other cell types. Previous immunolocalization studies place synexin at exocytotic sites in chromaffin cells, and we conclude that synexin is an atypical G protein that may be responsible for both detecting and mediating the Ca2+/GTP signal for exocytotic membrane fusion.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnert-Hilger G., Wegenhorst U., Stecher B., Spicher K., Rosenthal W., Gratz M. Exocytosis from permeabilized bovine adrenal chromaffin cells is differently modulated by guanosine 5'-[gamma-thio]triphosphate and guanosine 5'-[beta gamma-imido]triphosphate. Evidence for the involvement of various guanine nucleotide-binding proteins. Biochem J. 1992 Jun 1;284(Pt 2):321–326. doi: 10.1042/bj2840321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M., Rajmilevich G., Beaven M. A., Sagi-Eisenberg R. Activation of exocytosis by the heterotrimeric G protein Gi3. Science. 1993 Dec 3;262(5139):1569–1572. doi: 10.1126/science.7504324. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992 May;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. K., Scheller R. H. The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner M. A., Holz R. W. Kinetic analysis of secretion from permeabilized adrenal chromaffin cells reveals distinct components. J Biol Chem. 1992 Aug 15;267(23):16219–16225. [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K. W., Pollard H. B. Pertussis toxin stimulates delayed-onset, Ca2+-dependent catecholamine release and the ADP-ribosylation of a 40 kDa protein in bovine adrenal chromaffin cells. FEBS Lett. 1988 Jul 18;234(2):439–445. doi: 10.1016/0014-5793(88)80133-9. [DOI] [PubMed] [Google Scholar]
- Burns A. L., Magendzo K., Shirvan A., Srivastava M., Rojas E., Alijani M. R., Pollard H. B. Calcium channel activity of purified human synexin and structure of the human synexin gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3798–3802. doi: 10.1073/pnas.86.10.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns A. L., Magendzo K., Srivastava M., Rojas E., Parra C., de la Fuente M., Cultraro C., Shirvan A., Vogel T., Heldman J. Human synexin (annexin VII) polymorphisms: tissue specificity and expression in Escherichia coli. Biochem Soc Trans. 1990 Dec;18(6):1118–1121. doi: 10.1042/bst0181118. [DOI] [PubMed] [Google Scholar]
- Ceña V., Brocklehurst K. W., Pollard H. B., Rojas E. Pertussis toxin stimulation of catecholamine release from adrenal medullary chromaffin cells: mechanism may be by direct activation of L-type and G-type calcium channels. J Membr Biol. 1991 May;122(1):23–31. doi: 10.1007/BF01872736. [DOI] [PubMed] [Google Scholar]
- Chapman E. R., Hanson P. I., An S., Jahn R. Ca2+ regulates the interaction between synaptotagmin and syntaxin 1. J Biol Chem. 1995 Oct 6;270(40):23667–23671. doi: 10.1074/jbc.270.40.23667. [DOI] [PubMed] [Google Scholar]
- Creutz C. E., Pazoles C. J., Pollard H. B. Identification and purification of an adrenal medullary protein (synexin) that causes calcium-dependent aggregation of isolated chromaffin granules. J Biol Chem. 1978 Apr 25;253(8):2858–2866. [PubMed] [Google Scholar]
- Creutz C. E., Pazoles C. J., Pollard H. B. Self-association of synexin in the presence of calcium. Correlation with synexin-induced membrane fusion and examination of the structure of synexin aggregates. J Biol Chem. 1979 Jan 25;254(2):553–558. [PubMed] [Google Scholar]
- Creutz C. E. cis-Unsaturated fatty acids induce the fusion of chromaffin granules aggregated by synexin. J Cell Biol. 1981 Oct;91(1):247–256. doi: 10.1083/jcb.91.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darchen F., Zahraoui A., Hammel F., Monteils M. P., Tavitian A., Scherman D. Association of the GTP-binding protein Rab3A with bovine adrenal chromaffin granules. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5692–5696. doi: 10.1073/pnas.87.15.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Döring V., Schleicher M., Noegel A. A. Dictyostelium annexin VII (synexin). cDNA sequence and isolation of a gene disruption mutant. J Biol Chem. 1991 Sep 15;266(26):17509–17515. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Ferguson K. M., Higashijima T., Smigel M. D., Gilman A. G. The influence of bound GDP on the kinetics of guanine nucleotide binding to G proteins. J Biol Chem. 1986 Jun 5;261(16):7393–7399. [PubMed] [Google Scholar]
