Summary
The goal of quantitative analysis of computed tomography (CT) scans is to understand the anatomic structure that is responsible for the physiological function of the lung. While the gold standard for structural analysis requires the examination of tissue this is not practical in most studies. Quantitative CT allows a method to obtain valuable information on lung structure without having to remove tissue from the body thereby allowing longitudinal studies of chronic lung diseases. This review briefly discusses CT analysis of the lung and some of the sources of variation that can cause differences in the CT metrics used for analysis of lung disease. While there are many sources of variation the purpose of this review will show that if the study is properly designed to take into account these variations and the CT scanner is properly calibrated valuable information can be obtained from CT scans that should allow us to study the pathogenesis of lung disease and the effect of treatment.
Keywords: Quantitative CT, COPD, Asthma
Introduction
It is well understood that the underlying structure of the lung is a key determinant of the function of the lung. For example, changes in airway lumen diameter result in changes in the airflow pattern within the lung, usually measured as a reduction in forced expiratory flow. Moreover, changes to the structure of the lung parenchyma can affect the elastic properties of the lung thereby influencing lung function. What we know about the lung structure and its relation to function has been described to us through careful and detailed studies of anatomy. Historically, these data have been collected from resected and postmortem specimens and even today, the gold standard for lung structural analysis is the actual physical specimen. Examination of pathologic and histologic specimens is important but it has severe limitations as well. Obviously, the acquisition of tissue is invasive which limits the amount of tissue available (e.g. biopsies only from living subjects), where the tissue can be acquired from (e.g. bronchoscopic biopsies from larger airways), when the tissue can be acquired (e.g. resection for severe disease) and the amount of tissue that is usually available for quantification.
The advent of the computed tomography (CT) scanner in the 1980s revolutionized the way that lung anatomists study the lung. CT scans allowed the acquisition of images of the lung that were very similar in appearance to gross pathology but did not require the removal of tissue from the body and could, therefore, be obtained from living humans. Moreover, possibly one of the greatest advantages of CT scanner is that it not only obtains “pictures” of the lung but that these images are densitometry maps that provide useful information on the amount of tissue and air within the lung since the Hounsfield Unit (HU) scale is linearly correlated with gravimetric density within the biological range. In a series of papers using the Dynamic Spatial Reconstructor, an early x-ray tomographic device, Hoffman and colleagues showed that the volume of the lung could be accurately measured in living animals 1. Furthermore, these same investigators showed that the density of the lung could also be measured, at different lung volumes and body orientations to give some indication on how the lung density changed during inspiration 2. Other investigators showed that at suspended inspiration a density gradient could be measured within the gravity plane that compared very favourably to pleural pressure gradients that had been described using inhaled radioactive Xenon 3,4. Importantly, these and other studies have shown that as disease develops the density of the lung changes and that these density changes are related to lung function 5,6. Therefore, the superior resolution of CT images and the ability to obtain quantitative information helped propel CT densitometry analysis into the mainstream such that CT became a popular and central part of many lung structure/function studies in health and disease. Unfortunately, as these studies became more widespread it was realized that there were large sources of variation in the CT measurements of lung densitometry and anatomy such that data from different centers and studies is often not comparable 7. It is beyond the scope of this review to comment on all the sources of variation that can occur in studies of lung densitometry and it will not go into great detail on the physics behind the differences but what this document wishes to comment on is some of the major sources of variation in CT analysis which occur commonly in studies today. There are two main arms to CT analysis of lung structure; those focusing on the lung parenchyma which is usually measured using densitometry and those measuring the airway wall and lumen dimensions which involve complicated segmentation and measurement algorithms.
Lung Parenchyma
As mentioned briefly above, CT is excellent at measuring lung volume and density. However, probably the most widely used measurement of chronic lung disease is a measurement of low attenuation regions of the lung which correlate with pathologic measurements of emphysema. Two separate groups of investigators examined the x-ray attenuation values within a CT scan and compared threshold, or cut-off values to pathologic and histologic measurements of emphysema 8,9. One of these studies by Hayhurst and colleagues examined the entire frequency distribution curve and showed that by a cut-off value at the lowest 5th percentile point of the distribution curve was correlated with the extent of emphysema observed on resected specimens 8. They further refined and developed this percentile point by showing that it was correlated with airspace enlargement on histologic preparations of the resected tissue 10. In a similar study Muller and Miller examined various threshold values along the x-ray attenuation value scale and found that, using conventional axial images and 2mm slice thickness, -910 HU correlated with emphysematous holes greater than 5mm in diameter on gross pathology 9,11. There were other studies using CT of the lung around the same time, but it was really these two groups of studies that captured the imagination of investigators interested in chronic obstructive pulmonary disease and are mainly responsible for the use of CT in lung research today.
