Abstract
Disorders of phosphorus metabolism are independent risk factors for cardiovascular disease. Because excess dietary phosphorus intake is common in the general population and plays a central role in disturbances in phosphorus homeostasis, these findings have fueled interest in restricting phosphorus intake as a potential therapy for improving cardiovascular outcomes. Although experimental and observational data support this possibility, current limitations in the assessment of dietary phosphorus consumption in free-living populations and the lack of reliable biomarkers of the effects of dietary phosphorus on cardiovascular health pose major barriers to the design and conduct of trials assessing the efficacy of phosphorus restriction in improving cardiovascular health. Fibroblast growth factor 23 and Klotho are novel mediators of phosphorus metabolism that are tightly linked to dietary phosphorus intake and show promise as integrated biomarkers of phosphorus excess and its long-term health consequences. Advances in the understanding of how these hormones are associated with diet and phosphorus metabolism will likely bolster future efforts to assess the true health consequences of excess phosphorus intake and whether restricting phosphorus intake has salutary effects on cardiovascular health.
Introduction
Phosphorus is an essential micronutrient involved in a number of key biological processes. Disturbances in systemic phosphorus homeostasis have been associated with cardiovascular disease events and death, particularly among individuals with chronic kidney disease (CKD)4 (1–4). Although the mechanisms for these associations remain incompletely understood, a considerable body of data implicates local and systemic alterations in phosphorus metabolism in the pathogenesis of cardiovascular disease (5–10). Because excess dietary phosphorus intake is common in individuals consuming Westernized diets (11) and can lead to disturbances in phosphorus metabolism, these findings have fueled interest in dietary phosphorus restriction as a potential therapy for improving cardiovascular outcomes. Although intriguing, epidemiologic data linking dietary phosphorus intake and cardiovascular outcomes have been weak or inconclusive, in large part because of the difficulty in ascertaining phosphorus consumption in large cohort studies and the lack of reliable biomarkers of the effects of dietary phosphorus on cardiovascular health. The relatively recent discovery of novel mediators of phosphorus metabolism such as fibroblast growth factor 23 (FGF23) and Klotho may help to address these deficiencies and provide the necessary tools needed to examine the association of dietary phosphorus with cardiovascular outcomes. The focus of this section will be to review the evidence supporting this possibility and consider the next steps required to establish the efficacy and feasibility of dietary phosphorus restriction for cardiovascular protection.
Current Status of Knowledge
Role of diet in maintenance of phosphorus metabolism.
Serum phosphorus concentrations represent a highly dynamic balance of dietary phosphorus absorption, urinary phosphorus excretion, and exchanges with bone, soft tissue, and intracellular stores (11). The kidneys are the primary organs that regulate this balance by modulating urinary phosphorus excretion in response to changes in diet intake and bone/soft tissue turnover, with diet intake making up the majority of the obligate phosphorus load that the kidneys must eliminate on a daily basis to maintain phosphorus balance (12).
Dietary phosphorus is well absorbed across the entire intestinal tract by a combination of passive paracellular diffusion and active transport across luminal sodium-phosphorus cotransporters (13). Active phosphorus transport is primary regulated by the secosteroid hormone 1,25-dihydroxyvitamin D [1,25(OH)2D], which enhances intestinal phosphorus absorption by stimulating phosphorus transport across gut epithelial cells (14). Most circulating inorganic phosphorus is freely filtered in renal glomeruli and enters renal proximal tubules. Under typical dietary conditions, 80–90% of the filtered load is reabsorbed across sodium-phosphorus cotransporters in proximal tubular cells and the rest is excreted in the urine (15). Parathyroid hormone and FGF23 are the primary hormones that regulate the fraction of filtered phosphorus that is reabsorbed in renal proximal tubules by downregulating sodium-phosphorus cotransporters in renal proximal tubule cells (12, 16). FGF23 also limits dietary phosphorus absorption by lowering 1,25(OH)2D concentrations via inhibition of 25-hydroxyvitamin D-1α-hydroxylase and stimulation of 24-hydroxylase, the major catabolic pathway for 1,25(OH)2D (16).
The secretion of FGF23 is strongly influenced by dietary phosphorus intake (Fig. 1). Excess dietary phosphorus consumption results in increased FGF23 secretion as a compensatory response to help prevent hyperphosphatemia by enhancing urinary phosphorus excretion and limiting dietary phosphorus absorption (17–19). Similarly, the secretion of FGF23 is suppressed by dietary phosphorus restriction to preserve phosphorus balance by reducing phosphorus excretion in the urine and enhancing dietary phosphorus reabsorption. Consistent with this, animal and human studies have shown that restriction of dietary phosphorus absorption decreased FGF23, whereas oral phosphorus loading stimulated secretion of FGF23 in healthy volunteers (20–22). These findings indicate that FGF23 is pivotal for maintaining normal phosphorus homeostasis in response to changes in dietary phosphorus intake.
