Abstract
An array of examples of diastereoselective, phosphate tether-mediated ring-closing metathesis reactions, which highlight the importance of product ring size and substrate stereochemical compatibility, as well as complexity, is reported. Studies focus primarily on the formation of bicyclo[n.3.1]phosphates, involving the coupling of C2-symmetric dienediol subunits with a variety of simple, as well as complex alcohol cross-partners.
Keywords: phosphate tether, diastereotopic differentiation, ring-closing metathesis, bicyclic phosphate, natural products
Introduction
The development of atom-,[1] step-,[2] and redox-economical[3] methods to generate important subunits common to a variety of biologically-relevant natural products stands at the forefront of modern-day synthesis and drug discovery. In particular, tether-mediated methodologies which couple both simple and complex molecular fragments to access highly functionalized core intermediates represent some of the most facile and convergent pathways to accomplish this goal.[4] Over the past decade, we have investigated the use of phosphate tethers to access complex polyol fragments via the desymmetrization of 1,3-anti-diol subunits using ring-closing metathesis (RCM) and one-pot, sequential RCM/CM/hydrogenation protocols.[5] In this regard, reported studies have focused solely on the use of simple allyl alcohol coupling partners to synthesize bicyclo[4.3.1]-phosphates en route to the syntheses of bioactive natural products.[6] Despite obvious attributes, a thorough understanding of the behavior of phosphate tethers across a wide range of substrates represents a notable deficiency that impedes general applicability of the method—as well as extensive use—in the synthesis of biologically active small molecules. This stands in contrast to seminal works by Evans, Kobayashi, and others that have provided insight on the behavior of silicon tethers to RCM in a variety of systems.[4,7] Inspired by this shortcoming, as well as a surprising inability to access the targeted key fragment en route to the synthesis of dictyostatin (1) (Scheme 1), vide infra, ongoing efforts in our lab have focused on the exploration of phosphate-tether reactivity profiles across a spectrum of substrates. We herein report the preliminary results of a detailed study highlighting the stereochemical factors involved in phosphate tether-mediated diastereotopic differentiation of 1,3-anti-diol subunits (Figure 1).
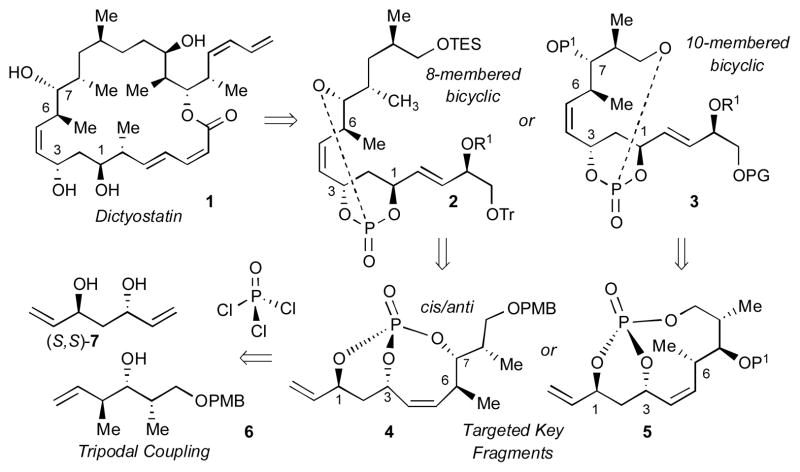
Scheme 1.
Proposed retrosynthesis of dictyostatin via a phosphate tether-mediated desymmetrization approach.
Figure 1.
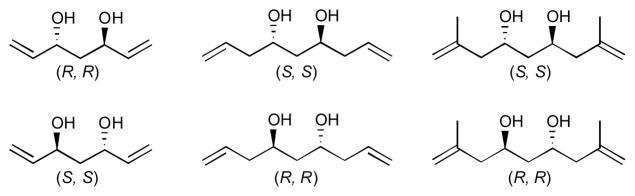
C2-symmetric 1,3-anti-diol substrates
Recent efforts towards synthesis of 1,3-anti-diol containing natural products utilizing phosphate tether-mediated selective reactions[5,6] led to application for dictyostatin, a marine macrolide with promising antitumor and anticancer activities.[8] Retrosynthetic analysis of fragments 2 or 3 (Scheme 1) revealed that 1 could, in theory, be constructed via a phosphate tether-mediated tripodal coupling of POCl3, C2-symmetric diene diol (S,S)-7 and olefin partner 6, followed by RCM to yield the bicyclo[5.3.1]- and [7.3.1]-phosphates 4 and 5, respectively.
