Abstract
Studies involving oro-facial asymmetries in nonhuman primates have largely demonstrated a right hemispheric dominance for communicative signals and conveyance of emotional information. A recent study on chimpanzee reported the first evidence of significant left-hemispheric dominance when using attention-getting sounds and rightward bias for species-typical vocalizations (Losin, Russell, Freeman, Meguerditchian, Hopkins & Fitch, 2008). The current study sought to extend the findings from Losin et al. (2008) with additional oro-facial assessment in a new colony of chimpanzees. When combining the two populations, the results indicated a consistent leftward bias for attention-getting sounds and a right lateralization for species-typical vocalizations. Collectively, the results suggest that both voluntary- controlled oro-facial and gestural communication might share the same left-hemispheric specialization and might have coevolved into a single integrated system present in a common hominid ancestor.
Keywords: Hemispheric specialization, Oro-facial asymmetry, Communicative behaviors, Gestural communication, Oro-facial communication, Emotions, Primates
1. Introduction
Since the 19th century, it has been well established that most of the language functions involved a functional dominance of the left hemisphere of the brain (e.g., Broca, 1865; Wernicke, 1874) especially for production, whereas perception of signals involves both the left and right hemispheres depending of the information processed from the signals (e.g., Hickok & Poeppel, 2007). Specifically, left-lateralization for language has been demonstrated at functional levels including hemispheric perfusion asymmetries within the middle cerebral arteries (e.g., Knecht et al., 2000) or regional asymmetries within the posterior frontal and temporal cortex and in subcortical areas (e.g., Indefrey & Levelt, 2000; Vigneau et al., 2006). In contrast, understanding the cerebral mechanisms underlying the production and perception of emotions in humans was relatively neglected until the 1970s (e.g., Borod, Haywood, & Koff, 1997). Two primary theories attempt to explain cerebral specialization for emotional processing. The first, and more popularly accepted theory, proposes that the right hemisphere is involved in perception and production of both positive and negative emotions (Borod et al., 1997). The second theory, known as the “valence theory”, argues that the right hemisphere is specialized for processing negative emotions whereas the left hemisphere is specialized for positive emotions (Davidson, 1995).
Cerebral specialization and population-level behavioral asymmetries were historically considered as a unique trait of human evolution (e.g., Crow, 2004). However, this latter view has been contested by a growing number of studies providing evidence of population-level brain and behavioral asymmetries in a wide range of vertebrates species (MacNeilage, Rogers, & Vallortigara, 2009; Rogers & Andrew, 2002; Vallortigara, Chiandetti, & Sovrano, 2011), particularly in nonhuman primates (Hopkins, 2007). Considering the phylogenetic proximity between human and nonhuman primates, investigating lateralization in nonhuman primates for cognitive processing, including communication and emotions, as well as the motor system, such as handedness and manual gestures, may provide relevant clues to determine the precursors of hemispheric specialization in humans (for reviews, see Hopkins, 2007; Hopkins & Vauclair, 2012; Vauclair, Fagot, & Dépy, 1999).
In primate species, natural communicative signals such as vocalizations, facial expressions and manual gestures are critical for the coordination of social activities and exchange between social partners. Thus, many behavioral studies have examined hemispheric specialization related to communicative gestures in nonhuman primates within the theoretical framework on the origin of left-hemispheric specialization for language (Hopkins et al., 2005; Meguerditchian, Gardner, Schapiro, & Hopkins, 2012). However, little research has been done on the lateralization of the oro-facial and vocal communicative systems while they may provide insight into cerebral asymmetries in nonhuman primates associated to communication and its potential continuities with hemispheric specialization for language.
In humans, during speech production, the right side of the mouth opens first and wider, reflecting the left hemispheric dominance for language control. In contrast, oro-facial expressions of emotion, such as smiling, elicit a left-hemimouth asymmetry (i.e., right hemisphere specialization, Graves & Landis, 1990). In nonhuman primates, a left oro-facial asymmetry (i.e. right hemisphere dominance) for species-specific vocalizations has been similarly reported in chimpanzees, marmosets, rhesus monkeys and baboons (Fernández-Carriba, Loeches, Morcillo, & Hopkins, 2002; Hauser, 1993; Hauser & Akre, 2001; Hook-Costigan & Rogers, 1998; Losin et al., 2008; Wallez & Vauclair, 2011). Right hemisphere lateralization for the production of vocalizations and facial expressions is consistent with the idea that these behaviors might be used in an emotional context rather than being used as a linguistic signal (Seyfarth & Cheney, 2003; Vauclair, 2003).