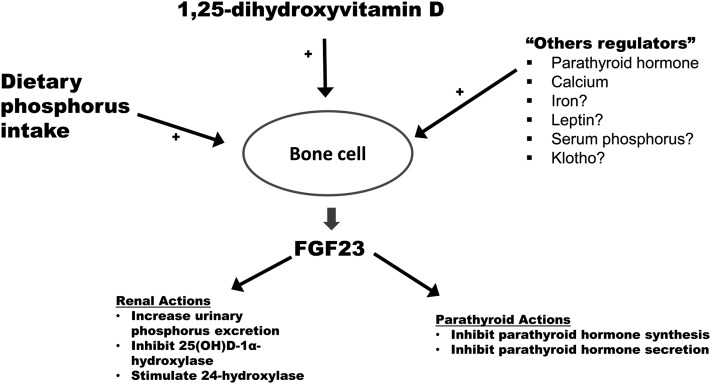
FIGURE 1.
Bone cells are the primary cells that synthesize and secrete FGF23. Increased dietary phosphorus absorption and increased 1,25(OH)2D concentrations are 2 of the best-characterized systemic stimuli (+) of FGF23 secretion. A number of other factors may also stimulate FGF23 secretion, including parathyroid hormone, calcium, iron, leptin, serum phosphorus, and circulating Klotho, though the evidence supporting the stimulatory effects of some of these systemic factors are controversial (denoted by question marks). FGF23 acts primarily in the kidneys and parathyroid glands. In the kidneys, FGF23 augments urinary phosphate excretion by downregulating sodium-phosphate cotransporters in renal proximal tubular cells. In addition, FGF23 inhibits the synthesis of 25-hydroxyvitamin D-1α-hydroxylase and upregulates 24-hydroxylase, both of which serve to decrease circulating 1,25(OH)2D concentrations. In the parathyroid glands, FGF23 inhibits both the synthesis and secretion of parathyroid hormone. Transmembrane Klotho is needed for FGF23 to bind to its receptor in the kidney and parathyroid glands with adequate affinity to effect signal transduction. FGF23, fibroblast growth factor 23; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Klotho plays a complementary role to FGF23 in mediating phosphorus balance. Klotho was first identified as an anti-aging protein, resulting in its being named after 1 of the 3 Greek goddesses that controlled the length of life, and exists in 2 major forms: a transmembrane form and a secreted form (23). The transmembrane form serves as the critical cofactor required for FGF23 to bind to its cognate receptor in the kidney and parathyroid glands with adequate affinity to effect signal transduction. The circulating form is derived from alternative RNA splicing or cleavage of the extracellular domain and has a variety of systemic effects, including regulation of phosphorus and calcium metabolism (23–25). Because transmembrane Klotho is needed for FGF23 to bind to fibroblast growth factor receptor (FGFR) with high affinity, decreases in Klotho expression contribute to lower FGF23 binding to FGFR, with attendant consequences such as lower urinary phosphorus excretion. The importance of this is evidenced by Klotho knockout mice that develop severe hyperphosphatemia, widespread vascular calcification, and premature death due to impaired regulation of phosphorus metabolism (26). Circulating Klotho also directly induces phosphaturia (25), indicating that Klotho regulates renal phosphorus handling independent of FGF23. These actions collectively support the notion that Klotho plays an important role in regulating phosphorus metabolism.
Disorders of phosphorus homeostasis and cardiovascular disease.
Phosphorus excess has been implicated in the pathogenesis of adverse cardiovascular outcomes. A large body of literature has shown that excess phosphorus promotes pathologic calcification of vascular media and heart valves (5, 6, 9, 10, 27), helps to induce cardiomyocyte hypertrophy (28–30), and impairs vascular reactivity by inhibiting NO synthesis in animals and humans (8, 31, 32). Together, these findings provide direct mechanistic evidence that excess phosphorus can initiate or accelerate cardiovascular disease. In addition, higher serum phosphorus was associated with inflammatory cytokines (33), and restriction of gut phosphorus absorption reduced biomarkers of inflammation in CKD patients (34–36), linking excess phosphorus intake to inflammation, itself an important mediator of cardiovascular disease.