However, as CT scanners proliferated in the market more researchers began to examine CT scan densitometry data under varying conditions and they started to report differences in quantification that were dependant on factors that were either physiologically based or scanner based. An example of a scanner based variation was the development of “high resolution” (HRCT), thin slice (1mm) and high spatial frequency reconstruction algorithms. In a series of papers Gevenois performed similar studies to Muller and Miller and related numerous HU thresholds to both gross and microscopic measurements of emphysema 12,13. These studies showed that the best correlation with emphysema was the -950 HU threshold 12. These data are important because they show that measurements of low attenuation regions are dependent on the CT scan parameters and that the threshold value is really an arbitrary value and needs validation with pathology.
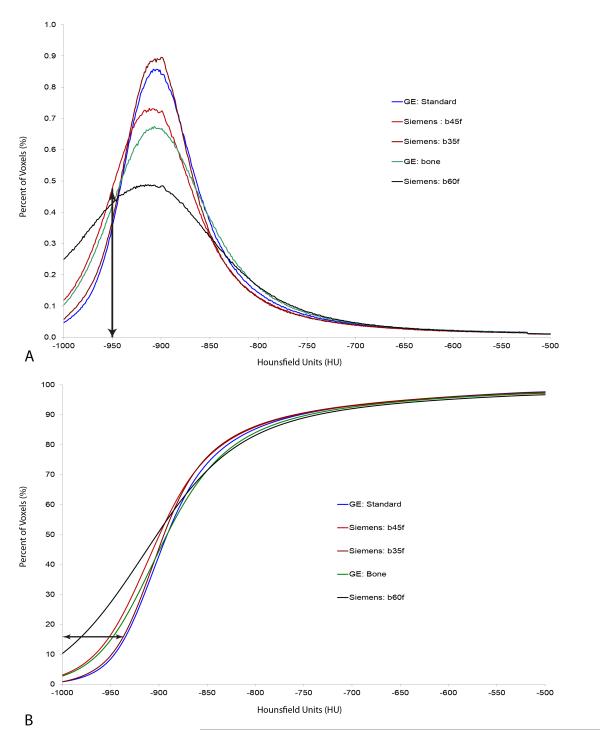
Other investigators have detailed numerous other sources of variation in density measurements including CT slice thickness, reconstruction kernel and radiation dose 14-16. In an early paper, Kemerinck shows that a thicker CT slice and a low spatial frequency reconstruction algorithm have the best density resolution of a foam phantom 17. While the mean density measured in the CT scan did not change in this study, the distribution curves of the two foams became sufficiently broadened so that it was no longer possible to separate the distinct foams from each other and the differences became even more problematic for human subjects 17. In this study one can clearly see that as the density resolution decrease the frequency distribution curve becomes broad enough that it will cause large changes in the measurements of low attenuating regions (emphysema) because both HU threshold and percentile cut-off are made at the extreme of the distribution curve. Boedeker described the effect of reconstruction kernel on low attenuation regions showing that the apparent extent of emphysema would increase as a sharper reconstruction kernel was used 15. This is also shown in Figure 1 where different reconstruction kernels from two different CT scanners result in quite different frequency distribution curves. Here is it can clearly be seen that the “extent of emphysema” will really depend on the kernel chosen to reconstruct the images. In another recent study, Madani et al showed that the measured extent of emphysema changes with slice thickness, with thinner slices demonstrating higher measured “emphysema” extent 14. While thicker slices probably give better density resolution, the advent of multi-slice CT scanners has almost completely obliterated the use of thicker slices. These new scanners make it so easy to obtain thin slice images of the thorax that they are pretty much the standard in CT studies today. While there may be drawbacks to using thicker slices they have their advantages as well because it is now possible to measure the lung parenchyma and airways on the same CT study (see below for sources of variation in airway measurements). However, while thin slices are the new standard, most investigators still use an lower spatial frequency reconstruction algorithm to try and keep image noise to a minimum, while still allowing good visualization of the anatomic features and edge detection for airway analysis.
