Abstract
This report explores aspects of developing obesity in two captive populations of common marmosets (Callithrix jacchus), a small primate with a short lifespan that may be of value in modeling chronic aspects of obesity acquisition and its lifetime effects. Two populations were examined. In study 1, body composition, lipid parameters, and glucose metabolic parameters were measured in a population of 64 adult animals. Animals classified as obese (>80th percentile relative fat based on sex) displayed both dyslipidemia (higher triglyceride and very low–density lipoprotein (VLDL)) and altered glucose metabolism (higher fasting glucose and HbA1c). Using operational definitions of atypical values for factors associated with metabolic syndrome in humans, five subjects (7.8%) had at least three atypical factors and five others had two atypical factors. A previously unreported finding in these normally sexually monomorphic primates was higher body weight, fat weights, and percent fat in females compared to males. In a second study, longitudinal weight data for a larger population (n = 210) were analyzed to evaluate the development of high weight animals. Differences in weights for animals that would exceed the 90th percentile in early adulthood were evident from infancy, with a 15% difference in weight between future-large weight vs. their future-normal weight litter mates as early as 4–6 months of age. The marmoset, therefore, demonstrates similar suites of obesity-related alterations to those seen in other primates, including humans, suggesting that this species is worthy of consideration for obesity studies in which its fast maturity, high fertility, relatively short lifespan, and small size may be of advantage.
Introduction
Animal models are vital to understand the mechanisms leading to and outcomes stemming from obesity. Rodent models (1–3) have provided the primary testing ground; however, rodent models have limitations when applying findings to humans (4) given phylogenetic differences in fat cell function and distribution (5), development and circadian rhythm of feeding behavior (6), and patterns of postnatal growth and its dependence upon milk vs. postweaning. Finally, while some therapeutic targets have correlated effects in mouse knockout models and humans, function of adipokines in rodents and humans can also differ dramatically as demonstrated for the influence of resistin on insulin sensitivity (7).
There are advantages, then, to examining the development and effects of adiposity in nonhuman primate models. Old World monkeys are the most commonly used primate models in most areas, including obesity and diabetes research (8–14), often showing striking similarities to human phenotypes. Drawbacks of using these species include special housing requirements and, in the case of macaques, potential zoonotic risks. Perhaps the most serious drawback is that the late maturity and long lifespan of these monkeys make following the course of long-term chronic conditions, such as obesity, difficult and expensive.
A relatively fast maturing primate would offer advantages in certain types of obesity studies. For example, evidence from animal models and epidemiological studies suggests that prenatal condition may predispose offspring to adult obesity and associated metabolic disorders (15,16). Common marmosets (Callithrix jacchus) offer the ability to model this process in a short-lived primate. Small (350–400 g) South American primates that are capable of producing twins or triplets every 5.5 months, marmosets are the most fertile of the anthropoid primates (17). Marmosets begin puberty before 1 year of age and reach sexual maturity at 1.7 years (18). The average lifespan for a captive marmoset is ∼6 years (17) and the maximum lifespan is ∼16 years. Marmosets can be considered aged at ∼8 years of age (18). Contrast this with the lifespan of a macaque or baboon, in which sexual maturity is reached at 3–4 years of age, the average lifespan often exceeds 15 years and old age is not reached until 20–25 years. In addition to the benefit offered by a fast life history, the routine production of marmoset twins offers the opportunity to have >1 infant exposed to the same uterine and postnatal family environments.
Marmosets show a secular trend toward larger size over captive generations (19; N. Schultz-Darken, personal communication). Wild marmosets average 320–336 g (refs. 20,21). Captive animals average from 283 to 530 g, depending upon the colony (22–25). The extent to which this trend represents changes in lean vs. fat weight is unknown. One published study of body composition in marmosets was based upon a relatively lean group of 20 animals, with an average weight of 342 g and average body fat of 8.34% (ref. 26).
This report explores aspects of developing obesity in two captive populations of common marmosets. For one population, cross-sectional data were available for an array of parameters related to defining obesity and its sequelae, including body composition, and circulating lipid and glucose metabolism parameters. The relations among these parameters were examined to determine whether marmosets display suites of associated traits that would make them a valuable additional model for metabolic syndrome, a syndrome encompassing change in the blood trafficking of both lipids and glucose, associated with diabetic and cardiovascular symptoms (27).
A second population provided longitudinal data on body weight from birth through early adulthood, allowing further exploration of possible sex differences in body weight and the relation of those differences to early weight growth. Humans are sexually dimorphic in a suite of characters related to fat distribution, accumulation and metabolism, and differences in adiposity are evident in childhood (28). While humans are unusual in the extreme degree of sexual dimorphism in relative amount of and distribution of body fat (29), many nonhuman primates also display higher relative fat in females and higher absolute and relative lean weight in males (30–32). However, the examination of sexual dimorphism in the amount and distribution of body fat in nonhuman primates has been largely limited to those large-bodied, terrestrial species that display a high level of overall sexual dimorphism in size, such as macaques and baboons. Marmosets do not display high levels of sexual dimorphism in size and are generally described as sexually monomorphic (20,21) relative to overall body weight. In our previously published study on body composition in this species, the relation of body fat to body weight was identical in males and females (26). However, as mentioned previously, the animals in this study were generally extremely lean. Preliminary observations (33) suggested that this monomorphism may not extend to accumulation of atypical amounts of adipose tissue, with females perhaps more likely than males to become obese in a captive setting. We therefore, re-examined whether there is a sex difference in absolute or relative degrees of adiposity in marmoset populations containing obese animals and, if so, how early in life these differences can be observed.