The clinical relevance of experimental data linking excess phosphorus with cardiovascular disease is supported by observational studies showing that elevated serum phosphorus is associated with vascular calcification in individuals across the spectrum of kidney function (37–39). In addition, studies have linked higher serum phosphorus concentrations with surrogate measures of vascular calcification such as increased arterial stiffness, higher left ventricular mass index, and carotid vessel disease (8, 40–46). Higher serum phosphorus concentrations have also been associated with kidney injury. Studies have shown that higher serum phosphorus concentrations were associated with faster kidney disease progression and higher risk of incident end-stage renal disease independently of established risk factors, including lower baseline estimated glomerular filtrate rate (eGFR) (47–51). Even among individuals with normal kidney function and serum phosphorus concentrations within the normal range, a higher serum phosphorus concentration at baseline was independently associated with higher risk of incident CKD (51), suggesting that excess serum phosphorus may impair renal health at all levels of kidney function.
Dietary phosphorus, serum phosphorus, and adverse outcomes: do the dots connect?
The experimental and epidemiologic data reviewed above provide biological plausibility for a pathophysiological link between dietary phosphorus excess and cardiovascular disease vis-à-vis increased serum phosphorus concentrations. However, to date, convincing evidence linking excess dietary phosphorus intake and cardiovascular disease has been weak at best. There are a number of reasons for this. First, with the possible exception of individuals with CKD, the magnitude of the effect of dietary phosphorus intake on serum phosphorus concentrations appears to be relatively small. In the largest observational study to examine this issue, de Boer et al. (52) examined the associations of dietary phosphorus intake with serum phosphorus in 15,513 participants of the Third NHANES. Utilizing 24-h dietary recall and 1-mo food frequency data, these investigators found a weak but significant association of dietary phosphorus intake with serum phosphorus concentrations. Specifically, they found that each 500-mg greater intake of phosphorus was associated with 0.03-mg/dL higher serum phosphorus concentrations after adjustment for age, sex, race/ethnicity, time of blood draw, and fasting status. When the authors coupled these data with a Spanish study showing similar results (53), they interpreted the data to suggest that dietary intake only minimally affects serum phosphorus concentrations, because phosphorus balance is tightly regulated within a narrow range despite wide variations in dietary intake. Taking this argument to its logical conclusion, this would serve to undercut the notion that excess dietary phosphorus intake contributes to cardiovascular disease by raising serum phosphorus concentrations, at least in individuals with normal kidney function.
Several limitations to these studies deserve consideration prior to drawing broader conclusions on the effect of dietary phosphorus intake on serum phosphorus. First, given that phosphorus-based food additives contribute substantially to total daily phosphorus intake in individuals consuming typical Westernized diets (54), a number of investigators have questioned whether dietary instruments used by NHANES and other large population-based studies adequately capture true dietary phosphorus intake, because standard dietary surveys may not properly account for the additive content of food (55). If not, then it is possible that the association of dietary phosphorus intake with serum phosphorus is weak because of incomplete ascertainment of dietary phosphorus intake and not because of a small magnitude of effect of excess dietary phosphorus intake on serum phosphorus.
In addition, observational studies such as NHANES primarily measured serum phosphorus concentrations in morning fasting samples. Serum phosphorus displays natural diurnal variation, with concentrations reaching a nadir in the morning and rising gradually throughout the day, with a peak concentration normally in the mid-afternoon (56). Detailed feeding studies involving healthy volunteers have demonstrated that, when measured in fasting morning blood samples, serum phosphorus concentrations were minimally different in participants undergoing dietary phosphorus loading compared with individuals consuming a standard phosphorus diet (56–58). However, when comparing mean serum phosphorus concentrations averaged during 24 h (capturing the diurnal variation throughout the day), phosphorus-loaded participants had significantly higher serum phosphorus than controls, primarily because of greater diurnal increases in serum phosphorus during afternoon/evening hours in the loaded group. These studies clearly demonstrate that dietary phosphorus loading can meaningfully increase time-averaged serum phosphorus concentrations, an effect that is missed in population-based studies, which normally only obtain single morning fasting serum phosphorus concentrations. It is important to note, however, that the magnitude of increase in time-averaged serum phosphorus observed in these studies was relatively modest (on the order of 0.5–1.0 mg/dL). Whether such small increases are enough to mediate cardiovascular disease is unclear and requires further study.
A second factor undermining the evidence linking dietary phosphorus intake with cardiovascular disease is the scarcity of epidemiologic data associating excess dietary phosphorus consumption itself (and not surrogate markers such as serum phosphorus) with adverse cardiovascular outcomes. In one of the largest studies examining the relation between dietary phosphorus intake and cardiovascular disease, Alonso et al. (59) analyzed the associations of diet phosphorus (as assessed by validated FFQs) with blood pressure at the baseline visit and incidence of hypertension in 13,444 participants from the Atherosclerosis Risk in Communities and the Multi-Ethnic Study of Atherosclerosis cohorts. These investigators found that, compared with individuals in the lowest quintile of phosphorus intake, those in the highest quintile had lower systolic and diastolic blood pressures after adjustment for potential confounders. Further, higher dietary phosphorus intake was associated with lower risk of development of future hypertension after adjustment for nondietary confounders, though this association was no longer significant after adjustment for dietary factors. These findings suggest that at best there is no association of dietary phosphorus intake with hypertension, with a possible signal for a protective effect of higher phosphorus intake against developing hypertension.
