Abstract
Iron status and immune response become impaired in situations that involve chronic inflammation, such as obesity or aging. Little is known, however, about the additional burden that obesity may place on the iron status and immune response in the elderly. This question is relevant given the rising numbers of elderly obese (BMI >30 kg/m2) individuals and the high prevalence of iron deficiency worldwide. Iron is necessary for proper function of both the innate and adaptive immune system. Hepcidin, a peptide hormone that regulates cellular iron export, is essential for the maintenance of iron homeostasis. Therefore, since immune cells require iron for proper function hepcidin may also play an important role in immune response. In this review, we summarize the evidence for hepcidin as a link between the fields of gerontology, obesity, iron biology, and immunology. We also identify several gaps in knowledge and unanswered questions pertaining to iron homeostasis and immunity in obese populations. Finally, we review studies that have shown the impact of weight loss, focusing on calorie restriction, iron homeostasis, and immunity. These studies are important both in elucidating mechanistic links between obesity and health impairments and identifying possible approaches to target immune impairment and iron deficiency as comorbidities of obesity.
Introduction
Over the past few decades research on iron has spanned different disciplines of the biological sciences. This nutrient is essential for red blood cell formation, immune function, fetal development, and physical and mental well-being. At the same time, however, anemia, defined by the WHO as hemoglobin (Hb)3 <12 g/dL for adult nonpregnant women and <13 g/dL for adult men (1), affects more than one-quarter of the world’s population (2), and iron deficiency is even more prevalent. For individuals older than 5 y with no chronic inflammation or chronic disease, iron deficiency is defined as a serum ferritin <15 μg/L (3). As explained in later sections, defining iron deficiency in individuals with chronic inflammation, such as the obese and the elderly, is less straightforward.
Iron supplementation has at times proven ineffective and even detrimental to health. Several studies have demonstrated that iron supplementation in populations at risk of certain infectious diseases may increase the risk of morbidity and mortality. Furthermore, excessive iron intake may cause iron toxicity because free iron is a potent pro-oxidant that may damage cells and tissues. On the other hand, certain conditions, such as obesity (BMI >30 kg/m2), chronic disease, and aging are associated with low iron status. Both aging and obesity are also associated with impaired immune response, in which iron plays an important role.
Hepcidin is a small peptide hormone essential for iron homeostasis and immune response. In this review we summarize existing evidence and identify gaps in knowledge regarding the increased risk in obese and elderly individuals for iron deficiency and immune response impairment. In addition, our objective is to review known associations among obesity, aging, immune response, and iron homeostasis through hepcidin. The convergence of these fields is relevant to the demographic and health-related changes that the general population is currently experiencing. We review the role that hepcidin plays in iron homeostasis and immune response. We then summarize current knowledge regarding chronic inflammation of obesity and its affect on iron status, immune response, and hepcidin. Finally, we address aging, immunosenescense, and iron deficiency and how increasing rates of obesity in the elderly may be placing this age group at an even higher risk of impaired immune response and iron deficiency.
Iron Homeostasis and Hepcidin
Maintenance of iron homeostasis is essential for proper cellular function. Adequate iron levels must meet the needs of different organs and tissues, but excess iron causes cellular damage. Iron is excreted in an unregulated manner, mainly through enterocyte sloughing and loss of bodily fluids and skin cells (4). Iron balance is primarily regulated by absorption through the intestine and iron export from cells. A thorough description of iron absorption, storage, transport, and metabolism can be found elsewhere (4, 5). Once in the enterocyte, iron can be stored by binding ferritin (5). If iron stored in enterocytes is not utilized, it is lost when the enterocyte is shed off the intestinal lumen. To be absorbed into the bloodstream, iron is exported through ferroportin, the only known iron exporter, located in the basolateral side of the enterocyte. To avoid oxidative damage, iron in circulation is bound by the carrier protein transferrin and delivered to target cells through the transferrin cycle (5, 6), which occurs mainly in erythroid precursor cells that have the highest iron demands and to a lesser extent in other cell types (4). Iron can be transported to other target tissues, such as liver, which is the main site for iron storage, bone marrow, and peripheral blood mononuclear cells (PBMCs), among others, to be used in important processes such as synthesis of Hb or Fe-S clusters in mitochondria. About 70% of iron in the body is used for Hb synthesis (7). When erythrocytes have reached the end of their life span, they are recycled by reticuloendothelial macrophages. The amount of iron recycled through this system is ∼25 mg/day (4). Almost all iron needed for erythropoiesis is provided by red blood cell recycling (7). Regulation of iron recycling and storage, together with iron absorption, is essential for iron homeostasis.
Given the damage that iron may cause through oxidation, but also its importance for cellular processes, iron levels must be tightly regulated. Iron homeostasis is regulated at different levels, either post-translationally (Fig. 1), or post-transcriptionally. Post-transcriptional regulation of proteins involved in iron homeostasis, such as ferritin and transferrin, has been extensively covered in other reviews (4, 5). Post-translational regulation of iron homeostasis occurs primarily through hepcidin, a small peptide hormone central to iron homeostasis that regulates cellular iron export (5). Hepcidin binds the membrane-associated iron exporter ferroportin (Fig. 1), which is expressed on enterocytes and macrophages, as well as other cells such as PBMCs. Upon binding, hepcidin induces internalization and degradation of ferroportin through tyrosine phosphorylation, ubiquitination, and lysosomal degradation. In this way, iron remains in intracellular stores and export into the plasma is reduced (4, 5). Iron export through ferroportin from enterocytes, macrophages, and PBMCs has been shown to be prevented by hepcidin (8–10), although recent evidence has suggested that there are mechanistic differences in the effect of hepcidin on enterocytes. It has been suggested that acute hepcidin exposure may induce downregulation of divalent metal transporter 1 (DMT1), rather than ferroportin, downregulation through proteosomal degradation, whereas chronic hepcidin exposure may induce downregulation of ferroportin (11). Furthermore, macrophages have been found to respond more rapidly to changes in hepcidin than enterocytes (12).
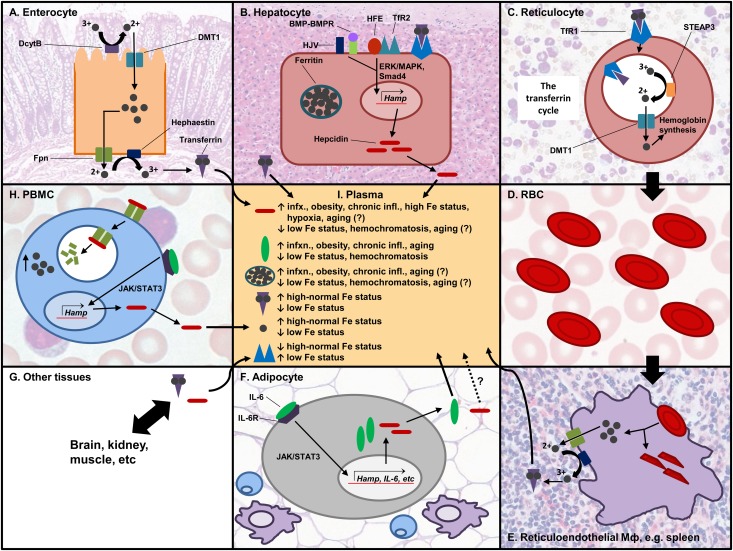
FIGURE 1.
Post-translational regulation of iron homeostasis. Iron is absorbed through the enterocyte (A). It is first reduced by DcytB and transported by DMT1 into the cell, and it is either stored or exported from the cell by the action of hephaestin and ferroportin. Two iron atoms bind transferrin, which transports iron to target tissues. (B) Iron can be stored in hepatocytes in the storage protein ferritin. The liver is the main producer of hepcidin, and its expression can be induced by HJV/BMP-BMP receptor, or by TfR2/HFE through the Smad4 or ERK/MAPK pathways, respectively. (C) In the bone marrow, reticulocytes take in transferrin-bound iron to make Hb. The TfR/transferrin-iron complex is internalized by endocytosis. Acidification of the endosome induces the release of iron from transferrin. Iron is reduced and exported into the cytoplasm and used for Hb synthesis in the mitochondria. TfR and transferrin are recycled back to the cell membrane. (D) RBCs transport the synthesized Hb and are involved in oxygen and CO2 exchange with different tissues. (E) Effete RBCs are recycled via the reticuloendothelial system. Macrophages phagocytose the RBCs and recycle the iron. (F) AT secretes adipokines, including pro-inflammatory cytokines such as IL-6 that induce hepcidin expression via the JAK/STAT3 pathway. It is not confirmed whether hepcidin made in AT goes into circulation. (G) Transferrin and hepcidin go to different tissues in the body that utilize iron. (H) PBMCs are one of the cell types that express ferroportin. Hepcidin binds ferroportin and induces its internalization and degradation, increasing the intracellular iron pool. (I) Different proteins are found in circulation, and their levels affect or are affected by iron status. AT, adipose tissue; DcytB, duodenal cytochrome B; DMT1, divalent metal transporter 1; Fpn, ferroportin; Hamp, hepcidin gene; Hb, hemoglobin; HJV, hemojuvelin; IL-6, interleukin 6; JAK/STAT3, Janus kinase/signal transducer and activator of transcription 3; PBMCs, peripheral blood mononuclear cells; RBCs, red blood cells; STEAP3, six-transmembrane epithelial antigen of the prostate 3; TfR, transferrin receptor. Source for histology images: Wheater's functional histology: a text and colour atlas (112).
Hepcidin is transcribed from the Hamp gene into an 84–amino acid preprohepcidin, which is then cleaved into a 60–amino acid prohepcidin, detectable in serum (13,14). This precursor is further processed into the mature bioactive form, a 25–amino acid peptide (13) containing 4 disulfide bonds. Hepcidin is expressed mainly by the liver (5), although more recent studies have shown that other cell types such as adipocytes (15) and PBMCs (10) also express hepcidin. Hamp expression is induced through different signaling pathways. Primarily, hepcidin expression is induced through the bone morphogenetic protein (BMP)/Smad pathway, which is activated by interaction between the BMP receptor (BMPR) and the coreceptor hemojuvelin (HJV). Matriptase-2 (also called TMPRSS6) inhibits hepcidin upregulation by cleaving HJV into soluble HJV and preventing activation of the BMP/Smad pathway (8). In addition, soluble HJV inhibits hepcidin expression by competing with membrane-bound HJV for the BMPR.
