Abstract
The liver plays a unique, central role in regulating lipid metabolism. In addition to influencing hepatic function and disease, changes in specific pathways of fatty acid (FA) metabolism have wide-ranging effects on the metabolism of other nutrients, extra-hepatic physiology, and the development of metabolic diseases. The high prevalence of nonalcoholic fatty liver disease (NAFLD) has led to increased efforts to characterize the underlying biology of hepatic energy metabolism and FA trafficking that leads to disease development. Recent advances have uncovered novel roles of metabolic pathways and specific enzymes in generating lipids important for cellular processes such as signal transduction and transcriptional activation. These studies have also advanced our understanding of key branch points involving FA partitioning between metabolic pathways and have identified new roles for lipid droplets in these events. This review covers recent advances in our understanding of FA trafficking and its regulation. An emphasis will be placed on branch points in these pathways and how alterations in FA trafficking contribute to NAFLD and related comorbidities.
Introduction
Hepatic steatosis is defined by the accumulation of TG in lipid droplets (LDs)4, commonly referred to as nonalcoholic fatty liver disease (NAFLD) in humans when alcohol is not a contributing factor. NAFLD can comprise simple steatosis or the more advanced nonalcoholic steatohepatitis, which is characterized by inflammation and hepatocyte injury that often is accompanied by fibrosis (1). The reported prevalence of NAFLD varies widely among limited studies with estimates showing that ~20–30% of the general population have NAFLD (1, 2), although numerous risk factors such as obesity (3–5), type 2 diabetes (6, 7), Hispanic ethnicity (2, 8), and male gender (9) increase its prevalence. NAFLD increases the risk of type 2 diabetes, dyslipidemia, hypertension, cardiovascular disease, chronic kidney disease, liver cirrhosis, hepatocellular carcinoma, and mortality (10–15). Unfortunately, there are limited effective treatment options for NAFLD. Lifestyle intervention involving weight loss, insulin-sensitizing agents, and vitamin E are the most recommended treatment options, but efficacy is limited due to patient adherence and safety concerns (16). Additionally, hepatic steatosis is not limited to humans and occurs in numerous other species. The most prevalent examples include obese cats, periparturient ruminants, especially dairy cows in negative energy balance, and poultry force-fed grain for foie gras production (17–19).
Ultimately, hepatic steatosis occurs due to an imbalance of TG synthesis and breakdown. An inherent difficulty in understanding the etiology of NAFLD is that there is no single pathway universally responsible for the development of steatosis. Rather, there is most likely heterogeneity in flux through individual pathways that ultimately results in the development of steatosis. These pathways are highly influenced by genetics, diet, environment (toxins, drugs, etc.), and the presence of specific diseases; thus, it is difficult to identify the cause of or optimal treatment for NAFLD.
Current Status of Knowledge
Pathways of fatty acid accretion
The liver derives fatty acids (FAs) from 3 primary sources: uptake of FFAs from the blood, chylomicron remnant uptake, and de novo lipogenesis (DNL). Each of these pathways is highly regulated; therefore, their contribution to the total liver FA pool is variable. In addition to these external or de novo sources of FAs, intracellular pathways such as TG hydrolysis and autophagy can also contribute to endogenous release of FAs. Alterations in any of these pathways influence both hepatic and whole-body energy metabolism and may contribute to disease development.
FA uptake and transport.
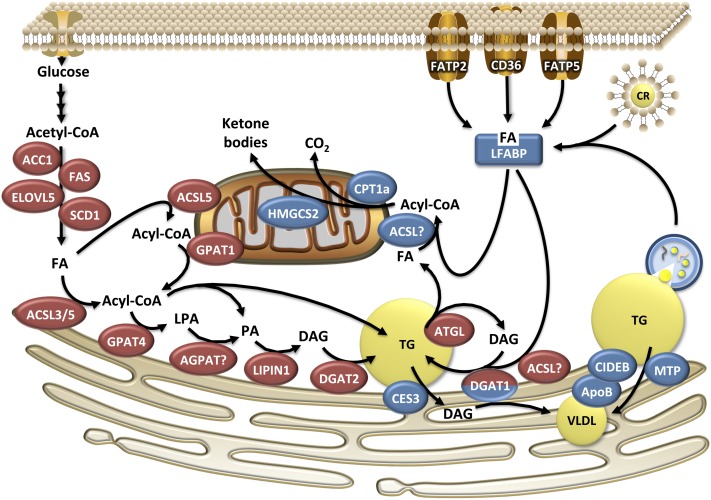
The liver extracts exogenous FFAs proportional to their concentration in the blood (20, 21). Studies employing arterial-venous balance and perfused rodent livers reveal that first-pass extraction of FFA is typically 20–30% (20, 21). There appears to be no difference in uptake among FA species with the exception of stearic acid (18:0), which has low or no hepatic net uptake, a finding that is conserved across numerous animal species (20, 22–25). Stable isotope studies in humans show that uptake of exogenous FFA is the single largest source of FA in stored hepatic TG and this contribution is further increased with fasting and NAFLD (26, 27). Increased visceral fat content is correlated with hepatic TG content (28–30) and has been implicated in NAFLD etiology. According to the portal vein theory, visceral adipose tissue releases FFA and cytokines into the portal vein, thereby exposing the liver to high concentrations of adipose-derived products. Yet visceral depots account for only 5–30% of hepatic FFA exposure (31) and the liver does not discriminate between FFA derived from different adipose depots. The importance of FFA derived from visceral adipose tissue to the portal vein theory and development of NAFLD remains to be determined. Clearance of chylomicron-remnant TG also contributes to the hepatic FA pool. In humans, chylomicron remnant uptake accounts for 15% of the liver FA pool during fasting and 25% during the fed state (26), and this contribution decreases with meal feeding compared with continuous feeding (32). Figure 1 illustrates the different possible routes of FA uptake and overviews the major pathways that contribute to hepatic FA trafficking.
FIGURE 1.
Pathways and enzymes involved in hepatic FA trafficking. Enzymes in red indicate catabolic pathways, whereas blue enzymes are involved in FA disposal. DGAT1 is bicolor, because it may be involved in the synthesis of cytosolic TG from exogenous FA and in VLDL synthesis. ACC1, acetyl-CoA carboxylase 1; ACSL, long chain acyl-CoA synthetase; AGPAT, sn-1-acyl-glycerol-3-phosphate acyltransferase; ATGL, adipose triglyceride lipase; CES, carboxylesterase; CIDEB, cell death-inducing DFF45-like effector B; CPT1a, carnitine palmitoyl transferase 1; CR, chylomicron remnant; DAG, diacylglycerol; DGAT, sn-1,2-diacylglycerol acyltransferase; ELOVL5, long chain fatty acid elongase 5; FA, fatty acid; FAS, fatty acid synthase; FATP, fatty acid transport protein; GPAT, glycerol-3-phosphate acyltransferase; HMGCS2, HMG-CoA synthetase 2; LFABP, liver fatty acid binding protein; LPA, lysophosphatidic acid; MTP, microsomal triglyceride transfer protein; PA, phosphatidic acid; SCD1, stearoyl-CoA desaturase 1.
The regulation of FA transport and uptake into the liver is complex and often debated. Clearly, there is strong evidence for protein-mediated transfer of FA across the plasma membrane. There are numerous proteins involved in the membrane transport of FA, including the family of FA transport proteins (FATPs) and the scavenger receptor CD36. Hepatic FA uptake is decreased in mice lacking FATP2 or FATP5 in the liver (33, 34). In addition, overexpression of CD36 in the liver increases hepatic FA uptake in vivo (35) and CD36 expression is increased in animal models of NAFLD and in humans with NAFLD (35–37). Surprisingly, CD36 knockout mice have normal rates of FA uptake, suggesting that CD36 is not required for FA uptake under normal conditions (38). Membrane proteins such as caveolins are also important contributors to FA influx, but their role has not been sufficiently characterized in the liver (39). An important factor that influences FA uptake is the subsequent activation of FAs to their acyl-CoA derivatives, thereby preventing efflux (40). Both ACSL and FATP family members possess acyl-CoA synthetase activity and play key roles in channeling exogenous FAs into specific metabolic pathways as discussed below. In addition to FA activation, impairments in downstream utilization of acyl-CoAs also can lead to reduced FA uptake [see (41) for review]. It should be noted that passive diffusion also contributes to hepatic uptake of FAs, but this process is a minor contributor to total FA influx (42, 43). Regardless of the exact mechanism, uptake of exogenous FAs is a major source of intracellular FAs in the liver.
FAs are intrinsically hydrophobic and, as a result, do not readily diffuse into the aqueous cytosol. Thus, intracellular binding proteins are responsible for shuttling FAs between organelles and facilitating their uptake and metabolism. The FA binding protein (FABP) family member that is the most highly expressed isoform in the liver is liver fatty acid binding protein (LFABP) (44). Ablation of LFABP in mice does not alter initial rates of FA uptake in hepatocytes, but after 30 s, FA uptake is reduced (45). This delayed uptake is suggestive of a mechanism whereby altered downstream metabolism, perhaps activation of FAs to acyl-CoAs, becomes impaired in the absence of LFABP. Once activated, acyl-CoAs are subsequently bound by liver acyl-CoA binding protein (46), although the contribution of this protein to hepatic FA uptake has not been well defined. The fate of FA taken up by the liver is dependent on a host of factors, including the structure of the FA, the rate of uptake, and influenced by the activity of the hepatocytes, which is influenced by activity of the enzymes involved in FA metabolism.
DNL.
The liver is a major site of DNL, but its contribution to whole-body lipogenesis varies across species. The liver is thought to be the primary source of DNL in poultry, fish, and humans (47–49); the latter is still controversial. Hepatic DNL has a moderate contribution to whole-body DNL in rodents and rabbits (50) and is low in ruminants, dogs, and pigs (50–52). DNL accounts for ~5% of hepatic FA during fasting and up to 30% after a meal (27, 53), although rates of DNL are highly variable among people (54).
Acetyl-CoA carboxylase (ACC) and FA synthase (FAS) are responsible for carrying out the committed steps of DNL. Two isoforms of ACC exist, with each differing in its physiological function. ACC1 is primarily cytosolic and produces malonyl-CoA that is largely used as a substrate by FAS for DNL. In contrast, ACC2 is localized on the mitochondria and produces a local pool of malonyl-CoA that inhibits carnitine palmitoyl-transferse 1 (CPT1; discussed below), although it may also be able to compensate for ACC1 when the latter is ablated (55–57). The terminal elongation of FAs by FAS produces palmitate (16:0) as its primary product (58). Subsequently, elongase and desaturate enzymes can act on de novo synthesized 16:0 to generate 16:1, 18:0, or 18:1. In the liver, stearoyl-CoA desaturase 1 (SCD1) is the primary desaturase responsible for the generation of C16:1 and C18:1 from SFAs (59), whereas long-chain FA elongase 5 appears to be the primary hepatic elongase for generating C18:0 and C18:1 (60). Additional elongases and desaturases likely also contribute to the elongation and desaturation of longer PUFAs (e.g., C18:2 and C18:3) (61, 62). Long chain acyl-CoA synthetase (ACSL) 3 and ACSL5 are involved in the activation of de novo synthesized FAs and regulate their subsequent incorporation into TG and phospholipids (63, 64).
