Abstract
In the present study we demonstrate highly sensitive detection of rare, aberrant cells in a population of wild-type human cells by combining a rolling circle-enhanced enzyme activity single molecule detection assay with a custom designed microfluidic device. Besides reliable detection of low concentrations of aberrant cells, the integrated system allowed multiplexed detection of individual enzymatic events at the single cell level. The single cell sensitivity of the presented setup relies on the combination of single-molecule rolling circle-enhanced enzyme activity detection with the fast reaction kinetics provided by a picoliter droplet reaction volume and subsequent concentration of signals in a customized drop-trap device. This setup allows the fast reliable analyses of enzyme activities in vast number of single cells thereby offering a valuable tool for basic research as well as theranostics.
Keywords: Single-molecule Detection, Microfluidics, Single Cell Analysis, Enzyme Activity, Rolling Circle Amplification
Reliable identification of rare cells in a cell population as well as quantitative enzyme detection at the single cell level poses great potential for basic research, diagnostic or therapeutic purposes. We recently reported a highly sensitive Rolling circle-Enhanced Enzyme Activity Detection (REEAD) assay for the specific detection of enzymatic DNA cleavage-ligation events at the single molecule level.1 This assay relies on the conversion of linear DNA sensors to circular products. Such products act as templates for isothermal Rolling Circle Amplification (RCA) resulting in ~103 tandem repeat products (RCPs), which are visualized at the single molecule level by hybridization of fluorescent probes. As opposed to thermal cycling enhancement procedures such as PCR2, isothermal RCA follows linear reaction kinetics and, hence, REEAD enables direct quantification of single enzymatic events simply by counting the number of RCP signals, which each represents a single DNA cleavage-ligation event (Figure 1a).1 Compared to other RCA-based detection systems specific towards nucleotide-3, 4, protein-5, 6 or small-molecule targets7, 8 (reviewed in9), the REEAD system possesses the unique capability of detecting the activity rather than merely the presence of a biomarker. Indeed, biomolecule activity, which ultimately defines the cell phenotype and may be the target of drug action10, is a more important determinant of cell characteristics/fate than biomolecule abundance. Moreover, by detecting enzyme activity rather than abundance, REEAD achieves high sensitivity owing to the numerous circular DNA products generated from each target molecule.
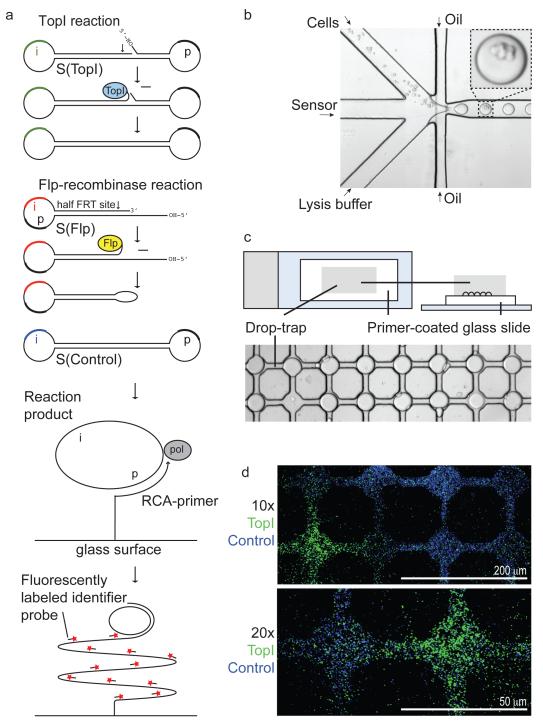
Figure 1. The combined REEAD-microfluidic experimental setup.
(a) S(TopI) and S(Flp) are each composed of an oligonucleotide that folds onto itself to allow cleavage-ligation by hTopI and Flp, respectively. These reactions circularize the substrates. S(TopI), S(Flp), and S(control) all contain a specific primer annealing p-element and a probe annealing i-element. The circles allow solid-support RCA generating ~103 tandem repeat RCPs that are visualized in a microscope at the single-molecule level by hybridization of fluorescent probes. (b) The microfluidic setup. Cells-to-be-analyzed, DNA substrate(s) and lysis buffer are, by competitions with oil, confined in picoliter droplets in which DNA circularization takes place. (c) The droplets are confined in a drop-trap on a primer-coated glass slide on which RCA takes place. (d) The result of measuring hTopI activity using five million cells/mL in the combined REEAD-microfluidic setup. As a positive control S(control) was applied together with S(TopI). hTopI and S(control) specific signals were visualized by FAM- (green) and Cy5- (blue) labeled probes, respectively.
