Abstract
Over the last century, the issue of brain lateralization in primates has been extensively investigated and debated, yet no previous study has reported eye preference in great apes. This study examined eye preference in 45 captive chimpanzees (Pan troglodytes) in response to various stimuli. Eye preference was assessed when animals looked through a hole that only accommodated one eye at an empty box, a mirror, a picture of a dog, a rubber snake, food biscuits, bananas, a rubber duck, and a video camera. Main effects of stimulus type were found for direction of eye preference, number of looks, and looking duration, but not for strength of eye preference. A left-eye bias was found for viewing the rubber snake and a right-eye bias was found for viewing the bananas, supporting theories that emotional valence may affect lateralized behaviors. In addition, a significant shift in eye preference took place from the initial look to subsequent looks when viewing the snake. These results are not consistent with previous reports of human eye preference and may reflect lateralization differences for emotional processing. No relationship between eye preference and previously recorded hand preference was found.
Keywords: Ocular dominance, Laterality, Apes, Emotion
Introduction
Structural and functional brain laterality were long believed to be unique characteristics of humans. However, research has shown that the brain lateralization extends out to many vertebrates (Vallortigara et al. 1999), including birds (Vallortigara et al. 1999), fish, reptiles, amphibians (Bisazza et al. 1998) and primates (Hopkins and Bard 1993), all exhibiting varying levels of laterality. There are two key expressions of brain laterality: motor laterality (asymmetry of movement and physical tasks) and perceptual laterality (asymmetries in ear or eye use). Motor laterality has been extensively studied in a variety of species, most commonly through research on manual functions such as handedness. Perceptual laterality has mainly been examined in species with laterally placed eyes (Vallortigara et al. 2001) as it is related to the reception and transmission of sensory information to the central nervous system. The two hemispheres do not receive sensory information from a single stimulus in the same proportions because the contralateral optic fibers have a larger diameter and faster conduction speed than the ipsilateral optic fibers in most mammal species, especially apes (Watson and Hanbury 2007; Jeffery 2001). In essence, the contralateral hemisphere receives monocular visual information faster and of a different quality than the isolateral hemisphere (Bishop et al. 1953). Although in some mammals uncrossed axons are intermingled with crossed axons while others remain segregated, this varies on a species-to-species basis (Jeffery 2001). Because of this, eye dominance may indicate lateralization in the processing of visual information.
Eye preference has been recorded in humans with 66.76 % of the research population displaying a right-eye preference for sighting tasks or the performance of monocular activities (Bourassa et al. 1996). Walls (1951) described various measures of eye preference from very simple methods, requiring the subject to hold up one finger or look through a tube, to more complex techniques utilizing specialized optometric equipment. More importantly, one must distinguish between eye dominance (the tendency to prefer visual input from one eye over the other), sensory dominance (related to binocular rivalry where perception alternates between images presented to each eye), and acuity dominance (concerned with differences in visual sharpness).
Visual laterality has been reported in a number of species including birds, reptiles and primates (Chapelain and Blois-Heulin 2008; Vallortigara et al. 2001; Bisazza et al. 1998) dating back to 1938 when the first primate eye preference study was published reporting a right-eye preference for looking through a tube at a piece of food by three immature capuchin monkeys (Kounin 1938). Almost 20 years later Cole (1957) reported a right-eye preference in seven adult pigtail macaques when viewing a food item through a tube. In contrast, a left-eye preference was found in a group of 19 immature rhesus macaques, and no preference was found for seven adult rhesus (Kruper et al. 1966). More recently, Rogers et al. (1994) measured eye preference in four adult female and two 1-month-old bushbabies. All subjects displayed left-eye dominance for viewing both the researcher and food through a grid. This eye preference was weakened when testing mother bushbabies as they viewed their babies being held by the tester, suggesting that arousal and/or the stimulus being viewed effected eye use. For red-capped mangabeys, the strength of eye preference has been correlated to food preference, implying a directional relationship where the more desirable food elicits a stronger eye preference (de Latude et al. 2009). Studies regarding the influence of stimulus suggest that the direction of eye preference depends not only on the stimulus, but also on the subject’s emotions toward it (Hook-Costigan and Rogers 1998).
