Abstract
Cyclodextrin derivatives can be utilized as anti-infectives with pore-forming proteins as the targets. The highly efficient selection of potent inhibitors was achieved because per-substituted cyclodextrins have the same symmetry as the target pores. Inhibitors of several bacterial toxins produced by B. anthracis, S. aureus, C. perfringens, C. botulinum and C. difficile were identified from a library of ~200 CD derivatives. It was demonstrated that multi-targeted inhibitors can be found using this approach and could be utilized for the development of broad-spectrum drugs against various pathogens.
Introduction
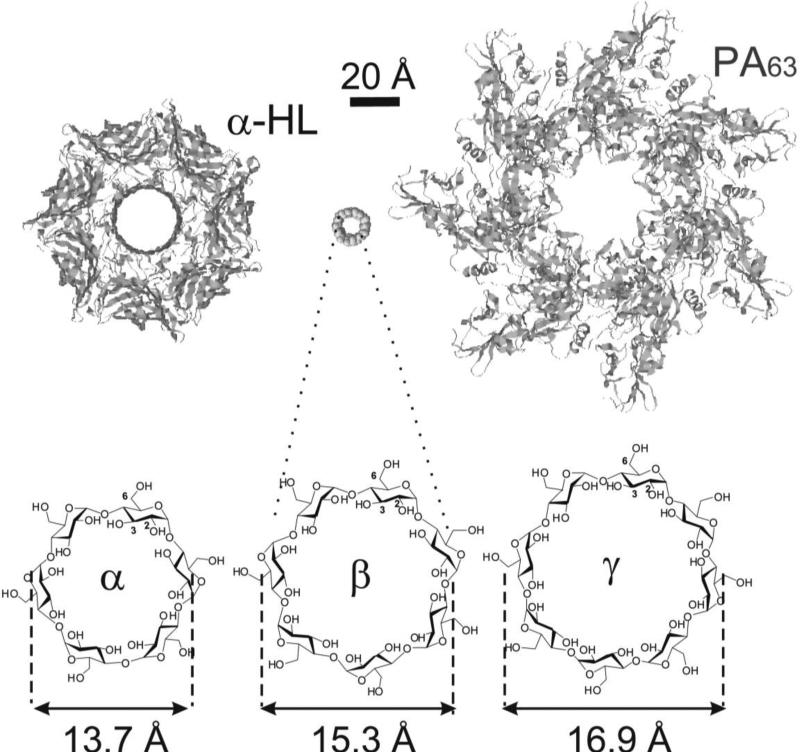
The α-, β-, and γ-cyclodextrins (α-, β-, and γ-CDs) are natural cyclic oligosaccharides, consisting of six, seven, and eight D-glucopyranose residues, respectively, linked by α-1,4 glycosidic bonds into a macrocycle [1] (Fig. 1).
Figure 1.
Schematic illustration of α-, β- and γ-cyclodextrin molecules in comparison with staphylococcal α-HL channel (left) and anthrax PA (right) prepore. The sizes of cyclodextrin molecules are taken from [1].
Cyclodextrins and their derivatives are known to encapsulate organic molecules in aqueous solutions and have been widely used in the pharmaceutical industry for decades to enhance the solubility, bioavailability and stability of drug molecules [2•, 3,4]. Many of the known cyclodextrins and their derivatives exhibit low toxicity and resistance to degradation by enzymes in biological fluids and have GRAS (generally regarded as safe) status from the FDA. The methods for selective modifications of cyclodextrins are very well developed and offer excellent opportunities for the synthesis of various derivatives [5].
CDs have been utilized for the encapsulation of antibiotics [6,7], but their direct use as anti-microbials was suggested only recently with bacterial pore-forming toxins as targets [8••, 9,10•,11•,12, 13•,14•,15•, 16-20].
Bacterial virulence factors, in general, are considered by many as valid targets for the discovery of new therapeutics [21]. It is known that many pathogens utilize the formation of transmembrane pores in target cells in the process of infection [22•,23]. They are important virulence factors and can serve as good targets for drug discovery. For example, the well-known anti-influenza drugs amantadine and rimantadine act by blocking the transmembrane channel formed by the viral protein M2 [24,25].
Table 1 shows some of the pore-forming proteins, both bacterial and viral, with known functions. They can act using different mechanisms of action. Making a pore in the membrane of the target cell may cause osmotic shock or it can lead to a change of pH, which is required for the pathogen's replication. In some bacteria, the transmembrane pores are used for the delivery of various enzymes inside the target cells that kill the cell. The precise mechanisms of action can be different, but the key step is the formation of the transmembrane pore.
Table 1.
Pore-forming proteins.
| Pore-Forming Protein | Pathogen |
|---|---|
| 1. Protective Antigen | Bacillus anthracis |
| 2. α-Hemolysin | Staphylococcus aureus |
| 3. ε-Toxin | Clostridium perfringens |
| 4. Toxin C2 | Clostridium botulinum |
| 5. Toxins A, B and CDT | Clostridium difficile |
| 6. Aerolysin | Aeromonas hydrophilia |
| 7. VacA | Helicobacter pylori |
| 8. p7 protein | Hepatitis C Virus |
| 9. Vpu | HIV |
| 10. M2 protein | Influenza virus |
A novel approach suggested by Karginov et al. [8•] utilizes the blocking of homooligomeric pores with molecules having the same symmetry as the pores and comparable dimensions. It was successfully tested on various bacterial toxins forming heptameric transmembrane pores with the use of β-cyclodextrin derivatives as pore blockers that had the same seven-fold symmetry (Fig. 1).