The first generation REEAD assays1, 11 are specific for the cancer relevant DNA-cleaving enzyme human topoisomerase I (hTopI) or the related Flp- and Cre recombinases, of which the DNA cleavage-ligation reaction (specifically measured by REEAD) is the direct target of anti-cancer chemotherapeutics10, or important in site-directed recombination technologies12, 13, respectively. These assays have proven extremely robust, fast, specific and capable of multiplexed single molecule detection of the target enzyme activities even in crude cell extracts.11 Moreover, as opposed to other published single molecule analysis techniques for members of the topoisomerase-family enzyme group relying on magnetic tweezers, optical trapping, or other specialized setups14-16 the REEAD assay is characterized by a high degree of simplicity, with low requirements to assay conditions and/or equipment.
In principle, the single-catalytic-event detection limit of REEAD should allow single cell analysis although the original setup of analyzing the samples in a ~9 mm2 area suffers from loss of sensitivity due to spreading of signals.1 The simplicity of RCA-based detection, however, makes REEAD integratable into customized devices for minimizing reaction volumes and concentrating signals. Consequently, Konry et al. recently demonstrated detection of the EpCAM cancer marker at the surface of single cells by combining RCA enhancement with microfluidics.5 Here, we present the integration of REEAD with a customized microfluidic setup17 for detection of the enzymatic content of one or few cells. This is achieved by enzyme reaction with DNA sensors in picoliter-sized droplets followed by concentration of signals in small cavities of a drop-trap device. Using this setup we demonstrate concentration independent detection of rare Flp recombinase expressing human cells on a background of wild-type cells and multiplexed detection of Flp recombinase and hTopI activities in single cells. This is, to our knowledge, the first example of multiplexed detection of individual enzymatic events in single cells. The presented technology, which outcompetes other known enzyme-activity detection assays with respect to sensitivity and ease by which it can be performed, may pave the road for analyses of cell-to-cell variations of putative importance for biological systems, including tumor growth and development of drug-resistance.18, 19 Note, that one of the detected enzymes, hTopI, is the sole cellular target of chemotherapeutics from the camptothecin family, which are routinely used in the treatment of colon-, ovarian- and small-cell lung cancers and a suggested important cancer-prognostic marker.20
RESULTS AND DISCUSSION
The DNA sensors S(TopI) or S(Flp) for hTopI or Flp recombinase REEAD were, as previously described11, each comprised of one oligonucleotide that was converted to a closed circle by a single hTopI or Flp recombinase cleavage-ligation event. As a positive control of RCA we used a pre-formed DNA circle (S(control)) (Figure 1a). To investigate whether REEAD could be integrated with the microfluidic setup (Figure 1b) HEK293 cells, to be analyzed for endogenous hTopI activity, were loaded into one channel, S(TopI) and S(control) into a second, and lysis buffer into a third channel of the microfluidic device. The merged aqueous streams were broken up by an oil stream to form a stable water-in-oil emulsion. The four components confined in the aqueous picoliter droplets flowed through a serpentine channel to ensure adequate mixing of the reagents (Figure 1b).21 Cell lysis released hTopI into the solution and allowed it to interact with and circularize S(TopI). After exit from the channel, the aqueous droplets were individually captured in cavities of a drop-trap (Figure 1c and Supporting Figure 1) and exsiccated on a DNA primer-coated glass slide. This allowed RCA of S(control) and circularized S(TopI). RCA of unreacted S(TopI) was prevented as previously described.1 The resulting RCPs were visualized at the single molecule level by microscopy upon annealing of fluorescent probes. As shown in Figure 1d, the combination of REEAD and microfluidics enabled multiplexed detection of S(control) (blue) and hTopI reacted S(TopI) (green) in a pattern matching the drop-trap cavities. In the presented experiment the microfluidic system was loaded with five million cells/mL. As estimated from the Poisson distribution (Supporting Figure 2) and confirmed experimentally (Supporting Figure 3) this cell density resulted in ~60% of droplets without cells and ~40% with one or more cells.22 Consistently, all drop-trap cavities contained equally distributed S(control) originating blue signals, while only a part of them contained green signals arising from circularized S(TopI).