Hepcidin gene expression is also regulated through transferrin receptor (TfR) signaling. Recent evidence suggested that the coreceptor hemochromatosis protein, a MHC class I-like protein (HFE) competes with iron-bound transferrin for TfR1 binding. When transferrin-bound iron is low, HFE binds TfR1 and hepcidin expression is not induced. When iron levels are high, transferrin binds TfR1 and HFE is displaced. It is believed HFE is then sequestered by TfR2, which has both HFE and transferrin allosteric binding sites. In this way, HFE binding to TfR2 may be the signal that induces the ERK/MAPK signaling cascade that leads to Hamp expression, but more research is needed (16). This system is intimately related to the BMP/Smad pathway, but the details have not been fully elucidated.
Chronic or acute inflammation also induces hepcidin expression (4, 5, 17). Studies in hepatocyte cell lines (18), mice (19), and humans (17) have shown that IL-6 upregulates hepcidin through the JAK/STAT3 pathway and STAT3 binding motif in the Hamp promoter. A study in humans showed that IL-6 or lipopolysaccharide (LPS) injections induced hypoferremia and were directly correlated with an increase in serum hepcidin (17).
Signals that inhibit hepcidin expression include erythropoiesis potentially through inactivation of the BMP/Smad pathway by BMPR binding protein TWSG1, and hypoxia through stimulation of erythropoietin production and perhaps also of hypoxia-inducible factor 1 and 2 (4, 5). Genetic deficiency of hepcidin leads to hemochromatosis, an iron overload disorder. Mutations in both hepcidin and its regulators lead to this disease. Class I hemochromatosis is characterized by mutations in the Hamp gene; class II hemochromatosis is due to defects in HFE, HJV, or TfR2; and class III hemochromatosis is due to defects in ferroportin, preventing binding of hepcidin and regulation of cellular iron export (5). HJV mutations are the most common cause of juvenile hemochromatosis, accounting for 95% of cases (8). Hemochromatosis is characterized by low and even undetectable hepcidin levels, iron overload, and iron deposition in liver, heart, and endocrine organs.
It has been suggested that no cross-talk occurs between the signals that regulate hepcidin (4, 20). Iron status, inflammation, and erythropoiesis seem to influence hepcidin expression independently from each other, with the strongest signal outweighing the others and determining hepcidin levels. This concept of competing signals is particularly important for populations, such as the elderly, that are at risk of both low iron status (a signal that downregulates hepcidin) and chronic inflammation (a signal that induces hepcidin).
Current Status of Knowledge
Innate and adaptive immune function are iron dependent and influenced by hepcidin
Both iron and hepcidin are essential in the innate and adaptive immune response. Iron levels have a direct impact not only on the host’s immunity but also on the pathogen. Hepcidin was originally identified as an antimicrobial peptide and was found to be essential for innate immunity. Evidence has suggested that hepcidin is also important for the adaptive immune response.
Iron excess or deficit affects the immune response.
Both iron deficiency and iron overload affect immune function. As reviewed in Oppenheimer et al. (21), iron deficiency affects neutrophil function, lymphoproliferation, cytokine production, and natural killer (NK) cell activity. On the other hand, numerous studies have shown the negative effect of iron overload through supplementation, disease, or blood transfusions on vulnerability to infection (7, 22–24). The most noteworthy study showing this evidence was conducted by Sazawal et al. (25) in Pemba, Tanzania. This study was discontinued before completion because of a substantial increase in mortality or the likelihood for children to be hospitalized in the group supplemented with iron and folic acid (25). Iron supplementation in malaria-endemic regions may increase the risk of malaria infection, but findings from different studies have not been uniform, with some studies showing increased malaria risk and others no increased risk and a decrease in anemia (26). Conversely, iron deficiency has been associated with protection against malaria (7). This topic remains controversial, and evidence suggests that the severity of malaria after iron supplementation depends on the host’s health and nutritional status before supplementation. HIV viral replication and risk of secondary opportunistic infections, such as tuberculosis, have also been shown to increase with iron supplementation (7, 27). Similarly, patients with iron overload as seen in hemochromatosis have increased susceptibility to infection (28). Therefore, iron supplementation not only enhances the host immune function, but it also provides an environment for pathogens to thrive, causing controversy regarding iron supplementation in regions with high risk of iron deficiency and infection. It is important to note that iron supplementation may have beneficial effects on immunity, and it has been shown to improve resistance to respiratory infection in children (21).
Iron and hepcidin in innate immunity.
Because iron is needed by most living organisms, one of the first lines of defense by the human body as a host is to deprive invading pathogens of the iron required for them to survive. In response to this defense, certain pathogens such as the malaria parasite, Plasmodium falciparum, have evolved to invade iron-rich pools in the host (23). Other modes of attack by invading pathogens include production of siderophores, molecules produced by bacteria to chelate any available free and transferrin-bound iron and deliver it to the bacteria (29). In fact, >500 known siderophores can accomplish this function (23).
The host’s immune system has developed different but equally ingenious strategies of defense, which mainly involve depriving pathogens of iron. One mechanism of defense is the production of lipocalin-2, which is expressed in neutrophils and epithelial cells and binds to siderophores, inhibiting their action (30). Lactoferrin, another protein used in immune defense, has high affinity for free iron during infection. In addition, hepcidin plays a central role in host defense against extracellular pathogens. Hepcidin was simultaneously identified as an antimicrobial peptide and a regulator of iron status (23). As mentioned previously, hepcidin is upregulated by pro-inflammatory cytokines, such as IL-6 and IL-1, through signaling pathways involving JAK/STAT3 and toll-like receptor 4 (31). Hepcidin upregulation upon infection by extracellular pathogens leads to decreased circulating iron levels and iron sequestration within the host’s cells. Theurl et al. (32) showed that hepcidin regulates ferroportin expression and intracellular iron content in an autocrine fashion in primary monocytes from patients with anemia of chronic disease and in the human monocyte cell line THP-1. In addition, they showed how hepcidin knockdown with RNAi reversed this process and resulted in decreased iron sequestration and increased ferroportin in the monocytes. It is important to note that ferroportin expression is also downregulated at the gene expression level in monocytes by inflammatory cytokines independently of hepcidin (33).
Immune system defense against intracellular pathogens requires a different approach. IFN-γ, a macrophage-activating cytokine expressed by Th1 lymphocytes and NK cells, downregulates expression of TfR1 in macrophage phagosome membranes, thereby decreasing iron pools and preventing iron acquisition by intracellular bacteria (29). Nairz et al. (34) showed how iron deprivation through this mechanism could prevent Salmonella typhimurium infection. IFN-γ also acts on macrophages to induce maturation and acidification of phagosomes (7). Furthermore, evidence has shown that IFN-γ induces hepcidin expression in lung epithelial cells (35). Another method of defense is through natural resistance-associated macrophage protein 2, or Nramp2 (also known as SLC11A1, a homolog to divalent metal transporter 1), which is expressed in the phagosomes of macrophages and neutrophils and exports divalent metal protons from the phagosome to the cytosol. Ferroportin is also expressed in macrophage phagosomes (36). In this way, intracellular bacteria, such as Mycobacteria, are unable to acquire iron within the invaded cells (29). Decreasing iron levels and manipulating iron pools in the host as a defense mechanism against iron-dependent pathogens have also been illustrated in studies showing that overexpression of hepcidin and iron supplementation can increase the risk of intracellular pathogen infection, such as Legionella pneumophila (37), and this increase in risk can be reversed by localized iron depletion.
Besides manipulating iron pools, a crucial aspect of innate immunity in which iron is involved is the oxidative burst, which is the production of reactive oxygen species (ROS) to damage invading pathogens. These ROS created within immune cells, mainly neutrophils and macrophages, are released into phagosomes containing the engulfed or intracellular pathogen (38). Iron is essential for the production of ROS, and it may be through hepcidin’s action that iron is accumulated within cells involved in oxidative burst, but the role of hepcidin in this process has not been studied in depth. Iron status and intracellular macrophage iron levels affect the efficiency of the oxidative burst (38). In summary, the literature reviewed here indicates the importance of maintaining an optimal iron balance specific to the type of infection and emphasizes the crucial role hepcidin plays in regulation of the immune response, starting with innate immunity but also, as more recently demonstrated, for adaptive immunity.
Iron and hepcidin in adaptive immunity.
An optimal iron status is necessary for adaptive immune response. Iron and expression of TfR are essential for lymphocyte activation and proliferation (39). Both iron deficiency and iron overload have been associated with impaired cell-mediated immunity in humans (40, 41). Iron deficiency has been shown to decrease lymphocyte counts and proliferation as well as IFN-γ and IL-2 production and to impair NK cell activity. Omara and Blakley (41) found that mice fed an iron-deficient diet had a lower delayed type hypersensitivity response, in which CD4+ lymphocytes are key players, than mice fed a normal or supplemented iron diet. Furthermore, mice fed an iron-deficient diet had lower concanavalin A–induced lymphoproliferation than the normal or supplemented diet groups. These results suggested that iron deficiency leads to impaired T-cell response. Another study showed that NK cell cytotoxicity was impaired in rats fed an iron-deficient diet, and adding IFN-γ in vitro rescued the loss of function (42).
Certain aspects of adaptive immunity are affected by iron overload. For example, Omara and Blakley (41) showed that contact sensitivity, a measure of lymphocyte response and immunoglobulin E (IgE) production, was impaired in mice fed high-iron diets with respect to those fed an iron-replete diet. In addition, high CD4-to-CD8 T-cell ratios, low numbers of CD28+ cells, and impaired CD8+ T-cell function have been reported in patients with hemochromatosis (39), although direct causality with iron overload cannot be proven in this scenario. Mouse knockout models of β2-microglobulin, a protein associated with MHC class I molecules, have an abnormal increase of iron absorption and increased plasma iron. Furthermore, β2-microglobulin and Rag2−/− mouse knockout models, the latter being a factor essential for B and T lymphocyte development, have immature lymphocytes and severe iron overload (39). In summary, this evidence suggests different effects of iron deficiency and iron overload on the adaptive immune response.