Anthrax toxins
First, this approach was tested on anthrax toxin, which plays a key role in the pathogenesis of Bacillus anthracis and is regarded as a potential bioterrorism tool. Currently, there is no effective treatment for inhalational anthrax beyond the administration of antibiotics shortly after exposure. However, time delay dramatically reduces the effectiveness of antibiotic treatment. In the 2001 mail-based attacks, 5 out of 11 patients succumbed to inhalational anthrax despite antibiotic therapy (CDC MMWR). Antibiotic administration is ineffective if provided after bacterial exposure has led to the production of sufficient levels of toxins to kill the host. Therefore, the development of direct anti-toxin therapeutics that can be provided after exposure as a supplement to traditional antibiotic intervention is crucial for the treatment of this disease.
The mechanism of anthrax intoxication has been intensively investigated and its main steps and details have been described in various reviews [26-28]. The two anthrax toxins: lethal toxin (LeTx) and edema toxin (EdTx), are formed by three different proteins: protective antigen (PA, 83 kD) either combines with lethal factor (LF, 90 kD) to form lethal toxin (LeTx), or with edema factor (EF, 89 kD) to form edema toxin (EdTx). A trans-membrane pore created by PA facilitates the transport across the cell membrane of LF and EF, both of which are enzymes targeting substrates within the cytosol. LF is a metalloprotease that cleaves mitogen-activated protein kinase kinase (MAPKK), triggering an intracellular signaling cascade, leading to the death of macrophages. EF is a calmodulin-dependent adenylate cyclase that causes edema and impairs neutrophil function.
According to the broadly accepted mechanism of anthrax toxin action, the original 83 kD form of PA (PA83) binds to one of the cell surface receptors: tumor endothelial marker-8 (called TEM8, ATR or ANTXR1) or capillary morphogenesis protein 2 (CMG2 or ANTXR2). Next, a furin-like protease removes the amino terminal 20 kD segment from PA83. The 63 kD form of PA (PA63) oligomerizes to form a heptameric prepore, binds to LF or EF, and the complex is trafficked into the endosome. The low pH in the endosomes causes conformational changes of the prepore, which leads to its conversion to a transmembrane pore followed by the translocation of LF and EF to the cytosol.
Significant progress has been achieved since the 2001 events in the discovery and development of new inhibitors of anthrax toxins using various approaches to block the critical steps of the intoxication mechanism. The most advanced products are the ones based on monoclonal antibodies against PA [29], some of which have passed preclinical and Phase I clinical trials. One product (Raxibacumab) has been delivered to the U.S. Strategic National Stockpile. In the therapeutic-intervention studies of this mAb in cynomolgus macaques challenged with anthrax (Ames) spores, the survival rate of the animals that received the antibody by slow i. v. injection at a dose of 40 mg/kg was only 64% (http://us.gsk.com/products/assets/us_raxibacumab.pdf). In addition, this approach utilizes high molecular weight proteins. Such inhibitors are less attractive as potential drugs in comparison with low molecular weight compounds, which are more desirable given their better bioavailability, lack of sensitivity to proteases and lower cost of production. In case of a bioterrorism attack, an orally or inhalationally available small molecule drug would be the first choice. Further efforts on the development of small molecule anthrax toxin inhibitors are required.
A number of groups are involved in the search for small molecule inhibitors of anthrax toxin but almost all of them are focused exclusively on the inhibition of LF protease activity. None of these compounds would inhibit EF. Recent studies show that edema factor may have a major role in shock during anthrax infection and that it may also be immunosuppressive [26].
Small molecule inhibitors of anthrax toxins based on β-cyclodextrin should inhibit both LeTx and EdTx since they would block the transmembrane pore formed by PA required for the delivery of LF and EF.
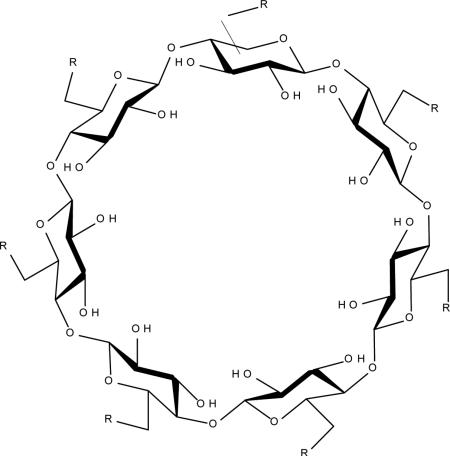
A library of ~ 200 β-CDs per-substituted at positions 6 (see Fig. 1) was synthesized. Most of the compounds carried positively charged groups since it was suggested that they could be more effective blockers of the PA pore because the lumen of the PA pore contains a significant number of negatively charged amino acids. The hydroxyls at positions 2 and 3 form hydrogen bonds and are required for keeping the molecule rigid, making the 6-OH group a favorable site for modifications.