Vocal production in nonhuman primates has been historically considered as involuntary signals associated to a specific emotional state and produced in response to specific stimuli (Lieberman, 1998). In addition, vocalizations are emitted by the signaler toward the whole social group, rather than directed to a single individual (Arbib, 2005). More recently, a class of novel idiosyncratic atypical sounds produced by some captive chimpanzees, called attention-getting vocalizations, have been described (see Marshall, Wrangham, & Arcadi, 1999 for the first description). Three attention-getting vocalizations have been identified including the “raspberry”, the “extended grunt” and the “kiss” (Hopkins, Taglialatela, & Leavens, 2007). The report of the use of attention getting vocalizations by chimpanzees has challenged the view of a lack of voluntary control over vocalizations and facial expressions, and that these behaviors are related to innate emotional responses. Indeed, it has been shown that attention-getting vocalizations are socially learned and voluntarily and selectively produced to capture the attention of an otherwise inattentive human (Hopkins et al., 2007; Hostetter, Russell, Freeman, & Hopkins, 2007; Taglialatela, Reamer, Schapiro, & Hopkins, in press). Because the signaler “intends” to influence a social partner by producing attention-getting vocalizations towards a human, it has been suggested that these sounds may be referential and intentionally produced (Hopkins et al., 2007), whereas the species-typical vocal repertoire of chimpanzees and of the rest of nonhuman primate species is likely used in relation to a specific emotional state (e.g., Goodall, 1986) and is dependent on a specific context (Pollick & De Waal, 2007).
Losin et al. (2008) assessed oro-facial asymmetries of learned sounds (raspberry and extended grunt) compared to species-typical vocalizations (food-bark and pant-hoot) to examine whether differences in hemispheric specialization of the neuropsychological mechanisms emerged. Interestingly, a rightward asymmetry (i.e. left hemisphere) was reported for the two atypical sounds (i.e., raspberry and extended grunt) whereas a significant leftward hemiface lateralization was found for species-typical vocalizations (i.e., the pant-hoot and the food-bark). The authors suggested that the left-hemisphere implication for oro-facial motor control during attention-getting sounds production could be a crucial stage for the evolution of more sophisticated motor systems that allowed for the emergence of human speech. However, while the involvement of the right-hemisphere for several species-typical vocalizations has been frequently reported in nonhuman primate species, little is known about the robustness finding of a left-hemisphere lateralization during attention-getting sounds production in chimpanzees.
Therefore, the current study proposed to assess oro-facial asymmetries during the production of species-typical vocalizations and attention-getting sounds in a new cohort of chimpanzees. Then, we increased the sample size of captive chimpanzee individuals and the sample number of still facial expressions by compiling our new sample data with the sample data set of Losin et al. (2008) in order to address the consistency of their previous findings regarding hemispheric specialization for the raspberry, extended grunt, pant-hoot and the food-bark. We also quantified oro-facial asymmetries for the “kiss” sound. Furthermore, we evaluated the hypothesis advanced by Losin et al. (2008) regarding a specific neuropsychological substrate for the production of emotional and intentional signals. To assess hemispheric specialization for expressions, the physical feature of each hemimouth area was calculated and statistics were performed on the entire set of data.
2. Results
First, we performed an independent samples t-test to control the consistency of the FAI measures from the sample of chimpanzees of Losin et al. (2008) and the new data set for each expression category. No significant difference between the two samples was noticed for the raspberry: t(62) = −1.22, p = .23; the extended food grunt: t(12) = −1.66, p = .12; the food-bark: t(55) = −0.12, p = .90 and the pant-hoot: t(51) = 1, p = .32. Thus, because the FAI values of the two samples of data were nearly identical, we subsequently used the combined sample for the following analyses.