Hepcidin’s role in adaptive immunity has been defined more recently, but more evidence is needed. Pinto et al. (10) showed that lymphocyte hepcidin production increased upon stimulation with anti– T-cell receptor (anti-CD3 and anti-CD28) antibody and that partial knockdown of hepcidin impaired T-cell proliferation. This effect was reversed by adding synthetic hepcidin peptide to the hepcidin-deficient lymphocytes. In addition, different subtypes of PBMCs were shown to express hepcidin and seem to play a role in iron homeostasis, because they express ferroportin and internalize iron in the presence of hepcidin. Furthermore, it has been suggested that regulation of hepcidin through the Smad and STAT3 pathways may play a role in Th17 responses (23).
Taken together, these findings suggest that iron homeostasis and immune response are closely related. The interaction between iron status and immune response is complex, implying that optimal iron status for immune response may depend on the nature of the pathogen. Iron plays an important role in both innate and adaptive immunity, but many questions remain unanswered, especially pertaining to hepcidin’s involvement in adaptive immunity.
Chronic inflammation of obesity is associated with high hepcidin and low iron status
Obesity and iron homeostasis.
In the past few decades, several reports have shown that obese adults (BMI >30 kg/m2) are at high risk of having low iron status (43–48). The first evidence came from Wenzel et al. (49) in 1962, showing lower serum iron in obese adolescents compared with normal weight adolescents, and other studies in children and adults around the world followed with consistent findings (Table 1).
TABLE 1.
Cross-sectional or iron supplementation studies reporting iron status in relation to obesity1
| Author | Year | Population | Relevant primary measurements | Key findings |
| Wenzel et al. (49) | 1962 | Adolescents 11–19 y (n = 355) (United States) | BMI, iron status (sFe, Hb) | Obese adolescents had lower sFe than lean adolescents. No difference in Hb was found. |
| Seltzer et al. (101) | 1963 | Children and adolescents 11–21 y (n = 321) (United States) | Iron status (sFe, Tsat, Hb, HCT, mean corpuscular Hb concentration), obesity defined using Wetzel Grid | sFe and Tsat were lower in obese vs. nonobese subjects. |
| Micozzi et al. (102) | 1989 | NHANES I: Adults 25–74 y (n = 13,834) (United States) | BMI, body composition (skinfold thickness, lean body mass, total fat mass), iron status (Hb, HCT, TIBC), serum albumin, total protein, and cholesterol | Body size was associated with lower sFe and Tsat, but higher Hb and HCT, in men and women. |
| Pinhas-Hamiel et al. (103) | 2003 | Children and adolescents (n = 321) (Israel) | BMI (defined overweight using BMI percentiles), iron deficiency (sFe < 8 μmol/L), and iron deficiency anemia (2 SD below mean for age and gender) | Iron deficiency was as follows: 4.4% in normal weight children, 12.1% in overweight children, and 38.8% in obese children. Iron deficiency anemia was more prevalent with higher BMI. |
| Nead et al. (104) | 2004 | NHANES III: children 2–16 y (n = 9698) (United States) | BMI (defined overweight using BMI percentiles), iron status (Tsat, erythrocyte protoporphyrin, ferritin) | Children at risk for being overweight (14%) and overweight children (10%) were twice as likely to be at risk for iron deficiency than normal-weight children. |
| Moayeri et al. (105) | 2006 | Overweight and obese children (n = 540) and normal weight children (n = 200) (Iran) | BMI, iron status (sFe, Tsat, ferritin, Hb) | The highest prevalence of iron deficiency (Tsat <16% and ferritin <12 ng/mL) was in obese children (7% of total) and overweight children (5.3% of total), with 2.5% iron deficiency in the normal-weight group. Iron deficiency was more prevalent in female subjects. |
| Lecube et al. (43) | 2006 | Postmenopausal, nondiabetic, obese women (n = 50) and nonobese healthy postmenopausal women (n = 50) (Spain) | BMI, iron status (sFe, Tsat, sTfR, ferritin, Hb, reticulocytes, sTfR/log ferritin), insulin resistance (HOMA-IR) | Obese women had lower iron status (higher sTfR and sTfR/log-ferritin). BMI was positively correlated with sTfR and sTfR/log-ferritin. Linear regression analysis showed BMI was a positive predictor of sTfR. Insulin resistance was not correlated with sTfR. |
| Yanoff et al. (45) | 2007 | Obese (n = 234) and nonobese adults (n = 172) (United States) | BMI, iron status (sFe, TfR, ferritin), iron intake, CRP, body composition | Iron status (sFe and sTfR) was lower and CRP and ferritin were higher in the obese group. About 25% of subjects in the obese group had iron deficiency. |
| Brotanek et al. (106) | 2007 | NHANES IV: Representative sample of children 1–3 y (n = 1641) (United States) | Weight-for-height percentiles, iron status (Tsat, erythrocyte protoporphyrin, ferritin) | Prevalence of iron deficiency was greatest in overweight subjects (20%) than those at risk for being overweight (8%) or individuals with normal weight (7%). When categorized by race, prevalence of iron deficiency was as follows: Hispanics (12%), whites (6%), and blacks (6%). Hispanic children were more likely to be overweight than other racial groups. |
| Ausk et al. (107) | 2008 | NHANES III BMI (kg/m2) categories: BMI ≤25 (n = 6,059), BMI >25 and ≤30 (n = 5,108), BMI >30 and ≤35 (n = 2,366), BMI >35 and < 40 (n = 850), and BMI ≥40 (n = 465) (United States) | BMI, iron status (sFe, Tsat, H, ferritin), CRP, dietary iron (24 h dietary recall questionnaire) | Serum ferritin increased with BMI, and Tsat and sFe decreased. Hb was not different across BMI categories. Anemia was not more prevalent in overweight or obese subjects. |
| Eckhardt et al. (108) | 2008 | Lean (BMI <25) or overweight and obese (BMI ≥25) women 18–49 y from Mexico (n = 11,965), Peru (n = 5078), and Egypt (n = 6841) | BMI, Hb, and anemia (Hb <12 g/dL); controlled for sociodemographic factors | Prevalence of women with BMI ≥25 was >50% in the 3 countries, with Egypt being the highest, at 77%. Odds ratio for anemia was greater in lean women in Egypt and Peru and was not different across BMI categories in Mexico. Total prevalence of anemia was between 20% and 30% in all 3 countries. |
| Menzie et al. (44) | 2008 | Obese (n = 207) and nonobese adults (n = 177) (United States) | BMI; dietary intake of iron; and factors that affect iron absorption, iron status (sFe, Tsat, Hb, ferritin), and body composition (DXA) | Iron status (sFe, Tsat) was lower in obese than in nonobese subjects. The obese group consumed more animal protein and heme iron and less vitamin C and calcium than the nonobese group. Total iron intake was not different between groups. Fat mass was a negative predictor of sFe. |
| Zimmermann et al. (51) | 2008 | Premenopausal women in Thailand (n = 92) and children in Morocco and India (n = 1688 for baseline studies and n = 727 for intervention studies) | BMI and iron absorption using an isotope-labeled reference meal (Thailand cohort). BMI Z-scores, iron status (Hb, ferritin, sTfR, and body iron stores) (baseline and intervention studies in Morocco and India) | In Thai cohort, 20% of subjects were iron deficient and 22% were overweight. Inflammation and lower iron absorption were associated with higher BMI Z-score. There was 42% iron deficiency and 6.3% overweight in Indian and Moroccan children. For these 2 cohorts, BMI was inversely related to iron status at baseline and less improvement in iron status after an iron-fortified diet. |
| Tussing-Humphreys et al. (109) | 2009 | NHANES III: female adolescents 12–17 y (n = 210) (United States) | BMI, iron status (sFe, Tsat, sTfR, Hb, mean cell volume, erythrocyte protoporphyrin), CRP, physical activity level (metabolic equivalent score), dietary intake | Overweight and obese subjects (BMI > 85th percentile) had more iron deficiency (31%) than lean subjects (14%). Groups did not differ in iron intake, age, physical activity, or time of first menarche. Both high BMI and CRP were predictors of lower iron status. Iron status was lower in the high-BMI group. |
| Cepeda-Lopez et al. (50) | 2011 | Mexican Nutrition Survey (1999): children (n = 1174) and women (n = 621) (Mexico) | BMI (women) or BMI Z-scores (children), iron status (Hb, sFe, Tsat), CRP, dietary intake of iron, and factors that affect iron absorption | Obesity was seen in 25% of women and 3.5% of children. There was higher risk for iron deficiency in obese women and children than in lean subjects. CRP was higher in the overweight and obese groups and was a negative predictor of iron status. Iron intake did not differ between lean and obese subjects. |
Studies reporting only hemoglobin and/or ferritin as measurement of iron status have been excluded. CRP, C-reactive protein; Hb, hemoglobin; HCT, hematocrit; sFe, serum iron; sTfR; soluble transferrin receptor; TIBC, total iron binding capacity; Tsat, transferrin saturation.
It was later observed that the identified association between obesity and low iron status was independent of iron intake or other dietary factors (44, 45, 50, 51). In fact, studies found that obese individuals become iron deficient despite having adequate iron consumption. Furthermore, it was observed that iron absorption was affected by obesity. A recent study related poor iron absorption to obesity and adiposity-related inflammation in women and children through the measurement of stable iron isotope incorporation (51). These groups were in transition countries and were less responsive to iron fortification than their non-obese counterparts. Iron status was negatively correlated with C-reactive protein (CRP) and BMI in women. In children, an inverse relation was found between BMI Z-score and body iron. In addition, change in body iron after iron supplementation was inversely related to BMI, suggesting that absorption was reduced in obese children. Another study found that obesity was a strong predictor of iron deficiency in obese women and children from Mexico, regardless of iron intake (50). Obese women were 2 times as likely as lean women, and obese children were 4 times as likely as lean children, to be iron deficient (50). In summary, these studies identified iron deficiency as a comorbidity of obesity in different age groups, independent of iron consumption.