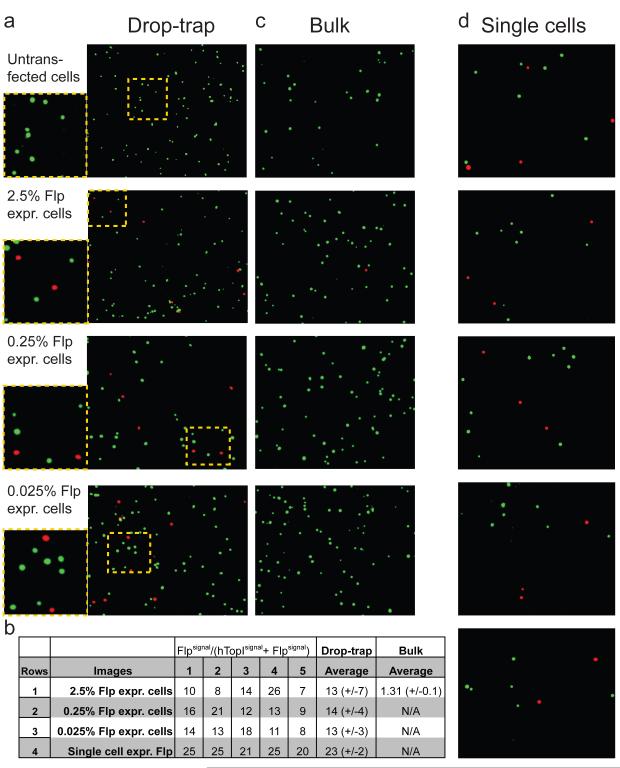
To investigate the feasibility of using the combined REEAD-microfluidic setup to detect rare cells in a cell population, we used HEK293 cells containing different proportions of Flp recombinase expressing cells as a model (Supporting Figure 4). Five million cells/mL containing 2.5%, 0.25% or 0.025% Flp recombinase expressing cells were loaded into the microfluidic device together with S(TopI), S(Flp) and lysis buffer as described above. After entrapment of droplets and RCA, circularized S(TopI) was visualized by green and circularized S(Flp) by red fluorescence, respectively. As evident from Figure 2a, red Flp recombinase specific signals could be detected on the background of green signals originating from endogenous hTopI activity present in all the cells. Moreover, although the number of drop-trap cavities containing red signals decreased with decreasing density of Flp recombinase expressing cells (the percentage of cavities containing red and green signals relative to cavities containing only green signals approximating the percentage of Flp recombinase expressing cells loaded to the system, data not shown) the average percentage of Flp recombinase specific red signals in the drop-trap cavities that did contain red signals was similar regardless the dilution of Flp recombinase expressing cells within the tested concentration range (Figure 2b). In contrast, the previously described “large-volume” bulk experimental setup1, 11 could not detect any Flp recombinase specific signals beyond the 2.5% dilution of Flp recombinase expressing cells (Figure 2c). Note, that as discussed below, the relatively large deviation of red signals present in individual drop-trap cavities (Figure 2b, rows 1-3) most probably is a consequence of non-uniform encapsulation of different mixtures of two or more (wild-type or Flp recombinase expressing) cells when high cell density was used (Supporting Figure 2 and 3).
Figure 2. Detection of enzyme activities in rare- or single cells.
(a) Five million cells/mL of HEK293 cells containing 2.5%, 0.25% or 0.25% Flp recombinase expressing cells were analyzed for Flp recombinase and hTopI activity using the REEAD-microfluidic setup. Drop-trap cavities containing red signals corresponding to Flp recombinase activity were selected. (b) Shows the percentage of red signals in five cavities of the drop-trap when five million cells/mL containing 2.5%, 0.25% or 0.25% Flp recombinase expressing cells were analyzed for Flp recombinase and hTopI activity (row 1-3) or when 0.5 million cells/mL containing 2.5% GFP-recombinase expressing cells were analyzed (row 4). (c) The result of analyzing the cell populations used in (a) for Flp recombinase and hTopI activity in the “large-volume” bulk assay setup. (d) Same as (a) except that 0.5 million cells/mL containing 2.5% Flp recombinase expressing cells was analyzed. hTopI and Flp recombinase specific signals were visualized by FAM- (green) and TAMRA- (red) labeled probes, respectively.