2.1. Hemispheric specialization for atypical sounds expressions
For the raspberry, according to the individual Asymmetry Quotient (AQ), 35 individuals showed a right- and 15 a left-hemimouth asymmetry. Only 6 subjects did not show oro-facial biases. This distribution differed significantly from chance based on a chi-square goodness-of-fit test, χ2 (2, N = 55) = 23.61; p < .001. A one-sample t-test on the mean FAI (M.FAI = .06, SD = .18) showed a significant rightward asymmetry for the raspberry (t(55) = 2.27; p = .025). For the kiss; (Nright = 6, Nleft = 5, Nno bias = 0) and the extended grunt (Nright = 7, Nleft = 5, Nno bias = 2), the distributions of oro-facial preferences did not significantly differ from chance based on a chi-square goodness-of-fit test, χ2 (2, N = 10) = 5.64; p = .06, χ2 (2, N = 13) = 2.71; p = .26, respectively. According to an ANOVA on the FAIs, no significant effect of sex was found for the raspberry (F(1, 54) = 0.35, p = .55), the extended grunt (F(1, 12) = 3.54, p = .08) and the kiss (F(1, 9) = 0.15, p = .71). According to Spearman’s rank correlation, no significant associations were found between age and the FAI measures: (rs(54) = −0.04, p = .74; rs(12) = 0.09, p = .76; rs(9) = −0.17, p = .61, respectively).
2.2. Hemispheric specialization for species-typical vocalizations
From the 57 individuals of which food-bark vocalizations were observed, the majority of subjects (N = 34) showed a left-side bias, fewer individuals (N = 15) showed a rightward asymmetry. Only eight individuals did not exhibit oro-facial biases. This distribution differed significantly from chance based on a chi-square goodness-of- fit test, χ2 (2, N = 57) = 19.05, p < .001). A one-sample t-test on the M.FAI of the subjects (M.FAI = −.05, SD = .15) indicated a significant degree of oro-facial bias at a group-level toward the left-side of the mouth (t(56) = −2.5; p = .01). For the pant-hoot, 27 individuals showed an oro-facial asymmetry toward the left-side of the mouth whereas18 showed a right-side asymmetry. Six individuals exhibited no asymmetry. This distribution differed significantly from chance based on a chi-square goodness-of-fit test, χ2 (2, N = 50) = 13.06; p < .001. However, the degree of oro-facial asymmetry of the subjects (M.FAI = −.015, SD = .25) was not significant according to a one sample t-test: t(50) = −0.42; p = .68. This absence of significant bias could be explained by the high variability in the degree of hemiface asymmetry between subjects. No influence of sex on the mean FAIs was found for the food-bark (F(2, 54) = 0.89, p = .42) and for the pant-hoot (F(1, 49) = 1.15, p = .29). Finally, no influence of age on the mean FAIs was found for the food-bark and the pant-hoot (respectively: rs(54) = 0.11, p = .4 and rs(49) = −0.009, p = .95).
3. Discussion
Our aim with this study was to test the validity of hemispheric specialization with regards to the functionality of vocalizations and facial expressions reported by Losin et al. (2008). Consistent patterns of lateralization were found for each category of vocalization between the two populations of chimpanzees investigated separately in the previous and present studies. When combining the two samples of subjects, a significant oro-facial bias toward the right-side of the mouth consistently occurred when chimpanzees produced raspberry sounds, and a significant leftward oro-facial asymmetry for the production of food-barks vocalizations (see Fig. 1). The current study confirms previous findings that differential cerebral processing occurs according to the function of the expression, namely, a left hemispheric dominance for the production of learned attention-getting sounds, and right hemispheric dominance for the production of species-typical vocalizations both at a group-level. Regarding the theory of the involvement of the right hemisphere for emotion processing (Borod et al., 1997), this latter finding is consistent with the view that the production of species-specific vocalizations might be related to a specific emotional state that involved a right-hemispheric dominance. Nevertheless, given that the species-specific vocalizations tested in the present study seem rather related to a positive context (presence of food) than to a negative context, no data are available so far for discussing the “valence theory” for emotions (Davidson, 1995). In other words, we cannot evaluate whether the variation of emotions (negative versus positive emotions) in vocal productions drives the direction of the hemispheric specialization (i.e., right or left hemisphere dominance, respectively) in chimpanzees.
Fig. 1.