Obesity is characterized by low-grade chronic inflammation, that is, an increase in circulating inflammatory molecules, such as IL-6 and CRP (52), which in turn is associated with chronic disease risk. In recent years, studies have shown an association between hepcidin, chronic inflammation, and low iron status in obese populations (15, 46, 53–57), identifying a possible mechanism by which iron status impairment occurs in the obese (Table 2). These studies consistently showed an association between serum hepcidin, inflammation, and low iron status. These associations have not been established in overweight (BMI ≥25 and <30 kg/m2) populations. Of note, Tussing-Humphreys et al. (46) found substantially higher serum hepcidin and CRP, together with lower iron status in obese versus lean women. Similar results were reported in other adult cohorts, as well as children (53, 55, 57), and pregnant women (54). As to the latter, we recently found higher hepcidin and CRP levels in obese pregnant women compared with lean pregnant women (54). Iron status markers did not differ between the mothers, but they did for the newborns, with lower transferrin saturation (Tsat) and serum iron in cord blood from the obese group. In addition, we found strong negative correlations between maternal BMI and cord blood iron status and between maternal serum hepcidin and cord blood iron status. In summary, these data suggest that hepcidin dysregulation causes iron status impairment in obese individuals. Further studies are needed to confirm these results and to elucidate the mechanism of hepcidin dysregulation in obesity.
TABLE 2.
Cross-sectional or iron supplementation studies reporting iron status, inflammation, and hepcidin in relation to obesity1
| Author | Year | Population | Relevant primary measurements | Key findings |
| Bekri et al. (15) | 2006 | Three groups of obese participants: obese (n = 8), obese with diabetes (n = 7), obese with NASH (n = 10); lean controls (n = 9) (France) | BMI, iron status (sFe, Tsat, Hb, ferritin), CRP, and IL-6. Hepcidin expression in visceral and subcutaneous AT and liver biopsies. Hepcidin expression with stimulation in human AT cultured explants. | In the obese group, 68% had low Tsat (<25%) and 24% had anemia. Hepcidin mRNA and protein were expressed in liver and AT (no difference between visceral and subcutaneous), with substantially higher expression in AT of obese patients. There was no effect of diabetes or NASH. AT CRP and IL-6 strongly correlated with AT hepcidin levels. In vitro studies showed hepcidin stimulation in AT upon treatment with IL-6. |
| Aeberli et al. (53) | 2009 | Lean (n = 33) and overweight children (n = 85) 6–14 y (Switzerland) | Iron status (sTfR, ferritin, body iron), iron intake, and bioavailability, hepcidin (n = 30), CRP, IL-6 (n = 68), and leptin | Overweight group had lower iron status, seen as a higher sTfR. Hepcidin, CRP, and IL-6 were higher in the overweight group. No difference in iron intake or bioavailability was observed. |
| Giudice et al. (55) | 2009 | Obese (n = 60) and lean children (n = 50) (Italy) | BMI and BMI Z-score, iron status (sFe, Tsat, Hb, ferritin), iron absorption (iron load test), serum hepcidin, leptin, and IL-6 | Iron status (sFe, Tsat) was lower in obese children. Hepcidin, IL-6, and leptin were higher in the obese group. Hepcidin was inversely correlated with iron absorption. |
| Tussing-Humphreys et al. (46) | 2009 | Obese (n = 20) and nonobese (n = 20) premenopausal women; groups matched for Hb (United States) | BMI, waist circumference, iron status (sFe, Tsat, ferritin, sTfR), serum hepcidin, IL-6, and CRP. Iron accumulation and hepcidin mRNA in AT and liver biopsies. Dietary iron and vitamin C. | Obese group had higher serum hepcidin, CRP, and sTfR. Hepcidin did not correlate with iron status in the obese group, but it did in the nonobese group. There was little iron accumulation in AT and liver of the obese subjects. Liver hepcidin mRNA strongly correlated with serum hepcidin. |
| Luciani et al. (56) | 2011 | Two groups of morbidly obese women: group 1 (n = 18), blood and AT samples; and group 2 (n = 16), blood samples for serum HJV. Lean control group (n = 9 women), blood and AT samples (France) | BMI, iron status (sFe, Tsat, ferritin, Hb), CRP. HJV expression in AT biopsies. Hepcidin expression after BMP stimulation in cultured adipocytes | Serum CRP and HJV were higher in the obese group than in the lean group. Iron status (SFe, Tsat, and Hb) was lower in obese groups. HJV, Hamp, and IL-6 mRNA were substantially higher in obese group than in lean group. HJV in AT correlated with BMI and AT hepcidin expression. Treatment of cultured adipocytes with BMP2 stimulated hepcidin expression. |
| Sanad et al. (57) | 2011 | Three groups of children 7y old: obese with iron deficiency anemia (n = 35), non-obese with iron deficiency anemia (n = 35), and non-obese healthy (n = 30). All groups were supplemented with iron for 3 mo (Egypt) | BMI, iron status (sFe, Tsat, transferrin, ferritin, Hb), CRP, serum hepcidin | Compared with nonobese healthy children, serum hepcidin was lower in nonobese anemic children and higher in anemic obese children. CRP was highest in the obese group. Iron status was not different between the anemic groups. After 3 mo of iron supplementation, serum hepcidin increased in nonobese anemic but not in the obese group. Iron status increased in both anemic groups but to a lesser extent in the obese group. In the obese group, hepcidin was inversely related to iron status. |
| Tussing-Humphreys et al. (60) | 2011 | Obese (n = 9) and lean (n = 9) adults (UK) | Plasma hepcidin in arterialized vs. venous blood. Arterio-venous differences were measured across subcutaneous AT | There was no difference between arterialized and venous blood. Authors concluded hepcidin in obesity is not secreted from AT to the plasma and does not contribute to hepcidin dysregulation. |
| Dao et al. (54) | 2012 | Obese (n = 15) and lean (n = 15) pregnant women. Cord blood from obese (n = 8) and lean (n = 9) group (United States) | BMI, and maternal and cord blood iron status (sFe, Tsat, ferritin, Hb, HCT), CRP, and IL-6 | Hepcidin, CRP, and IL-6 were higher in the obese group, but not in cord blood from this group. Iron status was not different between obese and lean women, but cord blood from the obese group had lower iron status (sFe, Tsat) than the lean group. Serum hepcidin and BMI in women were negatively correlated with cord blood iron status. |
AT, adipose tissue; CRP, C-reactive protein; Hb, hemoglobin; HCT, hematocrit; HJV, hemojuvelin; NASH, nonalcoholic steatohepatitis; TIBC, total iron binding capacity; Tsat, transferrin saturation; sFe, serum iron; sTfR; soluble transferrin receptor.
Some publications in the past few years have shown that adipose tissue (AT) may play an important role in hepcidin dysregulation in obesity (15, 56). Bekri et al. (15) showed that hepcidin expression is higher in AT of obese individuals (BMI >42 kg/m2) who had a high prevalence (68%) of iron deficiency, defined as a Tsat <25%, and anemia (24%), defined as Hb <8 mmol/L. The same group later showed that HJV may be a primary regulator of hepcidin in AT. They found substantially higher hepcidin, IL-6, and HJV expression in AT of obese subjects compared with lean subjects (56). BMI and AT HJV expression were correlated. When cultured adipocytes were treated with BMP2, which is downstream of HJV signaling, hepcidin expression was upregulated. AT may also contribute to hepcidin dysregulation in other tissues through adipokine secretion. Chung et al. (58) found that hepcidin in HuH17 human hepatoma cells was upregulated by leptin through the JAK/STAT3 pathway. Leptin is substantially upregulated in obesity because it is secreted by AT (59), and liver is the main producer of hepcidin (5), thus, increased adiposity may have an impact on hepatic hepcidin, but further research is needed.
The effect of obesity on AT hepcidin expression is not completely understood. Tussing-Humphreys et al. (60) recently found no differences between arterial and venous hepcidin across subcutaneous AT of obese and lean subjects. In addition, no differences were found between groups, suggesting that subcutaneous AT may not be a major contributor of hepcidin secretion into plasma. However, the study sample size was small, with only 9 subjects per group, and the subjects’ iron status and serum hepcidin levels spanned a wide range, making it difficult to draw definite conclusions. Also, important differences may exist between subcutaneous and visceral AT, although Bekri et al. (15) showed that hepcidin mRNA levels were not different between the two in morbidly obese patients. Together, these findings suggest a mechanistic link between low iron status and obesity through inflammation, where AT hepcidin expression may play an important role. More research is needed to determine the influence of AT on hepcidin levels and iron status. Further research on the impact of chronic hepcidin upregulation on iron absorption, a site of iron status regulation, and on the impact of obesity-associated hepcidin upregulation in other cell types, such as PBMCs, is needed.
Weight loss in obese people, inflammation, and iron status.
Weight loss studies, of both calorie restriction (CR) and restrictive bariatric surgery, are good approaches to studying the underlying mechanisms of iron status impairment and hepcidin dysregulation in obesity. To date, 2 intervention studies measuring the effect of weight loss on iron status, hepcidin, and inflammation have been conducted (Table 3). The first one, by Tussing-Humphreys et al. (47) showed that 6 mo after bariatric surgery obese women had a substantial decrease in systemic inflammation (measured as CRP and IL-6) and serum hepcidin as well as an improvement in iron status markers. The second study, by Amato et al. (61) found that obese children losing weight through CR experienced a decrease in serum IL-6 and hepcidin, accompanied by enhanced iron status. They also observed an improvement in iron absorption after weight loss. No studies on the impact of CR on hepcidin and iron status in adults are available. Bariatric surgery may not be a good model to study changes in iron status and hepcidin. Malabsorptive bariatric interventions are the most effective for weight loss, but as a result of the excision of a considerable portion of the small intestine, iron absorption is impaired. Iron deficiency and anemia are common complications in bariatric patients after surgery (62). Therefore, CR studies in adults are needed to determine the effect of weight loss on iron homeostasis and to uncover the underlying mechanisms of iron deficiency in obesity.