Results and Discussion
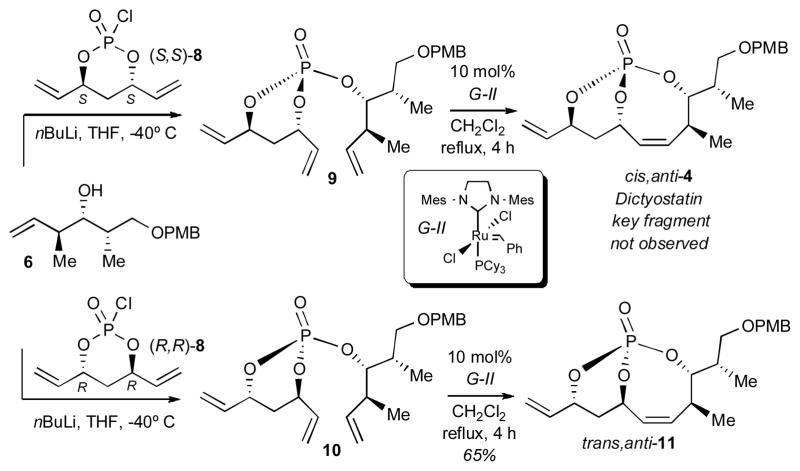
Initial studies focused on synthesis of the C1–C8 fragment of dictyostatin via formation of the Z-configured, bicyclo[5.3.1]-phosphate 4 from triene 9 [via monochlorophosphate (S,S)-8] (Scheme 2). However, the desired product 4 was not observed.[9] Interestingly, coupling of (R,R)-8 with 6 generated triene 10, which upon RCM yielded bicyclic phosphate 11 (dictyostatin diastereomeric subunit) in 65%. This result demonstrated that unforeseen factors were operative, which prompted efforts to carry out a detailed investigation of more complex tethers.
Scheme 2.
RCM to bicyclo[5.3.1]phosphate intermediates en route to key fragments of dictyostatin.
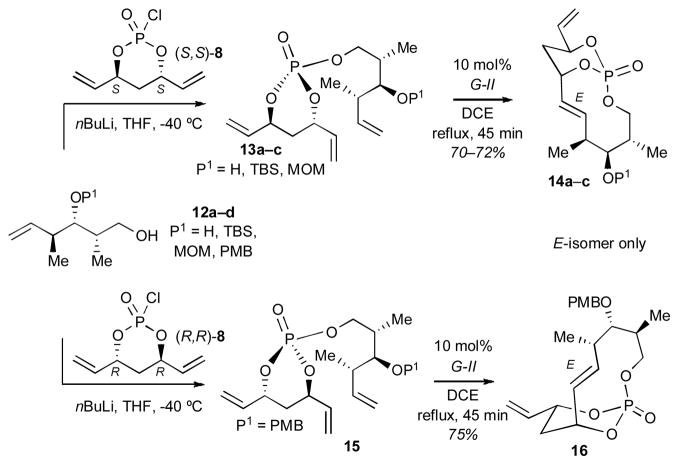
Studies were next focused on the synthesis of the Z-configured 10-membered subunit 5 (Scheme 1) via RCM with substrates bearing requisite “dictyostatin-like” olefin tether partners (Scheme 3). In this study, trienes 13a–c, possessing various substitutions at the C3 carbinol (P1 = H, TBS, and MOM), were synthesized. Subsequent RCM of 13a–c afforded excellent yields of E-configured bicyclo[7.3.1]phosphates 14a–c. In addition, the diastereomeric triene 15 [derived from (R,R)-8] also produced the RCM product 16 in good yield and with E-selectivity. Collectively, these results prompted us to carry out detailed RCM studies to various bicyclo[n.3.1]phosphates.
Scheme 3.
RCM to bicyclo[7.3.1]phosphate intermediates en route to key fragments of dictyostatin.