Figure 1.
This figure shows some representative frequency distribution curves (A) and cumulative distribution curves (B) for five different reconstruction algorithms from two CT manufacturers from a single subject (the GE scans were acquired 9 months before the Siemens scans). The vertical arrow in panel A shows the -950 HU threshold cut-off for low attenuation analysis of emphysema. The horizontal arrow in panel B shows the 15th percentile cut-off analysis for emphysema. Both of these panels show that the reconstruction algorithm can make significant differences in the “extent of emphysema” measured.
Recently there has been a significant push to reduce the dose at which CT scans are obtained. Numerous studies have looked at the reduced dose CT scans and found that the noise within the image increases, which while not having a large effect on mean lung density does have an effect on the measurement of low attenuation values. Yuan et al showed that the percentage of the lung occupied by low attenuation regions and the mean value of the lowest 15th percentile were both affected by CT dose with the lower dose scans producing “more emphysema” even though there were only minor changes in mean lung density 16. Figure 2 shows images obtained in Yuan study and here it can be seen that higher dose CT scans have much less visible noise in the images and the frequency distribution curve is much less broad for the higher dose CT scans. In the study mentioned above on slice thickness, Madani also showed more emphysema in lower dose CT scans although the difference between low and high dose scans was minimal when thicker slices were used 14. Once again these studies show that measurements of emphysema are very susceptible to noise in the CT image and any procedure that increases image noise will increase the low attenuation regions in the lung.
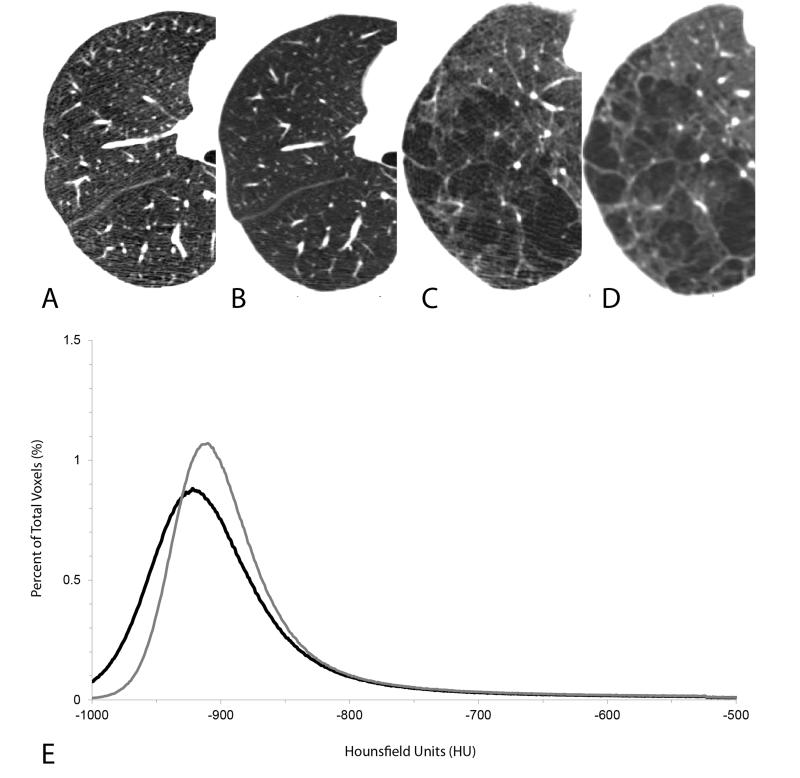
Figure 2.
This figure shows CT scans from two subjects scanned using a low dose CT scan (40 mAs) (panels A and C) and a clinical CT dose (120 mAs) (panels B and D). The increased noise in the low dose CT scans (A and C) can be seen as linear “streaks” or a more “mottled” image while the clinical dose CT scans show (B and D) show a “smoother” image. Panel E shows the frequency distribution of x-ray attenuation values from one subject and it can be seen that there is a difference in the extent of emphysema measured using either the threshold or percentile analysis (solid gray line ( ) clinical dose CT scan, solid black line ( ) low dose CT scan).