TABLE 3.
Studies on the impact of weight loss interventions on iron status, inflammation, and hepcidin1
| Author | Year | Population | Relevant primary measurements | Key findings |
| Ausk et al. (107) | 1997 | Severely obese women (n = 43) on 3 different very low-energy diets: lowest calorie, middle calorie supplemented with iron (27 mg/dL), and highest calorie (United States) | BMI, body composition (fat mass and fat-free mass), and iron status (Tsat, ferritin, Hb, HCT) | Initially, Tsat decreased by 30% in all groups, but only the iron-supplemented group had an increase in iron status back to normal levels. |
| Di Toro et al. (110) | 1997 | Obese children and adolescents (n = 55) on a 13-wk hypocaloric diet (n = 22) or a 10-wk (n = 33) protein sparing modified fast diet (Italy) | Ideal body weight (IBW), arm fat area, arm muscle area, iron status (sFe, Tsat, ferritin), dietary intake (24-h recall) | There was weight loss with both diets but no change in iron status. |
| Anty et al. (111) | 2008 | Morbidly obese women (n = 178) undergoing bariatric surgery with a 6-mo follow-up (n = 55) (France) | BMI, iron status (plasma iron, Tsat, ferritin, Hb), CRP | At baseline, there was a high prevalence of iron deficiency (53%, Tsat <20%), and 6% of the subjects were anemic (Hb < 12 g/dL). Six months after bariatric surgery there was a decrease in CRP and an increase in Tsat, from 18% to 25%. |
| Amato et al. (61) | 2010 | Obese children (n = 15) undergoing a 6-mo calorie-restricted diet (Italy) | BMI, iron status (sFe, Tsat, ferritin), iron absorption (iron load test), serum hepcidin, IL-6, and leptin | There was a decrease in BMI, serum hepcidin, and leptin and a trend for decrease in IL-6. Also, iron status (sFe, Tsat) and iron absorption increased after weight loss. Hepcidin was positively correlated with serum leptin. |
| Tussing-Humphreys et al. (47) | 2010 | Premenopausal obese women undergoing bariatric surgery (n = 20) with a 6-mo follow-up (United States) | BMI, waist circumference, iron status (sFe, Tsat, sTfR, ferritin, Hb, HCT), serum hepcidin, CRP, IL-6, dietary iron | At baseline, iron deficiency was seen in 45% of subjects (sTfR >28.1 nmol/L). There was a decrease in BMI, CRP, serum hepcidin, and sTfR. There were increases in Hb and HCT. Change in hepcidin was not associated with change in BMI. |
| Tussing-Humphreys et al. (71) | 2011 | Obese (n = 17) and lean (n = 19) premenopausal women. Outcomes were also measured in obese group 6-mo after bariatric surgery (United States) | BMI, waist circumference, iron status (sTfR, Hb), serum hepcidin, CRP, IL-6. Cytokine production (IL-6, IL-10, IL-22, IFN-γ, TNF-α) by WBC after stimulation with LPS or ZY | At baseline, cytokine production (IL-6, IFN-γ, and TNF-α) after stimulation with LPS or ZY was lower in the obese vs. lean women. After weight loss, IL-6 and TNF-α production in the obese group increased and became equal to the nonobese group. At baseline, IFN-γ ex vivo production in the obese group correlated negatively with serum hepcidin and positively with sTfR. |
AT, adipose tissue; CRP, C-reactive protein; Hb, hemoglobin; HCT, hematocrit; LPS, lipopolysaccharide; sFe, serum iron; sTfR; soluble transferrin receptor; TIBC, total iron binding capacity; Tsat, transferrin saturation; WBCs, white blood cells; ZY, zymosan.
Determination of iron status in obese populations
Iron status can be determined through several parameters in serum or plasma, including serum iron, total iron binding capacity (TIBC), Tsat, ferritin, soluble TfR (sTfR), and the ratio of sTfR to log-ferritin. For healthy individuals with no chronic or acute inflammation, serum ferritin is a good indicator of iron status. Another commonly used marker is sTfR, which is directly related to cellular iron stores: the higher the level of sTfR in circulation the lower the iron status (63). However, cutoff values and normal ranges for sTfR are variable between publications and assays (63), thus when sTfR is used as a measure of iron status, it is necessary to indicate assay specifications. Serum iron, TIBC, and Tsat are also effectively used to measure iron status. TIBC is a measure of total circulating transferrin levels, and Tsat is calculated by the following formula: Tsat = (serum iron/TIBC) × 100%. Hb is used to determine the presence of anemia.
Determining iron status in obese individuals is more complicated and controversial because low-grade chronic inflammation affects several markers of iron status. In addition, the definition of iron deficiency in the obese has not been consistent in different publications. Ferritin levels are affected by chronic inflammation (63), and ferritin expression in hepatocytes, macrophages, and adipocytes is induced by IL-1β and TNF-α (64), which are known to be upregulated in obesity. When iron sequestration occurs in cells without real iron deficiency, as may be the case with obese individuals, sTfR levels are normal. Tussing-Humphreys et al. (20) suggested that in obesity chronic inflammation leads to real iron deficiency as a result of long-term decreased iron absorption and unregulated iron loss. The qualifiers for iron deficiency in obese individuals may therefore be similar to those seen in anemia of chronic disease coexisting with real iron deficiency. However, this definition of iron deficiency has not always been the case in studies measuring iron status in obese populations, and further research is needed.
The criteria used by Muñoz et al. (62) to diagnose iron deficiency in obese patients are chronic inflammation (CRP >1), low Hb (<12 g/dL for women and <13 g/dL for men), low Tsat (<20%), normal to high ferritin (30–100 μg/L), sTfR above the normal range (range specified by manufacturer), and a high ratio of sTfR to log-ferritin (>2). For anemia of chronic disease without real iron deficiency, sTfR would be normal and ferritin would be >100 μg/L. Similarly, Tussing-Humphreys et al. (20) have defined iron status in obese individuals as unaffected or low Hb, unaffected or increased ferritin, decreased serum iron and Tsat, unaffected or increased TIBC, and high sTfR.
sTfR is not influenced by acute or chronic inflammation; instead, the level of sTfR reflects cellular iron requirements and is proportional to TfR on cell membranes (47). Therefore, sTfR is a marker that can be used to define true iron deficiency. The effect of weight loss through CR in obese individuals on sTfR has not been reported. In conclusion, for populations with chronic inflammation, such as those with obesity, it is necessary to take into account markers of chronic inflammation, specifically CRP, and a combination of iron status markers to define iron deficiency. In addition, establishing a set of criteria used consistently for the diagnosis of iron deficiency in obese individuals will be essential for the advancement of the field.
Immune function is altered in obesity
Obesity and immune response.
Several studies of high-fat–fed rodent models and obese humans have shown immune response impairment, including decreased macrophage, dendritic, and NKT cell function, and impaired lymphocyte response to mitogenic or antigenic stimulation (65, 66). Specifically, T-cell function is impaired as shown by decreased thymic output of naïve T-cells and lower lymphocyte proliferation, with evidence of altered circulating numbers of CD4+ and CD8+ T-cells (65–68). Evidence also shows T-cells skewed toward a Th1 phenotype with higher production of IFN-γ in humans (69, 70). These alterations in the immune system, which are associated with other obesity comorbidities such as type 2 diabetes, may explain the increased prevalence of susceptibility to infections in obese individuals (65, 66).
Iron homeostasis, although important for immune function as shown in previous sections of this review, has not been studied extensively together with immune response in obese individuals. Only 1 study has measured the effect of obesity and weight loss on iron status, hepcidin, and immune response simultaneously (71). This study found that despite higher serum levels of inflammatory cytokines, obese women had lower production of IL-6, TNF-α, and IFN-γ than lean women in PBMCs stimulated with LPS (71). It seems that even though basal production of inflammatory cytokines is higher in obese individuals, stimulated immune cell cytokine production is suppressed in these individuals compared with lean individuals. Although this might appear contradictory, it could indicate that the higher inflammation associated with obesity renders the immune cells less responsive to activation. This study found that serum hepcidin and sTfR were correlated with ex vivo production of IFN-γ upon stimulation of PBMCs. These results suggest that further research is needed to explore the role of hepcidin and iron status in immune dysregulation seen in obesity. Many of the studies conducted so far on the impact of obesity on immunity have small sample sizes and populations with mixed age groups and comorbidities, and studies that account for these factors are needed (65). In conclusion, immune function is impaired with obesity, but more information is needed to characterize this impairment in different age groups and link it to clinical outcomes. Chronic inflammation seems to desensitize the immune response when it encounters stimuli such as LPS. Whether hepcidin and iron play a role in this desensitization remains to be elucidated.
Weight loss in obese individuals and immune function.
In general, weight loss is associated with improved health. As little as 1% weight loss has been associated with a decrease in systemic inflammation (72). The most consistent systemic changes with weight loss are decreases in serum CRP and IL-6. Decreased inflammation after weight loss has been associated with improvement in insulin sensitivity and iron status, among other conditions (47, 73–75). Most weight loss studies have also shown an improvement in cell-mediated immune response measured as lymphocyte proliferation (65, 71, 76, 77). For example, Ahmed et al. (76) showed an improvement in lymphoproliferation after a 10 or 30% CR over 6 mo. This improvement was shown in whole blood stimulated with concanavalin A and phytohemagglutinin. Antigenic stimulation was improved in the 30% calorie-restricted group but not in the 10% calorie-restricted group. In addition, they found that delayed type hypersensitivity response, an in vivo measure of T-cell function, improved with CR. In this study, percent change in BMI was correlated with percent increase in lymphoproliferation. These findings show that CR can improve T-cell function. Given that not all studies have shown substantial and positive effects of weight loss on immune response, more research is needed.