Numerous studies have been published on various fish species that all report similar results; fish preferentially use the left eye when presented with a familiar object or pattern and preferentially use the right eye when viewing unfamiliar objects or patterns (Sovrano 2004; Sovrano et al. 1999, 2001; DeSanti et al. 2001; Bisazza et al. 1998). Both dolphins and horses have been reported to look at novel stimuli with the right eye (Ridgway 1986; De Boyer Des Roches et al. 2008). Several fish species also exhibit a left-eye preference for looking at their own image and a right-eye preference for inspecting a potential predator (DeSanti et al. 2001; Sovrano et al. 1999, 2001; Vanegas and Ito 1983). These data suggest an aspect of recognition or familiarity in the expression of eye preference, suggesting that lateralization is associated with species recognition, or recognition of familiarity. Dolphins were reported to gaze longer at unfamiliar than familiar humans, also suggesting a capacity to discriminate based on familiarity (Thieltges et al. 2011). Similar to findings for primates, the lateral preference may also be related to the emotional valence of the object being viewed. This can even be said for food preference; Brown and Magat (2011) reported a strong correlation between avian eye preference and foot preference when examining food items, with 11 out of 16 Australian parrot species showing significant preferences.
The 40-plus years of research on the hemispheric specialization of emotional processing in humans has produced multiple theoretical models. Two of the more relevant theories to the study of eye preference are the “Valence Model” and the “Approach-Withdrawal” model. According to the Valence Model, the experience and expression of positive emotions are produced in the left hemisphere and negative emotions are processed and expressed through the right hemisphere (Davidson 1992; Erhan et al. 1998). This model is based on multiple studies examining facial expressions and brain activity (via fMRI) while subjects observe emotional stimuli, in order to assess patterns of hemispheric activation in various brain regions (Davidson et al. 1990). The Approach-Withdrawal model states that the drives behind approach behaviors are primarily processed in the left hemisphere of the brain and those associated with withdrawal behaviors are processed in the right hemisphere (Demaree et al. 2005). Davidson and other independent researchers have produced a variety of evidence over the past 10 years indicating the two hemispheres of the brain are differentially responsible for specific positive and negative emotions.
The aim of this study was to investigate eye preference in response to objects with varying degrees of emotionality and familiarity in captive chimpanzees. Similar data have not been previously published for any great ape species. The influence of each stimulus on both directional preferences and strength of eye use was examined, as were any shifts in eye preference. We expected the chimpanzee subjects to display a right-eye preference for viewing familiar and emotionally neutral objects, similar to the preference shown by humans (Bourassa et al. 1996) and marmosets (Hook-Costigan and Rogers 1998). In addition, we hypothesized that eye preference would shift to either a weak left-eye preference or no preference when viewing threatening or unfamiliar objects.
Methods
Looking behavior by 45 adult chimpanzees (19 males and 26 females), ranging in age from 13 to 47 years (mean age of 28.8 years), was studied. The chimpanzee subjects were socially housed in groups of between four and 15 animals at the Michale E. Keeling Center for Comparative Medicine and Research of the University of Texas MD Anderson Cancer Center in Bastrop, Texas. All chimpanzees remained in their home enclosures (Primadomes® and open top corrals) for testing on all eight experimental conditions.
Eye preference was tested using a monocular viewing box (50.8 cm × 50.8 cm) made from white polycarbonate resin thermoplastic with a small-viewing hole centered on the front panel (1.2 cm in diameter). The purpose of the box was to force the subjects to view the test object using only one eye, while simultaneously concealing the object. The box was set on a plastic rolling cart at approximately standing eye level for the chimpanzee subjects (1.1 m). Each presentation began with separating the subject animal from the group. The subject was isolated in the indoor section of the enclosure while the remaining group members were in the outdoor enclosure to ensure that eye preference was not dependent on, or influenced by, social cues. Out of view of the subject, the experimenter placed the stimulus object in the box and then rolled the cart into view of the subject, flush against the mesh caging. The cart was placed directly opposite the metal door separating the subject from group members outside, thus ensuring that eye preference was not directionally influenced by any noise coming from the door. The experimenter remained directly behind the device in order to keep an accurate spoken commentary on eye use and not to influence directional looking. Forty-five subjects were tested every week day until they had been tested in each of eight conditions. Condition presentation was randomized and only one 15-minute trial was completed per animal per 24-hour period. At the end of each presentation, the subject was rewarded with a small slice of apple or 2 grapes, to ensure that the shifting process and trial remained positive experiences. The reward did not vary based on object presented and was only given after the viewing box was moved out of view. In the event that an animal did not approach the device, the trial was rerun at a later time, but only when the experimenter was sure the subject was not able to view the object the first time. A small web camera (Windows LifeCam VX-5000) was set up on top of the viewing box to record the eye used and the experimenter recorded a spoken commentary on the subject’s actions as well as the eye being used. We recorded data for all conditions from both video and the experimenter’s commentary.