TG synthesis.
The initial step in the formation of TG is the formation of lysophosphatidic acid from glycerol-3-phosphate and acyl-CoA catalyzed by sn-1-glycerol-3-phosphate acyltransferase (GPAT). Subsequently, sn-1-acyl-glycerol-3-phosphate acyltransferase (AGPAT) catalyzes the formation of phosphatidic acid, which can be further metabolized to generate phosphatidylinositol and phosphatidylglycerol or can be dephosphorylated by LIPIN proteins to produce 1,2-diacylglycerol (DAG). DAG is an important branch point in glyceroplipid synthesis, because it can be further acylated by sn-1,2-diacylglycerol acyltransferase (DGAT) to form TG, or phosphoethanolamine or phosphocholine head groups can be added to form phosphatidylethanolamine and phosphatidylcholine, respectively.
These pathways become more complex when the number of isoforms of each enzyme is considered. For example, there are 4 known isoforms of GPAT, 10 putative AGPATs, 3 LIPINs, and 2 DGATs that differ in tissue expression pattern, intracellular location, and substrate preference (65). As an example of the latter point, GPAT1, a major hepatic GPAT isoform, prefers SFAs as a substrate resulting in the high prevalence of SFAs in the sn-1 position of many glycerolipids (66, 67). Thus, the relative activity of different isoforms along with availability of specific FAs dictates the final composition of TG and phospholipids. Additionally, each of these enzymes most likely utilizes a physically distinct pool of substrate and forms a distinct pool of product due to the enzyme’s intracellular location and interaction with upstream/downstream proteins in a given pathway. Although both GPAT1 and GPAT4, the 2 major liver isoforms, can utilize exogenous FAs as substrates, only GPAT1 is required for the esterification of de novo synthesized FAs (68). Similar differences exist among the DGAT isoforms with DGAT2 utilizing FAs derived from DNL as its substrate, whereas DGAT1 preferentially reesterifies exogenous FAs onto DAG generated from lipolysis (69–71). This selective formation of TG also appears to have ramifications on VLDL secretion as discussed below.
It should also be noted that the liver expresses all 3 isoforms of monoacylglycerol acyltransferase (MGAT) and the expression of MGAT3 increases in individuals with NAFLD (72). The monoacylglycerol pathway was thought to exist primarily in enterocytes; however, these recent data suggest that the MGATs may contribute to hepatic FA metabolism as well.
FATP and ACSL isoforms also play an important role in activating FAs to be used for TG synthesis. Liver ablation of FATP2 or FATP5 lowers hepatic TG in models of NAFLD (33, 34). However, it has not been determined if this effect is due to decreased FA uptake or altered TG synthesis or hydrolysis. ACSL5, which is located on mitochondria and the endoplasmic reticulum (ER), increases FA uptake and partitions FAs toward TG and phospholipid synthesis in hepatocytes (64, 73).
A single mutation (I148M) in the gene patatin-like phospholipase domain containing A3 (PNPLA3) is the best known genetic predictor of NAFLD (74). This variant is more prevalent in certain minorities, including Hispanics, and likely accounts for the increased rates of NAFLD in this population (74, 75). PNPLA3 encodes the protein adiponutrin, which shares the greatest homology to the TG lipase adipose triglyceride lipase (ATGL; encoded by PNPLA2). Given the similarity to a known TG lipase, previous studies identified adiponutrin to have TG hydrolase activity; therefore, it was presumed that the I148M mutation would cause NAFLD as a result of reduced TG hydrolysis (76). However, mice lacking adiponutrin do not have hepatic steatosis or any overt phenotype, suggesting that other mechanisms are involved (77, 78). Subsequent work has revealed that adiponutrin is a lysophosphatidic acid acyltransferase (i.e., catalyzes the same reaction as the AGPAT enzymes discussed above) with a preference for unsaturated FAs (79). The I148M variant has increased acyltransferase activity compared with wild-type adiponutrin, suggesting that this mutation results in a gain-of-function leading to enhanced formation of phosphatidic acid, and subsequently TG, rather than a loss of function influencing TG hydrolysis (79). Given its prominent role as a genetic predictor of NAFLD, future work is needed to definitively elucidate the mechanism through which adiponutrin regulates TG metabolism and FA trafficking.
FA incorporation into TG serves an important role in preventing the accumulation of intracellular FAs or lipid metabolites that can cause liver injury and dysfunction (80). For example, DGAT2 overexpression causes steatosis in the absence of liver injury or insulin resistance (81), whereas DGAT2 knockdown reduces steatosis yet causes oxidative stress and fibrosis (82). The accumulation of intermediates involved in glycerolipid synthesis, such as phosphatidic acid and DAG enriched in SFAs, also impairs insulin signaling (83, 84). Moreover, the inability of FAs to be esterified into TG is responsible for the lipotoxicity associated with high levels of SFAs (85). Thus, dysregulation through one or more steps of the TG synthetic pathway can have detrimental effects on hepatic function.
LDs
Once formed, TGs are stored primarily in cytosolic LDs. The formation of LDs occurs largely on the membrane of the ER, although enzymes responsible for TG synthesis are also present on LDs and may play a role in LD expansion (86). Numerous models of LD biogenesis have been proposed, but the exact mechanism of LD formation and maturation is still under investigation [see (87) for review]. The hydrolysis of TG from LDs has received much attention in adipose tissue, but more recent research has shed light on the importance of this metabolic pathway in the liver. Studies from our laboratory and others have characterized several lipases, including ATGL, carboxylesterase 3 (CES3; also known as TGH) and carboxylesterase 1 (CES1) as important hepatic TG hydrolases with divergent effects on LD metabolism and FA partitioning. LD-associated proteins play an important structural role, but many are also directly involved in regulating lipolysis or interacting with other proteins to coordinate LD formation and lipolysis. The composition of the LD proteome changes with NAFLD and likely contributes to changes in FA metabolism (88, 89). Because of the divergence in effects of specific TG lipases and LD proteins, we will discuss them in the appropriate sections that follow.
Pathways of FA disposal
The liver disposes of FAs through either oxidation or secretion as VLDL. FA oxidation is highly variable and largely influenced by FA influx, whereas VLDL secretion exhibits much more subtle changes in response to FA influx and hormonal oscillations. Multiple branch points exist regarding the partitioning of FAs between these 2 pathways.
FA oxidation.
FAs can be catabolized through 3 distinct pathways of oxidation: α, ω, and β. ω-Oxidation occurs exclusively in microsomes and α- and β-oxidation can occur in peroxisomes and mitochondria. Mitochondrial β-oxidation is the primary catabolic pathway in the liver for most FAs, but peroxisomal β-oxidation is largely responsible for the initial oxidation of very long-chain (>20) FAs (90). Although mitochondrial β-oxidation is a major pathway of FA disposal in the liver, complete oxidation to CO2 is a minor contributor, with incomplete oxidation to ketone bodies being the primary end product of hepatic FA catabolism (91).
FAs must be activated to their acyl-CoA derivatives prior to their subsequent metabolism. ACSL1 is located on mitochondria and plays a major role in activating FAs destined for β-oxidation in heart and adipose (92, 93). In contrast, ACSL1 ablation in the liver results in only a small reduction in FA oxidation (94); the ACSL or FATP isoforms responsible for activating FAs for hepatic mitochondrial β-oxidation remain unknown. LFABP appears to play an important role in carrying exogenous FAs to the mitochondria. Mice that lack LFABP have reduced FA oxidation and impaired ketogenesis during fasting (95). LFABP also interacts with mitochondria (96) and, more specifically, CPT1a, the rate-limiting step in mitochondrial FA oxidation (97, 98). Malonyl-CoA, an intermediate in DNL, allosterically inhibits CPT1a to prevent FA oxidation during times of increased flux through DNL (99). This regulation forms the basis of the classic Randle Cycle that links carbohydrate metabolism and DNL to FA catabolism (100). There is also evidence that CPT1a reduces VLDL secretion, perhaps due to the disposal of FAs through oxidation, thereby limiting the availability of FAs for VLDL synthesis (101–103).
There are numerous studies showing that inhibiting esterification of FAs diverts them toward oxidation. ACSL5 knockdown diverts FAs from incorporation into TG and phospholipids toward β-oxidation (64). Similar to ACSL5, hepatic GPAT1 (68, 104, 105) and DGAT2 (106, 107) ablation results in increased partitioning of FAs toward oxidation at the expense of esterification. Thus, the flux through TG synthesis plays an important role in determining the rate of FA oxidation.
In addition to oxidation of exogenous FAs, hydrolysis of intracellular TG is also a source of FAs that can be used for energy production. Research from our laboratory and others shows that gene ablation or shRNA knockdown of hepatic ATGL reduces TG hydrolase activity and increases TG accumulation (108, 109). In addition, ATGL channels hydrolyzed FAs toward oxidation but has no effect on VLDL secretion (108, 109). The lack of effect of ATGL on VLDL secretion is surprising and clearly highlights LD catabolism as a branch point in hepatic FA trafficking. Consistent with the effects of ATGL, ablation of the LD proteins perilipin 2 or 5, which results in enhanced lipolysis, increases the oxidation of hydrolyzed FAs, whereas ablation of the ATGL co-activator comparative gene identification-58 impairs FA oxidation (110–112). ATGL-mediated lipolysis also regulates PPARα signaling as a means to coordinate downstream FA oxidation (108–110). PPARα is a transcription factor that is activated by FAs and in turn increases transcription of genes involved in FA oxidation (discussed below). Surprisingly, administration of the PPARα agonist, fenofibrate, did not normalize PPARα target gene expression or liver TG concentrations in mice treated with adenovirus harboring ATGL shRNA (108), suggesting that a more complex mechanism linking ATGL-catalyzed lipolysis to PPARα activation may exist. It is unclear if ATGL primarily drives FA oxidation through selective channeling, through increased oxidative capacity (driven by PPARα), or if both these mechanisms contribute to enhanced FA oxidation.
VLDL secretion.
Although considerable progress has been made in recent years into our understanding of VLDL synthesis and secretion, much remains to be determined. Current models suggest that there are distinct steps of TG addition during VLDL synthesis. The first step occurs in the ER where lipidation of the primordial VLDL particle is mandatory to prevent apoB degradation (113–115). This step requires microsomal triglyceride transfer protein (MTP), which facilitates the transfer of lipids onto the developing VLDL particle (116). The second step involves bulk lipidation of the VLDL from cytosolic LDs. The exact mechanism of how cytosolic lipids are transferred across the membrane is not known and the importance of MTP in this process is also unclear (117, 118). Early studies demonstrated that <30% of exogenous FAs are directly incorporated into VLDL, with the majority first transiting through the cytosolic LD droplet pool (70, 119, 120). TG incorporated into VLDL are not completely hydrolyzed from cytosolic LDs, but instead they are partially hydrolyzed to DAG (119, 121), pointing toward a role for TG lipases in regulating VLDL assembly.