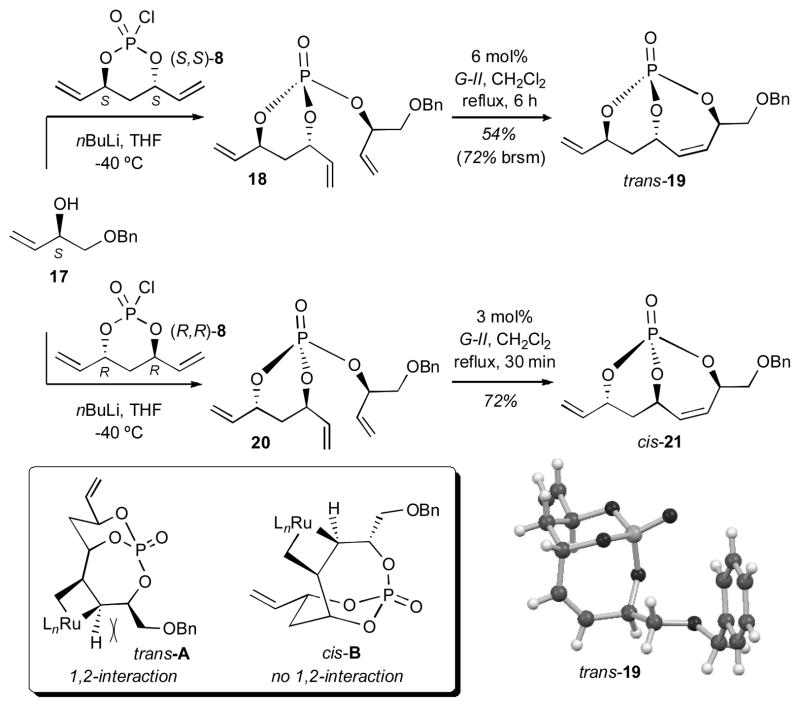
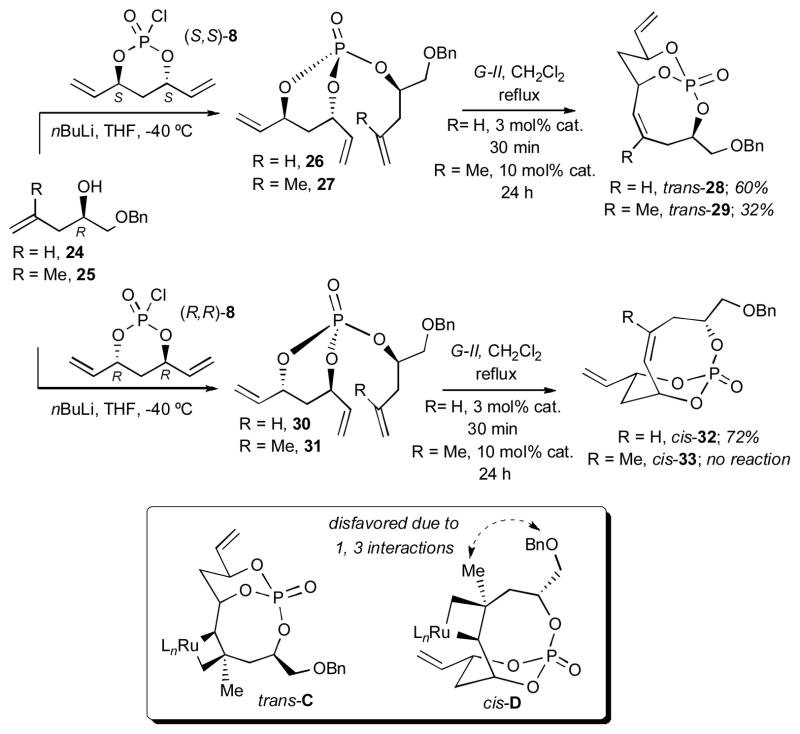
Investigations began with RCM studies of trienes 18 and 20 constructed from enantiomeric (S,S)-8 and (R,R)-8 en route to 7-membered bicyclo[4.3.1]phosphates trans-19 and cis-21, respectively (Scheme 4). In contrast to the elegant diastereoselective RCM studies by Evans and coworkers7 using Si-tethered dienes where only cis-substituted 7-membered products were observed, RCM of phosphate trienes 18 and 20, yielded both 7-membered products trans-19 and cis-21, albeit with different reaction rates and catalyst loadings (Scheme 4). In addition, the cis-diastereomer reacted at a much faster rate and with better yields. Presumably, a detrimental 1,2-steric interaction between the CH2OBn group and the metallocyclobutane in intermediate trans-A outlined in Scheme 4 is operative, thus slowing RCM for the trans-substituted case.