In a more recent study using the Osteoporotic Fractures in Men study cohort, Dominguez et al. (60) examined the relation between 24-h urinary phosphorus:creatinine ratio and fractional excretion of phosphorus with all-cause and cardiovascular disease-related death. A large part of the motivation for conducting this study was the hypothesis that urinary measures of phosphorus excretion may be more accurate markers of true dietary phosphorus consumption than estimates from dietary surveys. Nonetheless, in multivariable adjusted models, these investigators found no associations of urinary phosphorus excretion measures with all-cause or cardiovascular disease-related death, undermining the notion that dietary phosphorus intake associates with adverse cardiovascular outcome.
The association of dietary phosphorus and outcomes has also been examined in the population in which excess phosphorus intake should be most deleterious for cardiovascular health, namely those with underlying kidney disease. Using data from NHANES, Murtaugh et al. (61) examined the association of dietary phosphorus intake (using 24-h dietary recalls) and all-cause mortality in 1105 individuals with CKD defined as an eGFR <60 mL/(min · 1.73 m2). In unadjusted analyses, higher dietary phosphorus intake was associated with lower risk of death. However, after adjustment for demographics, comorbid conditions, eGFR, and other confounders, the inverse association of dietary phosphorus intake with mortality was attenuated and no longer significant.
In contrast to the studies above, Yamamoto et al. (62) demonstrated a direct association of phosphorus intake with cardiovascular disease. These investigators examined the association of dietary phosphorus intake (as assessed by FFQ) and left ventricular mass in 4494 participants of the Multi-Ethnic Study of Atherosclerosis. After adjustment for demographics, diet confounders, and established risk factors for left ventricular hypertrophy, they found that higher dietary phosphorus intake was associated with higher left ventricular mass index in both men and women and greater odds of left ventricular hypertrophy in women but not men. Although these findings suggest that excess dietary phosphorus intake may contribute to heart disease, when combined with the studies showing negative or reverse associations reviewed above, the plurality of current epidemiologic data does not support an association of excess dietary phosphorus intake with development of cardiovascular disease.
Diet phosphorus, FGF23, Klotho, and cardiovascular outcomes: the missing link?
While the evidence linking excess dietary phosphorus with adverse cardiovascular outcomes has been weak at best, it remains unclear whether this is because phosphorus consumption truly has a minimal impact on cardiovascular health or because prior studies have failed to adequately assess the primary exposure of interest, namely phosphorus intake. As mentioned above, assessment of dietary phosphorus intake in free-living adults is hampered by the lack of dietary instruments specifically designed to capture phosphorus in foods in all its forms, particularly inorganic sources from phosphorus-based food additives. Thus, it is quite possible that incomplete ascertainment of true phosphorus intake may at least partly explain the lack of evidence linking dietary phosphorus with poor outcomes. In addition, reliable markers of dietary phosphorus intake beyond dietary survey estimates are lacking. Serum phosphorus concentrations are poor biomarkers of dietary phosphorus intake, even when obtaining time-averaged measurements throughout the day; even though urinary phosphorus excretion should theoretically serve as a better marker of daily phosphorus absorption, it is unclear how well these measures capture actual dietary phosphorus intake, especially in older adults.
For these reasons, the discovery and characterization of newer markers of phosphorus balance such as FGF23 and Klotho may provide desperately needed alternative measures of phosphorus excess. As reviewed above, dietary phosphorus intake is one of the most important systemic modulators of FGF23 secretion, with oral phosphorus loading stimulating FGF23 secretion and diet phosphorus restriction doing the opposite. Although there are relatively few data concerning the impact of dietary phosphorus on Klotho expression, one recent study showed that renal Klotho expression increased in an animal model of CKD following severe dietary phosphorus restriction (63). Given that diminished Klotho expression has been associated with cardiovascular and kidney disease, this suggests that lower phosphorus consumption may be able to reverse the pathophysiological effects of decreased Klotho expression observed in kidney injury and other disease conditions (64).