Methods and Procedures
The subjects of the analyses described herein were part of two marmoset populations maintained at the Southwest National Primate Research Center in San Antonio, TX. All animal studies were reviewed and approved by Institutional Animal Care and Use Committees at the Southwest Foundation for Biomedical Research (host institution of the Southwest National Primate Research Center) and the University of Texas Health Science Center at San Antonio.
Study 1
Subjects
Study 1 was conducted on 64 adult animals (32 males, 32 nulliparous females) that originated from three different sources—two national primate research centers and one commercial vendor. These animals were assigned to ongoing projects assessing obesity propensity. They were singly housed and fed, in ad lib quantities, a commercial marmoset diet (Purina Mazuri gelled diet; primary ingredients: glucose, casein, ground wheat, corn flour, dehulled soybean meal, gelatin, porcine fat, dehydrated alfalfa meal, dried beet pulp, egg yolk solids, dried whey, soybean oil; 22% protein, 6% fat; 4.47 kcal/g dry weight) and 3–5 days per week were given one small slice of fruit or 1–3 raisins in the afternoon.
Data collection
After a habituation period of 4 months, weights, body composition, and blood samples were collected from each animal, within a 1-week period in August 2006. Animals were weighed, either through placing a scale into the animal's home cage or by removing the animal from its home cage in a catch box and weighing it in the catch box. All scales provided weights to the nearest gram and were calibrated every 6 months.
Estimates of lean and fat mass were obtained through quantitative magnetic resonance scans, using an EchoMRI unit (EchoMRI; Echo Medical Systems, Houston, TX) designed for marmosets, based on a previously designed rat systems. This system has been extensively validated for mice (34), and the detection methodology used in the marmoset system is identical to that used in the mouse system with only the volume of the homogenous magnetic region differing. Unsedated animals were placed in a plastic tube, which was then inserted into the magnetic chamber. Scans took <2 min, on average, for each animal.
Glucose metabolism (glucose, HbA1c) and lipid (high-density lipoprotein (HDL), low-density lipoprotein (LDL), very low–density lipoprotein (VLDL), and triglyceride) parameters were measured in blood samples collected into a heparinized syringe from unsedated animals that had been fasted overnight. At the time of blood draw, a drop was used to measure glucose using a FreeStyle glucometer and 10 μl of whole blood separated for use in assaying HbA1c. The remaining blood was processed for plasma collection. The plasma and the HbA1c samples were shipped to the laboratory of M. Paulik, GSK, Research Triangle, NC for analysis. All serum chemistry parameters were obtained utilizing the Olympus Au640 clinical chemistry analyzer (Olympus America Inc, Melville, NY) with the reactions run at 37 °C.
The relationship of morphometric parameters—body weight, lean weight, fat weight, and relative fat (total weight of fat based on magnetic resonance imaging divided by total body weight)—to sex and age were determined through a generalized linear model analysis, with sex as a main factor and age as a covariate.
In humans, obesity and other risk factors associated with increasing cardiovascular disease or diabetes risks are often defined by assessment in large-scale epidemiological studies or in consensus conferences of clinicians (35). Although information on actual body fat and its distribution is most desirable, for practical reasons human obesity has been variously defined relative to weight (>30% above average in relation to sex and age; BMI (weight/height2, with values >30 kg/m2 indicative of obesity), waist circumference (>102 cm in men or >88 cm in women), and only occasionally in relation to relative body fat (>25% in males and 30% in females representing obesity). To relate the findings from the marmoset population to assessments of human risk factors for metabolic syndrome, we used the following operational definitions for atypically high or low values of parameters identified as risk factors in various human metabolic syndrome models:
Total body fat or relative body fat >80th percentile (the top quintile of animals), defined separately for males (58.2 g or 14%) and females (73.4 g or 17%). The 80th percentile was chosen to differentiate animals that were 30% above the sex-specific mean relative body fat, making these results comparable to human studies and to operational definitions of fatness in other nonhuman primate studies (e.g., ref. 14).
Average HDL—1 s.d.: <42 mg/dl.
Average fasting glucose + 1 s.d: 219 mg/dl or average HbA1c + 1 s.d.: 5.5%.
Triglyceride concentration >400 mg/dl, representing the point at which the elongated portion of the high concentrations driving the non-normal distribution began.
HDL, glucose and triglyceride cutoff values were not sex specific because a comparison of the mean/median values for these parameters between males and females exceeding the 80th percentile of relative body fat revealed no significant sex differences.
Two analyses of the relations among these variables were conducted: (i) the mean or median concentration for metabolic and lipid parameters were compared in subjects exceeding vs. not exceeding the 80th percentile in body fat. Comparisons involving non-normally distributed variables (VLDL and triglyceride concentrations) were made using Mann– Whitney U tests while comparisons involving normally distributed variables (all others) were made using analyses of variance; (ii) the number and distribution of atypical factors displayed among the subjects was tallied and is presented in tabular form.