In contrast to the effects of ATGL on the partitioning of FAs to oxidative pathways, CES3 appears to be a major lipase involved in VLDL secretion. Global or hepatic ablation of CES3 impairs VLDL secretion without causing steatosis (122, 123). The lack of effect of CES3 on steatosis may be explained by increased rates of hepatic FA oxidation (123). Additionally, hepatic CES3 knockout mice have altered LD morphology, with more but smaller LDs and a delayed transfer of newly formed LDs to preformed LDs (124). CES3 localizes to the ER and appears in proximity to cytosolic LDs (124, 125). Although the exact mechanism defining how CES3 alters LD metabolism and VLDL synthesis remains unknown, it is clearly an important regulator of FAs destined for VLDL secretion. CES1, another member of the CES family, has recently been shown to play an important role in the hydrolysis of PUFAs from TG. Mice with global deletion of CES1 are obese, develop hepatic steatosis, and have increased lipogenesis and VLDL secretion (126). Mice lacking CES1 have TG that is specifically enriched in long-chain PUFAs, especially EPA (20:5) and DHA (22:6). The authors further show that the block in EPA and DHA hydrolysis from TG results in an upregulation of sterol response element binding protein-1c (SREBP-1c), a master transcriptional regulator of lipogenesis that is normally suppressed by long-chain PUFAs. Taken together, these data identify a novel role for CES1 in generating a specific pool of long-chain PUFAs that inhibit SREBP-1c to suppress lipogenesis and VLDL secretion. Thus, in addition to the channeling of hydrolyzed FAs, members of the CES family of lipases may also serve an important role in FA signaling to coordinate downstream metabolism.
Despite the fact that most cytosolic TG is hydrolyzed prior to its incorporation into VLDL, it is unclear if there are ever truly cytosolic LDs that are independent of the ER. Indeed, Wolinski (127) showed that all LDs in yeast are associated with the ER. If this model holds true, it would help our understanding of how ER luminal lipases involved in VLDL formation would have access to the LD to facilitate hydrolysis. Cell death-inducing DFF45-like effector B (CIDEB, also known as FSP27) is a protein that localizes to LDs and the ER (128) and has been shown to play a role in VLDL assembly. As evidence, CIDEB directly interacts with apoB and ablation of CIDEB reduces VLDL secretion (128). Additional studies show that CIDEB also localizes to the golgi and may be involved in the second lipidation step of VLDL formation (129).
DGAT1 is thought to be the primary acyltransferase responsible for the reesterification of DAG to TG that is destined for secretion (69, 70, 130). Indeed, hepatocytes possess DGAT activity on both the cytosolic and luminal sides of the ER (70, 131). DGAT1 also selectively uses FAs derived exogenously, rather than through DNL, as substrates and selectively channels these FAs into a pool of TG that is preferentially secreted as VLDL (70, 71). This finding may also explain why de novo synthesized FAs have a slower rate of secretion into VLDL compared with exogenous FAs in humans (132). The finding that FAs formed from DNL are more slowly secreted is surprising given that rates of DNL are typically associated with increased VLDL secretion, an observation noted across multiple species (133). It is still unclear, however, which ACSL or FATP isoform is responsible for the activation of FAs prior to DGAT1. Additionally, the role of carnitine acyltransferases in shuttling acyl-CoAs across the ER membrane for use by lumen DGAT activity has not been extensively characterized (134, 135).
Autophagy and FA metabolism
Autophagy, which becomes activated in response to cellular stress such as starvation, is an evolutionarily conserved mechanism to degrade and recycle damaged organelles. A major breakthrough in the field was the discovery that autophagy is also involved in the disposal of LDs, a process known as macrolipophagy (136). Accordingly, ablation of hepatic autophagy results in hepatic steatosis through decreased TG turnover (136). Although autophagy results in the catabolism of complex lipids, there is no net gain or loss of FAs, because FAs released by lysosomes remain within cells. Although autophagy is clearly an important regulator of hepatic metabolism, many questions remain regarding its role in lipid metabolism. For example, the metabolic fate of FA liberated by lysosomal lipases is largely unknown as are the factors that regulate this process; ablation of hepatic autophagy decreases FA oxidation but also suppresses VLDL secretion (136, 137). Also, it remains to be determined how LDs are recognized by autophagosomes to be endocytosed. It is presumed that specific LD-associated proteins target the LD for degradation, but these proteins have yet to be identified. Regardless, future research into autophagy and NAFLD and related diseases undoubtedly will advance our understanding of yet another pathway controlling hepatic FA trafficking.
Regulation of FA trafficking
Transcriptional regulation.
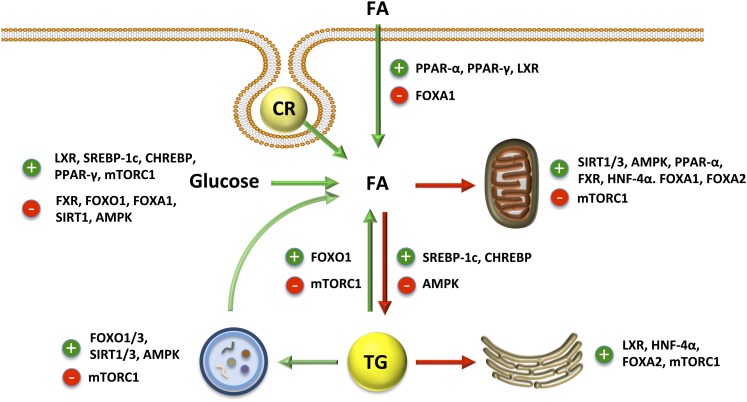
PPARα is perhaps the most well-characterized transcription factor involved in governing hepatic FA metabolism; see Figure 2 for an overview of hepatic FA trafficking regulation. PPARα induces the expression of many of the enzymes involved in peroxisomal and mitochondrial β-oxidation in addition to proteins involved in FA uptake (CD36), transport (LFABP, acyl-CoA binding protein), and activation (ACSL1) (138). PPARα binds numerous ligands, but long-chain PUFAs such as EPA appear to be the most robust activators (139). Formation of 16:0/18:1 phosphatidylcholine, which requires DNL for its synthesis, is also an activator of PPARα (140). PPARα is coactivated by peroxisome proliferator-activated receptor gamma, coactivator-1α (PGC-1α, which drives mitochondrial biogenesis and oxidative capacity through its interaction with other transcription factors such as estrogen-related receptor-α and nuclear respiratory factors 1 and 2 (141). Forkhead box protein O1 (FOXO1) is another transcription factor that binds PGC-1α and has been shown to induce ATGL expression, although the physiological consequences of this regulation on downstream FA metabolism have not been studied (142). In addition to its regulation by PPARα, hepatic FA uptake is also promoted by the liver X receptor (LXR) via changes in CD36 expression (143) and inhibited by FOXA1 via reduced FATP2 expression (144). There is also evidence that FOXA1, FOXA2, and hepatocyte nuclear factor-4α (HNF-4α) increase transcription of genes that promote hepatic FA oxidation and ketogenesis as a means to decrease hepatic TG content (144–148).
FIGURE 2.
Transcriptional and post-transcriptional regulation of hepatic FA trafficking. Green arrows indicate sources of intracellular FA and red arrows indicate routes of FA disposal. AMPK, AMP-activated protein kinase; CHREBP, carbohydrate response element binding protein; CR, chylomicron remnant; FA, fatty acid; FOXA/O, forkhead box protein; FXR, farnesoid X receptor; HNF-4α, hepatocyte nuclear factor-4α LXR, liver X receptor; mTORC1, mammalian target of rapamycin complex 1; SIRT1, sirtuin 1; SREBP, sterol regulatory element binding protein.
In general DNL and TG synthesis run in parallel and, as such, are controlled by similar mechanisms. Following a meal, nutrient flux to the liver and the insulin response to the meal signal to upregulate DNL and TG synthesis. SREBP1c is responsive to insulin signaling and upregulates ACC1/2, FAS, GPAT1, and other genes, including glycolytic genes that support lipid anabolism (149, 150). ER stress also increases SREBP1c activity leading to elevated DNL and may be an important contributor to the elevated DNL observed in individuals with NAFLD (151, 152). Glucose and/or fructose drive DNL by providing substrates for DNL and by upregulating carbohydrate response element binding protein (CHREBP), which in turn increases the transcription of genes involved in glycolysis, DNL, and TG synthesis (153–155). Fructose is especially lipogenic due to its very rapid flux into the liver and its ability to bypass key regulatory steps in glycolysis [see (156) for review]. In support, high-carbohydrate diets or sugars, especially fructose, induce DNL (54, 157). Both SREBP1c and CHREBP are target genes of LXR, thus making it an important regulator of DNL as well (158, 159). Farnesoid X receptor (FXR), which is activated by bile acids, antagonizes SREBP1c to decrease DNL (160) and promotes PPARα-mediated FA oxidation (161). Additional work has also shown PPARγ plays an important role in upregulating DNL especially in NAFLD (162–164). In contrast, FOXO1 and FOXA1 antagonize DNL (144, 165, 166).
The transcriptional regulation of VLDL secretion is less clear. LXR, HNF-4α, and FOXA2 promote VLDL secretion (145, 148, 167). Although SREBP1c and CHREBP do not upregulate genes involved in VLDL secretion, they do promote DNL, which contributes substrates for VLDL synthesis. As such, SREBP1c is required for increased VLDL production in diabetic or high-carbohydrate diet-fed mice (168). FOXO1 is a transcription factor that regulates both DNL and VLDL secretion. FOXO1 increases the expression of MTP and apoCIII and promotes VLDL secretion in some studies (169, 170). However, FOXO1 also inhibits DNL and has been shown to reduce VLDL secretion in other studies (171, 172). These studies employ mouse models that use different constitutively active forms of FOXO1, which may be a contributing factor to the discrepancies.
Transcriptional activation of autophagy is mediated largely by stress-responsive transcription factors such as p53 and nuclear factor-κB (173). The FOXO family of transcription factors is also induced by cellular stress and accordingly have been shown to promote autophagy (174, 175). However, little is known regarding the transcriptional regulation of lipophagy and how it contributes to hepatic FA metabolism.
Post-translational regulation.
As expected, FA metabolism is highly regulated by cellular energy status. AMP-activated protein kinase (AMPK) acts as a cellular energy gauge and in response to low cellular energy (↑AMP/ATP), it phosphorylates target proteins to increase or decrease their activity. AMPK-mediated phosphorylation of ACC1 and ACC2 (176–178) results in reduced activity and a lowering of malonyl-CoA levels. In addition to lowering rates of DNL, this modification of ACC2 also enhances FA oxidation through the blunting of malonyl-CoA–mediated inhibition of CPT1. Therefore, targeting one enzyme can reciprocally regulate both DNL and FA oxidation. Although direct phosphorylation has not been shown, AMPK increases malonyl-CoA decarboxylase activity, thus providing another mechanism through which AMPK lowers malonyl-CoA levels (179). AMPK inhibits the activity of GPAT1 in hepatocytes, resulting in a shuttling of FAs toward oxidation rather than esterification into TG (180). Finally, AMPK also regulates FA metabolism through changes in transcription thought largely to emanate from its phosphorylation and activation of PGC1α (181). Thus, taken as a whole, AMPK acts as a critical node in partitioning hepatic FA between anabolic and catabolic pathways.