Eight conditions were tested, with each presentation of the box representing a single condition. One of seven objects was placed inside the viewing box at a time, and each object was only presented once in order to preserve any pre-existing familiarity. An additional condition was tested where the box remained empty. Objects and related details are listed in Table 1. These objects reflect 3 categories based on emotional valence: food (a low quality food represented by biscuits versus a high-quality food of bananas), novelty (the novel duck and canine face picture versus the commonly seen video camera) and fear or curiosity inducing (a mirror and a plastic rattlesnake). Snakes are present in the wooded and grassy areas around the chimpanzee enclosures at Bastrop, thus the animals are familiar with them and frequently fear bark when snakes are visible. Most of the chimpanzees have seen dogs before, but not this specific breed, nor photographs of dogs. Mirrors are also commonly provided as part of a rotating enrichment schedule, so they are not a novel object.
Table 1.
Test conditions with details on objects and emotional valence
| Condition | Arousal level (high/low) | Familiarity level (novel/ familiar) | Object | Details |
|---|---|---|---|---|
| Empty | Low | Novel | None | Empty box presented |
| Snake | High | Familiar | Plastic diamondback rattlesnake | 114.3 cm long |
| Mirror | High | Familiar | Pedestal mirror | Mirror positioned 15.5 cm from hole for viewing reflection |
| Banana | High | Familiar | Three whole bananas | Fresh yellow bananas used with little to no bruising |
| Biscuits | Low | Familiar | 3 Purina chow biscuits | Placed on a brown paper towel positioned to see all three biscuits |
| Dog | Low | Novel | Picture of a canine face | 20.32 × 25.4 cm picture of a light colored golden retriever face |
| Duck | Low | Novel | Rubber duck | Yellow, 8.25 cm × 7.11 cm |
| Camera | Low | Familiar | Sony DCR-PC10 digital video camera | Black and silver, placed with LCD display closed, 20 × 6.35 cm |
Individual subject eye preferences for each condition were determined based on an eye use index (EI). Eye use indices for all animals were calculated to quantify the degree of lateral bias. This was done by subtracting the total number of left-eye uses (L) from the number of right-eye uses (R) and dividing by the total number of eye use instances (R + L):
EI values range from 1.0 (extreme right-eye preference) to −1.0 (extreme left-eye preference). The absolute value of the eye use index (absEI) was also calculated (absEI = |EI|); the absolute value of the EI score represents the strength of eye preference irrespective of direction and ranges from 0 (no eye preference) to 1.0 (extreme lateralization in one direction or the other). There was no minimum number of looks required for any condition. We use the term “look” to designate one instance of looking (e.g., approaching and looking into the viewing box), and all events were incorporated as data points. “First looks” were scored using only the first look of a bout of looking (after moving more than one body length away from the box) and recorded as independent data points, consistent with “bouts” measures of hand use (McGrew and Marchant 1997). The “initial look” of each condition was also examined, being the first look of each trial. By examining the initial look apart from subsequent looks, it was possible to examine eye use before and after the subject knew what was in the box and thus to evaluate how the condition directly affected eye use. Each look was coded as −1 (left eye) or 1 (right eye). The number of looks ranged from 2 to 27 per subject per condition, while the number of first looks ranged from 2 to 13. Subjects with only one look were excluded from the analysis in order to ensure comparison between the initial look and at least one subsequent look.
Results
Significant deviations from normality were found for all looking measures (EI and absEI) using a Kolmogorov–Smirnov test (p < .02), so nonparametric statistics were utilized for analysis. The null hypothesis for most analyses was that there would be no difference between conditions in either eye preference strength or direction.
Direction of eye preference
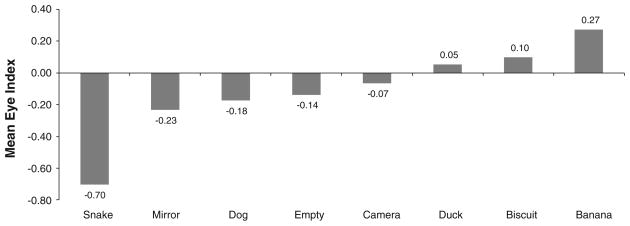
In order to evaluate the effect of stimulus on the direction of eye preference, we examined the EI, calculated using first looks, with a Friedman test (Fig. 1). The EI differed significantly across conditions (χ2 = 55.58, p < .001), with the snake being viewed mostly with the left eye (mean EI = −0.70) and the bananas being viewed mostly with the right eye (EI = 0.27). The left-eye preference for the snake condition was significantly greater than that for the mirror condition using a Wilcoxon matched pairs signed ranks test (Z = −3.638, p < .001, T = 101.5). The strongest directional eye preference was shown for viewing the snake.
Fig. 1.
Mean eye index per object presented. A significant difference in eye preference was found across conditions (χ2 = 55.58, p < .001)
Strength of eye preference
The absEI was also calculated, to determine differences in the strength of eye preference based on condition, but no significant effect was found (χ2 = 9.723, p > .05). This indicates that the only significant changes in eye preference, based on condition, were directional.