The library was screened for the ability to inhibit the cytotoxicity of LeTx in a cell-based assay utilizing mouse macrophage RAW 264.7 cells. The extraordinary effectiveness of this approach was demonstrated by the fact that several dozen of the synthesized compounds displayed inhibitory activity with IC50 values in the low- and sub-micromolar range [9,10•,19,20]. One of the inhibitors of LeTx was tested for its ability to block EdTx activity in CHO-K1 cells [10]. The compound inhibited EdTx in the same concentration range as LeTx providing additional support for the originally proposed mechanism of action, which involves the blocking of PA63 that is required for the delivery of both LF and EF into the cytoplasm.
The ability of structurally related α-, β- and γ-cyclodextrins carrying the same modifications to inhibit Bacillus anthracis lethal toxin was compared. It was found that both β- and γ-cyclodextrin derivatives effectively inhibited anthrax toxin action, whereas α-cyclodextrins were ineffective [20]. That could be related to the recently reported observation of PA octameric pores having the same eight-fold symmetry as γ-CD [30].
Several β-CD derivatives displaying inhibitory activity were tested for their ability to block conductance through PA channels incorporated into a bilayer lipid membrane. They blocked the PA pore at low nanomolar concentrations. Also, a correlation between the IC50 values obtained in cell-based and ion conductance experiments was observed despite the tremendous difference in these two methods [10]. This finding supports the concept that β-CDs inhibit anthrax toxins by means of blocking the PA channel.
The involvement of electrostatic interactions is suggested by the strong dependence of blockage parameters on salt concentration which screens out charges on both the blocker and the protein. Experiments with β-CD derivatives carrying extra aromatic groups performed with the F427A mutant of PA63, which lacks the functionally important “phenylalanine clamp” [31], demonstrated the potential role of phenylalanine 427 with regards to the PA channel interaction with the blockers [18].
To check if LeTx inhibitors can bind to the PA prepore, preliminary crystallographic studies were performed by Dr. Liddington at the Sanford-Burnham Medical Research Institute. The binding mode of the anthrax PA heptamer to compound AMBnTβCD (Table 2) was defined by crystallography at a resolution of ~3.5 Å using a synchrotron source. The inhibitor is observed to bind about half-way along the lumen axis, making polar and charged interactions between the benzylamine groups of AMBnTβCD and the 14 loops (2 per PA monomer) close to the region implicated in the prepore-to-pore conversion that creates the “phenylalanine clamp” (unpublished data).
Table 2.
Inhibitors of the cytotoxic activity of bacterial toxins.
| Compound | R | Targets | |
|---|---|---|---|
|
AMBnTβCD |
|
Anthrax toxins LeTx and EdTx C. botulinum C2 toxin C. perfringens iota toxin C. difficile toxins A, B and CDT |
| NPβCD |
|
Anthrax toxins LeTx and EdTx C. perfringens ε-toxin |
|
| ANBOβCD |
|
S. aureus alpha-toxin (α-hemolysin) |
Some additional insight on the mechanism of LeTx inhibition was obtained utilizing fluorescent tracers based on LFn, a catalytically inactive fragment of LF capable of PA-dependent binding and internalization into cells. With the use of fluorescent microscopy, it was demonstrated that a β-CD based blocker of PA did not prevent the formation of the PA prepore, it's binding to the cell surface nor the binding of the LFn based tracer, but did inhibit LFn translocation into cells [12].
These data show that, in addition to blocking the already formed transmembrane pore, β-CDs may bind to the pre-pore (probably to the same area of the amino acid sequence located close to the “phenylalanine clamp”) and prevent its transformation into the transmembrane channel.
Some of the most active inhibitors of LeTx were tested in vivo. The toxicity of these derivatives of β-cyclodextrin were tested in mice, rats and rabbits and it was found that the animals tolerated up to a 20 mg/kg dose of the compounds administered intravenously (unpublished data).
A preliminary evaluation of the pharmacokinetic properties of the cyclodextrin derivative AMBnTβCD was performed in rabbits using a competitive ELISA assay with antibodies against this compound [32]. The obtained results showed that the apparent half-life of elimination (t½) was 4.7 hours and the total plasma clearance of the compounds were approximately equal to the glomerular filtration rate, suggesting that the elimination occurred via the kidneys and most likely the compounds had limited distribution into the tissues, which is in agreement with previous reports for other cyclodextrins [2•].
The efficacy studies were performed first in Fischer 344 rats challenged with LeTx. It was demonstrated that β-CDs completely protected rats from a deadly dose of LeTx at as low a dose as 85 μg/rat [14•]. The protective properties of compound AMBnTβCD was tested in mice challenged with Bacillus anthracis (Sterne strain) in a delayed treatment study. The animals were infected with 10 LD50 of anthrax spores. At day 1 post-challenge, the mice received the compound (i.v.) at a dose of 2.5 mg/kg alone or in combination with the antibiotic ciprofloxacin (50 mg/kg, i.p.). AMBnTβCD and ciprofloxacin were administered once daily for 10 days. The combination treatment saved up to 90% of the mice while all untreated animals or those treated with antibiotic or antitoxin died [14•].