- Ferro-Novick S., Jahn R. Vesicle fusion from yeast to man. Nature. 1994 Jul 21;370(6486):191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G., Mignery G. A., Baumert M., Perin M. S., Hanson T. J., Burger P. M., Jahn R., Südhof T. C. rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1988–1992. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg E. J., Pollard H. B. Ba2+-induced ATP release from adrenal medullary chromaffin cells is mediated by Ba2+ entry through both voltage- and receptor-gated Ca2+ channels. Neuroscience. 1988 Nov;27(2):711–715. doi: 10.1016/0306-4522(88)90300-4. [DOI] [PubMed] [Google Scholar]
- Geppert M., Bolshakov V. Y., Siegelbaum S. A., Takei K., De Camilli P., Hammer R. E., Südhof T. C. The role of Rab3A in neurotransmitter release. Nature. 1994 Jun 9;369(6480):493–497. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- Gerke V. Identification of a homologue for annexin VII (synexin) in Dictyostelium discoideum. J Biol Chem. 1991 Jan 25;266(3):1697–1700. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D., Cockcroft S., Howell T. W., Nüsse O., Tatham P. E. The dual effector system for exocytosis in mast cells: obligatory requirement for both Ca2+ and GTP. Biosci Rep. 1987 May;7(5):369–381. doi: 10.1007/BF01362501. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D. GE: a GTP-binding protein mediating exocytosis. Annu Rev Physiol. 1990;52:591–606. doi: 10.1146/annurev.ph.52.030190.003111. [DOI] [PubMed] [Google Scholar]
- Heidelberger R., Heinemann C., Neher E., Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994 Oct 6;371(6497):513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- Heinemann C., Chow R. H., Neher E., Zucker R. S. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+. Biophys J. 1994 Dec;67(6):2546–2557. doi: 10.1016/S0006-3495(94)80744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldman E., Levine M., Raveh L., Pollard H. B. Barium ions enter chromaffin cells via voltage-dependent calcium channels and induce secretion by a mechanism independent of calcium. J Biol Chem. 1989 May 15;264(14):7914–7920. [PubMed] [Google Scholar]
- Hong K., Düzgüneş N., Ekerdt R., Papahadjopoulos D. Synexin facilitates fusion of specific phospholipid membranes at divalent cation concentrations found intracellularly. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4642–4644. doi: 10.1073/pnas.79.15.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Düzgüneş N., Papahadjopoulos D. Role of synexin in membrane fusion. Enhancement of calcium-dependent fusion of phospholipid vesicles. J Biol Chem. 1981 Apr 25;256(8):3641–3644. [PubMed] [Google Scholar]
- Howell T. W., Cockcroft S., Gomperts B. D. Essential synergy between Ca2+ and guanine nucleotides in exocytotic secretion from permeabilized rat mast cells. J Cell Biol. 1987 Jul;105(1):191–197. doi: 10.1083/jcb.105.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes L., Lledo P. M., Roa M., Vincent J. D., Henry J. P., Darchen F. The GTPase Rab3a negatively controls calcium-dependent exocytosis in neuroendocrine cells. EMBO J. 1994 May 1;13(9):2029–2037. doi: 10.1002/j.1460-2075.1994.tb06476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers G. A., Lee G., Pollard H. B. Immunolocalization of synexin (annexin VII) in adrenal chromaffin granules and chromaffin cells: evidence for a dynamic role in the secretory process. Cell Tissue Res. 1992 Aug;269(2):323–330. doi: 10.1007/BF00319624. [DOI] [PubMed] [Google Scholar]