Study 2
A set of longitudinal analyses were conducted on a second population consisting of offspring produced from a single marmoset breeding colony, begun in 1994 and continuously breeding since that time— see ref. 36 for details on the colony history and management. All animals were housed in typical marmoset family groups consisting of one breeding female, one breeding male and their offspring up to 2–4 years of age. This colony has been maintained on two base diets fed simultaneously—a purified gelled diet (Teklad Research Diets, Madison, WI; primary ingredients: lactalbumin, dextrin, sucrose, soybean oil; 15% protein, 5% fat, 4.0 kcal/g) and one of two commercial diets—either Purinary-Mazuri gelled diet (see Study 1) or ZuPreem canned diet (primary ingredients: cracked wheat, whole egg, soybean meal, sucrose, rice, vegetable oil, alfalfa meal; 22% protein, 6% fat; 4.84 kcal/g). This population also received supplements of fresh or dried fruit 3–5 times per week. The following data were available for >80% of individuals born into this colony who were reared to weaning: dam, sire, sex, birth date, litter size, birth weight, and average early adult weight (taken as the average of all weights between 17 and 24 months of age). Details on methods for weighing are provided in ref. 36.
A general linear model analysis was used to determine the relations of the following variables to early adult weight: sex, litter size, and birth year cohorts (as an estimator of secular trends). Dam identity was included as a random variable to correct for possible pseudo-replication effects inherent in individual dams contributing variable numbers of offspring to the population. The total population in this analysis was 210 individuals.
A subset of individuals from this population was chosen to further examine the relation of prepubescent growth in weight to early adult weight. The subset of subjects were selected as follows: (i) all litters with at least one individual who exceeded the 90th percentile of body weight as an early adult; (ii) each litter in the population immediately preceding or proceeding the birth of the above defined litter with sufficient early weight data to calculate early growth estimates. This selection process resulted in 49 animals from 29 litters born between 1998 and 2006: 10 male–female litters, 4 male–male litters, 6 female–female litters, four female singletons and five male singletons. Litter size refers, here to nursed litter size, not necessarily birth litter size. Weight data for each subject from birth to 9 months of age were used to calculate linear regression estimators. Marmoset growth is linear during this age period (36), reflected in an average r2 of 0.90 for the animals in this analysis. As compared to study 1, a more conservative approach was used to compare body sizes, given that only body weight was available. A generalized linear model was used to compare weights at 0, 2, 4, and 6 months for animals exceeding 90th percentile in weight vs. those under the 90th percentile by early adulthood. A more stringent weight criterion (i.e., 90th vs. 80th percentile) was chosen for this analysis, given that we had only weight measures in this population and could not, therefore, differentiate between fat and lean differences. In addition, paired t-tests were used to compare estimated 2-, 4-, and 6-month weights within litters.
All analyses were conducted using SPSS, version 15.0 (SPSS, Chicago, IL).
Results
Study 1
Table 1 provides descriptive statistics on the first population. Females were significantly older, on average than males and had significantly higher body weights, fat weight, and relative proportion of fat. These sex differences in body fat appear to be age-independent. With age as a covariate, sex differences remained significant at P < 0.009 to P < 0.0001. As an example of the age-independence of these sex differences in body fat, Table 1 also provides descriptive statistics for a subset of the population that includes only individuals between 3.5 and 5.5 years of age, an age range at which adult marmosets have reached a stable weight and are not yet displaying age-related weight decline (23). In this subset of individuals, males and females do not differ in age, yet the same sex differences in fat weight (t = 2.24, P = 0.035) and relative fat (t = 2.10, P = 0.048) are observed. Because lean weight did not differ between males and females, females had on average, 36% more relative fat than males.
Table 1. Body composition in males and females for the total population in study 1 and for an age-matched, prime adult-aged (3.5–5.5 years) subset of that population.
| Female total sample (n = 32) | Male total sample (n = 32) | Female 3.5–5.5 years (n = 19) | Male 3.5–5.5 years (n = 5) | |
|---|---|---|---|---|
| Age (years) | 4.8 ± 1.1 | 3.1 ± 1.4 | 4.5 ± 0.5 | 4.2 ± 0.7 |
| Body wt (g) | 411.9 ± 52.5 | 386.0 ± 47.6 | 415.4 ± 54.4 | 374 ± 31.6 |
| Lean wt (g) | 282.9 ± 36.9 | 274.1 ± 29.8 | 281.5 ± 38.4 | 267.8 ± 17.2 |
| Fat wt (g) | 55.5 ± 21.7 | 39.3 ± 23.2 | 57.5 ± 22.6 | 33.4 ± 14.8 |
| Fat wt/body wt | 0.132 ± 0.04 | 0.097 ± 0.05 | 0.136 ± 0.05 | 0.087 ± 0.03 |
Values in boldface differ between sexes. See text for specific information on analyses and alpha values.
Glucose metabolism variables were all significantly correlated with each other at P < 0.001 (plasma glucose × glucometer = 0.912; plasma glucose × HbA1c = 0.509; glucometer × HbA1c = 0.461). Lipid parameters were correlated as follows: cholesterol was positively correlated with HDL (0.485), LDL (0.574), and VLDL (0.594) at P < 0.001; triglyceride was negatively correlated with LDL (−0.277, P < 0.03) and VLDL (−0.797, P < 0.001).