To address the potential of the REEAD-microfluidic setup for single cell analysis, 0.5 million cells/mL containing 2.5% Flp recombinase-expressing cells were loaded into the system and the activity of Flp recombinase or hTopI detected. At this cell density no more than one cell was encapsulated in each droplet (Supporting Figure 2 and 3). Hence, the signals in each drop-trap cavity (Supporting Figure 1 and Figure 2d) represented the enzyme activities of a single cell. Figure 2d shows the result of encapsulating Flp recombinase expressing cells, where red signals correspond to circularized S(Flp) and green signals to circularized S(TopI) (originating from endogenous hTopI activity). However, cavities with green signals only, representing a cell without Flp recombinase expression were also observed (data not shown). The percentage of Flp recombinase originating signals (red) relative to all signals in single cells averaged 23 +/−2% (Figure 2b, row 4). As estimated from standard Western blotting analyses of nuclear extracts the expression levels of Flp recombinase and hTopI was comparable in the Flp recombinase expressing cells (data not shown). Hence, the relative low percentage of red signals in these cells most probably reflects a lower specific activity of Flp recombinase relative to hTopI at the utilized assay conditions. This is consistent with the fact that it takes two Flp recombinase monomers to form one cleavage competent active site (whereas one hTopI monomer suffices for cleavage) and a more processive reaction mode of Flp recombinase relative to hTopI.23 The low standard deviation of results shown in Figure 2d compared to Figure 2a highlights the high precision achievable with single cell encapsulation when using low cell density input compared to results obtained when five million cells/mL was used. Indeed, the lower average Flp recombinase activity measured in individual drop-trap cavities when using 5 million cells/mL (Figure 2b, rows 1-3) suggests encapsulation of more than one cell (including various mixtures of wild-type and Flp recombinase expressing cells) in most droplets analyzed. This contradicts the theoretical estimation of cells per droplet according to the Poisson distribution (Supporting Figure 2), which predict ~61% droplets without cells, ~30% droplets with one cell, and ~8% with two cells and may be the result of slight aggregation of cells at these high cell densities combined with a visual bias towards selecting the drop-trap cavities containing most signals for analysis.
CONCLUSION
In conclusion, the current study illustrates the highly sensitive detection of rare Flp recombinase expressing cells on a background of wild-type cells in a cell density-independent manner as well as the comprehensive detection of signals from hTopI or Flp recombinase activities in single cells. The single cell analyses capability of the integrated REEAD-microfluidic setup without doubt relies on the inherent single molecule detection capacity of REEAD combined with the fast kinetics of reactions confined in picoliter droplets and the concentration of signals provided by the customized drop-trap device.
The obtained results strongly suggest the feasibility of using the REEAD-microfluidic setup to detect diminutive numbers of aberrant cells in a population. This indeed may hold great promise for future diagnostic purposes when combined with REEAD sensors specific for relevant disease targets, e.g. DNA cleaving-ligation enzymes from various pathogens or abnormal human cells. To this end, sensors specific for pathogen topoisomerases are already under development. For more immediate use the integrated REEAD-microfluidics may prove a valuable high-throughput system for investigating variations between individual cells within populations, including their response to external factors such as radiation or drugs in e.g. high-throughput drug screenings setups. For such purposes the small volume requirements of the setup may be of advantage.
Single cell measurements of hTopI activity, which is the cellular target of several anti-cancer drugs and a suggested prognostic marker for cancer20, may provide important new information regarding tumor development. Indeed current knowledge suggests rare cancer stem cells, rather than the bulk cells of a given cancer to be the main determinant of drug response and development of resistance.18, 19
MATERIALS AND METHODS
Cell culture and transfections
Human embryonic kidney HEK293 cells were cultured in GIBCO’s Minimal Essential Medium (MEM) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals), 100 units/mL penicillin and 100 mg/mL streptomycin (Invitrogen) in a humidified incubator (5% CO2/95% air atmosphere at 37°C). Cells were harvested with 0.25% Trypsin-EDTA (GIBCO) and resuspended in Phosphate-buffered Saline (1xPBS, Cellgro), 1% Pluronic F-68 (Sigma-Aldrich), 0.1% BSA (Invitrogen). The cell densities were adjusted to 0.5-5 million cells/mL and used for enzyme activity detection in the microfluidic system.