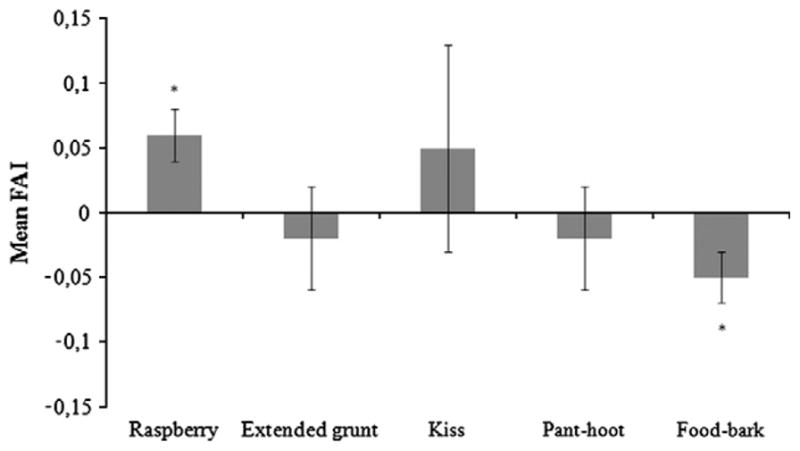
Oro-facial asymmetries for species-typical vocalizations and attentiongetting sounds. Means FAI for attention-getting sounds: raspberry (N = 56), extended grunt (N = 14) and kiss (N = 11) and species-typical vocalizations: panthoot (N = 55) and food-bark (N = 57) with SE. Negative values correspond to left hemiface asymmetries and positive scores indicate right ones. One sample t-test result: *p < .05.
Consistent rightward behavioral oro-facial asymmetry results during the production of learned atypical vocalizations support the interpretation by Losin et al. (2008) of greater left hemispheric involvement in these behaviors. In regards to the prevalence in which captive chimpanzees use learned vocalizations, Hopkins, Taglialatela, and Leavens (2011) reported that the raspberry vocalization was most often produced by contrast to the others attention-getting sounds. The authors suggest that the raspberry sound may be easier to produce than the other sounds. It is important to note that for the raspberry, a non-voiced sound signal, a chimpanzee only needs to learn novel oro-facial muscular movements for expelling air through the lips. In contrast, the production of extended grunt requires to learn also controlling the vocal apparatus including the vocal tract in order to produce a voiced sound. Recent comparative stereology and cytoarchitecture studies have demonstrated that chimpanzees have a greater potential for neural control of facial expressions in comparison with monkeys. Both chimpanzees and humans exhibited larger volumes of facial nuclei (Sherwood et al., 2005) and a wider Layer III in the face area of the primary motor cortex (Sherwood et al., 2003) in comparison to other nonhuman primates. Research on the cortical control of oro-facial movements is limited, although electrical stimulation of Brodmann’s area 44 in the frontal cortex has been shown to induce oro-facial movements in monkeys (Petrides, Cadoret, & Mackey, 2005). Thus, further investigation on the cortical substrate underlying the oro-facial system in nonhuman primates including the production of attention-getting sounds in chimpanzees are needed for better understanding the evolution of the control of oro-facial movements involved in spoken language.
Within this theoretical framework about the origins of language, in contrast with the “vocal origin of language” hypothesis (e.g., Snowdon, 2001; Zuberbühler, 2005), an alternative theory claims that language first evolved from manual communicative gestures (e.g., Corballis, 2002; Meguerditchian & Vauclair, 2008; Vauclair, 2004). For instance, findings from several studies that reported predominance of right-handedness for gestural communication in captive chimpanzees and in baboons have suggested a left cerebral specialization (Hopkins et al., 2005; Meguerditchian, Molesti, & Vauclair, 2011; Meguerditchian, Vauclair, & Hopkins, 2010). Furthermore, anatomical brain imaging studies have reported that right-handed chimpanzees for manual communicative gestures had greater leftward neuroanatomical asymmetries for the inferior frontal gyrus (IFG) and the planum temporale (PT) compared to left-handed subjects (Meguerditchian et al., 2012; Taglialatela, Cantalupo, & Hopkins, 2006). Given that the IFG and PT are known to overlap with Wernicke’s and Broca’s areas in humans, these findings suggested that the cerebral homologues of language areas in humans could be involved during gestural communication in chimpanzee.