The importance of these findings for cardiovascular health is supported by experimental and observational data showing that excess FGF23 and Klotho deficiency are strongly associated with cardiovascular disease and mortality. Higher FGF23 concentrations have been independently associated with higher risk of cardiovascular disease events (particularly those related to congestive heart failure) and death in individuals across the spectrum of kidney function (65–70). Though the mechanisms for these associations remain unclear, higher FGF23 concentrations are strongly associated with greater left ventricular mass and higher prevalence of left ventricular hypertrophy (71–73). In addition, FGF23 has been shown to induce hypertrophy of cardiomyocytes in vitro and in vivo (71), both through FGFR-mediated stimulation of hypertrophy and specific effects on calcium handling in the cardiomyocyte (74).
Klotho also has an important role in cardiovascular health. Circulating Klotho increases NO synthesis in endothelial cells (75, 76). The importance of this was shown in Klotho-deficient mice that had impaired vasodilation in response to acetylcholine challenge compared with wild-type controls (75). Further, overexpression of Klotho in an animal model of atherogenesis improved vascular endothelial dysfunction, increased NO production, and reduced elevated blood pressure (77), and Klotho knockdown accelerated vascular calcification, whereas overexpression of Klotho inhibited vascular calcification in an animal model of CKD (78). To date, epidemiologic data linking Klotho and adverse cardiovascular outcomes has lagged behind FGF23, and more studies are needed to determine whether experimental data linking Klotho with cardiovascular disease translates into worse outcomes in population-based studies.
Importantly, the magnitude and strength of the association of FGF23 with adverse outcomes have proven to be greater than that of serum phosphorus itself, suggesting that FGF23 may be a better biomarker of disturbances of phosphorus homeostasis in general. Given that dietary phosphorus is such a strong determinant of FGF23, this may suggest that excess FGF23 may better capture multiple risk elements related to excess phosphorus consumption and its downstream consequences, such as disturbances in total body phosphorus balance. Moreover, plasma FGF23 concentrations manifest much less random variation than other markers of phosphorus metabolism such as serum phosphorus concentrations (79), suggesting that FGF23 may be a more stable, long-term marker of phosphorus intake, akin to the difference between serum glucose and hemoglobin A1C (79). It is important to keep in mind, however, that a number of recent studies have shown that FGF23 may also be influenced by factors apart from dietary phosphorus intake, such as calcium and iron intake and disturbances in metabolic health (80), all of which are tightly linked with diet. Thus, future studies will need to determine whether specifically targeting dietary phosphorus intake can meaningfully reduce FGF23 concentrations in community-dwelling individuals and, if so, whether this has a salutary effect on cardiovascular health.
In conclusion, given the central role of dietary phosphorus intake in the pathogenesis of disturbances of phosphorus homeostasis and the strong link between disordered phosphorus metabolism and cardiovascular disease, restriction of phosphorus consumption may represent an effective intervention for mitigating adverse cardiovascular outcomes in the general population. Although both experimental and human data support this possibility, the lack of reliable biomarkers of phosphorus intake and, by extension, the dearth of appropriate targets for gauging the efficacy of dietary phosphorus restriction beyond serum phosphorus concentrations complicate the design and initiation of clinical trials to test this possibility. With the emergence of novel regulators of phosphorus metabolism that may be more specific markers of excess dietary phosphorus exposure like FGF23 and Klotho, exciting new avenues are emerging for assessing the efficacy of phosphorus restriction in individual patients. Given the markedly high phosphorus content of Westernized diets, these studies should be a high priority in future research.
Acknowledgments
The author read and approved the final manuscript.
Footnotes
Abbreviations used: CKD, chronic kidney disease; eGFR, estimated glomerular filtrate rate; FGF23, fibroblast growth factor 23; FGFR, fibroblast growth factor receptor; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Literature Cited
- 1.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–18 [DOI] [PubMed] [Google Scholar]
- 2.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D'Agostino RB, Sr, Gaziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–85 [DOI] [PubMed] [Google Scholar]
- 3.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–8 [DOI] [PubMed] [Google Scholar]