Plasmid pCAG-Flpe:GFP for expression of Flpe C-terminally tagged with green fluorescent protein (GFP) in human cells was from Addgene. Transient transfection of pCAG-Flpe:GFP into HEK293 cells was performed using Lipofectamine2000 (Invitrogen) and 8 μg plasmid DNA and was carried out in GIBCO’s Reduced Serum Medium (OPTI-MEM) according to the manufacturer’s instructions. 24 h after transfection, cells were harvested with 0.25% Trypsin-EDTA and resuspended in Phosphate-buffered Saline, 1% Pluronic F-68, 0.1% BSA. Transfected cells were mixed with non-transfected cells at the ratios stated in the text and the cell densities adjusted to five million cells/mL (for detection of rare cells) or 0.5 million cells/mL (for addressing the detection limit of the REEAD-microfluidic setup) and used for enzyme activity detection in the microfluidic system or in the “large-volume” bulk experimental setup.
Synthetic DNA Substrates, Probes, and Primers
Oligonucleotides for construction of the S(TopI), S(Flp), S(Control) substrates, the RCA-primer, and the fluorescently labeled identification probes for the three substrates were purchased from DNA Technology A/S. The sequences of all used the oligonucleotides have been published previously.11
Rolling circle Enhanced Enzyme Activity Detection (REEAD) in bulk setup
The single-molecule TopI and Flp activity assays were performed essentially as previously described11, except for the preparation of the cell extracts. In brief, mixtures of transfected and non-transfected HEK293 cells (described above) were incubated for 5 min in lysis buffer (20 mM Tris-HCL pH 7.5, 0.5 mM EDTA, 1 mM DTT, 1 mM PMSF, 0.2% Tween 20). Subsequently, S(TopI) and S(Flp) were added to the extract at a final concentration of 100 nM and incubation continued for 30 min at 37°C. RCA-based detection of circularized S(TopI) and S(Flp) in the samples was performed as previously published.11
Rolling circle Enhanced Enzyme Activity Detection (REEAD) in microfluidic system
The microfluidic setup consists of two devices: a flow-focusing droplet generator and a drop-trap. Both devices were fabricated by conventional soft lithography techniques24, casting and curing the PDMS prepolymer on a SU-8 3025 (MicroChem) master of a channel height at around 25 μm. PDMS prepolymer (Sylgard 184) was prepared in a 10 : 1 (base : curing agent) ratio and cured at 65°C for 1hr. Prior to the experiments, the channel was wetted with oil/surfactant (EA Surfactant, RainDance) for at least 15 min. Two syringe pumps (Harvard Apparatus) were used to control the flow rates of oil/surfactant and reagents independently, forming monodisperse water-in-oil droplets at a frequency of 0.8-1.5 kHz. The droplet volume and generation frequency was controlled by the flow rate ratio, determined by the competition between continuous phase (carrier fluid (FC-40 fluorocarbon oil (3M): the oil/surfactant, flow rate 22.5 μL/min) and disperse phase (aqueous reagents: cells, lysis buffer and substrates, flow rate 2.5 μL/min).
Cells, prepared as stated above, lysis buffer (20 mM Tris-HCL pH 7.5, 0.5 mM EDTA, 1 mM DTT, 1 mM PMSF, 0.2% Tween 20), and substrates (final concentration of 100 nM in the droplets) were loaded in each their channel in the microfluidic device and droplet generation initiated. The low salt lysis buffer was the most efficient in breaking cells open in the microfluidic setup and although a large part of hTopI and Flp recombinase may remain bound at the genomic DNA at low salt conditions the low salt lysis buffer increased efficiency of the assay compared to high salt buffers such as PBS. The generated droplets were harvested in eppendorf tubes and placed on a primer-printed glass slide (CodeLink Activated Slides from SurModics) prepared as previously described.11 The PDMS drop-trap was gently placed on top of the glass slide. The geometry of the drop-trap was designed according to the size of generated droplets. The droplets were left to exsiccate for 16 hours. Wash, RCA, and hybridization of probes were performed as previously described.11
Microscopy
Epifluorescent and bright field images were captured with an inverted fluorescence microscope (Axio Observer, Zeiss). Monocolor emission from each fluorophore was collected and filtered through appropriate filters and dichroics. Image processing and analysis was performed with MetaMorph (v.7.6.5).