Recently, research has reported that a subpopulation of captive chimpanzees produced manual gestures in conjunction with learned attention-getting sounds as a function of the human experimenter’s attentional state (Hostetter et al., 2007; Leavens, Hostetter, Wesley, & Hopkins, 2004). Interestingly, this subpopulation was observed to have an increased right hand preference for manual gestures when they were produced simultaneously with attention- getting vocalizations (Hopkins et al., 2005; Hostetter et al., 2007; Leavens & Hopkins, 1998; Leavens et al., 2004), indicating that both the oro-facial and gestural system might share the same left-hemispheric specialization. Moreover, using positron emission tomography (PET) studies reported an activation of the homologue of Broca’s area in the left hemisphere during the production of manual gestures used concurrently with learned atypical sounds in order to capture the attention of an experimenter (Taglialatela, Russell, Schaeffer, & Hopkins, 2008; Taglialatela, Russell, Schaeffer, & Hopkins, 2011). The authors assert that activation of cortical and subcortical areas during communicative signals is reminiscent of the neural mechanisms involved in the production of human language (Lieberman, 2007). These collective findings including the confirmation of the left hemisphere involvement for attention-getting sounds in the present study reappraised the “gestural origin of language” hypothesis. Both oro-facial and gestural communication in chimpanzees seems to share the same attention- getting functions and left-hemispheric lateralization and might be thus ultimately integrated into a single communicative system lateralized in the left-hemisphere. These neuropsychological correlates in chimpanzees provide additional supports to the “bimodal origin of language hypothesis” (McNeill, 1992).
In brief, our results demonstrate that hemispheric specialization is related to the function of the vocalization. Right hemispheric specialization was evident for species-typical vocalizations, which reinforces the idea that they are used in an emotional context. By contrast, a right hemiface asymmetry was found for atypical sounds suggesting a specific left-lateralized pattern for these communicative signals. The review of indirect behavioral and direct functional assays on hemispheric specialization of communicative signals allow us to suggest that in the course of the origin of language, voluntary oro-facial and gestural communicative signals might have coevolved into a single integrated communicative system in a common hominid ancestor.
4. Method
4.1. Subjects
The new cohort included 431 still images from 42 chimpanzees housed at the Michale E. Keeling Center for Comparative Medicine and Research (KCCMR) of The University of Texas MD Anderson Cancer Center in Bastrop, TX. The previous sample of data from Losin et al. (2008) was 267 still images from 69 chimpanzees housed at the Yerkes National Primate Research Center (YNPRC) in Atlanta, GA. For this current study, the both sample were combined and 698 still images from 111 individuals were used for analysis. The number of still images per subject ranged from 1 to 39 pictures (M = 6.29, SD = 6). For the learned sounds, 319 still images from 56 individuals producing the raspberry, 37 still images from 14 individuals producing the extended grunt, and 67 images from 11 individuals producing the kiss were collected. For species typical vocalizations, 160 still images from 57 individuals producing the food-bark, and 115 still images from 55 individuals producing the pant-hoot were included. The age range of the sample was 5–44 years (M = 20.7, SD = 9.8). Images from the YNPRC subjects were collected prior to 2008 and images from the KCCMR subjects were recorded between May 2008 and April 2009.
4.2. Behaviors of interest
The raspberry sound is a non-voiced, bilabial fricative in which the individual purses their lips and expels air out through these closed lips in a sputtering fashion. The kiss is produced in a manner similar to the raspberry, except that it requires the subject to purse or pucker their lips and inhale air. Finally, the extended grunt is a low frequency, broadband and atonal voiced sound. Concerning the species-typical vocalizations, two emotional valences were observed. The food-bark, a positively-valenced emotional behavior, is a repeated and often high-pitched bark produced by expelling air from the lungs with the lips slightly parted and mouth corners withdrawn. Finally, the pant-hoot, a negatively-valenced emotional behavior, consists of alternating “hoo” vocalizations, produced with forward-protruding rounded lips, and voiced inhalations, during which the mouth is open widest.
4.3. Apparatus and image analysis
Losin et al. (2008) provides an in depth description of the specifications of the camcorder used to record video sequences and of the video software used to capture still images. The contexts of video recording behavioral sequences were also described in Losin et al. (2008) as well as the entire procedural steps to assess oro-facial asymmetries.
4.4. Data analysis
Firstly, the direction of hemiface lateralization was determined for each subject in order to classify an individual with left, right or no hemispherical specialization for each behavior. A cut point value named Asymmetry Quotient (AQ), traditionally applied in the neuroanatomical literature for humans and apes (Cantalupo, Pilcher, & Hopkins, 2003) was used. It is based on the same formula used in the literature dealing with emotional oro-facial asymmetries. Individuals with an AQ score greater than .025 or less than −.025 showed respectively right- or left-hemimouth asymmetry. All other subjects were categorized as exhibiting no bias. Second, a Facial Asymmetry Index (FAI) indicating the degree of oro-facial lateralization for a given individual was computed based on the formula (R −L)/(R + L) where the R and the L were the values of the Right and Left hemimouth area measures. FAI values varied on a continuum from −.1 to 1, where negative values correspond to a left asymmetry and positive values to a right asymmetry.