- 4.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–33 [DOI] [PubMed] [Google Scholar]
- 5.Giachelli CM, Jono S, Shioi A, Nishizawa Y, Mori K, Morii H. Vascular calcification and inorganic phosphate. Am J Kidney Dis. 2001;38:S34–7 [DOI] [PubMed] [Google Scholar]
- 6.Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA. The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol. 2008;19:1092–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Marco GS, Hausberg M, Hillebrand U, Rustemeyer P, Wittkowski W, Lang D, Pavenstadt H. Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am J Physiol Renal Physiol. 2008;294:F1381–7 [DOI] [PubMed] [Google Scholar]
- 8.Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, Sato M, Nashiki K, Amo K, Yamamoto H, Higashi Y, et al. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 2009;20:1504–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau WL, Pai A, Moe SM, Giachelli CM. Direct effects of phosphate on vascular cell function. Adv Chronic Kidney Dis. 2011;18:105–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Abbadi MM, Pai AS, Leaf EM, Yang HY, Bartley BA, Quan KK, Ingalls CM, Liao HW, Giachelli CM. Phosphate feeding induces arterial medial calcification in uremic mice: role of serum phosphorus, fibroblast growth factor-23, and osteopontin. Kidney Int. 2009;75:1297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uribarri J. Phosphorus homeostasis in normal health and in chronic kidney disease patients with special emphasis on dietary phosphorus intake. Semin Dial. 2007;20:295–301 [DOI] [PubMed] [Google Scholar]
- 12.Berndt T, Kumar R. Phosphatonins and the regulation of phosphate homeostasis. Annu Rev Physiol. 2007;69:341–59 [DOI] [PubMed] [Google Scholar]
- 13.Sabbagh Y, Giral H, Caldas Y, Levi M, Schiavi SC. Intestinal phosphate transport. Adv Chronic Kidney Dis. 2011;18:85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katai K, Miyamoto K, Kishida S, Segawa H, Nii T, Tanaka H, Tani Y, Arai H, Tatsumi S, Morita K, et al. Regulation of intestinal Na+-dependent phosphate co-transporters by a low-phosphate diet and 1,25-dihydroxyvitamin D3. Biochem J. 1999;343:705–12 [PMC free article] [PubMed] [Google Scholar]
- 15.Murer H, Hernando N, Forster I, Biber J. Proximal tubular phosphate reabsorption: molecular mechanisms. Physiol Rev. 2000;80:1373–409 [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18:1637–47 [DOI] [PubMed] [Google Scholar]
- 17.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–9 [DOI] [PubMed] [Google Scholar]
- 18.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21:1187–96 [DOI] [PubMed] [Google Scholar]
- 19.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90:1519–24 [DOI] [PubMed] [Google Scholar]
- 20.Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL, Donahue SE, Asplin JR. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:257–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira RB, Cancela AL, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L, Carvalho AB, Jorgetti V, Canziani ME, Moyses RM. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5:286–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shigematsu T, Negi S. Combined therapy with lanthanum carbonate and calcium carbonate for hyperphosphatemia decreases serum FGF-23 level independently of calcium and PTH (COLC Study). Nephrol Dial Transplant. 2012;27:1050–4 [DOI] [PubMed] [Google Scholar]
- 23.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–30 [DOI] [PubMed] [Google Scholar]
- 24.Alexander RT, Woudenberg-Vrenken TE, Buurman J, Dijkman H, van der Eerden BC, van Leeuwen JP, Bindels RJ, Hoenderop JG. Klotho prevents renal calcium loss. J Am Soc Nephrol. 2009;20:2371–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51 [DOI] [PubMed] [Google Scholar]
- 27.Shroff RC, McNair R, Skepper JN, Figg N, Schurgers LJ, Deanfield J, Rees L, Shanahan CM. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol. 2010;21:103–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayus JC, Mizani MR, Achinger SG, Thadhani R, Go AS, Lee S. Effects of short daily versus conventional hemodialysis on left ventricular hypertrophy and inflammatory markers: a prospective, controlled study. J Am Soc Nephrol. 2005;16:2778–88 [DOI] [PubMed] [Google Scholar]
- 29.Galetta F, Cupisti A, Franzoni F, Femia FR, Rossi M, Barsotti G, Santoro G. Left ventricular function and calcium phosphate plasma levels in uraemic patients. J Intern Med. 2005;258:378–84 [DOI] [PubMed] [Google Scholar]
- 30.Neves KR, Graciolli FG, dos Reis LM, Pasqualucci CA, Moyses RM, Jorgetti V. Adverse effects of hyperphosphatemia on myocardial hypertrophy, renal function, and bone in rats with renal failure. Kidney Int. 2004;66:2237–44 [DOI] [PubMed] [Google Scholar]
- 31.Takeda E, Taketani Y, Nashiki K, Nomoto M, Shuto E, Sawada N, Yamamoto H, Isshiki M. A novel function of phosphate-mediated intracellular signal transduction pathways. Adv Enzyme Regul. 2006;46:154–61 [DOI] [PubMed] [Google Scholar]