A similar approach for the search for inhibitors of anthrax toxins has been developed using the design of polyvalent inhibitors consisting of multiple copies of an inhibiting peptide attached to β-cyclodextrin. It utilized the peptide HTSTYWWLDGAPC that inhibits the binding of LF and EF to PA. Seven copies of this peptide were attached to the β-cyclodextrin molecule via polyethylene glycol linkers of various lengths. Several of the heptavalent molecules inhibited the LeTx-induced death of RAW 264.7 cells at low nanomolar concentrations. One of the best inhibitors carrying the PEG11 linker was tested in vivo in Fischer 344 rats and it prevented 6 of 7 animals from becoming moribund when co-injected with LeTx at a 300 nmol dose of the peptide (~0.7 mg of the heptameric inhibitor per animal) [33].
S. aureus α-hemolysin
The broader applicability of this approach was tested using as a target α-hemolysin (α-HL), also known as α-toxin, of Staphylococcus aureus which is one of the key virulence factors produced by this bacteria. S. aureus is one of the most significant causes of serious hospital- and community-acquired bacterial infections, contributing to morbidity and mortality in individuals of all ages. Increasing antimicrobial resistance among S. aureus strains and the appearance of particularly virulent isolates of this pathogen within the community have rendered historic antimicrobial therapies obsolete, resulting in increased mortality and cost of care [34-37]. Novel approaches to both prevent and treat S. aureus-mediated infection, especially methicillin resistant S. aureus (MRSA), are necessary.
S. aureus relies on various virulence factors that act in concert to facilitate the survival and multiplication of the organism within the human host [38]. A number of earlier studies have highlighted the importance of S. aureus α-HL in disease and validated this protein as a viable target for the development of small molecule inhibitors [39-42].
α-Hemolysin is a single polypeptide chain with a molecular weight of 33 kDa. Water-soluble toxin monomers, secreted by S. aureus, bind to target membranes and oligomerize into a heptameric, nonlytic prepore complex. Subsequent conformational changes allow for the stem domain of the toxin to extend from the globular monomer through the host cell membrane. The coordinate insertion of the stem domains from each heptamer permit the formation of the lytic channel or pore that causes the loss of membrane integrity, culminating in cellular injury and eventual death. The pore formation also triggers secondary events within the host cell that could promote the development of pathologic conditions [43,44].
The crystallographic structure of the fully assembled α-hemolysin pore was resolved [45]. The diameter of the α-hemolysin channel lumen, which is 14 – 46 Å according to X-ray analysis data, is comparable with the outside diameter of β-CD – 15.3 Å [1].
The library of β-CD derivatives that was used to find inhibitors of anthrax toxins was screened against α-HL employing a standard rabbit erythrocyte hemolysis assay adapted to a 96-well plate format. Several compounds that inhibited α-hemolysin's cytotoxicity at low micromolar concentrations were identified [11•]. One compound (ANBOβCD, table 2) was tested using the human monocyte cell line THP-1 and it was found that this β-CD derivative protected the cells against α-HL cytotoxicity.
Similarly to above described LeTx studies, the ability of structurally related α-, β- and γ-cyclodextrins carrying the same modifications to inhibit α-HL were compared. It was found that both α- and γ-CD derivatives did not display any activity [20]. This difference in the activities of γ-CD derivatives against anthrax and staphylococcal toxins could be explained by the fact that in contrast to α-HL, PA presumably can form octameric pores [30]. These data show that size and conformation as well as a match in symmetry of the blocking molecule and pore play important roles in the activity of the inhibitors of pore-forming toxins.
Compound ANBOβCD (Table 2) was tested for its ability to block conductance through α-hemolysin channels incorporated into a bilayer lipid membrane. They blocked the α-HL pore at low micromolar concentrations but the mechanisms of blocking the PA and α-HL transmembrane pores were different. In contrast to the PA pore blockers, compound ANBOβCD blocked the α-HL channels irreversibly [11•]. For PA pore blockers, the binding was reversible and the involvement of electrostatic interactions is suggested by the strong dependence of blockage parameters on salt concentration.
Based on the preliminary computer modeling performed by Molsoft, LLC, compound ANBOβCD appears to be interacting with the α-HL pore via its t-butyl moiety which fits well into small pockets formed by the belt of hydrophobic side-chains of residues V149 and the main-chain surface of the beta-sheet in between. The charged amino-group of the ligand is not engaged in any direct receptor interactions and appears instead to stabilize a particular conformation of the side-chain moiety via hydrogen bonds (unpublished data).
To investigate whether the disruption of pore conductance and lytic function of α-HL by ANBOβCD results from an alteration in the heptameric state of the assembled toxin, the effects of this compound on α-HL oligomerization using [35S]methionine-labeled α-HL was examined. When the toxin was incubated with rabbit red blood cells in the presence of ANBOβCD, a stable oligomer formed in a fashion identical to that seen without treatment, suggesting that the inhibitor does not disrupt the toxin oligomerization, its binding to the cell membranes nor the formation of the heptameric transmembrane channel [15•]. Together, these data provide additional support to confirm that the principal mechanism by which ANBOβCD protects against α-HL-induced lysis is to block the transmembrane pore that is formed by the toxin. As a consequence, cell lysis is impaired, despite the fact that the toxin is membrane-bound in the fully functional oligomeric form.