In accordance with Losin et al.’s (2008) procedure, we controlled for the potential influence of orientation face toward the camera on the FAIs results. No asymmetry was observed after combining the entire data set for each behavior (raspberry: t(54) = 0.84, p = .4; extended grunt: t(12) = −0.8, p = .43; kiss: t(9) = 1.19, p = .25; food-bark: t(56) = 0.03, p = .98; pant-hoot: t(50) = 1.05, p = .3). A score of inter-rater reliability also was calculated in the previous study indicating a good rate of agreement between the experimenter and a naïve rater (Losin et al., 2008). To avoid possible variability in the measurement method, the same experimenter assessed the new set of data.
Acknowledgments
This research was supported by a French National Research Agency (ANR) grant, reference ANR-08-BLAN-0011_01. The chimpanzees at the KCCMR were supported by NIH-RR-15090 and additional funding was provided by NIH grants NS-42867, NS-73134, HD-56232 and HD-60563. The writing of this paper was made possible by a fellowship from the Medical Research Foundation to C. W.
References
- Arbib MA. From monkey-like action recognition to human language: An evolutionary framework for neurolinguistics. Behavioral and Brain Sciences. 2005;28(2):105–124. doi: 10.1017/s0140525x05000038. [DOI] [PubMed] [Google Scholar]
- Borod JC, Haywood CS, Koff E. Neuropsychological aspects of facial asymmetry during emotional expression: A review of the normal adult literature. Neuropsychology Review. 1997;7(1):41–60. doi: 10.1007/BF02876972. [DOI] [PubMed] [Google Scholar]
- Broca P. Sur le siège de la faculté du langage articulé. Bulletins de la Société d’Anthropologie de Paris. 1865;6(1):377–393. [Google Scholar]
- Cantalupo C, Pilcher DL, Hopkins WD. Are planum temporale and sylvian fissure asymmetries directly related? A MRI study in great apes. Neuropsychologia. 2003;41(14):1975–1981. doi: 10.1016/s0028-3932(02)00288-9. [DOI] [PubMed] [Google Scholar]
- Corballis M. From mouth to hand: The origins of language. Princeton: Princeton University Press; 2002. [Google Scholar]
- Crow T. Directional asymmetry is the key to the origin of modern Homo sapiens (the Broca-Annett axiom): A reply to Rogers’ review of the speciation of modern homo sapiens. Laterality. 2004;9(2):233–242. http://dx.doi.org/10.1080/13576500342000374. [Google Scholar]
- Davidson RJ. Cerebral asymmetry, emotion, and affective style. In: Hugdahl K, editor. Brain asymmetry. Cambridge, MA: MIT Press; 1995. pp. 361–387. [Google Scholar]
- Fernández-Carriba S, Loeches Á, Morcillo A, Hopkins WD. Asymmetry in facial expression of emotions by chimpanzees. Neuropsychologia. 2002;40(9):1523–1533. doi: 10.1016/s0028-3932(02)00028-3. http://dx.doi.org/10.1016/S0028-3932(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Goodall J. The chimpanzees of Gombe: Patterns of behavior. Cambridge, MA: Harvard University Press; 1986. [Google Scholar]
- Graves R, Landis T. Asymmetry in mouth opening during different speech tasks. International Journal of Psychology. 1990;25(2):179–189. [Google Scholar]
- Hauser MD. Right hemisphere dominance for the production of facial expression in monkeys. Science. 1993;261(5120):475–477. doi: 10.1126/science.8332914. http://dx.doi.org/10.1126/science.8332914. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Akre K. Asymmetries in the timing of facial and vocal expressions by rhesus monkeys: Implications for hemispheric specialization. Animal Behaviour. 2001;61(2):391–400. http://dx.doi.org/10.1006/anbe.2000.1588. [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hook-Costigan M, Rogers L. Lateralized use of the mouth in production of vocalizations by marmosets. Neuropsychologia. 1998;36(12):1265–1273. doi: 10.1016/s0028-3932(98)00037-2. http://dx.doi.org/10.1016/S0028-3932(98)00037-2. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. The evolution of hemispheric specialization in primates. Vol. 5. London: Elsevier/Academic Press; 2007. [Google Scholar]