- 32.Kööbi P, Vehmas TI, Jolma P, Kalliovalkama J, Fan M, Niemela O, Saha H, Kahonen M, Ylitalo P, Rysa J, et al. High-calcium vs high-phosphate intake and small artery tone in advanced experimental renal insufficiency. Nephrol Dial Transplant. 2006;21:2754–61 [DOI] [PubMed] [Google Scholar]
- 33.Navarro-González JF, Mora-Fernandez C, Muros M, Herrera H, Garcia J. Mineral metabolism and inflammation in chronic kidney disease patients: a cross-sectional study. Clin J Am Soc Nephrol. 2009;4:1646–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calò LA, Savica V, Piccoli A, Fusaro M, D'Angelo A, Davis PA. Reduction of hyperphosphatemia is related with the reduction of C-reactive protein in dialysis patients. Study in sevelamer-resistant dialysis patients treated with chitosan chewing gum as salivary phosphate binder. Ren Fail. 2011;33:11–4 [DOI] [PubMed] [Google Scholar]
- 35.Peres AT, Dalboni MA, Canziani ME, Manfredi SR, Carvalho JT, Batista MC, Cuppari L, Carvalho AB, Moyses RM, Guimaraes N, et al. Effect of phosphate binders on oxidative stress and inflammation markers in hemodialysis patients. Hemodial Int. 2009;13:271–7 [DOI] [PubMed] [Google Scholar]
- 36.Shantouf R, Budoff MJ, Ahmadi N, Tiano J, Flores F, Kalantar-Zadeh K. Effects of sevelamer and calcium-based phosphate binders on lipid and inflammatory markers in hemodialysis patients. Am J Nephrol. 2008;28:275–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol. 2009;20:381–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol. 2009;20:397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuttle KR, Short RA. Longitudinal relationships among coronary artery calcification, serum phosphorus, and kidney function. Clin J Am Soc Nephrol. 2009;4:1968–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ix JH, De Boer IH, Peralta CA, Adeney KL, Duprez DA, Jenny NS, Siscovick DS, Kestenbaum BR. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin J Am Soc Nephrol. 2009;4:609–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saab G, Whooley MA, Schiller NB, Ix JH. Association of serum phosphorus with left ventricular mass in men and women with stable cardiovascular disease: data from the Heart and Soul Study. Am J Kidney Dis. 2010;56:496–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutton-Tyrrell K, Venkitachalam L, Kanaya AM, Boudreau R, Harris T, Thompson T, Mackey RH, Visser M, Vaidean GD, Newman AB. Relationship of ankle blood pressures to cardiovascular events in older adults. Stroke. 2008;39:863–9 [DOI] [PubMed] [Google Scholar]
- 43.Onufrak SJ, Bellasi A, Cardarelli F, Vaccarino V, Muntner P, Shaw LJ, Raggi P. Investigation of gender heterogeneity in the associations of serum phosphorus with incident coronary artery disease and all-cause mortality. Am J Epidemiol. 2009;169:67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onufrak SJ, Bellasi A, Shaw LJ, Herzog CA, Cardarelli F, Wilson PW, Vaccarino V, Raggi P. Phosphorus levels are associated with subclinical atherosclerosis in the general population. Atherosclerosis. 2008;199:424–31 [DOI] [PubMed] [Google Scholar]
- 45.Kendrick J, Ix JH, Targher G, Smits G, Chonchol M. Relation of serum phosphorus levels to ankle brachial pressure index (from the Third National Health and Nutrition Examination Survey). Am J Cardiol. 2010;106:564–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meng J, Wassel CL, Kestenbaum BR, Collins TC, Criqui MH, Lewis CE, Cummings SR, Ix JH. Serum phosphorus levels and the spectrum of ankle-brachial index in older men: the Osteoporotic Fractures in Men (MrOS) study. Am J Epidemiol. 2010;171:909–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellasi A, Mandreoli M, Baldrati L, Corradini M, Di Nicolo P, Malmusi G, Santoro A. Chronic kidney disease progression and outcome according to serum phosphorus in mild-to-moderate kidney dysfunction. Clin J Am Soc Nephrol. 2011;6:883–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norris KC, Greene T, Kopple J, Lea J, Lewis J, Lipkowitz M, Miller P, Richardson A, Rostand S, Wang X, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol. 2006;17:2928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwarz S, Trivedi B, Kalanter-Zadeh K, Kovesdy C. Association of disorders of mineral metabolism with progression of chronic kidney disease. Clin J Am Soc Nephrol. 2006;1:825–31 [DOI] [PubMed] [Google Scholar]
- 50.Voormolen N, Noordzij M, Grootendorst DC, Beetz I, Sijpkens YW, van Manen JG, Boeschoten EW, Huisman RM, Krediet RT, Dekker FW. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant. 2007;22:2909–16 [DOI] [PubMed] [Google Scholar]
- 51.O'Seaghdha CM, Hwang SJ, Muntner P, Melamed ML, Fox CS. Serum phosphorus predicts incident chronic kidney disease and end-stage renal disease. Nephrol Dial Transplant. 2011;26:2885–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis. 2009;53:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mataix J, Aranda P, Lopez-Jurado M, Sanchez C, Planells E, Llopis J. Factors influencing the intake and plasma levels of calcium, phosphorus and magnesium in southern Spain. Eur J Nutr. 2006;45:349–54 [DOI] [PubMed] [Google Scholar]