Supplementary Material
ACKNOWLEDGEMENT
We thank RainDance Technologies for providing the EA Surfactant. This work was supported by NIH (HL89764), NSF EEC-0425626, the Danish Cancer Society, Dagmar Marshall’s Foundation, Dir. Einar Hansen og Vera Hansen’s Foundation, The Harboe Foundation, The Augustinus Foundation, Louis Hansen’s Foundation, The Hørslev Foundation, Fabrikant Einar Willumsen’s Foundation, Købmand Sven Hansen & hustru Ina Hansen’s Foundation, Dir. Emil Hertz & hustru Inger Hertz’ Foundation, Civilingeniør Frode Nygaard’s Foundation, Kong Christian den Tiende’s Foundation, The Gangsted Foundation, KU’s Foundation for Cancer research, Ludvid og Franciska Andersen’s Foundation, Arvid Nilsson’s Foundation, Frimodt-Heineke’s Foundation
Footnotes
Supporting Information Available: Including Figures of the drop-trap device, cell distribution in droplets and microscopic view of transfected cells. This material is available free of charge via the Internet at http://pubs.acs.org
REFERENCES
- 1.Stougaard M, Lohmann JS, Mancino A, Celik S, Andersen FF, Koch J, Knudsen BR. Single-Molecule Detection of Human Topoisomerase I Cleavage-Ligation Activity. ACS Nano. 2009;3:223–233. doi: 10.1021/nn800509b. [DOI] [PubMed] [Google Scholar]
- 2.Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H. Specific Enzymatic Amplification of DNA In Vitro: The Polymerase Chain Reaction. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- 3.Smolina I, Miller NS, Frank-Kamenetskii M. PNA-based Microbial Pathogen Identification and Resistance Marker Detection: an Accurate, Isothermal Rapid Assay Based on Genome-Specific Features. Artif DNA PNA XNA. 2010;1:1–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Larsson C, Koch J, Nygren A, Janssen G, Raap AK, Landegren U, Nilsson M. In Situ Genotyping Individual DNA Molecules by Target-primed Rolling-circle Amplification of Padlock Probes. Nat Methods. 2004;1:227–232. doi: 10.1038/nmeth723. [DOI] [PubMed] [Google Scholar]
- 5.Konry T, Smolina I, Yarmush JM, Irimia D, Yarmush ML. Ultrasensitive Detection of Low-Abundance Surface-Marker Protein Using Isothermal Rolling Circle Amplification In a Microfluidic Nanoliter Platform. Small. 2011;7:395–400. doi: 10.1002/smll.201001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Fung CW, Cho EJ, Ellington AD. Real-Time Rolling Circle Amplification for Protein Detection. Anal Chem. 2007;79:3320–3329. doi: 10.1021/ac062186b. [DOI] [PubMed] [Google Scholar]
- 7.Cho EJ, Yang L, Levy M, Ellington AD. Using a Deoxyribozyme Ligase and Rolling Circle Amplification to Detect a Non-Nucleic Acid Analyte, ATP. J Am Chem Soc. 2005;127:2022–2023. doi: 10.1021/ja043490u. [DOI] [PubMed] [Google Scholar]
- 8.Ma C, Wang W, Yang Q, Shi C, Cao L. Cocaine Detection Via Rolling Circle Amplification of Short DNA Strand Separated by Magnetic Beads. Biosens Bioelectron. 2011;26:3309–3312. doi: 10.1016/j.bios.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Conze T, Shetye A, Tanaka Y, Gu J, Larsson C, Goransson J, Tavoosidana G, Soderberg O, Nilsson M, Landegren U. Analysis of Genes, Transcripts, and Proteins Via DNA Ligation. Annu Rev Anal Chem (Palo Alto Calif) 2009;2:215–239. doi: 10.1146/annurev-anchem-060908-155239. [DOI] [PubMed] [Google Scholar]
- 10.Pommier Y. Topoisomerase I Inhibitors: Camptothecins and Beyond. Nat Rev Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 11.Andersen FF, Stougaard M, Jorgensen HL, Bendsen S, Juul S, Hald K, Andersen AH, Koch J, Knudsen BR. Multiplexed Detection of Site Specific Recombinase and DNA Topoisomerase Activities at the Single Molecule Level. ACS Nano. 2009;3:4043–4054. doi: 10.1021/nn9012912. [DOI] [PubMed] [Google Scholar]