AT inflammation (78, 79) and immune cell infiltration (80) have been shown to decrease with weight loss. However, AT remodeling during weight loss is not completely understood. A study by Capel et al. (81) presented AT gene profiling at different stages of weight loss in obese women: a 4-wk very low calorie diet, followed by a 2-mo weight stabilization period on a low-calorie diet and a 3- to 4-mo weight maintenance period. They showed that macrophage genes are initially upregulated during the very low calorie diet, together with a downregulation in genes involved in adipocyte metabolism. During the weight stabilization period they showed a reversed gene expression profile, with attenuation of macrophage gene expression and increase in adipocyte metabolic genes. Therefore, it is important to consider the stage of weight loss an individual is in when conducting CR studies. This study further showed a decrease in plasma CRP while AT macrophage markers were increasing in the energy restriction period, suggesting that adipose and systemic inflammation might, to a certain extent, be regulated separately.
In conclusion, evidence regarding the effect of weight loss on immune response and AT architecture seems to depend on the type and duration of the weight loss intervention, and more research is needed to further define these relations. In addition, the long-term effects of weight loss on immune response have not been determined (65).
Old age is characterized by increased adiposity and inflammation, impaired immune function, and low iron status
Old age is characterized by chronic inflammation and the decline of the immune response. Iron status is also impaired with aging, but the reason why is not completely understood. The prevalence of obesity in the elderly has increased at worrisome rates. With the expansion of the elderly population worldwide, expected to reach 20% of the world’s population by 2050 from a current 8% (82), and aging-associated morbidities placing a high burden on health care systems around the world, it is important to address issues such as iron status impairment in the obese elderly.
Obesity and increased adiposity are prevalent in the elderly.
Older adults are becoming obese at a global scale, with an estimate of >20% obese elderly in the United States by 2030 (83). Elderly obesity can lead to frailty and represents an increased challenge for mobility and strength because it leads to added fat mass to the already increasing levels of body fat seen with aging. Being elderly and overweight (BMI ≥25 and <30 kg/m2) but not obese is not associated with disease risk (84), but a meta-analysis showed that BMI >30 kg/m2 in older adults (≥65 y) was associated with a modest increase in the risk of mortality (85). Previously, intentional weight loss for older individuals was not recommended because it was considered to increase risk for disease and mortality (84). However, more recently, weight loss in elderly obese individuals has become a recommendation (86), as long as the approach includes both CR and increased physical activity. If this approach proves challenging because of the physical constraints and changes in body composition in the elderly, other approaches such as pharmacology and bariatric surgery can be used. Very few studies have been conducted to measure the effectiveness of weight loss on different health outcomes in obese elderly individuals. In their review, Witham and Avenell (84) stressed the need and importance for conducting such trials.
Aging, inflammation, and immune response.
The elderly experience low-grade chronic inflammation, characterized by an increase in circulating levels of IL-6 and CRP, among other inflammatory markers (87, 88). Higher IL-6 is an independent predictor of mortality and morbidity in older adults (87). In addition, old age is characterized by weakening of cell-mediated immunity, particularly that of T-cells (89, 90). Changes in the immune system observed with aging are substantial (88, 90). A consistent age-related defect observed across different models is the impaired ability of T-cells to proliferate and produce IL-2 in response to antigenic or mitogenic stimulation (89, 91).
Little is known about the effect of obesity on immune response in the elderly. A recent study showed that obesity negatively affects aging of the immune system, with acceleration of thymic involution, increase in perithymic AT, and reduction of T-cell precursors (92), narrowing the immune repertoire against antigens encountered later in life. Therefore, studies are needed to investigate the effect of obesity on the already declining elderly immune response.
Iron deficiency in the elderly.
The elderly have a high prevalence of anemia, defined by the WHO as Hb < 12 g/dL for women and <13 g/dL for men. Using this definition, anemia increases considerably after the age of 50 (93, 94). Moderate anemia and iron deficiency also increase with aging (94). Complications associated with anemia are cardiovascular disease, increased falls and fractures, and cognitive impairment. For example, a study found that the incidence of cognitive impairment was higher in anemic versus non-anemic men (55.6% vs. 34.4%) and women (47.5% vs. 40.1%) (95). A retrospective and observational study in nursing home residents and community-dwelling elderly individuals found that hospitalization as a result of hip fractures was more likely to occur in patients with anemia (30% in anemic vs. 13% in non-anemic) and that higher Hb was protective against falls resulting in fractures, showing that for every 1 g/dL increase in Hb, the risk decreased by 45% (96). With anemia, there is also decreased quality of life, increased frailty, and higher risk of mortality (94). A systematic review found that treating anemia was associated with increased quality of life in patients with cancer and renal disease (97). A more extensive analysis of the effects of iron deficiency and iron deficiency anemia can be found in a review by Eisenstaedt et al. (94).
The most common type of anemia in the elderly is anemia of chronic inflammation, caused by chronic conditions prevalent in older populations (98). According to studies using data from NHANES III, one-third of anemia cases in older people are caused by nutritional deficiencies of iron, folate, or vitamin B-12, with iron deficiency accounting for more than half of these deficiencies (93, 94). Low dietary iron, impaired iron absorption as a result of reduced stomach acid production, and gastrointestinal bleeding also contribute to iron deficiency anemia in older adults (93, 94, 98, 99). As with obesity, diagnosis of iron deficiency in the elderly is challenging because of the presence of chronic inflammation (99). Very limited information is available on the impact of obesity on iron homeostasis in the elderly. Recently, we found that elderly women with high BMI and waist circumference from low-income areas of Quito, Ecuador, had higher serum hepcidin and CRP than their lean counterparts (100, and unpublished data). Even though iron status was inversely related to CRP, it did not differ between women with high and normal BMI. Dietary iron, as well as intake of other micronutrients, was low in this population. Further research is necessary to determine the factors regulating iron status in elderly populations with a high prevalence of obesity accompanied by poor nutrition. Furthermore, evidence is needed on the effect of aging on hepcidin regulation and on the effect of weight loss on hepcidin, inflammation, and iron status in older obese individuals.
In this review we have summarized the research conducted thus far regarding the impact of obesity on iron status, inflammation, hepcidin, and immune response. We have described what is known about the role that iron and hepcidin play in immune response and identified gaps in research regarding iron homeostasis, immune function, obesity, and aging, where hepcidin may play an important role.
As a whole, the available evidence strongly suggests that chronic inflammation of obesity and aging may impair iron status through hepcidin and that hepcidin is not only crucial for iron homeostasis but also for immune response. Further research is needed in these areas: 1) the effect of inflammation of obesity in young and older individuals on iron homeostasis and immunity, 2) the role of hepcidin in the adaptive immune response, 3) the impact of weight loss on iron homeostasis and immune response in the obese, and 4) the effect of aging on these processes.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AT, adipose tissue; BMP, bone morphogenetic protein; BMPR, bone morphogenetic protein receptor; CR, calorie restriction; CRP, C-reactive protein; Hb, hemoglobin; HJV, hemojuvelin; NK, natural killer; PBMC, peripheral blood mononuclear cell; ROS, reactive oxygen species; sTfR, soluble transferrin receptor; TfR, transferrin receptor; TIBC, total iron binding capacity; Tsat, transferrin saturation.
Literature Cited
- 1.Nutritional anemias: report of a WHO scientific group. WHO Technical Report Series. Geneva, World Health Organization; 1968 [PubMed] [Google Scholar]
- 2.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12:444–54 [DOI] [PubMed] [Google Scholar]
- 3.World Health OrganizationIron deficiency anemia assessment, prevention and control. A guide for programme managers. Geneva: World Health Organization; 2001 [Google Scholar]
- 4.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38 [DOI] [PubMed] [Google Scholar]
- 5.Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112:219–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins HL. Withholding iron as a cellular defence mechanism–friend or foe? Eur J Immunol. 2008;38:1803–6 [DOI] [PubMed] [Google Scholar]
- 7.Doherty CP. Host-pathogen interactions: the role of iron. J Nutr. 2007;137:1341–4 [DOI] [PubMed] [Google Scholar]