Table 2 provides descriptive statistics for the obese (defined as a relative fat exceeding the 80th percentile) vs. nonobese animals in this population. Animals classified as obese did not differ from nonobese in age, lean weight, cholesterol, LDL, or HDL, but had significantly higher fat weight, fasting glucose, HbA1c, VLDL, and triglyceride concentrations.
Table 2. Metabolic and lipid parameters (mean ± s.d. or median) for marmosets defined as obese (>80th percentile relative fat) vs. nonobese (<80th percentile relative fat).
| Obese (N = 14) | Nonobese (N = 50) | Analysis | Test statistic | P | |
|---|---|---|---|---|---|
| Age (years) | 3.3 ± 1.4 | 4.2 ± 1.5 | ANOVA | 3.37 | 0.071 |
| Lean weight (g) | 291.7 ± 34.0 | 275.0 ± 34.4 | ANOVA | 2.59 | 0.113 |
| Fat weight (g) | 77.9 ± 12.0 | 36.6 ± 18.4 | ANOVA | 62.67 | 0.0001 |
| Glucose (mg/dl) | 202.3 ± 67.9 | 161.2 ± 38.6 | ANOVA | 8.61 | 0.005 |
| HbA1c (%) | 5.23 ± 0.86 | 4.52 ± 0.80 | ANOVA | 8.09 | 0.006 |
| Cholesterol (mg/dl) | 167.4 ± 70.2 | 151.2 ± 41.4 | ANOVA | 1.20 | 0.278 |
| HDL (mg/dl) | 71.6 ± 21.3 | 67.2 ± 27.4 | ANOVA | 0.31 | 0.579 |
| LDL (mg/dl) | 41.6 ± 25.4 | 59.8 ± 30.3 | ANOVA | 3.69 | 0.06 |
| VLDL (mg/dl) | 58.08 | 24.61 | Mann–Whitney | 91.5 | 0.0001 |
| Triglycerides (mg/dl) | 420.8 | 163.5 | Mann–Whitney | 167 | 0.003 |
Values in boldface differ between sexes.
HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low–density lipoprotein.
Out of 64 subjects, 28 (43.75%) had at least one variable that met the operational definitions of atypical metabolic syndrome risk factors (see Table 3). Of these 28 subjects, 64.3% (n = 18) had only one atypical factor, 17.8% (n = 5) had two, 14.3% (n = 4) had three, and 1 (3.6%) had four atypical factors, The most common risk factor was an elevated glucose metabolism parameter (either elevated fasting glucose concentration or elevated HbA1c); however, this variable was also the one least likely to be associated with others. Elevated triglyceride concentration was always associated with at least one other atypical factor and, in five of six cases, was associated with two or three additional factors—i.e., meeting the metabolic syndrome definition if these operationally defined factors translate into risks similar to those seen in humans.
Table 3. Distribution of atypical concentrations of factors used in defining human metabolic disease syndrome in 64 common marmosets.
| ↑ Triglyceride | ↓ HDL | ↑ Body fat | ↑ Glucose or ↑ HbA1c | No. of subjects |
|---|---|---|---|---|
| X | 4 | |||
| X | 6 | |||
| X | 8 | |||
| X | X | 1 | ||
| X | X | 4 | ||
| X | X | X | 1 | |
| X | X | X | 3 | |
| X | X | X | X | 1 |
A total of 28 marmosets (43.7%) displayed at least one atypical factor. Subjects in boldface displayed at least three atypical factors.
Study 2
The population in study 2 offered the opportunity to examine sex differences in weights for a larger population of males and females that were reared in the same colony and could be examined at the same age. Sex differences in early adult weight in study 2 mirrored those seen in study 1—females were significantly larger than males (mean ± s.e. for females = 395.10 ± 8.92 g; for males = 365.82 ± 5.92 g; F = 7.47, P < 0.007). It is possible that this sex difference in weight reflected a difference in overall body size, rather than a specific difference in body fat. Because we did not have measurements of body dimensions or quantification of lean vs. fat weight for this population, we were unable to address this question directly. We were able, a posteriori, to obtain weight and knee-heel length measurements in four male–female litters, ranging from 2.5 to 4.5 years of age, in which the female adult weight exceeded that of the male litter mate (female mean = 401.25 ± 73.4 vs. male mean = 369.0 ± 58.6 g). Although the females were significantly heavier than their male litter mates (paired t = 3.316, P = 0.052), their knee-heel lengths did not differ (female = 72.4 ± 1.54; male = 74.6 ± 1.0 mm; paired t = −1.84, P = 0.163), suggesting that the females were not likely larger in body length than were their male litter mates.
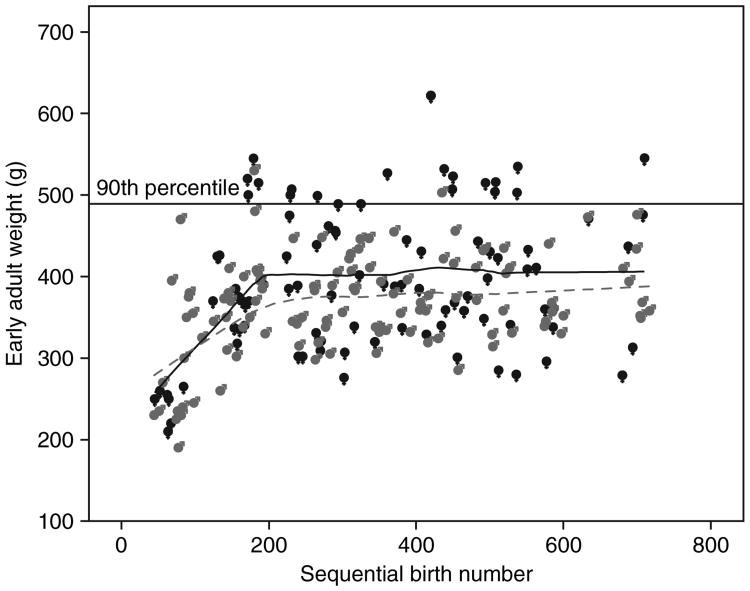
Figure 1 illustrates the early adult weights of all animals relative to their sequential birth order within the colony. In the early period of colony development, early adult weights were increasing linearly and were similar between males and females. However, once early adult weights began to stabilize, female weights were significantly higher than male weights.