- Hopkins WD, Russell J, Freeman H, Buehler N, Reynolds E, Schapiro SJ. The distribution and development of handedness for manual gestures in captive chimpanzees (Pan troglodytes) Psychological Science. 2005;16(6):487–493. doi: 10.1111/j.0956-7976.2005.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Taglialatela JP, Leavens DA. Chimpanzees differentially produce novel vocalizations to capture the attention of a human. Animal Behaviour. 2007;73(2):281–286. doi: 10.1016/j.anbehav.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Taglialatela JP, Leavens DA. Do Chimpanzees have voluntary control of their facial expressions and vocalizations? In: Vilain A, Abry C, Schwartz JC, Vaucair J, editors. Primate communication and human language: Vocalisation, gestures, imitation and deixis in humans and non-humans. Vol. 1. Amsterdam: John Benjamins Publishing Company; 2011. pp. 71–88. [Google Scholar]
- Hopkins WD, Vauclair J. Evolution of behavioral and brain asymmetries in primates. In: Tallerman M, Gibson K, editors. Handbook of language evolution. Oxford: Oxford University Press; 2012. pp. 184–197. [Google Scholar]
- Hostetter AB, Russell JL, Freeman H, Hopkins WD. Now you see me, now you don’t: Evidence that chimpanzees understand the role of the eyes in attention. Animal Cognition. 2007;10(1):55–62. doi: 10.1007/s10071-006-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The neural correlates of language production. In: Gazzaniga M, editor. The new cognitive neurosciences. Cambridge, Mass: MIT Press; 2000. pp. 845–865. [Google Scholar]
- Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD. Intentional communication by chimpanzees: A cross-sectional study of the use of referential gestures. Developmental Psychology. 1998;34(5):813–822. doi: 10.1037//0012-1649.34.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Hostetter AB, Wesley MJ, Hopkins WD. Tactical use of unimodal and bimodal communication by chimpanzees, Pan troglodytes. Animal Behaviour. 2004;67(3):467–476. [Google Scholar]
- Lieberman P. Speech evolution: Let barking dogs sleep. Behavioral and Brain Sciences. 1998;21(04):520–521. [Google Scholar]
- Lieberman P. The evolution of human speech. Current Anthropology. 2007;48(1):39–66. [Google Scholar]
- Losin EA, Russell J, Freeman H, Meguerditchian A, Hopkins WD, Fitch T. Left hemisphere specialization for oro-facial movements of learned vocal signals by captive chimpanzees. PloS One. 2008;3(6):e2529. doi: 10.1371/journal.pone.0002529. http://dx.doi.org/10.1371/journal.pone.0002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeilage PF, Rogers LJ, Vallortigara G. Origins of the left & right brain. Scientific American. 2009;301(1):60–67. doi: 10.1038/scientificamerican0709-60. [DOI] [PubMed] [Google Scholar]
- Marshall AJ, Wrangham RW, Arcadi AC. Does learning affect the structure of vocalizations in chimpanzees? Animal Behaviour. 1999;58(4):825–830. doi: 10.1006/anbe.1999.1219. [DOI] [PubMed] [Google Scholar]
- McNeill D. Hand and mind. Chicago: University of Chicago Press; 1992. [Google Scholar]
- Meguerditchian A, Gardner MJ, Schapiro SJ, Hopkins WD. The sound of one-hand clapping: Handedness and perisylvian neural correlates of a communicative gesture in chimpanzees. Proceedings of the Royal Society Biology. 2012;279:1959–1966. doi: 10.1098/rspb.2011.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguerditchian A, Molesti S, Vauclair J. Right-handedness predominance in 162 baboons for gestural communication: Consistency across time and groups. Behavioral Neuroscience. 2011;125(4):653–660. doi: 10.1037/a0023823. [DOI] [PubMed] [Google Scholar]
- Meguerditchian A, Vauclair J, Hopkins WD. Captive chimpanzees use their right hand to communicate with each other: Implications for the origin of the cerebral substrate for language. Cortex. 2010;46(1):40–48. doi: 10.1016/j.cortex.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguerditchian A, Vauclair J. Vocal and gestural communication in nonhuman primates and the question of the origin of language. In: Roska-Hardy LS, Neumann-Held EM, editors. Learning from animals? Examining the nature of human uniqueness. London: Psychology Press; 2008. pp. 61–85. [Google Scholar]