It was demonstrated that this α-HL inhibitor protects human alveolar A549 epithelial cells from S. aureus injury. To ensure the protection is in fact due to its ability to antagonize the toxin and not merely the result of an inhibitory effect on bacterial growth, the growth of S. aureus Newman in tryptic soy broth supplemented with either control PBSD or 5 μM of ANBOβCD was analyzed. It was shown that the presence of the compound did not alter bacterial growth kinetics. These results demonstrated the efficacy of compound ANBOβCD in protecting human alveolar epithelial cells against cell death caused by α-HL [15•].
The in vivo protective properties of compound ANBOβCD were tested in a pneumonia model of S. aureus infection in mice [15•]. It was evaluated whether a single dose of compound ANBOβCD would be able to prevent or treat S. aureus pneumonia when given at various times prior to and following infection. A single 10 mg/kg dose (i.v.) of the compound was given to mice at 2 hours prior to infection or 2, 6, or 12 h after infection with S. aureus. Mortality was then observed over a 72-h time course. Mortality was significantly reduced when the compound was given 2 h before or 2 h after infection, highlighting the role of this compound in mitigating the early stages of the disease. Treatment with repeated injections (2 h after infection and every 12 h thereafter) of compound ANBOβCD at a 10 mg/kg dose afforded complete protection against mortality caused by both the antibiotic sensitive Newman strain and the highly virulent MRSA USA 300 (LAC) strain [15•].
Compound ANBOβCD also significantly decreased the corneal pathology in rabbit eyes caused by S. aureus infection [46]. β-CD based inhibitors of α-HL can be potentially used for the treatment of S. aureus skin infections similarly to anti-α-HL monoclonal antibodies that were successfully tested in mice for immunoprophylaxis against staphylococcal skin and soft tissue infections [47,48].
C. perfringens ε-toxin
Epsilon toxin (ETX) of Clostridium perfringens is one of the most lethal bacterial toxins. It is considered as a potential biological weapon and is included in the list of category B priority agents. No specific therapy exists for ε-toxin; therefore, a great need exists for the development of effective therapeutics against this biodefense toxin.
Besides their biodefense importance, ETX-producing C. perfringens type B and D isolates are also important natural veterinary pathogens causing enterotoxemias in sheep, goats and other animals [49,50]. While antibiotics can kill or inhibit the growth of C. perfringens, they do not protect against the toxins, such as ETX, already present in the circulation. These observations indicate that therapeutic efforts should be directed against ETX to protect against natural type B and D infections.
ETX is produced as a 33 kDa prototoxin, which is relatively inactive in terms of toxicity. However, the ETX prototoxin can be quickly converted to a highly-lethal mature toxin by intestinal proteases, such as trypsin or chymotrypsin, which removes 13 amino acid residues from its N-terminus and 22 amino acids from its C-terminus [51].
ETX action begins when the toxin binds to a specific receptor of unknown identity. The bound toxin then uses lipid rafts to form heptameric pores in sensitive cells (e.g., Madin-Darby Canine Kidney [MDCK] cells) or artificial membranes [52]. ETX shows sequence homology with some other pore-forming toxins [51] and its 3D structure was solved by Basak's group [53].
A library of ~200 β-cyclodextrin derivatives was screened using the MTS bioreduction cell viability assay and the Roche xCELLigence System impedance measurement assay, which provides a real-time status of cellular activity. Several compounds that protected MDCK cells from ε-toxin-induced cytotoxicity in the low micromolar range were identified [54]. It is interesting to note, that one of the most active inhibitors of ε- toxin (NPβCD in Table 2) also inhibited anthrax LeTx.
Experiments on the blocking of ion conductance through the ETX pore were unsuccessful (unpublished data). Apparently, the cyclodextrin inhibitors bind only to the prepore form of the toxin and don't bind to the transmembrane pore. That causes the inhibition of the cytotoxic activity of ETX but not the blockage of ion conductance through the pore.
Other pore-forming toxins
It was discovered recently that one of the best blockers of anthrax LeTx (AMBnTβCD) also inhibited the cytotoxicity of binary actin-ADP-ribosylating C2 toxin from C. botulinum and iota toxin produced by C. perfringens, respectively. The inhibitor blocked the pores formed by the binding/translocation components of the toxins in artificial lipid bilayer membranes in vitro. It also inhibited membrane translocation of the enzyme moieties of the toxins through the pores across cell membranes and thereby the cellular uptake of the toxins in intact cells. The underlying molecular mechanism of the inhibitor action was identified, which seems to be widely comparable to the inhibition of anthrax toxin [13•,17].
Preliminary experiments on the ability of AMBnTβCD to inhibit C. difficile toxins CDT, TcdA and TcdB in Vero cells using the cell rounding assay were performed by Dr. Barth at the University of Ulm (Germany). These experiments showed that the cytopathic activity of all three toxins was neutralized by 20 μM AMBnTβCD (unpublished data).