There have been several studies performed on the effect that CT scanner manufacturer has on the measurements of emphysema 16,18-20. This is extremely important because of the number of large, multi-center studies that are taking place today. Some studies have shown that while many CT scanners are comparable 16,18 there can be significant differences in lung density measurements between scanners 18. Importantly, other studies have shown that even within a specific CT scanner there can be changes in the lung density measurement over time, most commonly due to incorrect calibration or changes in the x-ray tube 19,21,22. Recently using the sites of the COPDGene study, Sieren et al showed that most scanners obtained lung density measurements that were comparable with each other and over time but they noticed a few CT scanners that had significant temporal variations in their density measurements 19. This is similar to a previous study of Parr et al 21 which documented the same finding in a group of subjects and stresses the point that the precision of CT scanners cannot be taken for granted and scanners must be properly calibrated and tested over time to make sure that they are obtaining density measurements that are consistent and comparable 19,21,22. Also in the COPDGene study Zach and colleagues document differences in lung density in normal subjects between different scanners 20. They report that if they standardize for the CT attenuation values of tracheal air these differences are lessened and as part of a multivariate analysis do not reach significance. Once again these studies underline the fact that scanners, like any measuring device, need to be carefully observed and calibrated on a regular basis.
There have been numerous changes to CT scanners over the years as technology has pushed the field further. One of the most important changes has been the multi-detector row CT scanners. These new multi-slice CT scanners can acquire thin slice CT images of the thorax in well under 10 seconds but have introduced a new source of variation all their own. In a recent study Madani repeated earlier studies that compared quantitative pathology to CT emphysema measurements, both threshold and percentile cut-offs 23. They report that the threshold with the best correlation to pathology is -960 HU and the best percentile cutoff is the lowest 1st percentile 23. These are very nice studies and show how scanners have changed over the years and it is important to continually evaluate how you obtain measurements of low attenuation or emphysema. However, even though these data show that the -960 HU threshold may be a more precise threshold to assess emphysema, it has not gained traction in the literature. This failure is possibly due to the fact that the most common threshold in the literature now is the -950 HU cut-off and, while significantly different from -960 HU, does not seem to be different enough in the minds of investigators to change their studies. Moreover, the lowest 15th percentile is still the most commonly used percentile cut-off because investigators have shown that while the 15th percentile may not have the best correlation with pathology, it is a robust and reproducible value that provides valuable information across both time and different centers 24. Obviously, any threshold used is really just a “hallmark” of disease so consistency is the best approach. It appears that as long as scanners are properly calibrated and the images are acquired in a consistent and reproducible manner the data are useful in assessing disease and disease progression.
Many of the other sources of variation in CT emphysema measurements are related to biological or physiological differences. Obviously the deeper the level of inspiration during the scan results in a lower mean lung density and more low attenuation regions because there is more gas volume compared to tissue volume. Figure 3 shows the same subject at suspended full inspiration and expiration. It can clearly be seen from these images that the volume of the lung is larger at full inspiration (as it should be) and there are more low attenuating regions. This feature has been exploited in the extreme situation to assess “gas trapping” by applying a threshold cut-off at end expiration 25-27. The rationale for this approach is that at a low lung volume, functional residual capacity or end expiration, the lung should be relatively dense lung because it does not contain a large volume of air. Therefore, low attenuation regions at this level are thought to assess gas trapping due to small airway disease 25-27. This technique is turning out to be very informative for chronic lung disease because it provides information on lung structure below the resolution of the CT scanner. However, what must be remembered using this technique is that it is not defined yet as to whether these expiratory CT scans are acquired at functional residual capacity or residual volume and that this technique requires a specific conscious maneuver by the subject to get to a low lung volume, which can be quite variable depending on subject compliance or training. Furthermore, studies that rely on CT scans obtained at the traditional full inspiration must always keep in mind that differences in lung volume will result in differences in lung density, and even relatively small changes in lung volume may affect measurements of emphysema. Madani has reported that in both control and COPD subjects the extent of emphysema changes with lung volume 28. Stoel et al have assessed changes in both the threshold analysis and percentile density analysis with lung volume and report that lung volume can have large effects on the measurements of emphysema, particularly the threshold analysis29. For this reason they argue that volume correction should be applied in longitudinal studies of COPD, something that the Alpha-1 foundation recommends as well 24,29,30.
Figure 3.