- 8.Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci USA. 2005;102:1324–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laftah AH, Ramesh B, Simpson RJ, Solanky N, Bahram S, Schumann K, Debnam ES, Srai SK. Effect of hepcidin on intestinal iron absorption in mice. Blood. 2004;103:3940–4 [DOI] [PubMed] [Google Scholar]
- 10.Pinto JP, Dias V, Zoller H, Porto G, Carmo H, Carvalho F, de Sousa M. Hepcidin messenger RNA expression in human lymphocytes. Immunology. 2010;130:217–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brasse-Lagnel C, Karim Z, Letteron P, Bekri S, Bado A, Beaumont C. Intestinal DMT1 cotransporter is down-regulated by hepcidin via proteasome internalization and degradation. Gastroenterology. 2011;140(4):1261–1271.e1. [DOI] [PubMed] [Google Scholar]
- 12.Chaston T, Chung B, Mascarenhas M, Marks J, Patel B, Srai SK, Sharp P. Evidence for differential effects of hepcidin in macrophages and intestinal epithelial cells. Gut. 2008;57:374–82 [DOI] [PubMed] [Google Scholar]
- 13.Frazer DM, Anderson GJ. Hepcidin compared with prohepcidin: an absorbing story. Am J Clin Nutr. 2009;89:475–6 [DOI] [PubMed] [Google Scholar]
- 14.Malyszko J. Hepcidin assays: ironing out some details. Clin J Am Soc Nephrol. 2009;4:1015–6 [DOI] [PubMed] [Google Scholar]
- 15.Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Iannelli A, Staccini-Myx A, Casanova D, Ben Amor I, et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788–96 [DOI] [PubMed] [Google Scholar]
- 16.Goswami T, Andrews NC. Hereditary hemochromatosis protein, HFE, interaction with transferrin receptor 2 suggests a molecular mechanism for mammalian iron sensing. J Biol Chem. 2006;281:28494–8 [DOI] [PubMed] [Google Scholar]
- 17.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee P, Peng H, Gelbart T, Beutler E. The IL-6- and lipopolysaccharide-induced transcription of hepcidin in HFE-, transferrin receptor 2-, and beta 2-microglobulin-deficient hepatocytes. Proc Natl Acad Sci USA. 2004;101:9263–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy CN, Custodio AO, de Graaf J, Schneider S, Akpan I, Montross LK, Sanchez M, Gaudino A, Hentze MW, Andrews NC, et al. An Hfe-dependent pathway mediates hyposideremia in response to lipopolysaccharide-induced inflammation in mice. Nat Genet. 2004;36:481–5 [DOI] [PubMed] [Google Scholar]
- 20.Tussing-Humphreys L, Pusatcioglu C, Nemeth E, Braunschweig C. Rethinking iron regulation and assessment in iron deficiency, anemia of chronic disease, and obesity: introducing hepcidin. J Acad Nutr Diet. 2012;112:391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr. 2001;131(2S–2):616S–633S; discussion 633S–635S. [DOI] [PubMed] [Google Scholar]
- 22.Drakesmith H, Prentice A. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6:541–52 [DOI] [PubMed] [Google Scholar]
- 23.Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science. 2012;338:768–72 [DOI] [PubMed] [Google Scholar]
- 24.Schaible UE, Kaufmann SH. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–53 [DOI] [PubMed] [Google Scholar]
- 25.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–43 [DOI] [PubMed] [Google Scholar]
- 26.Prentice AM, Ghattas H, Doherty C, Cox SE. Iron metabolism and malaria. Food Nutr Bull. 2007; 28(4, Suppl)S524–39 [DOI] [PubMed] [Google Scholar]
- 27.de Monyé C, Karcher DS, Boelaert JR, Gordeuk VR. Bone marrow macrophage iron grade and survival of HIV-seropositive patients. AIDS. 1999;13:375–80 [DOI] [PubMed] [Google Scholar]
- 28.Khan FA, Fisher MA, Khakoo RA. Association of hemochromatosis with infectious diseases: expanding spectrum. Int J Infect Dis. 2007;11:482–7 [DOI] [PubMed] [Google Scholar]
- 29.Ganz T. Iron in innate immunity: starve the invaders. Curr Opin Immunol. 2009;21:63–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–21 [DOI] [PubMed] [Google Scholar]
- 31.Peyssonnaux C, Zinkernagel AS, Datta V, Lauth X, Johnson RS, Nizet V. TLR4-dependent hepcidin expression by myeloid cells in response to bacterial pathogens. Blood. 2006;107:3727–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, Sonnweber T, Eberwein L, Witcher DR, Murphy AT, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113:5277–86 [DOI] [PubMed] [Google Scholar]
- 33.Ludwiczek S, Aigner E, Theurl I, Weiss G. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood. 2003;101:4148–54 [DOI] [PubMed] [Google Scholar]
- 34.Nairz M, Fritsche G, Brunner P, Talasz H, Hantke K, Weiss G. Interferon-gamma limits the availability of iron for intramacrophage Salmonella typhimurium. Eur J Immunol. 2008;38:1923–36 [DOI] [PubMed] [Google Scholar]
- 35.Frazier MD, Mamo LB, Ghio AJ, Turi JL. Hepcidin expression in human airway epithelial cells is regulated by interferon-γ. Respir Res. 2011;12:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delaby C, Pilard N, Goncalves AS, Beaumont C, Canonne-Hergaux F. Presence of the iron exporter ferroportin at the plasma membrane of macrophages is enhanced by iron loading and down-regulated by hepcidin. Blood. 2005;106:3979–84 [DOI] [PubMed] [Google Scholar]
- 37.Paradkar PN, De Domenico I, Durchfort N, Zohn I, Kaplan J, Ward DM. Iron depletion limits intracellular bacterial growth in macrophages. Blood. 2008;112:866–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward RJ, Crichton RR, Taylor DL, Corte LD, Srai SK, Dexter DT. Iron and the immune system. J Neural Transm. 2011;118:315–28 [DOI] [PubMed] [Google Scholar]
- 39.Porto G, De Sousa M. Iron overload and immunity. World J Gastroenterol. 2007;13:4707–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joynson DH, Walker DM, Jacobs A, Dolby AE. Defect of cell-mediated immunity in patients with iron-deficiency anaemia. Lancet. 1972;2:1058–9 [DOI] [PubMed] [Google Scholar]
- 41.Omara FO, Blakley BR. The effects of iron deficiency and iron overload on cell-mediated immunity in the mouse. Br J Nutr. 1994;72:899–909 [DOI] [PubMed] [Google Scholar]
- 42.Spear AT, Sherman AR. Iron deficiency alters DMBA-induced tumor burden and natural killer cell cytotoxicity in rats. J Nutr. 1992;122:46–55 [DOI] [PubMed] [Google Scholar]
- 43.Lecube A, Carrera A, Losada E, Hernandez C, Simo R, Mesa J. Iron deficiency in obese postmenopausal women. Obesity (Silver Spring). 2006;14:1724–30 [DOI] [PubMed] [Google Scholar]
- 44.Menzie CM, Yanoff LB, Denkinger BI, McHugh T, Sebring NG, Calis KA, Yanovski JA. Obesity-related hypoferremia is not explained by differences in reported intake of heme and nonheme iron or intake of dietary factors that can affect iron absorption. J Am Diet Assoc. 2008;108:145–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yanoff LB, Menzie CM, Denkinger B, Sebring NG, McHugh T, Remaley AT, Yanovski JA. Inflammation and iron deficiency in the hypoferremia of obesity. Int J Obes (Lond). 2007;31:1412–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tussing-Humphreys LM, Nemeth E, Fantuzzi G, Freels S, Guzman G, Holterman AX, Braunschweig C. Elevated systemic hepcidin and iron depletion in obese premenopausal females. Int J Obes (Lond). 2010;18:1449–56 [DOI] [PubMed] [Google Scholar]
- 47.Tussing-Humphreys LM, Nemeth E, Fantuzzi G, Freels S, Holterman AX, Galvani C, Ayloo S, Vitello J, Braunschweig C. Decreased serum hepcidin and improved functional iron status 6 months after restrictive bariatric surgery. Obesity (Silver Spring). 2010;18:2010–6 [DOI] [PubMed] [Google Scholar]
- 48.Cheng HL, Bryant C, Cook R, O'Connor H, Rooney K, Steinbeck K. The relationship between obesity and hypoferraemia in adults: a systematic review. Obes Rev. 2012;13:150–61 [DOI] [PubMed] [Google Scholar]
- 49.Wenzel BJ, Stults HB, Mayer J. Hypoferraemia in obese adolescents. Lancet. 1962;2:327–8 [DOI] [PubMed] [Google Scholar]
- 50.Cepeda-Lopez AC, Osendarp SJ, Melse-Boonstra A, Aeberli I, Gonzalez-Salazar F, Feskens E, Villalpando S, Zimmermann MB. Sharply higher rates of iron deficiency in obese Mexican women and children are predicted by obesity-related inflammation rather than by differences in dietary iron intake. Am J Clin Nutr. 2011;93:975–83 [DOI] [PubMed] [Google Scholar]
- 51.Zimmermann MB, Zeder C, Muthayya S, Winichagoon P, Chaouki N, Aeberli I, Hurrell RF. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int J Obes (Lond). 2008;32:1098–104 [DOI] [PubMed] [Google Scholar]
- 52.Yudkin JS. Adipose tissue, insulin action and vascular disease: inflammatory signals. Int J Obes Relat Metab Disord. 2003;27: Suppl 3:S25–8 [DOI] [PubMed] [Google Scholar]
- 53.Aeberli I, Hurrell RF, Zimmermann MB. Overweight children have higher circulating hepcidin concentrations and lower iron status but have dietary iron intakes and bioavailability comparable with normal weight children. Int J Obes (Lond). 2009;33:1111–7 [DOI] [PubMed] [Google Scholar]
- 54.Dao MC, Sen S, Iyer C, Klebenov D, Meydani SN. Obesity during pregnancy and fetal iron status: is Hepcidin the link? J Perinatol. 2013;33:177–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.del Giudice EM, Santoro N, Amato A, Brienza C, Calabro P, Wiegerinck ET, Cirillo G, Tartaglione N, Grandone A, Swinkels DW, et al. Hepcidin in obese children as a potential mediator of the association between obesity and iron deficiency. J Clin Endocrinol Metab. 2009;94:5102–7 [DOI] [PubMed] [Google Scholar]
- 56.Luciani N, Brasse-Lagnel C, Poli M, Anty R, Lesueur C, Cormont M, Laquerriere A, Folope V, LeMarchand-Brustel Y, Gugenheim J, et al. Hemojuvelin: a new link between obesity and iron homeostasis. Int J Obes (Lond). 2011;19:1545–51 [DOI] [PubMed] [Google Scholar]
- 57.Sanad M, Osman M, Gharib A. Obesity modulate serum hepcidin and treatment outcome of iron deficiency anemia in children: a case control study. Ital J Pediatr. 2011;37:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chung B, Matak P, McKie AT, Sharp P. Leptin increases the expression of the iron regulatory hormone hepcidin in HuH7 human hepatoma cells. J Nutr. 2007;137:2366–70 [DOI] [PubMed] [Google Scholar]
- 59.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–5S [DOI] [PubMed] [Google Scholar]