Conclusions
Cyclodextrins derivatives can be effective blockers of pore-forming toxins and utilized as inhibitors of toxins and anti-infectives.
The use of molecules having the same symmetry as the target pores allowed for the highly efficient selection of potent inhibitors. This would be impossible with the standard high throughput screening of libraries of random compounds.
A number of inhibitors of various toxins have been identified from a small library of ~200 β-cyclodextrin derivatives. The list includes α–hemolysin of S. aureus, ε–toxin and ι–toxin produced by C. perfringens, C. botulinum C2 toxin and C. difficile toxins A, B and CDT.
It was demonstrated that one β-CD derivative can specifically block different pore-forming toxins simultaneously, meaning that this approach allows for the finding of multi-targeted inhibitors that could be used for the development of broad-spectrum drugs.
This approach may result in a pipeline of products since it can be used for the discovery of new therapeutics that may target a wide array of other bacterial and viral pathogens that utilize pore-forming proteins as virulence factors.
The three inhibitors of the various toxins that are presented in Table 2 were deposited at ATCC and are available for the scientific community (www.beiresources.org), catalog #'s NR-33151, NR-33152, NR-33153).
Highlights.
Cyclodextrins derivatives block pore-forming toxins and inhibit bacterial infection.
The symmetry match of blockers and target pores improved the selection of potent inhibitors.
Multi-targeted inhibitors were identified that could be developed into broad-spectrum drugs.
This approach can be used to find new drugs against pathogens producing pore-forming proteins.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem Rev. 1998:98:1743–1753. doi: 10.1021/cr970022c. [DOI] [PubMed] [Google Scholar]
- 2•.Stella VJ, He Q. Cyclodextrins. Toxicol Pathol. 2008;36:30–42. doi: 10.1177/0192623307310945. [This review outlines properties of cyclodextrins in regards to their pharmaceutical applications.] [DOI] [PubMed] [Google Scholar]
- 3.Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins: basic science and product development. J Pharm Pharmacol. 2010;62:1607–1621. doi: 10.1111/j.2042-7158.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- 4.Uekama K, Hirayama F, Irie T. Cyclodextrin drug carrier systems. Chem Rev. 1998;98:2045–2076. doi: 10.1021/cr970025p. [DOI] [PubMed] [Google Scholar]
- 5.Khan AR, Forgo P, Stine KJ, D'Souza VT. Methods for selective modification of cyclodextrins. Chem Rev. 1998;98:1977–1996. doi: 10.1021/cr970012b. [DOI] [PubMed] [Google Scholar]
- 6.Aleem O, Kuchekar B, Pore Y, Late S. Effect of beta-cyclodextrin and hydroxypropyl beta-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir. J Pharm Biomed Anal. 2008;47:535–540. doi: 10.1016/j.jpba.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Song W, Yu X, Wang S, Blasier R, Markel DC, Mao G, Shi T, Ren W. Cyclodextrin-erythromycin complexes as a drug delivery device for orthopedic application. Int J Nanomedicine. 2011;6:3173–3186. doi: 10.2147/IJN.S23530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Karginov VA, Nestorovich EM, Moayeri M, Leppla SH, Bezrukov SM. Blocking anthrax lethal toxin at the protective antigen channel by using structure-inspired drug design. Proc Natl Acad Sci U S A. 2005;102:15075–15080. doi: 10.1073/pnas.0507488102. [This is the first article demonstrating that cyclodextrin derivatives can be used as inhibitors of bacterial toxins.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karginov VA, Yohannes A, Robinson TM, Fahmi NE, Alibek K, Hecht SM. β-Cyclodextrin derivatives that inhibit anthrax lethal toxin. Bioorg Med Chem. 2006;14:33–40. doi: 10.1016/j.bmc.2005.07.054. [DOI] [PubMed] [Google Scholar]
- 10•.Karginov VA, Nestorovich EM, Yohannes A, Robinson TM, Fahmi NE, Schmidtmann F, Hecht SM, Bezrukov SM. Search for Cyclodextrin-Based Inhibitors of Anthrax Toxins: Synthesis, Structural Features, and Relative Activities. Antimicrob Agents Chemother. 2006;50:3740–3753. doi: 10.1128/AAC.00693-06. [This paper presents the detailed descriptions of the synthesis of various cyclodextrin derivatives, identification of inhibitors of anthrax toxin and their relative activities.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Karginov VA, Nestorovich EM, Schmidtmann F, Robinson TM, Yohannes A, Fahmi NE, Bezrukov SM, Hecht SM. Inhibition of S. aureus α-hemolysin and B. anthracis lethal toxin by βcyclodextrin derivatives. Bioorg Med Chem. 2007;15:5424–5431. doi: 10.1016/j.bmc.2007.05.058. [The first identification of cyclodextrin based inhibitors of S. aureus α-hemolysin.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Backer MV, Patel V, Jehnings BT, Claffey KP, Karginov VA, Backer JM. Inhibition of anthrax protective antigen outside and inside the targeted cell. Antimicrob Agents Chemother. 