This figure shows three-dimensional reconstructions of CT scans from one subject obtained at end expiration (A) and suspended inspiration (B). The three-dimensional analysis of low attenuation regions using the -950 HU threshold analysis is shown in panels C and D. It can clearly be seen that the lung is larger at end inspiration (6032 mL) compared to end expiration (3988 mL) (B), particularly noticeable in the apical region and the diaphragmatic surface, and that there are more highlighted regions indicating more low attenuation regions (21% inspiration vs. 14% expiration) (D). This figure gives an indication of the difference that lung volume can make for quantitative densitometry analysis of the lung.
Another form of biological variation is smoking status. A recent study by Ashraf shows that subjects that quit smoking during a longitudinal CT study of emphysema have an increase in the emphysema measurement 31. They suggest this is possibly due to the reduction in soot or tar and/or inflammation within the lung due to smoking cessation. This is a very interesting finding and one that must be taken into account during longitudinal studies and some longitudinal studies are correcting for acute changes in smoking status 32.
Finally, one of the more recent advances in CT scanning has been the use of iterative reconstruction techniques. In the push to reduce dose to the patient scanner manufactures have been developing new techniques to improve image quality while keeping dose down. The analysis of lungs using these new iterative reconstructions is still in its infancy but because anything that changes the noise within the image will change the measurement of emphysema these techniques can have a large effect on the emphysema measurement. In a recent publication Mets et al has shown that iterative reconstruction techniques will “de-noise” the image and, therefore, change the emphysema measurement 33. Interestingly, while even the 15th percentile measurement was significantly different from the filtered back projection reconstruction, the difference was consistent across all levels of iterative-to-filtered back projection suggesting that while the values were different they were consistently different 33. There is still a lot of work that needs to be done in this area yet, but it is obvious that as iterative reconstruction techniques gain traction in the imaging field they will change the way that lung density is assessed.
Airways
Just like measurements of lung density, there are numerous factors that affect the measurement of airway dimensions using CT scans. Unlike lung densitometry measurements, the airway measurements are the most complicated and possibly the hardest to control across all studies. As has been recently reviewed, one of the biggest variants in airway measurements is the algorithm used to measure the airways 34,35. The measurement of airways does not rely on simple measurements of x-ray attenuation, but must use a complicated algorithm to segment the airway lumen and wall from the surrounding lung parenchyma. The detection and measurement of the airway lumen is relatively simple because of the contrast between tissue density (high) and air density (low) but the measurement of the airway wall is complicated by the ability of the airway algorithm to locate the external boundary of the airway wall when the density difference to the surrounding lung parenchyma and the bronchovascular bundle is not so different. To date there are many airway algorithms in use within the field and while many of them have their advantages and disadvantages there is no “gold standard” for analysis. For example, probably the most commonly used algorithm in the literature has been the full width at half maximum algorithm 34,35. This is the simplest algorithm to implement in most centers and most studies however; it has been clearly shown to have numerous problems 36. Airway measurement algorithm development is still an open area of research and it is beyond the scope of this review to comment on specific airway algorithms but it is important to remember that they will have a large effect on the airway measurements 35. Since there is so much variation in airway algorithms, what is probably the most important fact to remember is that for airway analysis studies the same algorithm should be used for all subjects across the entire study 35. This approach will allow a researcher to comment on airway wall dimensions in a given study but may also limit the comparability of results across studies that use widely different algorithms. Of course the largest source of variation in airway measurements will be the size of the airway and the resolution of the CT scanner 20,37. Studies have shown that small airways are the most interesting to measure but also have the most error in airway wall measurement 38. Therefore investigators have limited their analysis to larger airways 38 and have tried to assess small airway function using other parameters such as gas trapping on expiratory CT scans 25-27.
There are also numerous biological factors that affect airway measurements as well. Bakker et al has shown a correlation between lung volume and airway lumen area such that larger lung volumes cause the lumen of airways to be larger 39. Moreover, the recent study by Zach and colleagues, mentioned above, shows that there are multiple factor that affect airway measurements including many of the same parameters that affect lung density such as body mass index, lung volume and, not surprisingly, the field of view (voxel size) of the CT image 20.