The sirtuin family of deacetylases has garnered considerable research attention regarding their role in regulating energy metabolism. Similar to AMPK, the sirtuins function as sensors of the cellular energy state. The activity of sirtuins is increased by NAD+, which is required for their activity. Of the family of sirtuins, SIRT1 and SIRT3 have been the most characterized in regards to their roles in hepatic FA metabolism. SIRT1-mediated deacetylation results in inactivation of SREBP1c (182, 183) and activation of FXR (184). The net result of these modifications is a decrease in lipogenic gene expression leading to reduced flux through the DNL pathway. SIRT1 also increases PGC1α/PPARα signaling. SIRT1 deacetylates and activates PGC1α (185) and interacts with and regulates PPARα as well (186); direct SIRT1-mediated deacetylation of PPARα has yet to be shown. SIRT1 also activates FOXA1, to promote FA oxidation and ketogenesis (187), and FOXO1, which increases ATGL expression to increase lipolysis (142, 188). In addition, SIRT1 induces autophagy in numerous cell types to further promote FA catabolism (189), but this has not been studied in the liver. Consistent with its role in promoting FA oxidation, SIRT1 expression is inhibited by CHREBP and induced by the cAMP-response element binding protein, which is activated by β-adrenergic signaling (e.g., fasting or exercise) (190). The role of specific targets of SIRT3 has been less characterized, but one known target involved in FA metabolism is HMG-CoA synthetase 2 (HMGCS2), the rate-limiting step in ketogenesis. Upon activation, SIRT3 deacetylates and activates HMGCS2 to increase ketone body synthesis (191). The importance of this regulation in hepatic and whole-body lipid metabolism has not been characterized; however, absence of HMGCS2 causes hypoketotic hypoglycemia and fatty liver in humans fed low-carbohydrate diets (192, 193), suggesting that this enzyme plays a major role in FA disposal and linking FA catabolism to gluconeogenesis.
In opposition to AMPK and SIRT1/3, mammalian target of rapamycin complex 1 (mTORC1) is activated in response to insulin signaling and nutrients, especially amino acids. Activation of mTORC1 increases phosphorylation of LIPIN1, which blocks the LIPIN1-mediated inhibition of SREBP-1c, resulting in enhanced DNL (194). Recent studies also suggest that mTORC1 inhibits PPARα activity through its activation of nuclear receptor co-repressor 1, a PPARα repressor (195). In addition, mTORC1 inhibits ATGL-mediated lipolysis in adipose tissue, which is mediated by the transcription factor early growth response protein 1 (196); the importance of this regulation in the liver has not been determined. Finally, mTORC1 is a potent inhibitor of autophagy adding yet another layer through which it antagonizes FA catabolism (197).
Insulin signaling also regulates VLDL secretion. Acute insulin signaling inhibits VLDL secretion in part through enhanced apoB degradation (198). In contrast, elevated and sustained insulin signaling, such as in insulin resistance, promotes VLDL secretion (199). There is much debate regarding the specific nodes of insulin signaling that regulate this process, although recent data suggest that mTORC1 may be involved in the rise in VLDL secretion during insulin resistance (200).
Clinical importance of intracellular hepatic FA trafficking
NAFLD is closely associated with obesity, insulin resistance, and type 2 diabetes (2, 201). A hallmark of insulin resistance is elevated serum FFA concentrations (202). As a result of increased FFA exposure, the liver of insulin-resistant individuals has increased rates of FFA uptake, which is likely a major contributor to the development of NAFLD (203, 204). Coinciding with changes in FFA uptake, individuals with NAFLD or NASH exhibit altered hepatic FA composition. A consistent finding is that PUFAs, especially n–3 long-chain FAs such as EPA and DHA, in total hepatic lipids or TG are reduced in individuals with NAFLD or NASH (205–207) the mechanisms leading to these changes in FA content remain unknown. Given its importance in FA catabolism, impairments in mitochondrial β-oxidation have been implicated as a causative factor in the development of steatosis (208–210). Undoubtedly, there are situations where this is the case; however, additional evidence suggests that NAFLD coincides with increased rates of FA oxidation in humans and mice (211–216). It is also well established that participants with NAFLD or insulin resistance have increased rates of VLDL secretion (199, 217, 218). Taken together, these data suggest that the liver attempts to compensate for the increased FA flux by increasing FA disposal through both secretion and oxidation. In addition, a growing body of literature shows that DNL is increased in the liver and reciprocally decreased in adipose tissue in insulin-resistant and type 2 diabetic individuals (157, 219–222). Finally, autophagy is downregulated by high-fat–induced hepatic steatosis (136), suggesting that the decrease in this route of lipid disposal may also contribute to NAFLD etiology.
With so many pathways that contribute to steatosis, it is unclear which pathway should be targeted. Numerous studies that have targeted DNL inhibition have observed marked improvements in hepatic steatosis, insulin resistance, and obesity resistance (223). Increasing the expression of wild-type or a malonyl-CoA–insensitive CPT1a mutant enhances hepatic FA oxidation and decreases steatosis (101–103). Enhancing autophagy alleviates steatosis and liver injury following high-fat feeding in mice (224). Is decreasing hepatic FFA uptake a good idea, or will it divert FFA to other tissues such as heart and muscle, where dysfunction will ensue? Is it a good idea to increase VLDL secretion as a means of disposing hepatic FA, or would the consequences of increasing serum TG outweigh the benefits of decreasing steatosis? Perhaps the pathway that should be targeted depends upon the specific disease (e.g., NAFLD alone or with complications). Little is known in regards to how the above individual pathways contribute to the progression of NAFLD to steatohepatitis or hepatocellular carcinoma or more systemic disease like Type 2 diabetes. Thus, a more tailored approach of targeting individual pathways based on the desired outcomes is warranted.
TG defines NAFLD and is tightly correlated with the comorbidities of steatosis; however, TG itself is widely considered to be inert and not involved in the complications of NAFLD. Instead, FAs may directly trigger hepatic dysfunction through their incorporation into and accumulation of metabolites such as DAG and ceramides (83). Alternatively, products resulting from FA metabolism, such as reactive oxygen species, may also link NAFLD to its comorbidities (225). Although FA metabolites are important signaling molecules, it is important to recognize that FAs, or their acyl-CoA derivatives, are also signaling molecules. FAs/acyl-CoAs can serve as ligands for transcription factors and as allosteric regulators of numerous enzymes involved in energy metabolism (65). These functions are dependent upon the structure of the FA/acyl-CoA and the pathway and enzyme responsible for its formation.
Clearly, there is no single metabolite or pathway that is responsible for NAFLD or its progression to more detrimental manifestations. Rather, variability in diet (e.g., carbohydrate content), genetics (e.g., PNPLA3 variants), metabolic state (e.g., insulin resistance), and other environmental factors all converge and lead to the single, but very heterogeneous, disease known as NAFLD.
In conclusion, hepatic FA trafficking is a complex process with many levels of regulation. Although tremendous progress towards our understanding of hepatic FA metabolism has been made in recent years, many pathways and enzymes that contribute to FA trafficking remain understudied or perhaps even unknown. Additionally, more studies in humans evaluating the flux through each pathway and its contribution to hepatic and whole-body FA trafficking in different metabolic states and disease models are needed. Given the prevalence of NAFLD and its associated comorbidities and the lack of effective treatment options, hepatic FA metabolism will remain an important research focus as we strive toward effective preventative or therapeutic options.
Acknowledgments
The author acknowledges Dr. Brian Finck for his critical review of this manuscript. The sole author had responsibility for all parts of this manuscript.
Footnotes
Abbreviations used: ACC, acetyl-CoA carboxylase; ACSL, long chain acyl-CoA synthetase; AGPAT, sn-1-acyl-glycerol-3-phosphate acyltransferase; AMPK, AMP-activated protein kinase; ATGL, adipose triglyceride lipase; CES, carboxylesterase; CHREBP, carbohydrate response element binding protein; CIDEB, cell death-inducing DFF45-like effector B; CPT1, carnitine palmitoyl-transferse 1; DAG, diacylglycerol; DGAT, diacylglycerol acyltransferase; DNL, de novo lipogenesis; ER, endoplasmic reticulum; FA, fatty acid; FAS, fatty acid synthase; FATP, fatty acid transport protein; FOXA/O, forkhead box protein; FXR, farnesoid X receptor; GPAT, glycerol-3-phosphate acyltransferase; HMGCS2, HMG-CoA synthetase 2; HNF-4α, hepatocyte nuclear factor-4α LD, lipid droplet; LFABP, liver fatty acid binding protein; LXR, liver X receptor; MGAT, monoacylglycerol acyltransferase; mTORC1, mammalian target of rapamycin complex 1; MTP, microsomal triglyceride transfer protein; NAFLD, nonalcoholic fatty liver disease; PGC-1α, peroxisome proliferator-activated receptor gamma, coactivator 1-α PNPLA3, phospholipase domain containing A3; SCD1, stearoyl-CoA desaturase 1; SREBP, sterol regulatory element binding protein.