Initial look analysis
In order to evaluate changes in eye preference within condition, the initial look was compared to the second look using a McNemar’s test. The only condition in which the eye preference changed significantly from the initial look to the second was the snake (Z = −3.46, p = .001). Eleven subjects initially looked with their right eye while 27 used their left (for a mean EI of −0.42), but only two of the 36 second looks were with the right eye (mean EI of −0.84). We also compared the initial look to the EI of each condition (reflecting overall eye use), using a Wilcoxon test. Only changes for the bananas (Z = −2.105, p = .001, T = 76.50) and snake (Z = −2.02, p = .001, T = 34) were significant. In total, 30 of the 38 subjects who viewed the snake had negative EI scores, reflecting a strong directional preference for left-eye use. The opposite was true for the banana where 33 of the total 45 subjects initially viewed the object with the right eye (mean EI = 0.47) then slowly began using the left eye more often (mean EI = 0.27). Across all first looks for the banana, 29 subjects had positive EI scores and 11 had negative. This reflects a shift away from right-eye use as looking progressed. By examining the initial look apart from subsequent looks, we were able to determine which eye was used before the subject knew the contents of the box and then after, thus reflecting how the condition directly affected eye use.
Number of first looks and viewing duration
The number of first looks (viewing “bouts”), and viewing duration, also differed significantly across conditions (Figs. 2, 3). Significant differences in the total number of first looks across all subjects and conditions were found (Friedman test; χ2 = 164.226, p < .001), ranging from 76 looks (both camera and duck conditions) to 272 looks (mirror condition). Cumulative viewing duration varied significantly based on condition (χ2 = 147.72, p < .001), ranging from 6.2 s for viewing the mirror to 2.3 s for the snake. The mirror was viewed significantly more often (Fig. 2), and also significantly longer (Fig. 3), than every other condition.
Fig. 2.
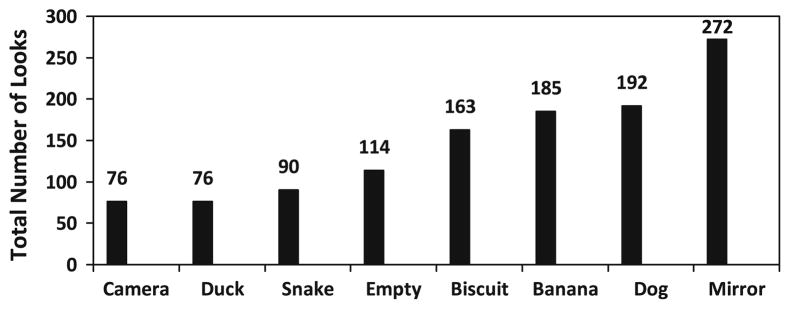
Total number of looks per object presented. A significant difference in total number of looks was found across conditions (χ2 = 164.226, p <.001), with the mirror being viewed significantly more than the camera (Wilcoxon signed ranks test, Z = −5.589, p < .001, T = 0), duck (Z = −5.737, p < .001, T = 5), snake (Z = −5.353, p < .001, T = 19), empty (Z = −5.041, p < .001, T = 36.50), biscuit (Z = −4.353, p < .001, T = 79), banana (Z = −2.881, p = .005, T = 196.50), and dog (Z = −3.394, p < .001, T = 108.50)
Fig. 3.
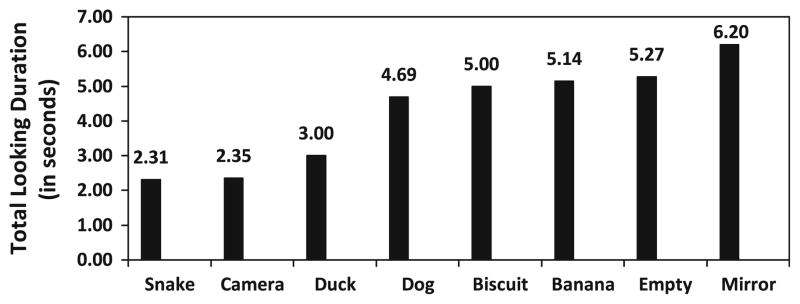
Total looking duration, in seconds, across all looks significantly differed between conditions (χ2 = 147.72, p < .001). The mirror was viewed significantly longer than the snake (Z = −5.471, p < .001, T = 33), camera (Z = −5.744, p < .001, T = 9), duck (Z = −5.693, p < .001, T = 13.50), dog (Z = −5.966, p < .001, T = 1.50), biscuit (Z = −3.73, p < .001, T = 154), banana (Z = −2.441, p = .015, T = 286), and empty conditions (Z = −2.453, p = .014, T = 201.50)
Categorical analysis
Analyses were also conducted based on general categories of emotional valence (see Fig. 1): foods versus non-foods, low arousal versus high arousal and novel versus familiar. The EI and AbsEI for each category were compared for significant differences in strength of eye preference and direction. It was found that the eye preference for viewing foods differed significantly from that for non-food items (Wilcoxon test: z = −1.317, p = .001, T = 220.50), but this was mostly driven by the strong preference, already noted, for viewing the banana. Food conditions were viewed more with the right eye (mean EI = 0.18) than non-food conditions (mean EI = −0.21). Similar results were found for non-food high arousal conditions (snake and mirror), which were viewed significantly more with the left eye than the right (Wilcoxon test: z = −4.167, p < .001, T = 101.50). The mean EI for these conditions was −0.45, while that of the remaining conditions was 0.02.