Scheme 4.
Bicyclo[4.3.1]phosphate series.
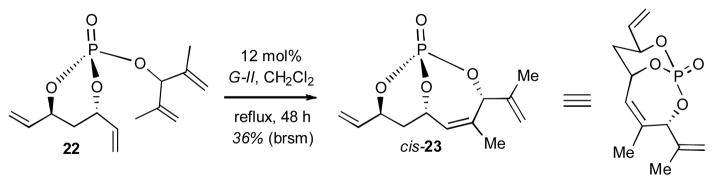
In order to ascertain additional information regarding the aforementioned 1,2-interaction, a double diastereotopic differentiation investigation of triene 22 was initiated using RCM. RCM of triene 22 [derived from the coupling of monochlorophosphate (S,S)-8 with 2,4-dimethylpenta-1,4-dien-3-ol] exclusively produced the kinetically favored cis-substituted bicyclo[4.3.1]phosphate 23 via an intermediate lacking a 1,2-interaction as in intermediate cis-B.
Studies were next directed to the 8-membered RCM reactions of trienes 26 and 30 derived from the coupling of alcohol (R)-24 (R = H) with monochlorophosphates (S,S)-8 and (R,R)-8, respectively. Gratifyingly, both 8-membered bicyclo-[5.3.1] phosphates, trans-28 and cis-32, respectively, were formed with good yields (Scheme 6). In similar fashion, the trienes 27 and 31 were synthesized from methyl-substituted alcohol (R)-25 (R = Me). When subjected to the RCM reaction only the trans-29 formed, albeit in only 32% yield, while no cis-product formation 33 was observed. A plausible explanation for these results is highlighted with trans-C and cis-D intermediates, whereby the metallocyclobutane would most likely form on the exo-face presumably due to the concave structure of the bicyclic phosphate and the size of the Ru-complex. Based on this assumption, in the case of the formation of cis-33, an unfavorable 1,3-interaction[10] between the vinylic CH3 and CH2OBn groups impedes RCM. However, for trans-29, no such interaction exists, thus RCM proceeds, albeit in moderate yield.
Scheme 6.
Bicyclo[5.3.1]phosphate series.
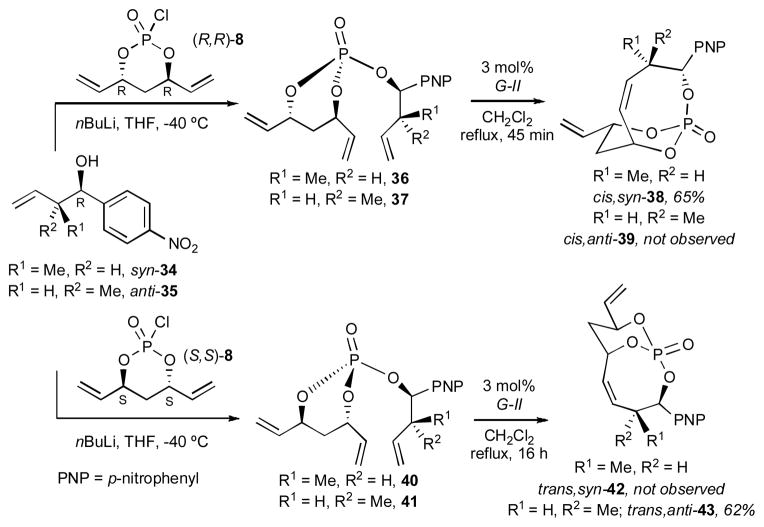
Comparative analysis of the non-allylic substituted trienes 26 and 30 that readily underwent RCM (Scheme 6), to trienes 9 and 10, bearing allylic Me-substitution (Scheme 3), allowed us to conclude that the stereochemistry of allylic substitution was the deciding factor in the successful formation of the desired product. Thus, additional studies were conducted for the RCM reactions of trienes derived from monochlorophosphates (R,R)-8/(S,S)-8 and substituted homoallyl alcohols syn-34 and anti-35 (Scheme 7). Product formations were observed only for cis,syn-38 and trans,anti-43, which is consistent with the previously mentioned preliminary results for dictyostatin, vide supra.