Literature Cited
- 1.Wattacheril J, Chalasani N. Nonalcoholic fatty liver disease (NAFLD): is it really a serious condition? Hepatology. 2012;56:1580–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD. Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95 [DOI] [PubMed] [Google Scholar]
- 3.Boza C, Riquelme A, Ibanez L, Duarte I, Norero E, Viviani P, Soza A, Fernandez JI, Raddatz A, Guzman S, et al. Predictors of nonalcoholic steatohepatitis (NASH) in obese patients undergoing gastric bypass. Obes Surg. 2005;15:1148–53 [DOI] [PubMed] [Google Scholar]
- 4.Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45:600–6 [DOI] [PubMed] [Google Scholar]
- 5.Gholam PM, Flancbaum L, Machan JT, Charney DA, Kotler DP. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol. 2007;102:399–408 [DOI] [PubMed] [Google Scholar]
- 6.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85 [DOI] [PubMed] [Google Scholar]
- 7.Leite NC, Salles GF, Araujo ALE, Villela-Nogueira CA, Cardoso CRL. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113–9 [DOI] [PubMed] [Google Scholar]
- 8.Wagenknecht LE, Scherzinger AL, Stamm ER, Hanley AJG, Norris JM, Chen YD, Bryer-Ash M, Haffner SM, Rotter JI. Correlates and heritability of nonalcoholic fatty liver disease in a minority cohort. Obesity (Silver Spring). 2009;17:1240–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen ZW, Chen L, Dai H, Chen J, Fang L. Relationship between alanine aminotransferase levels and metabolic syndrome in nonalcoholic fatty liver disease. J Zhejiang Univ Sci B. 2008;9:616–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–23 [DOI] [PubMed] [Google Scholar]
- 11.Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–8 [DOI] [PubMed] [Google Scholar]
- 12.Bonora E, Targher G. Increased risk of cardiovascular disease and chronic kidney disease in NAFLD. Nat Rev Gastroenterol Hepatol. 2012;9:372–81 [DOI] [PubMed] [Google Scholar]
- 13.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384–91 [DOI] [PubMed] [Google Scholar]
- 14.Otgonsuren M, Stepanova M, Gerber L, Younossi ZM. Anthropometric and clinical factors associated with mortality in subjects with nonalcoholic fatty liver disease. Dig Dis Sci. 2013;58:1132–40 [DOI] [PubMed] [Google Scholar]
- 15.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–44 [DOI] [PubMed] [Google Scholar]
- 16.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609 [DOI] [PubMed] [Google Scholar]
- 17.Armstrong PJ, Blanchard G. Hepatic lipidosis in cats. Vet Clin North Am Small Anim Pract. 2009;39:599–616 [DOI] [PubMed] [Google Scholar]
- 18.Bobe G, Young JW, Beitz DC. Invited review: pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J Dairy Sci. 2004;87:3105–24 [DOI] [PubMed] [Google Scholar]
- 19.Hermier D. Lipoprotein metabolism and fattening in poultry. J Nutr. 1997;127:S805–8 [DOI] [PubMed] [Google Scholar]
- 20.Hagenfeldt L, Wahren J, Pernow B, Räf L. Uptake of individual free fatty acids by skeletal muscle and liver in man. J Clin Invest. 1972;51:2324–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aydin A, Sokal JE. Uptake of plasma free fatty acids by the isolated rat liver: effect of glucagon. Am J Physiol. 1963;205:667–70 [DOI] [PubMed] [Google Scholar]
- 22.Mashek DG, Grummer RR. Short communication: net uptake of nonesterified long chain fatty acids by the perfused caudate lobe of the caprine liver. J Dairy Sci. 2003;86:1218–20 [DOI] [PubMed] [Google Scholar]
- 23.Trout DL, Estes EH., Jr Factors affecting liver uptake of serum free stearic acid-1–C14. Am J Physiol. 1962;203:1024–8 [DOI] [PubMed] [Google Scholar]
- 24.Thompson GE, Darling KF. The hepatic uptake of individual free fatty acids in sheep during noradrenaline infusion. Res Vet Sci. 1975;18:325–7 [PubMed] [Google Scholar]
- 25.Bruce JS, Salter AM. Metabolic fate of oleic acid, palmitic acid and stearic acid in cultured hamster hepatocytes. Biochem J. 1996;316:847–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrows BR, Parks EJ. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J Clin Endocrinol Metab. 2006;91:1446–52 [DOI] [PubMed] [Google Scholar]
- 27.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fishbein MH, Mogren C, Gleason T, Stevens WR. Relationship of hepatic steatosis to adipose tissue distribution in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2006;42:83–8 [PubMed] [Google Scholar]
- 29.Eguchi Y, Eguchi T, Mizuta T, Ide Y, Iwakiri R, Hisatomi A, Ozaki I, Yamamoto K, Kitajima Y, Kawaguchi Y, et al. Visceral fat accumulation and insulin resistance are important factors in nonalcoholic fatty liver disease. J Gastroenterol. 2006;41:462–9 [DOI] [PubMed] [Google Scholar]
- 30.Fenkci S, Rota S, Sabir N, Akdag B. Ultrasonographic and biochemical evaluation of visceral obesity in obese women with non-alcoholic fatty liver disease. Eur J Med Res. 2007;12:68–73 [PubMed] [Google Scholar]
- 31.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrows BR, Timlin MT, Parks EJ. Spillover of dietary fatty acids and use of serum nonesterified fatty acids for the synthesis of VLDL-triacylglycerol under two different feeding regimens. Diabetes. 2005;54:2668–73 [DOI] [PubMed] [Google Scholar]
- 33.Doege H, Baiillie RA, Ortegon AM, Tsang B, Wu Q, Punreddy S, Hirsch D, Watson N, Gimeno RE, Stahl A. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology. 2006;130:1245–58 [DOI] [PubMed] [Google Scholar]
- 34.Falcon A, Doege H, Fluitt A, Tsang B, Watson N, Kay MA, Stahl A. FATP2 is a hepatic fatty acid transporter and peroxisomal very long-chain acyl-CoA synthetase. Am J Physiol Endocrinol Metab. 2010;299:E384–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koonen DPY, Jacobs RL, Febbraio M, Young ME, Soltys CLM, Ong H, Vance DE, Dyck JRB. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes. 2007;56:2863–71 [DOI] [PubMed] [Google Scholar]
- 36.Greco D, Kotronen A, Westerbacka J, Puig O, Arkkila P, Kivilouto T, Laitinen S, Kolak M, Fisher RM, Hamsten A, et al. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1281–7 [DOI] [PubMed] [Google Scholar]
- 37.Mitsuyoshi H, Yasui K, Harano Y, Endo M, Tsuji K, Minami M, Itoh Y, Okanoue T, Yoshikawa T. Analysis of hepatic genes involved in the metabolism of fatty acids and iron in nonalcoholic fatty liver disease. Hepatol Res. 2009;39:366–73 [DOI] [PubMed] [Google Scholar]
- 38.Coburn CT, Knapp FF, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275:32523–9 [DOI] [PubMed] [Google Scholar]
- 39.Pohl J, Ring A, Ehehalt R, Herrmann T, Stremmel W. New concepts of cellular fatty acid uptake: role of fatty acid transport proteins and of caveolae. Proc Nutr Soc. 2004;63:259–62 [DOI] [PubMed] [Google Scholar]
- 40.Black PN, DiRusso CC. Vectorial acylation: linking fatty acid transport and activation to metabolic trafficking. Novartis Found Symp. 2007;286:127–38 [DOI] [PubMed] [Google Scholar]
- 41.Mashek DG, Coleman RA. Cellular fatty acid uptake: the contribution of metabolism. Curr Opin Lipidol. 2006;17:274–8 [DOI] [PubMed] [Google Scholar]
- 42.Rajaraman G, Roberts MS, Hung D, Wang GQ, Burczynski FJ. Membrane binding proteins are the major determinants for the hepatocellular transmembrane flux of long-chain fatty acids bound to albumin. Pharm Res. 2005;22:1793–804 [DOI] [PubMed] [Google Scholar]
- 43.Stump DD, Nunes RM, Sorrentino D, Isola LM, Berk PD. Characteristics of oleate binding to liver plasma membranes and its uptake by isolated hepatocytes. J Hepatol. 1992;16:304–15 [DOI] [PubMed] [Google Scholar]
- 44.Bass NM. Fatty acid-binding protein expression in the liver: its regulation and relationship to the zonation of fatty acid metabolism. Mol Cell Biochem. 1990;98:167–76 [DOI] [PubMed] [Google Scholar]
- 45.Newberry EP, Xie Y, Kennedy S, Han X, Buhman KK, Luo J, Gross RW, Davidson NO. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J Biol Chem. 2003;278:51664–72 [DOI] [PubMed] [Google Scholar]
- 46.Knudsen J, Jensen MV, Hansen JK, Faergeman NJ, Neergaard TB, Gaigg B. Role of acylCoA binding protein in acylCoA transport, metabolism and cell signaling. Mol Cell Biochem. 1999;192:95–103 [DOI] [PubMed] [Google Scholar]
- 47.Goodridge AG, Ball EG. Lipogenesis in the pigeon: in vivo studies. Am J Physiol. 1967;213:245–9 [DOI] [PubMed] [Google Scholar]
- 48.Lin H, Romsos DR, Tack PI, Leveille GA. Influence of dietary lipid on lipogenic enzyme activities in coho salmon, Oncorhynchus kisutch (Walbaum). J Nutr. 1977;107:846–54 [DOI] [PubMed] [Google Scholar]
- 49.Shrago E, Glennon JA, Gordon ES. Comparative aspects of lipogenesis in mammalian tissues. Metabolism. 1971;20:54–62 [DOI] [PubMed] [Google Scholar]
- 50.Ballard FJ, Hanson RW, Kronfeld DS. Gluconeogenesis and lipogenesis in tissue from ruminant and nonruminant animals. Fed Proc. 1969;28:218–31 [PubMed] [Google Scholar]
- 51.O’Hea EK, Leveille GA. Significance of adipose tissue and liver as sites of fatty acid synthesis in the pig and the efficiency of utilization of various substrates for lipogenesis. J Nutr. 1969;99:338–44 [DOI] [PubMed] [Google Scholar]
- 52.Baldner GL, Flatt RE, Shaw RN, Beitz DC. Fatty acid biosynthesis in liver and adipose tissue from dogs. Comp Biochem Physiol B. 1985;82:153–6 [DOI] [PubMed] [Google Scholar]
- 53.Timlin MT, Parks EJ. Temporal pattern of de novo lipogenesis in the postprandial state in healthy men. Am J Clin Nutr. 2005;81:35–42 [DOI] [PubMed] [Google Scholar]
- 54.Parks EJ, Skokan LE, Timlin MT, Dingfelder CS. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr. 2008;138:1039–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoehn KL, Turner N, Swarbrick MW, Wilks D, Preston E, Phua Y, Joshi H, Furler SM, Larance M, Hegarty BD, et al. Acute or chronic upregulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity. Cell Metab. 2010;11:70–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–6 [DOI] [PubMed] [Google Scholar]
- 57.Harada N, Oda Z, Hara Y, Fujinami K, Okawa M, Ohbuchi K, Yonemoto M, Ikeda Y, Ohwaki K, Aragane K, et al. Hepatic de novo lipogenesis is present in liver-specific ACC1-deficient mice. Mol Cell Biol. 2007;27:1881–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aarsland A, Wolfe RR. Hepatic secretion of VLDL fatty acids during stimulated lipogenesis in men. J Lipid Res. 1998;39:1280–6 [PubMed] [Google Scholar]
- 59.Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab. 2009;297:E28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Green CD, Ozguden-Akkoc CG, Wang Y, Jump DB, Olson LK. Role of fatty acid elongases in determination of de novo synthesized monounsaturated fatty acid species. J Lipid Res. 2010;51:1871–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bjermo H, Risérus U. Role of hepatic desaturases in obesity-related metabolic disorders. Curr Opin Clin Nutr Metab Care. 2010;13:703–8 [DOI] [PubMed] [Google Scholar]