Eye use and handedness
In order to examine laterality more broadly, EI and absEI scores were compared with previously reported handedness measures for the same individual chimpanzees (Braccini et al. 2010) using Spearman’s rank correlation. Three handedness measures were tested; relaxed posture tool use, supported bipedal tool use, and unsupported bipedal tool use. The handedness indices (HI and absHI) for each posture tended to be correlated with the eye use indices (EI and absEI) for each condition, but neither yielded significant results after applying a Bonferroni correction.
Sex differences
Collapsing across all conditions, no significant sex differences were found, with males exhibiting an average EI of 0.10 and females an average EI of −0.10 (Mann–Whitney U = 71.50, Z = −1.21, p > .05). In the empty box and mirror conditions, subtle but significant sex differences were found. Females exhibited a stronger eye preference than males for the empty box (Mann–Whitney U = 2,883.50; Z = −2.525, p = .012), with a mean EI of −0.18 for females and −0.08 for males. In the mirror condition, females looked significantly more often than males (Mann–Whitney U = 7,805.50; Z = −2.022, p = .043), with females looking at the mirror 141 times and males 131 times.
Discussion
This study, the first to report eye preference in any great ape species, reports directional eye preference in response to various stimuli in captive chimpanzees. Our results show (1) a difference from initial first eye use to subsequent eye use for both banana and snake conditions away from a right-eye preference (2) a group level bias for viewing the snake with the left eye and the banana with the right eye, and (3) more and longer looks at the mirror than any other object.
We found group level right-eye preference for high-quality food items, bananas, but not for any of the neutral stimuli (such as the empty box, biscuits, or video camera). These results are consistent with previous reports using low arousal stimuli with nonhuman primates (Cercocebus torquatus torquatus: de Latude et al. 2009; Callithrix jacchus: Hook-Costigan and Rogers 1998; Otolemur garnettii: Rogers et al. 1994). The absence of significant laterality when viewing low arousal stimuli could be due to the lack of emotional relevance connected to the object. Bananas are a known favorite food for the chimpanzees at the Keeling Center, frequently eliciting food barks and visible excitement (hand slapping, bouncing, and play faces). During banana trials, eight food barks were recorded from various subjects upon the initial look at the bananas in the viewing box. The plastic rattlesnake also elicited vocalizations, with 12 instances of alarm calls or whimpering after the initial look. On three occasions, the subject looked to the experimenter for reassurance, either panting or reaching out, after the initial look. Since snakes are present at the Keeling Center, these subjects were familiar with them and have been known to fear call in response to snakes (Braccini, personal observation).
Braccini and Caine (2009) have posited a connection between handedness, fear, and exploration in marmosets relating to both the Valence Model and the Approach-Withdrawal model. The Valence Model theorizes that the expression and experience of positive emotions are generated in the left hemisphere and negative emotions are processed and expressed through the right hemisphere (Davidson 1992; Erhan et al. 1998). In the case of eye preference, the emotional valence of the object being viewed could possibly influence the eye used, but not without knowledge of the object and its emotional relevance. This would entail approaching and initially viewing an object with one eye, evaluating the emotional reaction to the object, and then taking the second look with a different eye, as we report here for both the snake and banana conditions. These objects elicited the most emotional response of all the objects used and reflected both positive and negative extremes. The subjects’ previous knowledge or experience with snakes would be expected to elicit a negative emotional response. Bananas, being a preferred food item, should elicit a positive emotional response.