Figure 1.
Early adult weights of marmosets from one colony (study 2), 1994 through 2004 vs. sequential birth number, in which animal no. 36 represents the first birth in the colony—females = ♀ in black; males = ♂ in gray.
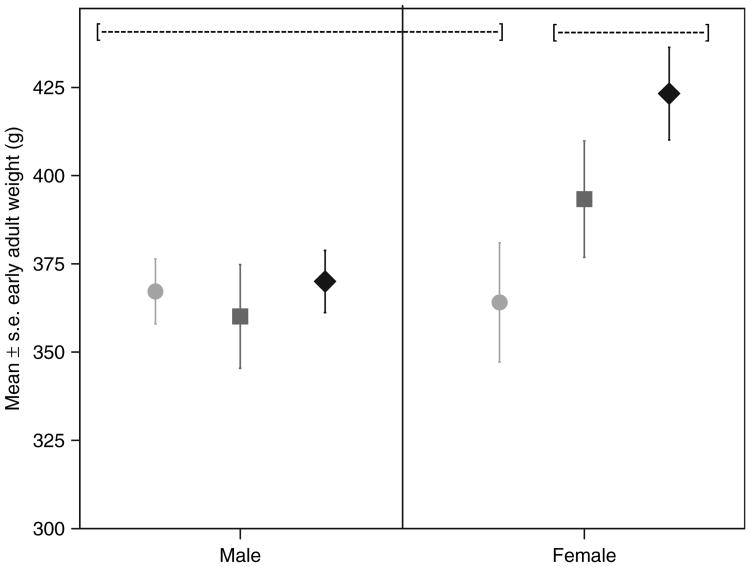
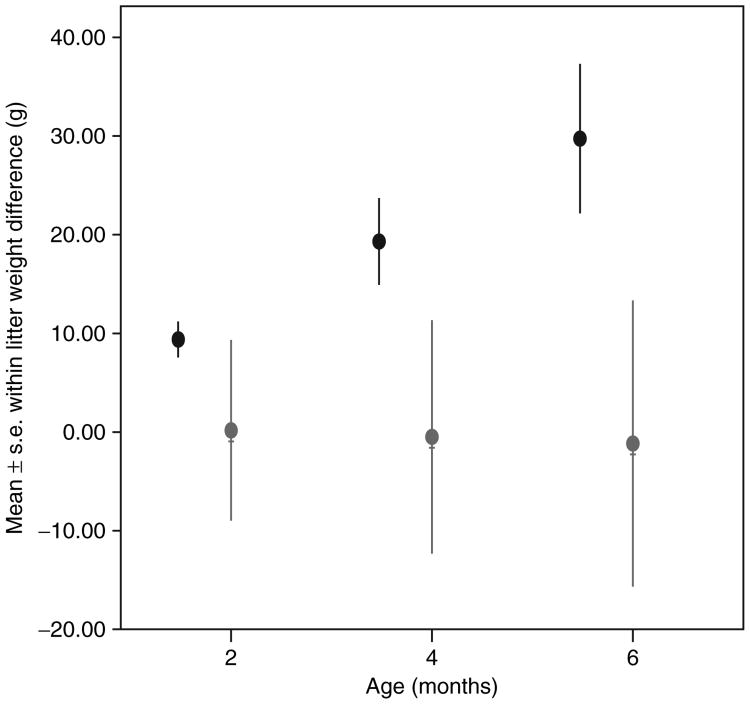
Early adult weight (17–24 months of age) was significantly related to birth weight in females, but not in males (Figure 2). Individuals who exceeded the 90th percentile in early adult weight were significantly larger than those that did not at 0, 2, 4, and 6 months of age (birth F = 9.854, P < 0.004; 2 months F = 12.77, P < 0.001; 4 months F = 13.45, P <0.001; 6 months F = 12.96, P < 0.001; see Table 4). The difference at birth, although statistically significant, was quite small (3%). The percent differences increased over infancy to ∼15%. The difference in early adulthood was 33.9%. Ten obese individuals had a litter mate—in only one case (one female–female litter) did both litter members exceed the 90th percentile in weight at early adulthood. Figure 3 illustrates the within-litter weight differences at 2–6 months for six male–female litters and three female–female litters in which at least one litter member exceeded the 90th percentile in early adulthood. Females litter mates tended to have similar growth trajectories, even if they differentiated in weight postinfancy, while males in male–female litters had a lower growth slope in infancy than did their sisters. There was no female–male litter in which the male exceeded the female in early infant growth or in early adult weight. These growth patterns resulted in an average early adult weight difference between female litter mates of 35 g (n = 3, range 20–60) vs. an average difference of 98.3 g (n = 6, range 43–162) between female–male litter mates.