- 62.Jump DB. Mammalian fatty acid elongases. Methods Mol Biol. 2009;579:375–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bu SY, Mashek MT, Mashek DG. Suppression of long chain acyl-CoA synthetase 3 decreases hepatic de novo fatty acid synthesis through decreased transcriptional activity. J Biol Chem. 2009;284:30474–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bu SY, Mashek DG. Hepatic long-chain acyl-CoA synthetase 5 mediates fatty acid channeling between anabolic and catabolic pathways. J Lipid Res. 2010;51:3270–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coleman RA, Mashek DG. Mammalian triacylglycerol metabolism: synthesis, lipolysis, and signaling. Chem Rev. 2011;111:6359–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Monroy G, Rola FH, Pullman ME. A substrate- and position-specific acylation of sn-glycerol 3-phosphate by rat liver mitochondria. J Biol Chem. 1972;247:6884–94 [PubMed] [Google Scholar]
- 67.Dircks LK, Sul HS. Mammalian mitochondrial glycerol-3-phosphate acyltransferase. Biochim Biophys Acta. 1997;1348:17–26 [DOI] [PubMed] [Google Scholar]
- 68.Wendel AA, Cooper DE, Ilkayeva OR, Muoio DM, Coleman RA. Glycerol-3-phosphate acyltransferase (GPAT)-1, but not GPAT4, incorporates newly synthesized fatty acids into triacylglycerol and diminishes fatty acid oxidation. J Biol Chem. Epub 2013. Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qi J, Lang W, Geisler JG, Wang P, Petrounia I, Mai S, Smith C, Askari H, Struble GT, Williams R, et al. The use of stable isotope-labeled glycerol and oleic acid to differentiate the hepatic functions of DGAT1 and -2. J Lipid Res. 2012;53:1106–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wurie HR, Buckett L, Zammit VA. Diacylglycerol acyltransferase 2 (DGAT2) acts upstream of DGAT1, and utilises nascent diglycerides and de novo synthesised fatty acids in HepG2 cells. FEBS J. 2012;279:3033–47 [DOI] [PubMed] [Google Scholar]
- 71.Villanueva CJ, Monetti M, Shih M, Zhou P, Watkins SM, Bhanot S, Farese RV. Specific role for acyl CoA:Diacylglycerol acyltransferase 1 (Dgat1) in hepatic steatosis due to exogenous fatty acids. Hepatology. 2009;50:434–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hall AM, Kou K, Chen Z, Pietka TA, Kumar M, Korenblat KM, Lee K, Ahn K, Fabbrini E, Klein S, et al. Evidence for regulated monoacylglycerol acyltransferase expression and activity in human liver. J Lipid Res. 2012;53:990–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mashek DG, McKenzie MA, Van Horn CG, Coleman RA. Rat long chain acyl-CoA synthetase 5 increases fatty acid uptake and partitioning to cellular triacylglycerol in McArdle-RH7777 cells. J Biol Chem. 2006;281:945–50 [DOI] [PubMed] [Google Scholar]
- 74.Romeo S, Kozlitina J, Zing C, Petsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goran MI, Walker R, Le KA, Mahurka S, Vikman S, Davis JN, Spruijt-Metz D, Weigensberg MJ, Allayee H. Effects of PNPLA3 on liver fat and metabolic profile in Hispanic children and adolescents. Diabetes. 2010;59:3127–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, Cohen JC, Hobbs HH. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285:6706–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Basantani MK, Sitnick MT, Cai L, Brenner DS, Gardner NP, Li JZ, Schoiswohl G, Yang K, Kumari M, Gross RW, et al. Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J Lipid Res. 2011;52:318–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen W, Chang B, Li L, Chan L. Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology. 2010;52:1134–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kumari M, Schoiswohl G, Chitraju C, Paar M, Cornaciu I, Rangrez AY, Wongsiriroj N, Nagy HM, Ivanova PT, Scott SA, et al. Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Cell Metab. 2012;15:691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leamy AK, Egnatchik RA, Young JD. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog Lipid Res. 2013;52:165–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78 [DOI] [PubMed] [Google Scholar]
- 82.Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–74 [DOI] [PubMed] [Google Scholar]
- 83.Nagle CA, Klett EL, Coleman RA. Hepatic triacylglycerol accumulation and insulin resistance. J Lipid Res. 2009;50:S74–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang C, Wendel AA, Keogh MR, Harris TE, Chen J, Coleman RA. Glycerolipid signals alter mTOR complex 2 (mTORC2) to diminish insulin signaling. Proc Natl Acad Sci USA. 2012;109:1667–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilfling F, Wang H, Haas JT, Krahmer N, Gould TJ, Uchida A, Cheng JX, Graham M, Christiano R, Frohlich F, et al. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev Cell. 2013;24:384–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brasaemle DL, Wolins NE. Packaging of fat: an evolving model of lipid droplet assembly and expansion. J Biol Chem. 2012;287:2273–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fujii H, Ikura Y, Arimoto J, Sugioka K, Iezzoni JC, Park SH, Naruko T, Itabe H, Kawada N, Caldwell SH, et al. Expression of perilipin and adipophilin in nonalcoholic fatty liver disease; relevance to oxidative injury and hepatocyte ballooning. J Atheroscler Thromb. 2009;16:893–901 [DOI] [PubMed] [Google Scholar]
- 89.Straub BK, Stoeffel P, Heid H, Zimbelmann R, Schirmacher P. Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology. 2008;47:1936–46 [DOI] [PubMed] [Google Scholar]
- 90.Mannaerts GP, Van Veldhoven PP, Casteels M. Peroxisomal lipid degradation via beta- and alpha-oxidation in mammals. Cell Biochem Biophys. 2000;32:73–87 [DOI] [PubMed] [Google Scholar]
- 91.Havel RJ. Caloric homeostasis and disorders of fuel transport. N Engl J Med. 1972;287:1186–92 [DOI] [PubMed] [Google Scholar]
- 92.Ellis JM, Li LO, Wu PC, Koves TR, Ilkayeva O, Stevens RD, Watkins SM, Muoio DM, Coleman RA. Adipose acyl-CoA synthetase-1 directs fatty acids toward beta-oxidation and is required for cold thermogenesis. Cell Metab. 2010;12:53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ellis JM, Mentock SM, Depetrillo MA, Koves TR, Sen S, Watkins SM, Muoio DM, Cline GW, Taegtmeyer H, Shulman GI, et al. Mouse cardiac acyl coenzyme a synthetase 1 deficiency impairs fatty acid oxidation and induces cardiac hypertrophy. Mol Cell Biol. 2011;31:1252–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li LO, Ellis JM, Paich HA, Wang S, Gong N, Altshuller G, Thresher RJ, Koves TR, Watkins SM, Muoio DM, et al. Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. J Biol Chem. 2009;284:27816–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Erol E, Kumar LS, Cline GW, Shulman GI, Kelly DP, Binas B. Liver fatty acid binding protein is required for high rates of hepatic fatty acid oxidation but not for the action of PPARalpha in fasting mice. FASEB J. 2004;18:347–9 [DOI] [PubMed] [Google Scholar]
- 96.Bordewick U, Heese M, Borchers T, Robenek H, Spener F. Compartmentation of hepatic fatty-acid-binding protein in liver cells and its effect on microsomal phosphatidic acid biosynthesis. Biol Chem Hoppe Seyler. 1989;370:229–38 [DOI] [PubMed] [Google Scholar]
- 97.Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F. Liver fatty acid-binding protein and obesity. J Nutr Biochem. 2010;21:1015–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Woldegiorgis G, Bremer J, Shrago E. Substrate inhibition of carnitine palmitoyltransferase by palmitoyl-CoA and activation by phospholipids and proteins. Biochim Biophys Acta. 1985;837:135–40 [DOI] [PubMed] [Google Scholar]
- 99.McGarry JD, Mannaerts GP, Foster DW. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977;60:265–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–9 [DOI] [PubMed] [Google Scholar]
- 101.Akkaoui M, Cohen I, Esnous C, Lenoir V, Sournac M, Girard J, Prip-Buss C. Modulation of the hepatic malonyl-CoA–carnitine palmitoyltransferase 1A partnership creates a metabolic switch allowing oxidation of de novo fatty acids. Biochem J. 2009;420:429–38 [DOI] [PubMed] [Google Scholar]
- 102.Stefanovic-Racic M, Perdomo G, Mantell BS, Sipula IJ, Brown NF, O'Doherty RM. A moderate increase in carnitine palmitoyltransferase 1a activity is sufficient to substantially reduce hepatic triglyceride levels. Am J Physiol Endocrinol Metab. 2008;294:E969–77 [DOI] [PubMed] [Google Scholar]
- 103.Orellana-Gavaldà JM, Herrero L, Malandrino MI, Paneda A, Sol Rodrizuez-Pena M, Petry H, Asins G, Van Deventer S, Hegardt FG, Serra D. Molecular therapy for obesity and diabetes based on a long-term increase in hepatic fatty-acid oxidation. Hepatology. 2011;53:821–32 [DOI] [PubMed] [Google Scholar]
- 104.Lindén D, William-Olsson L, Ahnmark A, Ekroos K, Hallberg C, Sjogren HP, Becker B, Svensson L, Clapham JC, Oscarsson J, et al. Liver-directed overexpression of mitochondrial glycerol-3-phosphate acyltransferase results in hepatic steatosis, increased triacylglycerol secretion and reduced fatty acid oxidation. FASEB J. 2006;20:434–43 [DOI] [PubMed] [Google Scholar]
- 105.Yazdi M, Ahnmark A, William-Olsson L, Snaith M, Turner N, Osla F, Wedin M, Asztely AK, Elmgren A, Bohlooly-Y M, et al. The role of mitochondrial glycerol-3-phosphate acyltransferase-1 in regulating lipid and glucose homeostasis in high-fat diet fed mice. Biochem Biophys Res Commun. 2008;369:1065–70 [DOI] [PubMed] [Google Scholar]
- 106.Yu XX, Murray SF, Pandey SK, Booten SL, Bao D, Song XZ, Kelly S, Chen S, McKay R, Monia BP, et al. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology. 2005;42:362–71 [DOI] [PubMed] [Google Scholar]
- 107.Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, Kim S, Distefano A, Samuel VT, Neschen S, et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem. 2007;282:22678–88 [DOI] [PubMed] [Google Scholar]
- 108.Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53:116–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu JW, Wang SP, Alvarez F, Casavant S, Gauthier N, Abed L, Soni KG, Yang G, Mitchell GA. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology. 2011;54:122–32 [DOI] [PubMed] [Google Scholar]
- 110.Sapiro JM, Mashek MT, Greenberg AS, Mashek DG. Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity. J Lipid Res. 2009;50:1621–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guo F, Ma Y, Kadegowda AKG, Xie P, Liu G, Liu X, Miao H, Ou J, Su X, Zheng Z, et al. Deficiency of liver Comparative Gene Identification-58 causes steatohepatitis and fibrosis in mice. J Lipid Res. 2013;54:2109–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown JM, Betters JL, Lord C, Ma Y, Han X, Yang K, Alger HM, Melchior J, Sawyer J, Shah R, et al. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J Lipid Res. 2010;51:3306–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fisher EA, Pan M, Xhen X, Wu X, Wang H, Jamil H, Sparks JD, Williams KJ. The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. J Biol Chem. 2001;276:27855–63 [DOI] [PubMed] [Google Scholar]
- 114.Sakata N, Wu X, Dixon JL, Ginsberg HN. Proteolysis and lipid-facilitated translocation are distinct but competitive processes that regulate secretion of apolipoprotein B in Hep G2 cells. J Biol Chem. 1993;268:22967–70 [PubMed] [Google Scholar]