Scheme 7.
Effect of methyl substitution in RCM to bicyclo[5.3.1]phosphates.
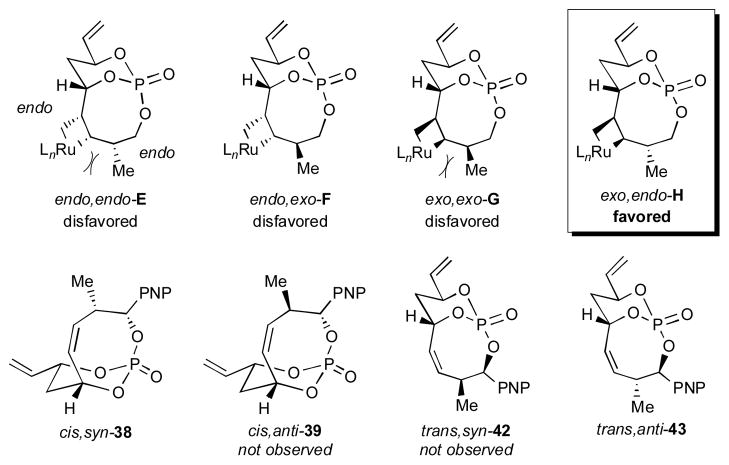
A plausible model was developed in order to provide insight for the observed stereochemical outcomes seen in the bicyclo[5.3.1]phosphate cases, in which the stereochemistry of allylic substitution is the critical factor (Figure 2). When considering the metallocyclobutane intermediates, the previous assumption that metallocyclobutane formation occurs on the exocyclic face eliminates the intermediates, endo,endo-E and endo,exo-F. Inspection of the remaining two intermediates, exo,exo-G and exo,endo-H, reveals an unfavorable steric interaction between the exo-Me and the required exo-metallocyclobutane in the case of exo,exo-G, which impedes the formation of the resultant bicyclic phosphate. Thus, only when the allylic Me is endo and the formed metallocyclobutane is exo is the intermediate energetically accessible such that the reaction can proceed to completion. This trend analysis therefore accounts for the selectivity.
Figure 2.
Proposed transition states and accessible products.
For experimental confirmation of the proposed intermediates in Figure 2, we synthesized triene 45a from (±)-2-methylbut-3-en-1-ol and the monochlorophosphate (S,S)-8 in order to perform a double diastereotopic differentiation experiment (Scheme 8). Subsequent RCM reaction exclusively generated bicyclo[5.3.1]phosphate diastereomer 46, which was confirmed by X-ray crystallography, along with unreacted diastereomeric triene 45b.[11] X-ray crystallography analysis[12] confirmed the endo orientation of the allylic methyl group in the bicyclic phosphate 46, thus supporting our proposed favorable intermediate exo,endo-H shown in Figure 2.
Scheme 8.
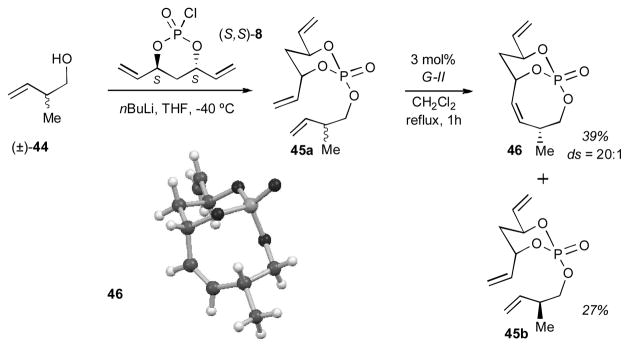
Experimental confirmation for above proposed intermediate exo,endo-H.