- 54.Gutíerrez OM. Sodium- and phosphorus-based food additives: persistent but surmountable hurdles in the management of nutrition in chronic kidney disease. Adv Chronic Kidney Dis. 2013;20:150–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calvo MS, Uribarri J. Contributions to total phosphorus intake: all sources considered. Semin Dial. 2013;26:54–61 [DOI] [PubMed] [Google Scholar]
- 56.Portale AA, Halloran BP, Morris RC., Jr Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for the renal production of 1,25-dihydroxyvitamin D. J Clin Invest. 1987;80:1147–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calvo MS, Kumar R, Heath H., III Elevated secretion and action of serum parathyroid hormone in young adults consuming high phosphorus, low calcium diets assembled from common foods. J Clin Endocrinol Metab. 1988;66:823–9 [DOI] [PubMed] [Google Scholar]
- 58.Kemi VE, Karkkainen MU, Lamberg-Allardt CJ. High phosphorus intakes acutely and negatively affect Ca and bone metabolism in a dose-dependent manner in healthy young females. Br J Nutr. 2006;96:545–52 [PubMed] [Google Scholar]
- 59.Alonso A, Nettleton JA, Ix JH, de Boer IH, Folsom AR, Bidulescu A, Kestenbaum BR, Chambless LE, Jacobs DR., Jr Dietary phosphorus, blood pressure, and incidence of hypertension in the atherosclerosis risk in communities study and the multi-ethnic study of atherosclerosis. Hypertension. 2010;55:776–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dominguez JR, Kestenbaum B, Chonchol M, Block G, Laughlin GA, Lewis CE, Katz R, Barrett-Connor E, Cummings S, Orwoll ES, et al. Relationships between serum and urine phosphorus with all-cause and cardiovascular mortality: the Osteoporotic Fractures in Men (MrOS) Study. Am J Kidney Dis. 2013;61:555–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murtaugh MA, Filipowicz R, Baird BC, Wei G, Greene T, Beddhu S. Dietary phosphorus intake and mortality in moderate chronic kidney disease: NHANES III. Nephrol Dial Transplant. 2012;27:990–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamamoto KT, Robinson-Cohen C, de Oliveira MC, Kostina A, Nettleton JA, Ix JH, Nguyen H, Eng J, Lima JA, Siscovick DS, et al. Dietary phosphorus is associated with greater left ventricular mass. Kidney Int. 2013;83:707–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang S, Gillihan R, He N, Fields T, Liu S, Green T, Stubbs JR. Dietary phosphate restriction suppresses phosphaturia but does not prevent FGF23 elevation in a mouse model of chronic kidney disease. Kidney Int. 2013;84:713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu MC, Kuro-o M, Moe OW. The emerging role of Klotho in clinical nephrology. Nephrol Dial Transplant. 2012;27:2650–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, Konig P, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–8 [DOI] [PubMed] [Google Scholar]
- 66.Gutíerrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutierrez OM, Steigerwalt S, He J, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seiler S, Reichart B, Roth D, Seibert E, Fliser D, Heine GH. FGF-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrol Dial Transplant. 2010;25:3983–9. [DOI] [PubMed] [Google Scholar]
- 69.Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009;24:2792–6 [DOI] [PubMed] [Google Scholar]
- 70.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22:1913–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gutíerrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546–51 [DOI] [PubMed] [Google Scholar]
- 74.Touchberry CD, Green TM, Tchikrizov V, Mannix JE, Mao TF, Carney BW, Girgis M, Vincent RJ, Wetmore LA, Dawn B, et al. FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am J Physiol Endocrinol Metab. 2013;304:E863–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-o M, et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248:324–9 [DOI] [PubMed] [Google Scholar]
- 76.Nagai R, Saito Y, Ohyama Y, Aizawa H, Suga T, Nakamura T, Kurabayashi M, Kuroo M. Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell Mol Life Sci. 2000;57:738–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276:767–72 [DOI] [PubMed] [Google Scholar]
- 78.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243–55 [DOI] [PubMed] [Google Scholar]
- 79.Isakova T, Gutierrez OM, Wolf M. A blueprint for randomized trials targeting phosphorus metabolism in chronic kidney disease. Kidney Int. 2009;76:705–16 [DOI] [PubMed] [Google Scholar]
- 80.Gutíerrez OM. Fibroblast growth factor 23, Klotho, and disordered mineral metabolism in chronic kidney disease: unraveling the intricate tapestry of events and implications for therapy. J Ren Nutr. 2013;23:250–4 [DOI] [PMC free article] [PubMed] [Google Scholar]