- 12.Bolusani S, Ma CH, Paek A, Konieczka JH, Jayaram M, Voziyanov Y. Evolution of Variants of Yeast Site-Specific Recombinase Flp that Utilize Native Genomic Sequences as Recombination Target Sites. Nucleic Acids Res. 2006;34:5259–5269. doi: 10.1093/nar/gkl548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Otin AL, Guillou F. Mammalian Genome Targeting Using Site-Specific Recombinases. Front Biosci. 2006;11:1108–1136. doi: 10.2741/1867. [DOI] [PubMed] [Google Scholar]
- 14.Koster DA, Croquette V, Dekker C, Shuman S, Dekker NH. Friction and Torque Govern the Relaxation of DNA Supercoils by Eukaryotic Topoisomerase IB. Nature. 2005;434:671–674. doi: 10.1038/nature03395. [DOI] [PubMed] [Google Scholar]
- 15.Charvin G, Strick TR, Bensimon D, Croquette V. Tracking Topoisomerase Activity at the Single-Molecule Level. Annu Rev Biophys Biomol Struct. 2005;34:201–219. doi: 10.1146/annurev.biophys.34.040204.144433. [DOI] [PubMed] [Google Scholar]
- 16.van Mameren J, Peterman EJ, Wuite GJ. See Me, Feel Me: Methods to Concurrently Visualize and Manipulate Single DNA Molecules and Associated Proteins. Nucleic Acids Res. 2008;36:4381–4389. doi: 10.1093/nar/gkn412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho YP, Grigsby CL, Zhao F, Leong KW. Tuning Physical Properties of Nanocomplexes Through Microfluidics-assisted Confinement. Nano Lett. 2011;11:2178–2182. doi: 10.1021/nl200862n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvero AB, Chen R, Fu HH, Montagna M, Schwartz PE, Rutherford T, Silasi DA, Steffensen KD, Waldstrom M, Visintin I, Mor G. Molecular Phenotyping of Human Ovarian Cancer Stem Cells Unravels the Mechanisms for Repair and Chemoresistance. Cell Cycle. 2009;8:158–166. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haraguchi N, Ohkuma M, Sakashita H, Matsuzaki S, Tanaka F, Mimori K, Kamohara Y, Inoue H, Mori M. CD133+CD44+ Population Efficiently Enriches Colon Cancer Initiating Cells. Ann Surg Oncol. 2008;15:2927–2933. doi: 10.1245/s10434-008-0074-0. [DOI] [PubMed] [Google Scholar]
- 20.Postma C, Koopman M, Buffart TE, Eijk PP, Carvalho B, Peters GJ, Ylstra B, van Krieken JH, Punt CJ, Meijer GA. DNA Copy Number Profiles of Primary Tumors as Predictors of Response to Chemotherapy in Advanced Colorectal Cancer. Ann Oncol. 2009;20:1048–1056. doi: 10.1093/annonc/mdn738. [DOI] [PubMed] [Google Scholar]
- 21.Vincent ME, Liu W, Haney EB, Ismagilov RF. Microfluidic Stochastic Confinement Enhances Analysis of Rare Cells by Isolating Cells and Creating High Density Environments for Control of Diffusible Signals. Chem Soc Rev. 2010;39:974–984. doi: 10.1039/b917851a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edd JF, Di Carlo D, Humphry KJ, Koster S, Irimia D, Weitz DA, Toner M. Controlled Encapsulation of Single-Cells into Monodisperse Picolitre Drops. Lab Chip. 2008;8:1262–1264. doi: 10.1039/b805456h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen JW, Lee J, Jayaram M. DNA Cleavage in Trans by the Active Site Tyrosine during Flp Recombination: Switching Protein Partners Before Exchanging Strands. Cell. 1992;69:647–658. doi: 10.1016/0092-8674(92)90228-5. [DOI] [PubMed] [Google Scholar]
- 24.Qin D, Xia Y, Whitesides GM. Soft Lithography for Micro- and Nanoscale Patterning. Nat Protoc. 2010;5:491–502. doi: 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.