Figure 2.
Relation between birth weight and early adult weight in males vs. females, with birth weights segmented into low, (circles), medium (squares), and high (diamonds). Groups that do not share a dotted line ([---]) differ from one another at P < 0.05.
Table 4. Weights (mean ± s.d.) through infancy and early adulthood for animals over (n = 13) or below (n = 36) the 90th percentile of early adult weight.
| Age (months) | >90th percentile | <90th percentile | % Difference |
|---|---|---|---|
| 0 | 31.6 ± 3.0 | 29.9 ± 2.7 | 3.0 |
| 2 | 120.4 ± 12.0 | 105.1 ± 10.0 | 13.6 |
| 4 | 202.8 ± 12.0 | 175.9 ± 15.5 | 14.9 |
| 6 | 285.1 ± 16.2 | 246.8 ± 23.4 | 15.5 |
| 24 | 514.6 ± 36.1 | 383.3 ± 45.2 | 33.9 |
Figure 3.
Average within-litter weight differences for six female–male litters (circles) and three female–female litters (♀, in gray) in which at least one member exceeded the 90th percentile in early adult weight.
Discussion
The results of these studies indicate that marmoset monkeys, in common with macaques (8–11,13), baboons (12), and vervet monkeys (14), spontaneously develop high body weight and high relative fat concentrations in captivity. The causes of the secular trend of increased body weight in marmosets (19, this study) have not been determined. The basic forms of caging and husbandry has remained the same throughout this colony's history. The population described in study 2 was maintained on the same base diets until 2006 when we switched from the ZuPreem to the Purina Mazuri base diet (reflecting changes in weaning and early adult diet for births numbered ≥520—see Figure 2), while continuing to feed the purified diet throughout. No striking change in adult weights, growth rates, or body composition was noted in association with this change.
A previously unreported finding in this species was the sex differences in propensity to develop large body weight and higher relative fat weight—i.e., to become overweight or obese. Marmosets are generally described as sexually monomorphic in terms of body size. We previously reported no sex differences in fat weight (estimated by deuterium- oxide-labeled water dilution) relative to lean weight or total body weight in a sample of 20 subjects, representative of the adult population from the early years of the Southwest National Primate Research Center breeding colony (26). Though fat and lean estimation methods differed in the two studies, a comparison of (26) and study 1 of this paper suggests that trends in larger body mass and fat mass in marmosets show a striking sex difference. Average male weight was 6.0% higher and fat mass did not differ between the two studies. In contrast, average female weight was 28.7% higher in study 1 than in ref. 26 and fat weight was increased by 141% (22.99–55.48 g). There was no sex difference in lean weight in ref. 26 or in this study. Therefore, female marmosets, in common with many other mammals including humans and other nonhuman primates show a higher propensity than males to store fat, but males marmosets do not show the lean weight sex difference often seen in other species—i.e., males having higher absolute lean weight.
Growth data suggest that sex differences in propensity to obesity begin early in life. Female birth weight was significantly related to adult size but this was not true for males. A sex difference in relative fat preceding puberty has been frequently described in humans (e.g., refs. 37–42 but see refs. 43–45). Future studies will determine the extent to which these early life weight differences in the marmoset reflect lean vs. fat weight accumulation. Although no systematic examination of neonatal body composition in marmosets has been conducted, studies from other nonhuman primates (46) as well as our anecdotal findings from necropsy of stillborn, normal weight marmoset infants suggests that marmoset neonates are unlikely to have large stores of body fat. Humans are unusual among primates in terms of having substantial amounts of adipose tissue at birth (46). Exploring how rapidly marmoset infants accumulate adipose tissue following birth and the variance in that trait is part of an ongoing project in our laboratory.
In study 1, animals classified as obese had altered lipid parameters (higher triglyceride and VLDL) and altered glucose metabolism (higher HbA1c, indicating a higher degree of ongoing hyperglycemia). Using our operationally defined risk factors, 7.8% of subjects fit the ATP III definition of metabolic syndrome, with at least three risk factors. An additional 7.8% had two risk factors. We were unable in this study to monitor blood pressure, a factor that we will add to future studies. Also of interest in future studies is to determine whether or not exposure to higher caloric density diets will increase the percentage of subjects meeting this operational definition of metabolic syndrome.
Fasting glucose (168 mg/dl) in nonobese subjects was high compared to larger-bodied primates but similar to values previously reported for marmosets (47,48). The fact that high fasting glucose was the single variable most likely to occur without association with any other atypical values, suggests that some of these glucose concentrations may be elevated in response to stress due to capture or handling; however, similar average values are reported for sedated and unsedated marmosets (47). HbA1c and fasting glucose concentrations are significantly positively correlated, suggesting that fasting glucose concentration may still be a valid measure of hyperglycemia.