Summary
In summary, CT analysis of the lung has become a standard in lung research. CT has become an important tool because it allows investigators to examine the structure of the lung that is responsible for lung function without having to remove tissue from the subject. This is extremely valuable for longitudinal studies that attempt to understand disease pathogenesis and response to therapeutics. Unfortunately, there are many sources of variation in the analysis of CT scans and researchers must take all of these into account very carefully. This review is not a comprehensive collection of the sources of variation but has tried to focus on some of the major ones that are described in the literature. Some of these are also summarized in Table 1. Even though there many sources of variation in quantitative analyses of CT scans they are still a powerful tool in lung research. The most important thing to remember is that the CT scan is a measuring device, and like all measuring devices it must be properly calibrated before use. It is for these reasons that more attention is being paid to the standardization of CT scanners and techniques and the reason that organizations such as the Radiological Society of North America have formed a Quantitative Imaging Biomarkers Alliance (QIBA) working group around CT in asthma and COPD. The goal of QIBA, in general, is to bring quantitative imaging into mainstream usage for studies of disease and intervention. However, QIBA realizes that for quantitative imaging biomarkers to be successful they must be rigorously standardized. Therefore, QIBA has established several working groups such as the asthma and COPD group to define the features necessary for the quantification of CT scans that make it easy to perform at all centers and that will make data comparable across studies. It is the belief of all investigators in this field that, when properly controlled, quantitative imaging can produce reliable and valuable data for the analysis of lung structure that can be used to study the pathogenesis of disease and the effect of therapeutic interventions.
Table 1.
This table lists some of the parameters that have been shown to have effects on lung densitometry, emphysema and airway measurements. The list is not exhaustive but some references are given as an example of the validation for each parameter. See the text for a more complete description of the factors listed. It should be noted that some of these parameters can interact with each other to make the effect size even greater
| Mean Lung Density |
Emphysema | Airways | References | |
|---|---|---|---|---|
| CT Scanner | +/− or + | +/− or + | 16,18-20 | |
|
Reconstruction
Algorithm |
+/− | +++ | +++ | 15,17,40 |
| Slice Thickness | +/− | +++ | +++ | 14,17 |
| CT Dose | +/− | +++ | 14,16,40 | |
|
Iterative
Reconstruction |
+/− | +++ | Uncertain | 33 |
| Body Mass Index | +/− | + for high BMI | + | 20 |
| Field of View | +/− | +/− | +++ | 20 |
|
Volume of
Inspiration |
+++ | +++ | +++ | 24,28,29,39 |
Key: +/−: minimal effect, +: significant effect, + + + : large effect
Acknowledgements
Special thanks to Dr Ren Yuan and Ms Tara Candido for help with the preparation of the figures.
Conflicts of Interest and Source of Funding Harvey Coxson received $4800 in the years 2008 - 2012 for serving on the steering committee for the ECLIPSE project for GSK. In addition HC was the co-investigator on two multi-center studies sponsored by GSK and has received travel expenses to attend meetings related to the project. HC has three contract service agreements with GSK (including the ECLIPSE study) to quantify the CT scans in subjects with COPD and a service agreement with Spiration Inc. to measure changes in lung volume in subjects with severe emphysema. HC was the recipient of a GSK Clinical Scientist Award in 2010. Dr Coxson is supported by a Roberta R. Miller Fellowship in Thoracic Imaging from the British Columbia Lung Association and was also supported, in part, by the University of Pittsburgh COPD SCCOR NIH 1P50 HL084948 and R01 HL085096 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD to the University of Pittsburgh. HC has ongoing research funding from CIHR Team Grant CIF# 97687.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoffman EA, Sinak LJ, Robb RA, et al. Noninvasive quantitative imaging of shape and volume of lungs. J Appl Physiol. 1983;54:1414–1421. doi: 10.1152/jappl.1983.54.5.1414. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman EA. Effect of body orientation on regional lung expansion: a computed tomographic approach. J Appl Physiol. 1985;59:468–480. doi: 10.1152/jappl.1985.59.2.468. [DOI] [PubMed] [Google Scholar]