- 115.Yao Z, Tran K, McLeod RS. Intracellular degradation of newly synthesized apolipoprotein B. J Lipid Res. 1997;38:1937–53 [PubMed] [Google Scholar]
- 116.Shelness GS, Ingram MF, Huang XF, DeLozier JA. Apolipoprotein B in the rough endoplasmic reticulum: translation, translocation and the initiation of lipoprotein assembly. J Nutr. 1999;129:S456–62 [DOI] [PubMed] [Google Scholar]
- 117.Raabe M, Veniant MM, Sullivan MA, Zlot CH, Bjorkegren J, Nielsen LB, Wong JS, Hamilton RL, Young SG. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J Clin Invest. 1999;103:1287–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pan M, Liang Js J, Fisher EA, Ginsberg HN. The late addition of core lipids to nascent apolipoprotein B100, resulting in the assembly and secretion of triglyceride-rich lipoproteins, is independent of both microsomal triglyceride transfer protein activity and new triglyceride synthesis. J Biol Chem. 2002;277:4413–21 [DOI] [PubMed] [Google Scholar]
- 119.Yang LY, Kuksis A, Myher JJ, Steiner G. Origin of triacylglycerol moiety of plasma very low density lipoproteins in the rat: structural studies. J Lipid Res. 1995;36:125–36 [PubMed] [Google Scholar]
- 120.Yang LY, Kuksis A, Myher JJ, Steiner G. Contribution of de novo fatty acid synthesis to very low density lipoprotein triacylglycerols: evidence from mass isotopomer distribution analysis of fatty acids synthesized from [2H6]ethanol. J Lipid Res. 1996;37:262–74 [PubMed] [Google Scholar]
- 121.Lankester DL, Brown AM, Zammit VA. Use of cytosolic triacylglycerol hydrolysis products and of exogenous fatty acid for the synthesis of triacylglycerol secreted by cultured rat hepatocytes. J Lipid Res. 1998;39:1889–95 [PubMed] [Google Scholar]
- 122.Wei E, Wen Ali Y, Lyon J, Wang H, Nelson R, Dolinsky VW, Dyck JR, Mitchell G, Korbutt GS, Lehner R. Loss of TGH/Ces3 in mice decreases blood lipids, improves glucose tolerance, and increases energy expenditure. Cell Metab. 2010;11:183–93 [DOI] [PubMed] [Google Scholar]
- 123.Lian J, Wei E, Wang SP, Quiroga AD, Li L, Pardo AD, van der Veen J, Sipione S, Mitchell GA, Lehner R. Liver specific inactivation of carboxylesterase 3/Triacylglycerol hydrolase decreases blood lipids without causing severe steatosis. Hepatology. 2012;56:2154–62 [DOI] [PubMed] [Google Scholar]
- 124.Wang H, Wei E, Quiroga AD, Sun X, Touret N, Lehner R. Altered lipid droplet dynamics in hepatocytes lacking triacylglycerol hydrolase expression. Mol Biol Cell. 2010;21:1991–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gilham D, Alam M, Gao W, Vance DE, Lehner R. Triacylglycerol hydrolase is localized to the endoplasmic reticulum by an unusual retrieval sequence where it participates in VLDL assembly without utilizing VLDL lipids as substrates. Mol Biol Cell. 2005;16:984–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Quiroga AD, Li L, Trötzmüller M, Nelson R, Proctor SD, Köfeler H, Lehner R. Deficiency of carboxylesterase 1/esterase-x results in obesity, hepatic steatosis, and hyperlipidemia. Hepatology. 2012;56:2188–98 [DOI] [PubMed] [Google Scholar]
- 127.Wolinski H, Kolb D, Hermann S, Koning RI, Kohlwein SD. A role for seipin in lipid droplet dynamics and inheritance in yeast. J Cell Sci. 2011;124:3894–904 [DOI] [PubMed] [Google Scholar]
- 128.Ye J, Li JZ, Liu Y, Li X, Yang T, Ma X, Li Q, Yao Z, Li P. Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B. Cell Metab. 2009;9:177–90 [DOI] [PubMed] [Google Scholar]
- 129.Li X, Ye J, Zhou L, Gu W, Fisher EA, Li P. Opposing roles of cell death-inducing DFF45-like effector B and perilipin 2 in controlling hepatic VLDL lipidation. J Lipid Res. 2012;53:1877–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liang JJ, Oelkers P, Guo C, Chu P-C, Dixon JL, Ginsberg HN, Sturley SL. Overexpression of human diacylglycerol acyltransferase 1, acyl-coa:cholesterol acyltransferase 1, or acyl-CoA:cholesterol acyltransferase 2 stimulates secretion of apolipoprotein B-containing lipoproteins in McA-RH7777 cells. J Biol Chem. 2004;279:44938–44 [DOI] [PubMed] [Google Scholar]
- 131.Waterman IJ, Price NT, Zammit VA. Distinct ontogenic patterns of overt and latent DGAT activities of rat liver microsomes. J Lipid Res. 2002;43:1555–62 [DOI] [PubMed] [Google Scholar]
- 132.Vedala A, Wang W, Neese RA, Christiansen MP, Hellerstein MK. Delayed secretory pathway contributions to VLDL-triglycerides from plasma NEFA, diet, and de novo lipogenesis in humans. J Lipid Res. 2006;47:2562–74 [DOI] [PubMed] [Google Scholar]
- 133.Pullen DL, Liesman JS, Emery RS. A species comparison of liver slice synthesis and secretion of triacylglycerol from nonesterified fatty acids in media. J Anim Sci. 1990;68:1395–9 [DOI] [PubMed] [Google Scholar]
- 134.Abo-Hashema KA, Cake MH, Lukas MA, Knudsen J. Evaluation of the affinity and turnover number of both hepatic mitochondrial and microsomal carnitine acyltransferases: relevance to intracellular partitioning of acyl-CoAs. Biochemistry. 1999;38:15840–7 [DOI] [PubMed] [Google Scholar]
- 135.Broadway NM, Saggerson ED. Inhibition of liver microsomal carnitine acyltransferases by sulphonylurea drugs. FEBS Lett. 1995;371:137–9 [DOI] [PubMed] [Google Scholar]
- 136.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Skop V, Cahová M, Papáčková Z, Páleníčková E, Daňková H, Baranowski M, Zabielski P, Zdychová J, Zídková J, Kazdová L. Autophagy-lysosomal pathway is involved in lipid degradation in rat liver. Physiol Res. 2012;61:287–97 [DOI] [PubMed] [Google Scholar]
- 138.Mandard S, Müller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci. 2004;61:393–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pawar A, Jump DB. Unsaturated fatty acid regulation of peroxisome proliferator-activated receptor alpha activity in rat primary hepatocytes. J Biol Chem. 2003;278:35931–9 [DOI] [PubMed] [Google Scholar]
- 140.Chakravarthy MV, Lodhi IJ, Yin L, Malapaka RR, Xu HE, Turk J, Semenkovich CF. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell. 2009;138:476–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93:S884–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chakrabarti P, Kandror KV. FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. J Biol Chem. 2009;284:13296–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.He J, Lee JH, Febbraio M, Xie W. The emerging roles of fatty acid translocase/CD36 and the aryl hydrocarbon receptor in fatty liver disease. Exp Biol Med. 2011;236:1116–21.d [DOI] [PubMed] [Google Scholar]
- 144.Moya M, Benet M, Guzmán C, Tolosa L, García-Monzón C, Pareja E, Castell JV, Jover R. Foxa1 reduces lipid accumulation in human hepatocytes and is down-regulated in nonalcoholic fatty liver. PLoS ONE. 2012;7:e30014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Martinez-Jimenez CP, Kyrmizi I, Cardot P, Gonzalez FJ, Talianidis I. Hepatocyte nuclear factor 4alpha coordinates a transcription factor network regulating hepatic fatty acid metabolism. Mol Cell Biol. 2010;30:565–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yin L, Ma H, Ge X, Edwards PA, Zhang Y. Hepatic hepatocyte nuclear factor 4α is essential for maintaining triglyceride and cholesterol homeostasis. Arterioscler Thromb Vasc Biol. 2011;31:328–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wolfrum C, Asilmaz E, Luca E, Friedman JM, Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432:1027–32 [DOI] [PubMed] [Google Scholar]
- 148.Wolfrum C, Stoffel M. Coactivation of Foxa2 through Pgc-1beta promotes liver fatty acid oxidation and triglyceride/VLDL secretion. Cell Metab. 2006;3:99–110 [DOI] [PubMed] [Google Scholar]
- 149.Kim JB, Sarraf P, Wright M, Yao KM, Mueller E, Solanes G, Lowell BB, Spiegelman BM. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J Clin Invest. 1998;101:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang D, Sul HS. Upstream stimulatory factor binding to the E-box at -65 is required for insulin regulation of the fatty acid synthase promoter. J Biol Chem. 1997;272:26367–74 [DOI] [PubMed] [Google Scholar]
- 151.Ferré P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab. 2010;12:83–92 [DOI] [PubMed] [Google Scholar]
- 152.Lee A-H, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ma L, Robinson LN, Towle HC. ChREBP*Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem. 2006;281:28721–30 [DOI] [PubMed] [Google Scholar]
- 154.Yamashita H, Takenoshita M, Sakurai M, Bruick RK, Henzel WJ, Shillinglaw W, Arnot D, Uyeda K. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci USA. 2001;98:9116–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guha P, Aneja KK, Shilpi RY, Haldar D. Transcriptional regulation of mitochondrial glycerophosphate acyltransferase is mediated by distal promoter via ChREBP and SREBP-1. Arch Biochem Biophys. 2009;490:85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nomura K, Yamanouchi T. The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. J Nutr Biochem. 2012;23:203–8 [DOI] [PubMed] [Google Scholar]
- 157.Schwarz J-M, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr. 2003;77:43–50 [DOI] [PubMed] [Google Scholar]
- 158.Higuchi N, Kato M, Shundo Y, Tajiri H, Tanaka M, Yamashita N, Kohjima M, Kotoh K, Nakamuta M, Takayanagi R, et al. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol Res. 2008;38:1122–9 [DOI] [PubMed] [Google Scholar]
- 159.Cha J-Y, Repa JJ. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem. 2007;282:743–51 [DOI] [PubMed] [Google Scholar]
- 160.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Goto T, Kim Y-I, Funakoshi K, Teraminami A, Uemura T, Hirai S, Lee J-Y, Makishima M, Nakata R, Inoue H, et al. Farnesol, an isoprenoid, improves metabolic abnormalities in mice via both PPARα-dependent and -independent pathways. Am J Physiol Endocrinol Metab. 2011;301:E1022–32 [DOI] [PubMed] [Google Scholar]
- 162.Zhang Y-L, Hernandez-Ono A, Siri P, Weisberg S, Conlon D, Graham MJ, Crooke RM, Huang L-S, Ginsberg HN. Aberrant hepatic expression of PPARgamma2 stimulates hepatic lipogenesis in a mouse model of obesity, insulin resistance, dyslipidemia, and hepatic steatosis. J Biol Chem. 2006;281:37603–15 [DOI] [PubMed] [Google Scholar]
- 163.Schadinger SE, Bucher NL, Schreiber BM, Farmer SR. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am J Physiol Endocrinol Metab. 2005;288:E1195–205 [DOI] [PubMed] [Google Scholar]
- 164.Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, Nicol CJ, Vinson C, Gonzalez FJ, Reitman ML. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268–76 [DOI] [PubMed] [Google Scholar]
- 165.Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem. 2006;281:10105–17 [DOI] [PubMed] [Google Scholar]