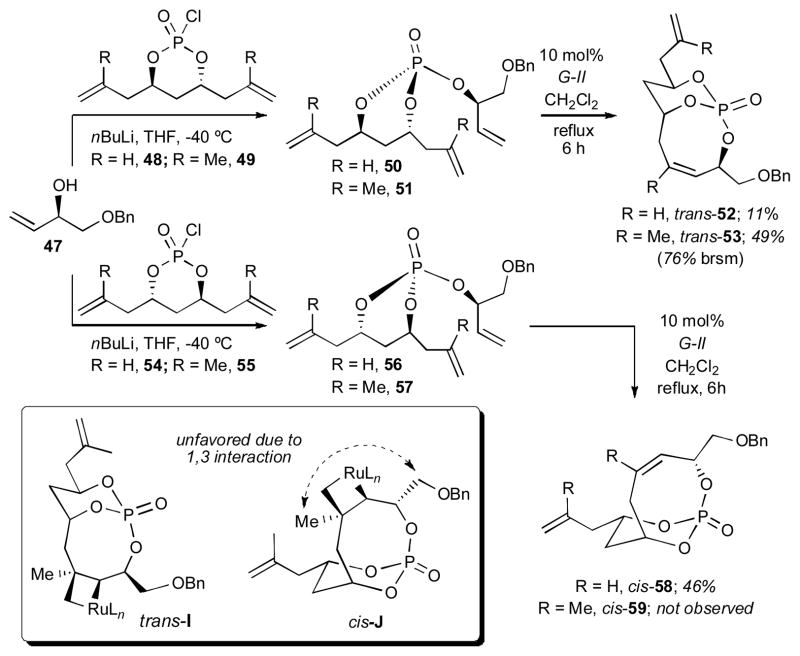
Next, we extended the 8-membered RCM studies to include coupling of the homologated monochlorophosphates 48, 49, 54 and 55 with allylic alcohol 47 (Scheme 9). Trienes 50 and 56, derived from unsubstituted monochlorophosphates 48 and 54 (R = H), respectively, participated in the RCM reaction although with poor yield for the trans-substituted bicyclo[5.3.1]phosphate 52 compared to the cis-substituted bicyclo[5.3.1]phosphate 58. The lowered yield of trans-52 was presumable due to an unfavorable 1,2-interaction between metallocyclobutane and –CH2OBn in the intermediate trans-I. In the cases of methyl-substituted homologated trienes 51 and 57, no cis RCM product 59 was observed due to a highly unfavorable 1,3-interaction (syn-pentane) between –CH3 and –CH2OBn in intermediate cis-J.[10]
Scheme 9.
RCM to bicyclo[5.3.1]phosphates with homologated 1,3-anti-dienediol.
Conclusion
In conclusion, we have detailed the phosphate tether-mediated diastereotopic differentiation of C2-symmetric dienediol subunits via RCM. Experimental outcomes were shown to be highly dependent upon various parameters, including the concave nature of the bicyclic phosphate, the stereochemistry within each coupling partner and ring size. Plausible metallocyclobutane-containing intermediates for RCM reactions to bicyclo[4.3.1]- and [5.3.1]-phosphates are proposed to rationalize observed experimental outcomes. In addition, notable trans-products in the bicyclo[4.3.1]phosphate series, as well as exclusive E-selectivity in the bicyclo[7.3.1]phosphate series, were also observed. Further applications in the synthesis of polyketide natural products, along with RCM studies towards other bicyclo[n.3.1]phosphates, are in order and will be reported in due course.