- 3.Denison DM, Morgan MDL, Millar AB. Estimation of regional gas and tissue volumes of the lung in supine man using computed tomography. Thorax. 1986;41:620–628. doi: 10.1136/thx.41.8.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coxson HO, Mayo JR, Behzad H, et al. Measurement of lung expansion with computed tomography and comparison with quantitative histology. J Appl Physiol. 1995;79:1525–1530. doi: 10.1152/jappl.1995.79.5.1525. [DOI] [PubMed] [Google Scholar]
- 5.Millar AB, Fromson B, Strickland BA, et al. Computed tomography based estimates of regional gas and tissue volume of the lung in supine subjects with chronic airflow limitation or fibrosing alveolitis. Thorax. 1986;41:932–939. doi: 10.1136/thx.41.12.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coxson HO, Hogg JC, Mayo JR, et al. Quantification of idiopathic pulmonary fibrosis using computed tomography and histology. Am J Respir Crit Care Med. 1997;155:1649–1656. doi: 10.1164/ajrccm.155.5.9154871. [DOI] [PubMed] [Google Scholar]
- 7.Stoel BC, Vrooman HA, Stolk J, et al. Sources of error in lung densitometry with CT. Invest Radiol. 1999;34:303–309. doi: 10.1097/00004424-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Hayhurst MD, Flenley DC, McLean A, et al. Diagnosis of pulmonary emphysema by computerized tomography. Lancet. 1984;2:320–322. doi: 10.1016/s0140-6736(84)92689-8. [DOI] [PubMed] [Google Scholar]
- 9.Müller NL, Staples CA, Miller RR, et al. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest. 1988;94:782–787. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 10.Gould GA, MacNee W, McLean A, et al. CT measurements of lung density in life can quantitate distal airspace enlargement--an essential defining feature of human emphysema. ARRD. 1988;137:380–392. doi: 10.1164/ajrccm/137.2.380. [DOI] [PubMed] [Google Scholar]
- 11.Miller RR, Müller NL, Vedal S, et al. Limitations of computed tomography in the assessment of emphysema. ARRD. 1989;139:980–983. doi: 10.1164/ajrccm/139.4.980. [DOI] [PubMed] [Google Scholar]
- 12.Gevenois PA, de Maertelaer V, De Vuyst P, et al. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152:653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 13.Gevenois PA, De Vuyst P, de Maertelaer V, et al. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154:187–192. doi: 10.1164/ajrccm.154.1.8680679. [DOI] [PubMed] [Google Scholar]
- 14.Madani A, De Maertelaer V, Zanen J, et al. Pulmonary Emphysema: Radiation Dose and Section Thickness at Multidetector CT Quantification--Comparison with Macroscopic and Microscopic Morphometry. Radiology. 2007;243:250–257. doi: 10.1148/radiol.2431060194. [DOI] [PubMed] [Google Scholar]
- 15.Boedeker KL, McNitt-Gray MF, Rogers SR, et al. Emphysema: effect of reconstruction algorithm on CT imaging measures. Radiology. 2004;232:295–301. doi: 10.1148/radiol.2321030383. [DOI] [PubMed] [Google Scholar]
- 16.Yuan R, Mayo JR, Hogg JC, et al. The Effects of Radiation Dose and CT Manufacturer on Measurements of Lung Densitometry. Chest. 2007;132:617–623. doi: 10.1378/chest.06-2325. [DOI] [PubMed] [Google Scholar]
- 17.Kemerink GJ, Kruize HH, Lamers RJS, et al. Density resolution in quantitative computed tomography of foam and lung. Medical Physics. 1996;23:1697–1708. doi: 10.1118/1.597757. [DOI] [PubMed] [Google Scholar]
- 18.Stoel BC, Bakker ME, Stolk J, et al. Comparison of the sensitivities of 5 different computed tomography scanners for the assessment of the progression of pulmonary emphysema: a phantom study. Invest Radiol. 2004;39:1–7. doi: 10.1097/01.rli.0000091842.82062.a3. [DOI] [PubMed] [Google Scholar]
- 19.Sieren JP, Newell JD, Judy PF, et al. Reference standard and statistical model for intersite and temporal comparisons of CT attenuation in a multicenter quantitative lung study. Med Phys. 2012;39:5757–5767. doi: 10.1118/1.4747342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zach JA, Newell JD, Jr., Schroeder J, et al. Quantitative computed tomography of the lungs and airways in healthy nonsmoking adults. Invest Radiol. 2012;47:596–602. doi: 10.1097/RLI.0b013e318262292e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parr DG, Stoel BC, Stolk J, et al. Influence of calibration on densitometric studies of emphysema progression using computed tomography. Am J Respir Crit Care Med. 2004;170:883–890. doi: 10.1164/rccm.200403-326OC. [DOI] [PubMed] [Google Scholar]