In summary, the marmoset demonstrates the similar suites of body composition, glucose metabolism alterations and lipid alterations as are seen in other nonhuman primates and in humans, suggesting that this species is worthy of consideration for obesity studies in which its fast maturity, high fertility, relatively short lifespan, and small size may be of advantage. Marmoset twins offer the opportunity to have >1 infant exposed to the same uterine and postnatal family environments. Future studies may also take advantage of the fact that the infants are easily fostered between parents.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Levin B, Dunn-Meynell D, Balkan B, Keesey R. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273:R725–R730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 3.Speakman J, Hambly C, Mitchell S, Krol E. Animal models of obesity. Obes Rev. 2007;8(1):55–61. doi: 10.1111/j.1467-789X.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 4.Spurlock M, Gabler N. The development of porcine models of obesity and the metabolic syndrome. J Nutr. 2008;138:397–402. doi: 10.1093/jn/138.2.397. [DOI] [PubMed] [Google Scholar]
- 5.Levin B, Govek E. Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol. 1998;275:R1374–R1379. doi: 10.1152/ajpregu.1998.275.4.R1374. [DOI] [PubMed] [Google Scholar]
- 6.Henrichs S. Mouse feeding behavior: ethology, regulatory mechanisms and utility for mutant phenotyping. Behav Brain Res. 2001;125:81–88. doi: 10.1016/s0166-4328(01)00287-x. [DOI] [PubMed] [Google Scholar]
- 7.Arner P. Resistin: yet another adipokine tells us that men are not mice. Diabetologia. 2005;48:2203–2205. doi: 10.1007/s00125-005-1956-3. [DOI] [PubMed] [Google Scholar]
- 8.Kemnitz J. Obesity in macaques: spontaneous and induced. Adv Vet Sci Comp Med. 1984;28:81–114. doi: 10.1016/b978-0-12-039228-5.50009-7. [DOI] [PubMed] [Google Scholar]
- 9.Bodkin N, Hannah J, Ortmeyer H, Hansen B. Central obesity in rhesus monkeys: association with hyperinsulinemia, insulin resistance and hypertriglyceridemia? Int J Obes Relat Metab Disord. 1993;17:53–61. [PubMed] [Google Scholar]
- 10.Hansen B, Bodkin N. Primary prevention of diabetes mellitus by prevention of obesity in monkeys. Diabetes. 1993;42:1809–1814. doi: 10.2337/diab.42.12.1809. [DOI] [PubMed] [Google Scholar]
- 11.West D, York B. Dietary fat, genetic predisposition and obesity: lessons from animal models. Am J Clin Nutr. 1998;67:505S–512S. doi: 10.1093/ajcn/67.3.505S. [DOI] [PubMed] [Google Scholar]
- 12.Comuzzie A, Cole C, Martin L, et al. The baboon as a nonhuman primate model for the study of the genetics of obesity. Obes Res. 2003;11:75–80. doi: 10.1038/oby.2003.12. [DOI] [PubMed] [Google Scholar]
- 13.Wagner J, Kavanagh K, Ward G, et al. Old world primate models of type 2 diabetes mellitus. ILAR J. 2006;47:259–271. doi: 10.1093/ilar.47.3.259. [DOI] [PubMed] [Google Scholar]
- 14.Kavanagh K, Fairbanks L, Bailey J, et al. Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obesity (Silver Spring) 2007;15:1666–1674. doi: 10.1038/oby.2007.199. [DOI] [PubMed] [Google Scholar]
- 15.Oiken E, Gillman M. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 16.Baird J, Fisher D, Lucas P, et al. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331:929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tardif S, Smucny D, Abbott D, et al. Reproduction in captive common marmosets (Callithrix jacchus) Comp Med. 2003;53:365–368. [PubMed] [Google Scholar]
- 18.Abbott D, Barnett D, Colman R, Yamamoto ME, Schultz-Darken NJ. Aspects of common marmoset basic biology and life history important for biomedical research. Comp Med. 2003;53:339–350. [PubMed] [Google Scholar]
- 19.Allison D, Newton W, Beasley M. Unexplained increases in marmoset body weights: a parallel obesity epidemic? Obesity. 2007;15:A121. [Google Scholar]
- 20.Ford S, Davis L. Systematics and body size: implications for feeding adaptations in New World monkeys. Am J Phys Anthropol. 1992;88:415–468. doi: 10.1002/ajpa.1330880403. [DOI] [PubMed] [Google Scholar]
- 21.Araujo A, Arruda M, Alencar A, et al. Body weight of wild and captive common marmosets. Int J Primatol. 2000;21:317–324. [Google Scholar]
- 22.Abbott D, Hearn J. Physical, hormonal and behavioural aspects of sexual development in the marmoset monkey, Callithrix jacchus. J Reprod Fertil. 1978;53:155–166. doi: 10.1530/jrf.0.0530155. [DOI] [PubMed] [Google Scholar]
- 23.Poole T, Evans S. Reproduction, infant survival and productivity of a colony of common marmosets (Callithrix jacchus) Lab Anim. 1982;16:88–97. doi: 10.1258/002367782780908760. [DOI] [PubMed] [Google Scholar]
- 24.Peters V, Guerra M. Growth of marmoset monkeys Callithrix jacchus in captivity. Folia Primatol (Basel) 1998;69:266–272. doi: 10.1159/000021636. [DOI] [PubMed] [Google Scholar]
- 25.Tardif S, Power M, Oftedal O, Power R, Layne D. Lactation, maternal behavior and infant growth in common marmoset monkeys (Callithrix jacchus): effects of maternal size and litter size. Behav Ecol Sociobiol. 2001;51:17–25. [Google Scholar]