- Lledo P. M., Vernier P., Vincent J. D., Mason W. T., Zorec R. Inhibition of Rab3B expression attenuates Ca(2+)-dependent exocytosis in rat anterior pituitary cells. Nature. 1993 Aug 5;364(6437):540–544. doi: 10.1038/364540a0. [DOI] [PubMed] [Google Scholar]
- Monck J. R., Fernandez J. M. The exocytotic fusion pore and neurotransmitter release. Neuron. 1994 Apr;12(4):707–716. doi: 10.1016/0896-6273(94)90325-5. [DOI] [PubMed] [Google Scholar]
- Morgan A., Burgoyne R. D. A role for soluble NSF attachment proteins (SNAPs) in regulated exocytosis in adrenal chromaffin cells. EMBO J. 1995 Jan 16;14(2):232–239. doi: 10.1002/j.1460-2075.1995.tb06996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A., Burgoyne R. D. A synthetic peptide of the N-terminus of ADP-ribosylation factor (ARF) inhibits regulated exocytosis in adrenal chromaffin cells. FEBS Lett. 1993 Aug 23;329(1-2):121–124. doi: 10.1016/0014-5793(93)80206-a. [DOI] [PubMed] [Google Scholar]
- Morgan A. Exocytosis. Essays Biochem. 1995;30:77–95. [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol. 1988 Jan;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Zucker R. S. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993 Jan;10(1):21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- Nir S., Stutzin A., Pollard H. B. Effect of synexin on aggregation and fusion of chromaffin granule ghosts at pH 6. Biochim Biophys Acta. 1987 Oct 2;903(2):309–318. doi: 10.1016/0005-2736(87)90221-5. [DOI] [PubMed] [Google Scholar]
- O'Connor V., Augustine G. J., Betz H. Synaptic vesicle exocytosis: molecules and models. Cell. 1994 Mar 11;76(5):785–787. doi: 10.1016/0092-8674(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Oberhauser A. F., Monck J. R., Balch W. E., Fernandez J. M. Exocytotic fusion is activated by Rab3a peptides. Nature. 1992 Nov 19;360(6401):270–273. doi: 10.1038/360270a0. [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi M., Kameyama K., Kawae N., Takeda K., Muramatsu S., Kumakura K. Regulatory role of the GTP-binding protein, G(o), in the mechanism of exocytosis in adrenal chromaffin cells. J Neurochem. 1992 Jun;58(6):2275–2284. doi: 10.1111/j.1471-4159.1992.tb10974.x. [DOI] [PubMed] [Google Scholar]
- Okano K., Monck J. R., Fernandez J. M. GTP gamma S stimulates exocytosis in patch-clamped rat melanotrophs. Neuron. 1993 Jul;11(1):165–172. doi: 10.1016/0896-6273(93)90280-5. [DOI] [PubMed] [Google Scholar]
- Pak C. C., Krumbiegel M., Blumenthal R., Raviv Y. Detection of influenza hemagglutinin interaction with biological membranes by photosensitized activation of [125I]iodonaphthylazide. J Biol Chem. 1994 May 20;269(20):14614–14619. [PubMed] [Google Scholar]
- Peveri P., Heyworth P. G., Curnutte J. T. Absolute requirement for GTP in activation of human neutrophil NADPH oxidase in a cell-free system: role of ATP in regenerating GTP. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2494–2498. doi: 10.1073/pnas.89.6.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard H. B., Burns A. L., Rojas E. Synexin (annexin VII): a cytosolic calcium-binding protein which promotes membrane fusion and forms calcium channels in artificial bilayer and natural membranes. J Membr Biol. 1990 Aug;117(2):101–112. doi: 10.1007/BF01868677. [DOI] [PubMed] [Google Scholar]
- Pollard H. B., Rojas E. Ca2+-activated synexin forms highly selective, voltage-gated Ca2+ channels in phosphatidylserine bilayer membranes. Proc Natl Acad Sci U S A. 1988 May;85(9):2974–2978. doi: 10.1073/pnas.85.9.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard H. B., Rojas E., Pastor R. W., Rojas E. M., Guy H. R., Burns A. L. Synexin: molecular mechanism of calcium-dependent membrane fusion and voltage-dependent calcium-channel activity. Evidence in support of the "hydrophobic bridge hypothesis" for exocytotic membrane fusion. Ann N Y Acad Sci. 1991;635:328–351. doi: 10.1111/j.1749-6632.1991.tb36503.x. [DOI] [PubMed] [Google Scholar]