2006;1:245–251. doi: 10.1128/AAC.00983-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13•.Bezrukov SM, Liu X, Karginov VA, Wein AN, Leppla SH, Popoff MR, Barth H, Nestorovich EM. Interactions of High-Affinity Cationic Blockers with the Translocation Pores of B. anthracis, C. botulinum, and C. perfringens Binary Toxins. Biophys J. 2012;103:1208–1217. doi: 10.1016/j.bpj.2012.07.050. [This article presents the first demonstration that a β-CD derivative can inhibit toxins produced by various bacteria and investigates the mechanisms of its action.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Moayeri M, Robinson TM, Leppla SH, Karginov VA. In vivo efficacy of β-cyclodextrin derivatives against anthrax lethal toxin. Antimicrob Agents Chemother. 2008;52:2239–2241. doi: 10.1128/AAC.00009-08. [A paper presenting data on in vivo activity of β-CD based anthrax toxin inhibitors.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Ragle BE, Karginov VA, Wardenburg JB. Prevention and treatment of Staphylococcus aureus pneumonia with a β-cyclodextrin derivative. Antimicrob Agents Chemother. 2010;54:298–304. doi: 10.1128/AAC.00973-09. [This article provides the first demonstration of the in vivo activities of cyclodextrin based inhibitors of S. aureus α-hemolysin and investigates the mechanisms of their action.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nestorovich EM, Karginov VA, Berezhkovskii AM, Bezrukov SM. Blockage of Anthrax PA63 Pore by a Multi-Charged High-Affinity Toxin Inhibitor. Biophysical Journal. 2010;99:134–143. doi: 10.1016/j.bpj.2010.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nestorovich EM, Karginov VA, Popoff MR, Bezrukov SM, Barth H. Tailor-made β-cyclodextrin derivative blocks the translocation pores of binary exotoxins from Clostridium botulinum and Clostridium perfringens and protects mammalian cells from intoxication. PLoS ONE. 2011;6:e23927. doi: 10.1371/journal.pone.0023927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nestorovich EM, Karginov VA, Berezhkovskii AM, Parsegian VA, Bezrukov SM. Kinetics and thermodynamics of binding reactions as exemplified by anthrax channel blockage with a cationic cyclodextrin derivative. Proc Natl Acad Sci U S A. 2012;109:18453–18458. doi: 10.1073/pnas.1208771109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Díaz-Moscoso A, Méndez-Ardoy A, Ortega-Caballero F, Benito JM, Ortiz Mellet C, Defaye J, Robinson TM, Yohannes A, Karginov VA, García Fernández JM. Symmetry Complementarity-Guided Design of Anthrax Toxin Inhibitors Based on β-Cyclodextrin: Synthesis and Relative Activities of Face-Selective Functionalized Polycationic Clusters. ChemMedChem. 2011;6:181–192. doi: 10.1002/cmdc.201000419. [DOI] [PubMed] [Google Scholar]
- 20.Yannakopoulou K, Jicsinszky L, Aggelidou C, Mourtzis N, Robinson TM, Yohannes A, Nestorovich EM, Bezrukov SM, Karginov VA. Symmetry requirements for the effective blocking of pore-forming toxins: Comparative study with α-, β- and γ-cyclodextrin derivatives. Antimicrob Agents Chemother. 2011;55:3594–3597. doi: 10.1128/AAC.01764-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alksne LE, Projan SJ. Bacterial virulence as a target for antimicrobial chemotherapy. Curr Opin Biotechnol. 2000;6:625–636. doi: 10.1016/s0958-1669(00)00155-5. [DOI] [PubMed] [Google Scholar]
- 22•.Bischofberger M, Iacovache I, van der Goot FG. Pathogenic pore-forming proteins: function and host response. Cell Host Microbe. 2012;12:266–275. doi: 10.1016/j.chom.2012.08.005. [A recent review on pore-forming proteins.] [DOI] [PubMed] [Google Scholar]
- 23.Nieva JL, Madan V, Carrasco L. Viroporins: structure and biological functions. Nat Rev Microbiol. 2012;10:563–574. doi: 10.1038/nrmicro2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hay AJ, Wolstenholme AJ, Skehel JJ, Smith MH. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985;4:3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamb RA, Zebedee SL, Richardson CD. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985;40:627–633. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Sherer K, Cui X, Eichacker PQ. New insights into the pathogenesis and treatment of anthrax toxin-induced shock. Expert Opin Biol Ther. 2007;7:843–854. doi: 10.1517/14712598.7.6.843. [DOI] [PubMed] [Google Scholar]
- 27.Young JA, Collier RJ. Anthrax toxin: receptor-binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- 28.Moayeri M, Leppla SH. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol Aspects Med. 2009;30:439–455. doi: 10.1016/j.mam.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneemann A, Manchester M. Anti-toxin antibodies in prophylaxis and treatment of inhalation anthrax. Future Microbiol. 2009;4:35–43. doi: 10.2217/17460913.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kintzer KF, Thoren KL, Sterling HJ, Dong KC, Feld GK, Tang II, Zhang TT, Williams ER, Berger JM, Krantz BA. The protective antigen component of anthrax toxin forms functional octameric complexes. J Mol Biol. 2009;392:614–629. doi: 10.1016/j.jmb.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krantz BA, Melnyk RA, Zhang S, Juris SJ, Lacy DB, Wu Z, Finkelstein A, Collier RJ. A phenylalanine clamp catalyzes protein translocation through the anthrax toxin pore. Science. 2005;309:777–781. doi: 10.1126/science.1113380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karginova E, Robinson T, Karginov V. “Pharmacokinetic Evaluation of Cyclodextrin Derivatives using an ELISA Based Assay”.. 16th International Cyclodextrins Symposium.; Tianjin, China. May 6-10, 2012. [Google Scholar]