The related Approach-Withdrawal model theorizes that the drive behind approach behaviors are primarily processed in the left hemisphere of the brain and those associated with withdrawal behaviors from the right hemisphere (Demaree et al. 2005). If an object was repeatedly viewed, or viewed in long duration, one could theorize that it was not eliciting a negative emotional response worthy of withdrawal or retreat. The opposite would also be true, potentially explaining the shorter total viewing duration, fewer total looks, and significant left-eye preference for the snake condition; the subjects were actively avoiding looking at the snake, activating the right hemisphere. Since a change in eye preference was found from the initial look to subsequent looks for the two most emotionally evocative objects, the data presented here support both of these theories: emotional valence clearly plays a differential role in chimpanzee eye preference.
For other nonhuman primates, emotion also seems to play a distinctive role in eye preference. Hook-Costigan and Rogers (1998) reported a right-eye preference in marmosets for viewing food or neutral stimuli and an absence of dominance or left preference for viewing negative stimuli, consistent with the results reported here. Rogers et al. (1994) studying small-eared bushbabies theorized that eye choice is based on the nature of the visual stimulus. Eye preference shifted away from a left-eye preference when showing subjects their babies in comparison with a right-eye bias for viewing a novel stimulus. Both the novel object and the presentation of the baby increased arousal rates, potentially altering the eye preference exhibited. In the current study, chimpanzees also exhibited various levels of arousal while exhibiting different eye preferences. The positive arousal for viewing the bananas with the right eye and the negative for viewing the snake with the left eye suggests the same preference or influence of emotion on eye preference.
In humans, eye dominance is thought to be associated with specialization of the contralateral hemisphere for language (Bryden 1988). In most individuals, there is a left hemisphere specialization for language and right-eye dominance. Left-eye dominant individuals then make greater use of the right hemisphere and are found to show superior abilities to decode nonverbal cues (Domangue 1984). It has been reported that a majority of humans exhibit a right-eye preference for looking at neutral stimuli (Reiss and Reiss 1997). Since it has also been suggested that emotion is lateralized in the human brain (Davidson et al. 1990), the data presented here appear to be more in line with the human data than those presented for other nonhuman primates.
These findings have interesting implications for the evolution of human lateralization. Research in the past few decades has provided increasing evidence that brain lateralization may have appeared early in evolution, probably beginning with perceptual processes (Rogers 2002; Vallortigara and Rogers 2005; Chapelain and Blois-Heulin 2008). Given the recent data indicating cerebral lateralization in a wide variety of vertebrate species, it seems very likely that some degree of laterality was present in our ancestors long before the evolution of language, or of dexterous human hands used for making tools (Bisazza etv al. 1998). This speaks against some established theories regarding the evolution of human brain lateralization, language, and hand use (McGrew and Marchant 1997). Evidence of brain lateralization for perceptual functions in a wide variety of vertebrates (Vallortigara 2000), along with great apes as documented here, suggests that human hemispheric specialization may have initially evolved from eye preference, or perceptual laterality, and then later influenced manual laterality (although it is remains possible that both evolved independently).
Either way there appear to be evolutionary advantages to being lateralized. Magat and Brown (2009) reported that strongly lateralized Australian parrots outperformed less lateralized individuals in a variety of tasks, with the relationship becoming stronger with the demands of the task. This suggests some association between lateralization and fitness-related abilities, which may begin to explain the prevalence of perceptual lateralization across species.
This study revealed a lateralized preference in eye use for captive chimpanzees at the group level. Strong preferences were found only for the two most emotionally arousing objects: a rubber snake, and a bunch of bananas. These preferences suggest an important role for emotion in determining eye use in chimpanzees, providing support for the valence theory of hemispheric specialization of emotions (Davidson 1992; Erhan et al. 1998).
Acknowledgments
The authors would like to thank Shilpa Mody for her assistance in data collection as well as the entire staff at the Michale E. Keeling Center for Comparative Medicine and Research of The University of Texas MD Anderson Cancer Center. Support for this project came from NIH/NCRR U42-RR15090. All procedures were conducted in accordance with all relevant federal, state, and local guidelines and were approved by the UTMDACC IACUC. The KCCMR has been fully accredited by AAALAC-International since 1979.
Contributor Information
Stephanie N. Braccini, Email: braccini@stlzoo.org, Centre for Social Learning and Cognitive Evolution and Scottish Primate Research Group, School of Psychology, University of St Andrews, St Andrews KY16 9JP, UK. Saint Louis Zoo, One Government Drive, St Louis, MO 63110, USA
Susan P. Lambeth, Department of Veterinary Sciences, The University of Texas MD Anderson Cancer Center, Bastrop, TX 78602, USA
Steven J. Schapiro, Department of Veterinary Sciences, The University of Texas MD Anderson Cancer Center, Bastrop, TX 78602, USA. Department of Experimental Medicine, University of Copenhagen, Copenhagen, Denmark
W. Tecumseh Fitch, Email: tecumseh.fitch@univie.ac.at, Department of Cognitive Biology, School of Life Sciences, University of Vienna, 14 Althanstrasse, Vienna, Austria. School of Psychology, University of St Andrews, St Andrews KY16 9JP, UK.