- 60.Tussing-Humphreys L, Frayn KN, Smith SR, Westerman M, Dennis AL, Nemeth E, Thomson J, Pusatcioglu C. Subcutaneous adipose tissue from obese and lean adults does not release hepcidin in vivo. ScientificWorldJournal. 2011;11:2197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amato A, Santoro N, Calabro P, Grandone A, Swinkels DW, Perrone L, Miraglia Del Giudice E. Effect of body mass index reduction on serum hepcidin levels and iron status in obese children. Int J Obes (Lond). 2010;34:1772–4 [DOI] [PubMed] [Google Scholar]
- 62.Muñoz M, Botella-Romero F, Gomez-Ramirez S, Campos A, Garcia-Erce JA. Iron deficiency and anaemia in bariatric surgical patients: causes, diagnosis and proper management. Nutr Hosp. 2009;24:640–54 [PubMed] [Google Scholar]
- 63.Skikne BS. Serum transferrin receptor. Am J Hematol. 2008;83:872–5 [DOI] [PubMed] [Google Scholar]
- 64.Rogers JT. Ferritin translation by interleukin-1and interleukin-6: the role of sequences upstream of the start codons of the heavy and light subunit genes. Blood. 1996;87:2525–37 [PubMed] [Google Scholar]
- 65.Karlsson EA, Beck MA. The burden of obesity on infectious disease. Exp Biol Med (Maywood). 2010;235:1412–24 [DOI] [PubMed] [Google Scholar]
- 66.Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71:298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nieman DC, Henson DA, Nehlsen-Cannarella SL, Ekkens M, Utter AC, Butterworth DE, Fagoaga OR. Influence of obesity on immune function. J Am Diet Assoc. 1999;99:294–9 [DOI] [PubMed] [Google Scholar]
- 68.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pacifico L, Di Renzo L, Anania C, Osborn JF, Ippoliti F, Schiavo E, Chiesa C. Increased T-helper interferon-gamma-secreting cells in obese children. Eur J Endocrinol. 2006;154:691–7 [DOI] [PubMed] [Google Scholar]
- 70.Svec P, Vasarhelyi B, Paszthy B, Korner A, Kovacs L, Tulassay T, Treszl A. Do regulatory T cells contribute to Th1 skewness in obesity? Exp Clin Endocrinol Diabetes. 2007;115:439–43 [DOI] [PubMed] [Google Scholar]
- 71.Tussing-Humphreys L, Pini M, Ponemone V, Braunschweig C, Fantuzzi G. Suppressed cytokine production in whole blood cultures may be related to iron status and hepcidin and is partially corrected following weight reduction in morbidly obese pre-menopausal women. Cytokine. 2011;53:201–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klimcakova E, Kovacikova M, Stich V, Langin D. Adipokines and dietary interventions in human obesity. Obes Rev. 2010;11:446–56 [DOI] [PubMed] [Google Scholar]
- 73.Bougoulia M, Triantos A, Koliakos G. Effect of weight loss with or without orlistat treatment on adipocytokines, inflammation, and oxidative markers in obese women. Hormones (Athens). 2006;5:259–69 [DOI] [PubMed] [Google Scholar]
- 74.Kopp HP, Kopp CW, Festa A, Krzyzanowska K, Kriwanek S, Minar E, Roka R, Schernthaner G. Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients. Arterioscler Thromb Vasc Biol. 2003;23:1042–7 [DOI] [PubMed] [Google Scholar]
- 75.Tchernof A, Nolan A, Sites CK, Ades PA, Poehlman ET. Weight loss reduces C-reactive protein levels in obese postmenopausal women. Circulation. 2002;105:564–9 [DOI] [PubMed] [Google Scholar]
- 76.Ahmed T, Das SK, Golden JK, Saltzman E, Roberts SB, Meydani SN. Calorie restriction enhances T-cell-mediated immune response in adult overweight men and women. J Gerontol A Biol Sci Med Sci. 2009;64:1107–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tanaka S, Isoda F, Ishihara Y, Kimura M, Yamakawa T. T lymphopaenia in relation to body mass index and TNF-alpha in human obesity: adequate weight reduction can be corrective. Clin Endocrinol (Oxf). 2001;54:347–54 [PubMed] [Google Scholar]
- 78.Clément K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JD, et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18:1657–69 [DOI] [PubMed] [Google Scholar]
- 79.Moschen AR, Molnar C, Geiger S, Graziadei I, Ebenbichler CF, Weiss H, Kaser S, Kaser A, Tilg H. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 2010;59:1259–64 [DOI] [PubMed] [Google Scholar]
- 80.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–86 [DOI] [PubMed] [Google Scholar]
- 81.Capel F, Klimcakova E, Viguerie N, Roussel B, Vitkova M, Kovacikova M, Polak J, Kovacova Z, Galitzky J, Maoret JJ, et al. Macrophages and adipocytes in human obesity: adipose tissue gene expression and insulin sensitivity during calorie restriction and weight stabilization. Diabetes. 2009;58:1558–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dao MC, Meydani SN. Micronutrient status, immune response and infectious disease in elderly of less developed countries. Sight Life Mag. 2009;3:6–15 [PMC free article] [PubMed] [Google Scholar]
- 83.Houston DK, Nicklas BJ, Zizza CA. Weighty concerns: the growing prevalence of obesity among older adults. J Am Diet Assoc. 2009;109:1886–95 [DOI] [PubMed] [Google Scholar]
- 84.Witham MD, Avenell A. Interventions to achieve long-term weight loss in obese older people: a systematic review and meta-analysis. Age Ageing. 2010;39:176–84 [DOI] [PubMed] [Google Scholar]
- 85.Janssen I, Mark AE. Elevated body mass index and mortality risk in the elderly. Obes Rev. 2007;8:41–59 [DOI] [PubMed] [Google Scholar]
- 86.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–34 [DOI] [PubMed] [Google Scholar]
- 87.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–12 [DOI] [PubMed] [Google Scholar]
- 88.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–99 [DOI] [PubMed] [Google Scholar]
- 89.Nikolich-Zugich J. T cell aging: naive but not young. J Exp Med. 2005;201:837–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–4 [DOI] [PubMed] [Google Scholar]
- 92.Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA, Dixit VD. Obesity accelerates thymic aging. Blood. 2009;114:3803–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patel KV. Epidemiology of anemia in older adults. Semin Hematol. 2008;45:210–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eisenstaedt R, Penninx BW, Woodman RC. Anemia in the elderly: current understanding and emerging concepts. Blood Rev. 2006;20:213–26 [DOI] [PubMed] [Google Scholar]
- 95.Argyriadou S, Vlachonikolis I, Melisopoulou H, Katachanakis K, Lionis C. In what extent anemia coexists with cognitive impairment in elderly: a cross-sectional study in Greece. BMC Fam Pract. 2001;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dharmarajan TS, Norkus EP. Mild anemia and the risk of falls in older adults from nursing homes and the community. J Am Med Dir Assoc. 2004;5:395–400 [DOI] [PubMed] [Google Scholar]
- 97.Ross SD, Fahrbach K, Frame D, Scheye R, Connelly JE, Glaspy J. The effect of anemia treatment on selected health-related quality-of-life domains: a systematic review. Clin Ther. 2003;25:1786–805 [DOI] [PubMed] [Google Scholar]
- 98.Tussing-Humphreys L, Braunschweig C. Anemia in postmenopausal women: dietary inadequacy or nondietary factors? J Am Diet Assoc. 2011;111:528–31 [DOI] [PubMed] [Google Scholar]
- 99.Rimon E, Levy S, Sapir A, Gelzer G, Peled R, Ergas D, Sthoeger ZM. Diagnosis of iron deficiency anemia in the elderly by transferrin receptor-ferritin index. Arch Intern Med. 2002;162:445–9 [DOI] [PubMed] [Google Scholar]
- 100.Dao MC, Sempertegui F, Estrella B, Hamer D, Tucker K, Rodriguez A, Dallal G, Griffiths J, Meydani SN. Hepcidin in elderly Ecuadorians: A link between inflammation, BMI, and iron status. FASEB J. 2011;(25):779.1. [Google Scholar]
- 101.Seltzer CC, Mayer J. Serum iron and iron-binding capacity in adolescents. II. comparison of obese and nonobese subjects. Am J Clin Nutr. 1963;13:354–61 [DOI] [PubMed] [Google Scholar]
- 102.Micozzi MS, Albanes D, Stevens RG. Relation of body size and composition to clinical biochemical and hematologic indices in US men and women. Am J Clin Nutr. 1989;50:1276–81 [DOI] [PubMed] [Google Scholar]
- 103.Pinhas-Hamiel O, Newfield RS, Koren I, Agmon A, Lilos P, Phillip M. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int J Obes Relat Metab Disord. 2003;27:416–8 [DOI] [PubMed] [Google Scholar]
- 104.Nead KG, Halterman JS, Kaczorowski JM, Auinger P, Weitzman M. Overweight children and adolescents: a risk group for iron deficiency. Pediatrics. 2004;114:104–8 [DOI] [PubMed] [Google Scholar]
- 105.Moayeri H, Bidad K, Zadhoush S, Gholami N, Anari S. Increasing prevalence of iron deficiency in overweight and obese children and adolescents (Tehran Adolescent Obesity Study). Eur J Pediatr. 2006;165:813–4 [DOI] [PubMed] [Google Scholar]
- 106.Brotanek JM, Gosz J, Weitzman M, Flores G. Iron deficiency in early childhood in the United States: risk factors and racial/ethnic disparities. Pediatrics. 2007;120:568–75 [DOI] [PubMed] [Google Scholar]
- 107.Ausk KJ, Ioannou GN. Is obesity associated with anemia of chronic disease? A population-based study. Obesity (Silver Spring). 2008;16:2356–61 [DOI] [PubMed] [Google Scholar]
- 108.Eckhardt CL, Torheim LE, Monterrubio E, Barquera S, Ruel MT. The overlap of overweight and anaemia among women in three countries undergoing the nutrition transition. Eur J Clin Nutr. 2008;62:238–46 [DOI] [PubMed] [Google Scholar]
- 109.Tussing-Humphreys LM, Liang H, Nemeth E, Freels S, Braunschweig CA. Excess adiposity, inflammation, and iron-deficiency in female adolescents. J Am Diet Assoc. 2009;109:297–302 [DOI] [PubMed] [Google Scholar]
- 110.Di Toro A, Marotta A, Todisco N, Ponticiello E, Collini R, Di Lascio R, Perrone L. Unchanged iron and copper and increased zinc in the blood of obese children after two hypocaloric diets. Biol Trace Elem Res. 1997;57:97–104 [DOI] [PubMed] [Google Scholar]
- 111.Anty R, Dahman M, Iannelli A, Gual P, Staccini-Myx A, Amor IB, Luciani N, Saint-Paul MC, Huet PM, Sadoul JL, et al. Bariatric surgery can correct iron depletion in morbidly obese women: a link with chronic inflammation. Obes Surg. 2008;18:709–14 [DOI] [PubMed] [Google Scholar]
- 112.Young B. Wheater's functional histology: a text and colour atlas. 5th ed. Philadelphia: Churchill Livingstone/Elsevier; 2006 [Google Scholar]