- 33.Joshi A, Kate S, Poon V, Mondal D, Boggara MB, Saraph A, Martin JT, McAlpine R, Day R, Garcia AE, Mogridge J, Kane RS. Structure-based design of a heptavalent anthrax toxin inhibitor. Biomacromolecules. 2011;12:791–796. doi: 10.1021/bm101396u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 35.Chambers HF, DeLeo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:2464–2474. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otto M. MRSA virulence and spread. Cell Microbiol. 2012;14:1513–1521. doi: 10.1111/j.1462-5822.2012.01832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandenesch F, Lina G, Henry T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front Cell Infect Microbiol. 2012;2:12. doi: 10.3389/fcimb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel AH, Nowlan P, Weavers ED, Foster T. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect Immun. 1987;55:3103–3110. doi: 10.1128/iai.55.12.3103-3110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji Y, Marra A, Rosenberg M, Woodnutt G. Regulated antisense RNA eliminates alpha-toxin virulence in Staphylococcus aureus infection. J Bacteriol. 1999;181:6585–6590. doi: 10.1128/jb.181.21.6585-6590.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bubeck-Wardenburg J, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun. 2007;75:1040–1044. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bubeck-Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205:287–294. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menestrina G, Serra MD, Prévost G. Mode of action of beta-barrel pore-forming toxins of the staphylococcal alpha-hemolysin family. Toxicon. 2001;39:1661–1672. doi: 10.1016/s0041-0101(01)00153-2. [DOI] [PubMed] [Google Scholar]
- 44.Montoya M, Gouaux E. Beta-barrel membrane protein folding and structure viewed through the lens of alpha-hemolysin. Biochim Biophys Acta. 2003;1609:19–27. doi: 10.1016/s0005-2736(02)00663-6. [DOI] [PubMed] [Google Scholar]
- 45.Song L, Hobaugh MR, Shustak C, Cheley S, Bayl ey H, Gouaux JE. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 1996;274:1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 46.Caballero AR, McCormick C, Karginov V, O'Callaghan R. Analysis of the effectiveness of three chemical inhibitors of alpha-toxin in the treatment of S. aureus experimental keratitis.. World Cornea Congress VI; Boston, MA. April 7-9.p. 2010. [Google Scholar]
- 47.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis. 2010;202:1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tkaczyk C, Hua L, Varkey R, Shi Y, Dettinger L, Woods R, Barnes A, MacGill RS, Wilson S, Chowdhury P, Stover CK, Sellman BR. Identification of anti-alpha toxin monoclonal antibodies that reduce the severity of Staphylococcus aureus dermonecrosis and exhibit a correlation between affinity and potency. Clin Vaccine Immunol. 2012;19:377–385. doi: 10.1128/CVI.05589-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Songer JG. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9:216–234. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smedley JG, 3rd, Fisher DJ, Sayeed S, Chakrabarti G, McClane BA. The enteric toxins of Clostridium perfringens. Rev Physiol Biochem Pharmacol. 2004;152:183–204. doi: 10.1007/s10254-004-0036-2. [DOI] [PubMed] [Google Scholar]
- 51.Popoff MR. Epsilon toxin: a fascinating pore-forming toxin. FEBS J. 2011;278:4602–4615. doi: 10.1111/j.1742-4658.2011.08145.x. [DOI] [PubMed] [Google Scholar]
- 52.Miyata S, Minami J, Tamai E, Matsushita O, Shimamoto S, Okabe A. Clostridium perfringens epsilon-toxin forms a heptameric pore within the detergent-insoluble microdomain of Madin-Darby canine kidney cells and rat synaptosomes. J Biol Chem. 2002;277:39463–39468. doi: 10.1074/jbc.M206731200. [DOI] [PubMed] [Google Scholar]
- 53.Cole AR, Gibert M, Popoff M, Moss DS, Titball RW, Basak AK. Clostridium perfringens epsilon toxin shows structural similarity to the pore-forming toxin aerolysin. Nat Struct Mol Biol. 2004;11:797–798. doi: 10.1038/nsmb804. [DOI] [PubMed] [Google Scholar]
- 54.Boley M, Robinson T, Jicsinszky L, Karginov V, Langenbach K. “In-vitro Inhibition of Clostridium perfringens Epsilon Toxin Using Beta-cyclodextrin Derivatives Provides a Potential Strategy for Therapeutic Development”.. 52d Interscience Conference on Antimicrobial Agents and Chemotherapy.; San Francisco. September 9-12, 2012. [Google Scholar]