- Pollard H. B., Rojas E., Pastor R. W., Rojas E. M., Guy H. R., Burns A. L. Synexin: molecular mechanism of calcium-dependent membrane fusion and voltage-dependent calcium-channel activity. Evidence in support of the "hydrophobic bridge hypothesis" for exocytotic membrane fusion. Ann N Y Acad Sci. 1991;635:328–351. doi: 10.1111/j.1749-6632.1991.tb36503.x. [DOI] [PubMed] [Google Scholar]
- Raynal P., Pollard H. B. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994 Apr 5;1197(1):63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Rojas E., Pollard H. B. Membrane capacity measurements suggest a calcium-dependent insertion of synexin into phosphatidylserine bilayers. FEBS Lett. 1987 Jun 8;217(1):25–31. doi: 10.1016/0014-5793(87)81235-8. [DOI] [PubMed] [Google Scholar]
- Rosenboom H., Lindau M. Exo-endocytosis and closing of the fission pore during endocytosis in single pituitary nerve terminals internally perfused with high calcium concentrations. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5267–5271. doi: 10.1073/pnas.91.12.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Shirvan A., Srivastava M., Wang M. G., Cultraro C., Magendzo K., McBride O. W., Pollard H. B., Burns A. L. Divergent structure of the human synexin (annexin VII) gene and assignment to chromosome 10. Biochemistry. 1994 Jun 7;33(22):6888–6901. doi: 10.1021/bi00188a019. [DOI] [PubMed] [Google Scholar]
- Srivastava M., Zhang-Keck Z. Y., Caohuy H., McPhie P., Pollard H. B. Novel isoforms of synexin in Xenopus laevis: multiple tandem PGQM repeats distinguish mRNAs in specific adult tissues and embryonic stages. Biochem J. 1996 Jun 15;316(Pt 3):729–735. doi: 10.1042/bj3160729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzin A. A fluorescence assay for monitoring and analyzing fusion biological membrane vesicles in vitro. FEBS Lett. 1986 Mar 3;197(1-2):274–280. doi: 10.1016/0014-5793(86)80341-6. [DOI] [PubMed] [Google Scholar]
- Stutzin A., Cabantchik Z. I., Lelkes P. I., Pollard H. B. Synexin-mediated fusion of bovine chromaffin granule ghosts. Effect of pH. Biochim Biophys Acta. 1987 Nov 27;905(1):205–212. doi: 10.1016/0005-2736(87)90024-1. [DOI] [PubMed] [Google Scholar]
- Südhof T. C. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995 Jun 22;375(6533):645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Thomas P., Wong J. G., Lee A. K., Almers W. A low affinity Ca2+ receptor controls the final steps in peptide secretion from pituitary melanotrophs. Neuron. 1993 Jul;11(1):93–104. doi: 10.1016/0896-6273(93)90274-u. [DOI] [PubMed] [Google Scholar]
- Thomas R., Pfeuffer T. Photoaffinity labeling of GTP-binding proteins. Methods Enzymol. 1991;195:280–286. doi: 10.1016/0076-6879(91)95173-h. [DOI] [PubMed] [Google Scholar]
- Vitale N., Mukai H., Rouot B., Thiersé D., Aunis D., Bader M. F. Exocytosis in chromaffin cells. Possible involvement of the heterotrimeric GTP-binding protein G(o). J Biol Chem. 1993 Jul 15;268(20):14715–14723. [PubMed] [Google Scholar]
- White J. M. Membrane fusion. Science. 1992 Nov 6;258(5084):917–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- Zhang-Keck Z. Y., Burns A. L., Pollard H. B. Mouse synexin (annexin VII) polymorphisms and a phylogenetic comparison with other synexins. Biochem J. 1993 Feb 1;289(Pt 3):735–741. doi: 10.1042/bj2890735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang-Keck Z. Y., Srivastava M., Kozak C. A., Caohuy H., Shirvan A., Burns A. L., Pollard H. B. Genomic organization and chromosomal localization of the mouse synexin gene. Biochem J. 1994 Aug 1;301(Pt 3):835–845. doi: 10.1042/bj3010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Rüden L., Neher E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science. 1993 Nov 12;262(5136):1061–1065. doi: 10.1126/science.8235626. [DOI] [PubMed] [Google Scholar]