- 166.Deng X, Zhang W. O-Sullivan I, Williams JB, Dong Q, Park EA, Raghow R, Unterman TG, Elam MB. FoxO1 inhibits sterol regulatory element-binding protein-1c (SREBP-1c) gene expression via transcription factors Sp1 and SREBP-1c. J Biol Chem. 2012;287:20132–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Basciano H, Miller A, Baker C, Naples M, Adeli K. LXRalpha activation perturbs hepatic insulin signaling and stimulates production of apolipoprotein B-containing lipoproteins. Am J Physiol Gastrointest Liver Physiol. 2009;297:G323–32 [DOI] [PubMed] [Google Scholar]
- 168.Moon Y-A, Liang G, Xie X, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Brown MS, Goldstein JL, Horton JD. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab. 2012;15:240–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kamagate A, Qu S, Perdomo G, Su D, Kim DH, Slusher S, Meseck M, Dong HH. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest. 2008;118:2347–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wu K, Cappel D, Martinez M, Stafford JM. Impaired-inactivation of FoxO1 contributes to glucose-mediated increases in serum very low-density lipoprotein. Endocrinology. 2010;151:3566–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Haeusler RA, Han S, Accili D. Hepatic FoxO1 ablation exacerbates lipid abnormalities during hyperglycemia. J Biol Chem. 2010;285:26861–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Qiang L, Tsuchiya K, Kim-Muller J-Y, Lin HV, Welch C, Accili D. Increased atherosclerosis and endothelial dysfunction in mice bearing constitutively deacetylated alleles of Foxo1 gene. J Biol Chem. 2012;287:13944–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pietrocola F, Izzo V, Niso-Santano M, Vacchelli E, Galluzzi L, Maiuri MC, Kroemer G. Regulation of autophagy by stress-responsive transcription factors. Semin Cancer Biol. Epub 2013. May 30. [DOI] [PubMed] [Google Scholar]
- 174.van der Vos KE, Eliasson P, Proikas-Cezanne T, Vervoort SJ, van Boxtel R, Putker M, van Zutphen IJ, Mauthe M, Zellmer S, Pals C, et al. Modulation of glutamine metabolism by the PI(3)K–PKB–FOXO network regulates autophagy. Nat Cell Biol. 2012;14:829–37 [DOI] [PubMed] [Google Scholar]
- 175.Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, Wang D, Feng J, Yu L, Zhu W-G. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol. 2010;12:665–75 [DOI] [PubMed] [Google Scholar]
- 176.Munday MR, Campbell DG, Carling D, Hardie DG. Identification by amino acid sequencing of three major regulatory phosphorylation sites on rat acetyl-CoA carboxylase. Eur J Biochem. 1988;175:331–8 [DOI] [PubMed] [Google Scholar]
- 177.Davies SP, Sim AT, Hardie DG. Location and function of three sites phosphorylated on rat acetyl-CoA carboxylase by the AMP-activated protein kinase. Eur J Biochem. 1990;187:183–90 [DOI] [PubMed] [Google Scholar]
- 178.Winder WW, Wilson HA, Hardie DG, Rasmussen BB, Hutber CA, Call GB, Clayton RD, Conley LM, Yoon S, Zhou B. Phosphorylation of rat muscle acetyl-CoA carboxylase by AMP-activated protein kinase and protein kinase A. J Appl Physiol. 1997;82:219–25 [DOI] [PubMed] [Google Scholar]
- 179.Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, Saha AK. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem. 2002;277:32571–7 [DOI] [PubMed] [Google Scholar]
- 180.Muoio DM, Seefeld K, Witters LA, Coleman RA. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J. 1999;338:783–91 [PMC free article] [PubMed] [Google Scholar]
- 181.Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA. 2007;104:12017–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ponugoti B, Kim D-H, Xiao Z, Smith Z, Miao J, Zang M, Wu S-Y, Chiang C-M, Veenstra TD, Kemper JK. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem. 2010;285:33959–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Walker AK, Yang F, Jiang K, Ji J-Y, Watts JL, Purushotham A, Boss O, Hirsch ML, Ribich S, Smith JJ. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24:1403–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kemper JK, Xiao Z, Ponugoti B, Miao J, Fang S, Kanamaluru D, Tsang S, Wu S-Y, Chiang C-M, Veenstra TD. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 2009;10:392–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–8 [DOI] [PubMed] [Google Scholar]
- 186.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.von Meyenn F, Porstmann T, Gasser E, Selevsek N, Schmidt A, Aebersold R, Stoffel M. Glucagon-induced acetylation of Foxa2 regulates hepatic lipid metabolism. Cell Metab. 2013;17:436–47 [DOI] [PubMed] [Google Scholar]
- 188.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–63 [DOI] [PubMed] [Google Scholar]
- 189.Ng F, Tang BL. Sirtuins’ modulation of autophagy. J Cell Physiol. 2013;228:2262–70. [DOI] [PubMed] [Google Scholar]
- 190.Noriega LG, Feige JN, Canto C, Yamamoto H, Yu J, Herman MA, Mataki C, Kahn BB, Auwerx J. CREB and ChREBP oppositely regulate SIRT1 expression in response to energy availability. EMBO Rep. 2011;12:1069–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shimazu T, Hirschey MD, Hua L, Dittenhafer-Reed KE, Schwer B, Lombard DB, Li Y, Bunkenborg J, Alt FW, Denu JM, et al. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 2010;12:654–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Aledo R, Zschocke J, Pié J, Mir C, Fiesel S, Mayatepek E, Hoffmann GF, Casals N, Hegardt FG. Genetic basis of mitochondrial HMG-CoA synthase deficiency. Hum Genet. 2001;109:19–23 [DOI] [PubMed] [Google Scholar]
- 193.Thompson GN, Hsu BY, Pitt JJ, Treacy E, Stanley CA. Fasting hypoketotic coma in a child with deficiency of mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase. N Engl J Med. 1997;337:1203–7 [DOI] [PubMed] [Google Scholar]
- 194.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, et al. mTOR Complex 1 regulates Lipin 1 Localization to control the SREBP pathway. Cell. 2011;146:408–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–4 [DOI] [PubMed] [Google Scholar]
- 196.Chakrabarti P, Kim JY, Singh M, Shin Y-K, Kim J, Kumbrink J, Wu Y, Lee M-J, Kirsch KH, Fried SK. Insulin inhibits lipolysis in adipocytes via the evolutionary conserved mTORC1-Egr1-ATGL-mediated pathway. Mol Cell Biol. 2013;33:3659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273:3963–6 [DOI] [PubMed] [Google Scholar]
- 198.Sparks JD, Sparks CE. Insulin regulation of triacylglycerol-rich lipoprotein synthesis and secretion. Biochim Biophys Acta. 1994;1215:9–32 [DOI] [PubMed] [Google Scholar]
- 199.Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab. 2011;22:353–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ai D, Baez JM, Jiang H, Conlon DM, Hernandez-Ono A, Frank-Kamenetsky M, Milstein S, Fitzgerald K, Murphy AJ, Woo CW, et al. Activation of ER stress and mTORC1 suppresses hepatic sortilin-1 levels in obese mice. J Clin Invest. 2012;122:1677–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Watanabe S, Yaginuma R, Ikejima K, Miyazaki A. Liver diseases and metabolic syndrome. J Gastroenterol. 2008;43:509–18 [DOI] [PubMed] [Google Scholar]
- 202.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ravikumar B, Carey PE, Snaar JEM, Deelchand DK, Cook DB, Neely RDG, English PT, Firbank MJ, Morris PG, Taylor R. Real-time assessment of postprandial fat storage in liver and skeletal muscle in health and type 2 diabetes. Am J Physiol Endocrinol Metab. 2005;288:E789–97 [DOI] [PubMed] [Google Scholar]
- 204.Jonkers RAM, van Loon LJC, Nicolay K, Prompers JJ. In vivo postprandial lipid partitioning in liver and skeletal muscle in prediabetic and diabetic rats. Diabetologia. 2013;56:618–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Allard JP, Aghdassi E, Mohammed S, Raman M, Avand G, Jalali P, Kandasamy T, Prayitno N, Sherman M, et al. Nutritional assessment and hepatic fatty acid composition in non-alcoholic fatty liver disease (NAFLD): a cross-sectional study. J Hepatol. 2008;48:300–7 [DOI] [PubMed] [Google Scholar]
- 206.Ayuso E, Mingozzi F, Bosch F. Production, purification and characterization of adeno-associated vectors. Curr Gene Ther. 2010;10:423–36 [DOI] [PubMed] [Google Scholar]
- 207.Araya J, Rodrigo R, Videla LA, Thielemann L, Orellana M, Pettinelli P, Poniachik J. Increase in long-chain polyunsaturated fatty acid n–6/n–3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci Lond Engl. 2004;106:635–43 [DOI] [PubMed] [Google Scholar]
- 208.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:92–103 [DOI] [PubMed] [Google Scholar]
- 210.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–53 [DOI] [PubMed] [Google Scholar]
- 211.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–92 [DOI] [PubMed] [Google Scholar]
- 212.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, Rizzetto M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–42 [DOI] [PubMed] [Google Scholar]
- 213.Hodson L, McQuaid SE, Humphreys SM, Milne R, Fielding BA, Frayn KN, Karpe F. Greater dietary fat oxidation in obese compared with lean men: an adaptive mechanism to prevent liver fat accumulation? Am J Physiol Endocrinol Metab. 2010;299:E584–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Iozzo P, Bucci M, Roivainen A, Någren K, Järvisalo MJ, Kiss J, Guiducci L, Fielding B, Naum AG, Borra R, et al. Fatty acid metabolism in the liver, measured by positron emission tomography, is increased in obese individuals. Gastroenterology. 2010;139:846–56 [DOI] [PubMed] [Google Scholar]
- 215.Satapati S, Sunny NE, Kucejova B, Fu X, He TT, Méndez-Lucas A, Shelton JM, Perales JC, Browning JD, Burgess SC. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J Lipid Res. 2012;53:1080–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14:804–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Adiels M, Taskinen M-R, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, Vehkavaara S, Häkkinen A, Olofsson S-O, Yki-Järvinen H, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49:755–65 [DOI] [PubMed] [Google Scholar]
- 218.Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Flannery C, Dufour S, Rabøl R, Shulman GI, Petersen KF. Skeletal muscle insulin resistance promotes increased hepatic de novo lipogenesis, hyperlipidemia, and hepatic steatosis in the elderly. Diabetes. 2012;61:2711–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Eissing L, Scherer T, Tödter K, Knippschild U, Greve JW, Buurman WA, Pinnschmidt HO, Rensen SS, Wolf AM, Bartelt A, et al. De novo lipogenesis in human fat and liver is linked to ChREBP-β and metabolic health. Nat Commun. 2013;4:1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–85 [DOI] [PubMed] [Google Scholar]
- 222.Beaven SW, Matveyenko A, Wroblewski K, Chao L, Wilpitz D, Hsu TW, Lentz J, Drew B, Hevener AL, Tontonoz P. Reciprocal regulation of hepatic and adipose lipogenesis by liver x receptors in obesity and insulin resistance. Cell Metab. 2013;18:106–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lin C-W, Zhang H, Li M, Xiong X, Chen X, Chen X, Dong XC, Yin X-M. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J Hepatol. 2013;58:993–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Begriche K, Massart J, Robin M-A, Bonnet F, Fromenty B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology.. [DOI] [PubMed] [Google Scholar]