- Petrides M, Cadoret G, Mackey S. Orofacial somatomotor responses in the macaque monkey homologue of Broca’s area. Nature. 2005;435(7046):1235–1238. doi: 10.1038/nature03628. [DOI] [PubMed] [Google Scholar]
- Pollick AS, de Waal FBM. Ape gestures and language evolution. Proceedings of the National Academy of Sciences (USA) 2007;104:8184–8189. doi: 10.1073/pnas.0702624104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L, Andrew R, editors. Comparative vertebrate lateralization. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Seyfarth RM, Cheney DL. Meaning and emotion in animal vocalizations. Annals of the New York Academy of Sciences. 2003;1000(1):32–55. doi: 10.1196/annals.1280.004. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Hof PR, Holloway RL, Semendeferi K, Gannon PJ, Frahm HD, et al. Evolution of the brainstem orofacial motor system in primates: A comparative study of trigeminal, facial, and hypoglossal nuclei. Journal of Human Evolution. 2005;48(1):45–84. doi: 10.1016/j.jhevol.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Holloway RL, Gannon PJ, Semendeferi K, Erwin JM, Zilles K, et al. Neuroanatomical basis of facial expression in monkeys, apes, and humans. Annals of the New York Academy of Sciences. 2003;1000(1):99–103. doi: 10.1196/annals.1280.021. [DOI] [PubMed] [Google Scholar]
- Snowdon CT. From primate communication to human language. In: deWaal FBM, editor. Tree of origin: What primate behavior can tell us about human social evolution. Cambridge, MA: Harvard University Press; 2001. pp. 193–227. [Google Scholar]
- Taglialatela JP, Cantalupo C, Hopkins WD. Gesture handedness predicts asymmetry in the chimpanzee inferior frontal gyrus. NeuroReport. 2006;17(9):923. doi: 10.1097/01.wnr.0000221835.26093.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela JP, Reamer L, Schapiro SJ, Hopkins WD. Social learning of a communicative signal in captive chimpanzees. Biology Letters. doi: 10.1098/rsbl.2012.0113. in press. http://dx.doi.org/10.1098/rsbl.2012.0113. [DOI] [PMC free article] [PubMed]
- Taglialatela JP, Russell JL, Schaeffer JA, Hopkins WD. Communicative signaling activates Broca’s’ homolog in chimpanzees. Current Biology. 2008;18(5):343–348. doi: 10.1016/j.cub.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela JP, Russell JL, Schaeffer JA, Hopkins WD. Chimpanzee vocal signaling points to a multimodal origin of human language. PloS One. 2011;6(4):e18852. doi: 10.1371/journal.pone.0018852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallortigara G, Chiandetti C, Sovrano VA. Brain asymmetry (animal) Wiley Interdisciplinary Reviews: Cognitive Science. 2011;2(2):146–157. doi: 10.1002/wcs.100. http://dx.doi.org/10.1002/wcs.100. [DOI] [PubMed] [Google Scholar]
- Vauclair J. Would humans without language be apes? In: Toomela A, editor. Cultural guidance in the development of the human mind. Vol. 7. Greenwich, CT: Ablex Publishing Corporation; 2003. pp. 9–26. [Google Scholar]
- Vauclair J. Lateralization of communicative signals in nonhuman primates and the hypothesis of the gestural origin of language. Interaction Studies. 2004;5(3):365–386. [Google Scholar]
- Vauclair J, Fagot J, Dépy D. Nonhuman primates as models of hemispheric specialization. In: Haug M, Whalen RE, editors. Animal models of human emotion and cognition. New York: APA Books; 1999. pp. 247–256. [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, et al. Meta-analysing left hemisphere language areas: Phonology, semantics, and sentence processing. NeuroImage. 2006;30(4):1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wallez C, Vauclair J. Right hemisphere dominance for emotion processing in baboons. Brain and Cognition. 2011;75(2):164–169. doi: 10.1016/j.bandc.2010.11.004. http://dx.doi.org/10.1016/j.bandc.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Der aphasische symptomen-complex: Eine psychologische studie auf anatomischer basis. Breslau: Cohn & Weigert; 1874. [Google Scholar]
- Zuberbühler K. The phylogenetic roots of language. Evidence from primate communication and cognition. Psychological Science. 2005;14(3):126–130. [Google Scholar]