References
- Bisazza A, Rogers LJ, Vallortigara G. The origins of cerebral asymmetry: a review of evidence of behavioural and brain lateralization in fishes, amphibians, and reptiles. Neurosci Biobehav Rev. 1998;22:411–426. doi: 10.1016/s0149-7634(97)00050-x. [DOI] [PubMed] [Google Scholar]
- Bishop PO, Jeremy D, Lance JW. The optic nerve: properties of a central tract. J Physiol Lond. 1953;121:415–432. doi: 10.1113/jphysiol.1953.sp004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa DC, McManus IC, Bryden MP. Handedness and eye-dominance: a meta-analysis of their relationship. Laterality. 1996;1:5–34. doi: 10.1080/713754206. [DOI] [PubMed] [Google Scholar]
- Braccini SN, Caine NG. Hand preference predicts reactions to novel foods and predators in marmosets (Callithrix geoffroyi) J Comp Psychol. 2009;123:18–25. doi: 10.1037/a0013089. [DOI] [PubMed] [Google Scholar]
- Braccini S, Lambeth S, Schapiro S, Fitch WT. Bipedal tool use strengthens chimpanzee hand preferences. J Hum Evol. 2010;58:234–241. doi: 10.1016/j.jhevol.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, Magat M. Cerebral lateralization determines hand preferences in Australian parrots. Biol Lett. 2011;7:496–498. doi: 10.1098/rsbl.2010.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden MP. An overview of the dichotic listening procedure and its relation to cerebral organization. In: Hugdahl KE, editor. Handbook of dichotic listening: theory, methods and research. Wiley; Oxford: 1988. pp. 1–43. [Google Scholar]
- Chapelain AS, Blois-Heulin C. Lateralization for visual processes: eye preference in Campbell’s monkeys. Anim Cogn. 2008;12:11–19. doi: 10.1007/s10071-008-0164-1. [DOI] [PubMed] [Google Scholar]
- Cole J. Laterality in the use of the hand, foot, and eye in monkeys. J Comp Psychol. 1957;50:296–299. doi: 10.1037/h0046343. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Emotion and affective style: hemispheric substrates. Psychol Sci. 1992;3:39–43. [Google Scholar]
- Davidson RJ, Ekman P, Saron CD, Senulis JA, Friensen WV. Approach-withdrawal and cerebral asymmetry: emotional expression and brain physiology. J Pers Soc Psychol. 1990;58:330–341. [PubMed] [Google Scholar]
- De Boyer Des Roches A, Richards-Yris MA, Henry S, Ezzaouïa M, Hausberger M. Laterality and emotions: visual laterality in the domestic horse (Equus caballus) differs with objects’ emotional value. Physiol Behav. 2008;94:487–490. doi: 10.1016/j.physbeh.2008.03.002. [DOI] [PubMed] [Google Scholar]
- de Latude M, Demange M, Bec P, Blois-Heulin C. Visual laterality responses to different emotive stimuli by red-capped mangabeys, Cercocebus torquatus torquatus. Anim Cogn. 2009;12:31–42. doi: 10.1007/s10071-008-0166-z. [DOI] [PubMed] [Google Scholar]
- Demaree HA, Everhart E, Youngstrom EA, Harrison DW. Brain lateralization of emotional processing: historical roots and a future incorporating “dominance”. Behav Cogn Neurosci Rev. 2005;4:3–20. doi: 10.1177/1534582305276837. [DOI] [PubMed] [Google Scholar]
- DeSanti A, Sovrano VA, Bisazza A, Vallortigara G. Mosquitofish display differential left- and right-eye use during a mirror-image scrutiny and predator-inspection responses. Anim Behav. 2001;61:305–310. [Google Scholar]
- Domangue BB. Sensation seeking and cognitive complexity. Percept Motor Skill. 1984;59:749–750. doi: 10.2466/pms.1984.58.1.3. [DOI] [PubMed] [Google Scholar]
- Erhan H, Borod JC, Tenke CE, Bruder GE. Identification of emotion in a dichotic listening task: event-related brain potential and behavioural findings. Brain Cogn. 1998;37:286–307. doi: 10.1006/brcg.1998.0984. [DOI] [PubMed] [Google Scholar]
- Hook-Costigan MA, Rogers LJ. Eye preference in common marmosets (Callithrix jacchus): influence of age, stimulus, and hand preference. Laterality. 1998;3:109–130. doi: 10.1080/713754297. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA. The ontogeny of lateralized behavior in nonhuman primates with special reference to chimpanzees (Pan troglodytes) In: Ward JP, Hopkins WD, editors. Primate laterality: current behavioral evidence of primate asymmetries. Springer; New York: 1993. pp. 251–265. [Google Scholar]