- 26.Power R, Power M, Layne D, et al. Relations among measures of body composition, age and sex in the common marmoset monkey (Callithrix jacchus) Comp Med. 2001;51:218–223. [PubMed] [Google Scholar]
- 27.Eckel R, Grundy S, Zimmet P. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 28.Power M, Schulkin J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: possible evolutionary origins. Br J Nutr. 2008;99:931–940. doi: 10.1017/S0007114507853347. [DOI] [PubMed] [Google Scholar]
- 29.Pond C. The biological origins of adipose tissue in humans. In: Morbeck M, Galloway A, Zihlman A, editors. The Evolving Female: A Life-History Perspective. Princeton University Press; Princeton, NJ: 1997. pp. 147–162. [Google Scholar]
- 30.Wailke B, Goodner D, Koerker E, Chideckel EW, Kalnasy LW. Assessment of obesity in pigtailed monkeys. J Med Primatol. 1977;6:151–162. doi: 10.1159/000459737. [DOI] [PubMed] [Google Scholar]
- 31.Rutenberg G, Coehlo A, Lewis S, Carey KD, McGill J. Body composition in baboons: evaluating a morphometric method. Am J Primatol. 1987;12:275–285. doi: 10.1002/ajp.1350120305. [DOI] [PubMed] [Google Scholar]
- 32.McFarland R. Female primates: fat or fit? In: Morbeck M, Galloway A, Zihlman A, editors. The Evolving Female: A Life-History Perspective. Princeton University Press; Princeton, NJ: 1997. pp. 163–176. [Google Scholar]
- 33.Tardif S, Power M, Paulik M. Gender and obesity in the marmoset monkey (Callithrix jacchus) Obesity. 2007;15:A122. [Google Scholar]
- 34.Tinsley F, Taicher G, Heiman M. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes Res. 2004;12:150–160. doi: 10.1038/oby.2004.20. [DOI] [PubMed] [Google Scholar]
- 35.Grundy S, Brewer H, Cleeman J, et al. Definition of metabolic syndrome: report of the National Heart, Lung and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 36.Tardif S, Bales K. Relations among birth condition, maternal condition and postnatal growth in captive common marmosets (Callithrix jacchus) Am J Primatol. 2004;62:83–94. doi: 10.1002/ajp.20009. [DOI] [PubMed] [Google Scholar]
- 37.Fukuyama S, Inaoka T, Matsumura Y, et al. Anthropometry of year 5–19old Tongan children with special interest in the high prevalence of obesity among adolescent girls. Ann Hum Biol. 2005;32:714–723. doi: 10.1080/03014460500273275. [DOI] [PubMed] [Google Scholar]
- 38.Buison A, Ittenbach R, Stallings V, Zemel B. Methodological agreement between two-compartment body-composition methods in children. Am J Hum Biol. 2006;18:470–480. doi: 10.1002/ajhb.20515. [DOI] [PubMed] [Google Scholar]
- 39.Whelton H, Harrington J, Crowley E, et al. Prevalence of overweight and obesity on the island of Ireland: results from the North South survey of children's height, weight and body mass index 2002. BMC Public Health. 2007;7:187. doi: 10.1186/1471-2458-7-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanigorski A, Bell A, Kremer P, Swinburn B. High childhood obesity in an Australian population. Obesity (Silver Spring) 2007;15:1908–1912. doi: 10.1038/oby.2007.226. [DOI] [PubMed] [Google Scholar]
- 41.Blair N, Thompson J, Black P, Becroft D, et al. Risk factors for obesity in 7-year-old children: the Auckland birthweight collaborative study. Arch Dis Child. 2007;92:866–871. doi: 10.1136/adc.2007.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishimura R, Sano H, Matsudaira T, et al. Childhood obesity and its relation to serum adiponectin and leptin: a report from a population-based study. Diabetes Res Clin Pract. 2007;76:245–250. doi: 10.1016/j.diabres.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 43.Davis C, Flickinger B, Moore D, et al. Prevalence of cardiovascular risk factors in schoolchildren in a rural Georgia community. Am J Med Sci. 2005;330:53–59. doi: 10.1097/00000441-200508000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Haque F, de la Rocha A, Horbul B, Desroches P, Orrell C. Prevalence of childhood obesity in northeastern Ontario: a cross-sectional study. Can J Diet Pract Res. 2006;67:143–147. doi: 10.3148/67.3.2006.143. [DOI] [PubMed] [Google Scholar]
- 45.Bua J, Olsen L, Sorensen T. Secular trends in childhood obesity in Denmark during 50 years in relation to economic growth. Obesity (Silver Spring) 2007;15:977–985. doi: 10.1038/oby.2007.603. [DOI] [PubMed] [Google Scholar]
- 46.Kuzawa C. Adipose tissue in human infancy and childhood: an evolutionary perspective. Am J Phys Anthropol. 1998;(27):177–209. doi: 10.1002/(sici)1096-8644(1998)107:27+<177::aid-ajpa7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 47.McNees D, Lewis R, Ponzio B, Sis RF, Stein FJ. Blood chemistry of the common marmoset (Callithrix jacchus) maintained in an indoor-outdoor environment: primate comparisons. Primates. 1984;25:103–109. [Google Scholar]
- 48.Clarke J. The common marmoset (Callithrix jacchus) ANZCCART News. 1994;7:1–8. [Google Scholar]