- 22.Stoel BC, Bode F, Rames A, et al. Quality Control in Longitudinal Studies with Computed Tomographic Densitometry of the Lungs. Proc Am Thorac Soc. 2008;5:929–933. doi: 10.1513/pats.200804-039QC. [DOI] [PubMed] [Google Scholar]
- 23.Madani A, Zanen J, de Maertelaer V, et al. Pulmonary Emphysema: Objective Quantification at Multi-Detector Row CT - Comparison with Macroscopic and Microscopic Morphometry. Radiology. 2006;238:1036–1043. doi: 10.1148/radiol.2382042196. [DOI] [PubMed] [Google Scholar]
- 24.Dirksen A. Monitoring the Progress of Emphysema by Repeat Computed Tomography Scans with Focus on Noise Reduction. Proc Am Thorac Soc. 2008;5:925–928. doi: 10.1513/pats.200804-033QC. [DOI] [PubMed] [Google Scholar]
- 25.Wan ES, Hokanson JE, Murphy JR, et al. Clinical and radiographic predictors of GOLD-unclassified smokers in the COPDGene study. Am J Respir Crit Care Med. 2011;184:57–63. doi: 10.1164/rccm.201101-0021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busacker A, Newell JD, Jr., Keefe T, et al. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest. 2009;135:48–56. doi: 10.1378/chest.08-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mets OM, Buckens CF, Zanen P, et al. Identification of chronic obstructive pulmonary disease in lung cancer screening computed tomographic scans. JAMA. 2011;306:1775–1781. doi: 10.1001/jama.2011.1531. [DOI] [PubMed] [Google Scholar]
- 28.Madani A, Van Muylem A, Gevenois PA. Pulmonary emphysema: effect of lung volume on objective quantification at thin-section CT. Radiology. 2010;257:260–268. doi: 10.1148/radiol.10091446. [DOI] [PubMed] [Google Scholar]
- 29.Stoel BC, Putter H, Bakker ME, et al. Volume Correction in Computed Tomography Densitometry for Follow-up Studies on Pulmonary Emphysema. Proc Am Thorac Soc. 2008;5:919–924. doi: 10.1513/pats.200804-040QC. [DOI] [PubMed] [Google Scholar]
- 30.Coxson HO. Quantitative chest tomography in COPD research: chairman’s summary. Proc Am Thorac Soc. 2008;5:874–877. doi: 10.1513/pats.200810-118QC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashraf H, Lo P, Shaker SB, et al. Short-term effect of changes in smoking behaviour on emphysema quantification by CT. Thorax. 2011;66:55–60. doi: 10.1136/thx.2009.132688. [DOI] [PubMed] [Google Scholar]
- 32.Coxson HO, Dirksen A, Edwards LD, et al. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE study. The Lancet Respiratory Medicine. 2013 doi: 10.1016/S2213-2600(13)70006-7. [DOI] [PubMed] [Google Scholar]
- 33.Mets OM, Willemink MJ, de Kort FP, et al. The effect of iterative reconstruction on computed tomography assessment of emphysema, air trapping and airway dimensions. Eur Radiol. 2012;22:2103–2109. doi: 10.1007/s00330-012-2489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coxson HO. Quantitative Computed Tomography Assessment of Airway Wall Dimensions: Current Status and Potential Applications for Phenotyping Chronic Obstructive Pulmonary Disease. Proc Am Thorac Soc. 2008;5:940–945. doi: 10.1513/pats.200806-057QC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pare PD, Nagano T, Coxson HO. Airway imaging in disease: gimmick or useful tool? J Appl Physiol. 2012;113:636–646. doi: 10.1152/japplphysiol.00372.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reinhardt JM, Hoffman EA. Quantitative pulmonary imaging: spatial and temporal considerations in high-resolution CT. Acad Radiol. 1998;5:539–546. doi: 10.1016/s1076-6332(98)80205-5. [DOI] [PubMed] [Google Scholar]
- 37.Nakano Y, Whittall KP, Kalloger SE, et al. Development and validation of human airway analysis algorithm using multidetector row CT. Proc SPIE. 2002;4683:460–469. [Google Scholar]
- 38.Nakano Y, Wong JC, de Jong PA, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171:142–146. doi: 10.1164/rccm.200407-874OC. [DOI] [PubMed] [Google Scholar]
- 39.Bakker ME, Stolk J, Reiber JH, et al. Influence of inspiration level on bronchial lumen measurements with computed tomography. Respir Med. 2012;106:677–686. doi: 10.1016/j.rmed.2011.11.013. [DOI] [PubMed] [Google Scholar]