- Jeffery G. Architecture of the optic chiasm and the mechanisms that sculpt its development. Psychol Rev. 2001;81:1393–1414. doi: 10.1152/physrev.2001.81.4.1393. [DOI] [PubMed] [Google Scholar]
- Kounin JS. Laterality in monkeys. J Genet Psychol. 1938;52:375–393. [Google Scholar]
- Kruper DC, Boyle BE, Patton RA. Eye and hand preference in rhesus monkeys. Psychon Sci. 1966;5:277–278. [Google Scholar]
- Magat M, Brown C. Laterality enhances cognition in Australian parrots. Proc R Soc B. 2009;276:4155–4162. doi: 10.1098/rspb.2009.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: current issues in and meta-analysis of the behavioral laterality of hand function in nonhuman primates. Yearb Phys Anthrop. 1997;40:201–232. [Google Scholar]
- Reiss M, Reiss G. Ocular dominance: some family data. Laterality. 1997;2:7–16. doi: 10.1080/713754254. [DOI] [PubMed] [Google Scholar]
- Ridgway SH. Physiological observations on the dolphin brain. In: Schusterman R, Thomas J, Wood F, editors. Dolphin cognition and behavior: a comparative approach. Erlbaum; Hillsdale: 1986. [Google Scholar]
- Rogers LJ. Advantages and disadvantages of lateralisation. In: Rogers LJ, Andrew RJ, editors. Comparative vertebrate lateralization. Cambridge University Press; Cambridge: 2002. [Google Scholar]
- Rogers LJ, Ward JP, Stafford D. Eye dominance in the small-eared bushbaby (Otolemur garnettii) Neuropsychologia. 1994;32:257–264. doi: 10.1016/0028-3932(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Sovrano VA. Visual lateralization in response to familiar and unfamiliar stimuli in fish. Behav Brain Res. 2004;152:385–391. doi: 10.1016/j.bbr.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Sovrano VA, Rainoldi C, Bisazza A, Vallortigara G. Roots of brain specializations: preferential left-eye use during mirror-image inspection in six species of teleost fishes. Behav Brain Res. 1999;106:175–180. doi: 10.1016/s0166-4328(99)00105-9. [DOI] [PubMed] [Google Scholar]
- Sovrano VA, Bisazza A, Vallortigara G. Lateralization of response to social stimuli in fishes: a comparison between different methods and species. Physiol Behav. 2001;74:237–244. doi: 10.1016/s0031-9384(01)00552-2. [DOI] [PubMed] [Google Scholar]
- Thieltges H, Lemasson A, Kuczaj S, Böye M, Blois-Heulin C. Visual laterality in dolphins when looking at (un)familiar humans. Anim Cogn. 2011;14:303–308. doi: 10.1007/s10071-010-0354-5. [DOI] [PubMed] [Google Scholar]
- Vallortigara G. Comparative neuropsychology of the dual brain: a stroll through animals’ left and right perceptual worlds. Brain Lang. 2000;73:189–219. doi: 10.1006/brln.2000.2303. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav Brain Sci. 2005;28:575–589. doi: 10.1017/S0140525X05000105. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Regolin L, Pagni P. Detour behaviour, imprinting and visual lateralization in chicks. Cogn Brain Res. 1999a;7:307–320. doi: 10.1016/s0926-6410(98)00033-0. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Rogers LJ, Bisazza A. Possible evolutionary origins of cognitive brain lateralization. Brain Res Rev. 1999b;30:164–175. doi: 10.1016/s0165-0173(99)00012-0. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Cozzutti CC, Tommasi L, Rogers LJ. How birds use their eyes: opposite left-right specialization for the lateral and frontal visual hemifield in the domestic chick. Curr Biol. 2001;11:29–33. doi: 10.1016/s0960-9822(00)00027-0. [DOI] [PubMed] [Google Scholar]
- Vanegas H, Ito H. Morphological aspects of the teleostean visual system. Brain Res Rev. 1983;6:117–137. doi: 10.1016/0165-0173(83)90036-x. [DOI] [PubMed] [Google Scholar]
- Walls GL. Theory of ocular dominance. Arch Ophthalmol. 1951;45:387–412. doi: 10.1001/archopht.1951.01700010395005. [DOI] [PubMed] [Google Scholar]
- Watson SL, Hanbury DB. Prosimian primates as models of laterality. In: Hopkins WD, editor. The evolution of hemispheric specialization in primates. Elsevier; London: 2007. pp. 229–250. [Google Scholar]