Experimental Section
(4S,6S)-2-(((2S,3S,4S)-1-((4-methoxybenzyl)oxy)-2,4-dimethylhex-5-en-3-yl)oxy)-4,6-divinyl-1,3,2-dioxaphosphinane 2-oxide (9)
To a solution of alcohol 6 (0.152 g, 0.575 mmol) in THF (2.9 mL), at −40 °C under argon, was added n-BuLi (2.5 M, 0.551 mmol), dropwise. The mixture was allowed to stir for 5 minutes, at which point a solution of phosphate monochloride (S,S)-8 (0.100 g, 0.479 mmol) in THF (1 mL) was slowly added to the reaction vessel via cannulation. The mixture stirred at −40 °C for 2 hours (monitored by TLC) and was quenched with 3 mL of aqueous NH4Cl (sat.). The biphasic solution was separated, and the aqueous layer was extracted EtOAc (3 × 5 mL). The combined organic layers were washed with brine, dried (Na2SO4), and concentrated under reduced pressure. Purification via flash chromatography (silica, 2:1 Hexanes/EtOAc) provided 0.172 g (82% yield) of triene product 9, as a colorless oil.13 Optical Rotation: [α]D = +23.0 (c = 0.1, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.28 (d, J = 8.0 Hz, 2H), 6.87 (d, J = 8.1 Hz, 2H), 6.01 (ddd, J = 16.7, 10.8, 5.7 Hz, 1H), 5.96–5.78 (m, 2H), 5.45 (d, J = 17.1 Hz, 1H), 5.38 (d, J = 17.2 Hz, 1H), 5.29 (d, J = 10.6 Hz, 2H), 5.10–5.02 (m, 3H), 4.97–4.90 (m, 1H), 4.60–4.55 (m, 1H), 4.49 (d, J = 11.5 Hz, 1H), 4.36 (d, J = 11.5 Hz, 1H), 3.81 (s, 3H), 3.41 (dd, J = 9.0, 7.1 Hz, 1H), 3.32 (dd, J = 8.9, 7.1 Hz, 1H), 2.50 (dq, J = 13.8, 6.9 Hz, 1H), 2.18–2.11 (m, 1H), 2.06 (ddd, J = 18.5, 14.0, 6.0 Hz, 2H), 1.07 (d, J = 6.8 Hz, 3H), 0.99 (d, J = 6.8 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 159.0, 140.0, 135.4 (d, JCP = 4.0 Hz), 135.3 (d, JCP = 7.6 Hz), 130.7, 129.4, 117.7, 117.1, 115.6, 113.7, 85.0 (d, JCP = 7.4 Hz), 77.3 (d, J = 6.8 Hz), 75.5 (d, JCP = 6.2 Hz), 72.8 (d, JCP = 6.0 Hz), 72.5, 55.3, 41.2 (d, JCP = 3.5 Hz), 36.1 (d, JCP = 3.9 Hz), 35.2 (d, JCP = 7.3 Hz), 17.5, 11.9; 31P NMR (162 MHz, CDCl3) δ −7.01; FTIR (neat): 2952, 2949, 1615, 1547, 1252, 1234, 1009, 845, 741 cm−1; HRMS: cald. for C23H33O6PNa (M+Na)+ 459.1912; found 459.1902 (TOF MS ES+).
(1R,3S,4S,7R,9R,Z)-3-((S)-1-((4-methoxybenzyl)oxy)propan-2-yl)-4-methyl-9-vinyl-2,10,11-trioxa-1-phosphabicyclo[5.3.1]undec-5-ene 1-oxide (trans,anti-11)
To a flask containing triene phosphate 10 (0.080 g, 0.183 mmol) in CH2Cl2 (dry, degassed, 26 mL), equipped with an argon inlet and reflux condenser, was added portion wise (ImesH2)(PCy3)(Cl)2Ru=CHPh (G-II)14 (16 mg, 0.018 mmol, 10 mol%), and the reaction mixture was heated to reflux. Upon completion (monitored by TLC), the reaction was cooled to room temperature and concentrated under reduced pressure. Purification via flash chromatography (silica, 2:1 Hexanes/EtOAc) provided 61 mg (65% yield) of the bicyclic phosphate 11 as a colorless oil.15 Optical Rotation: [α]D = −7.3 (c = 0.16, CHCl3);1H NMR (500 MHz, CDCl3) δ 7.26 (d, J = 8.5 Hz, 2H), 6.87 (d, J = 8.5 Hz, 2H), 5.87 (dddd, J = 17.2, 10.6, 5.4, 2.0 Hz, 1H), 5.49 (ddd, J = 11.6, 8.2, 2.8 Hz, 1H), 5.43 (ddd, J = 17.2, 1.2, 1.2 Hz, 1H), 5.39 (d, J = 11.9 Hz, 1H), 5.27 (ddd, J = 10.6, 1.2, 1.1 Hz, 1H), 4.98 (dd, J = 11.8, 5.4 Hz, 1H), 4.48 (s, 2H), 4.06 (ddd, J = 29.1, 11.5, 3.3 Hz, 1H), 3.80 (s, 3H), 3.71 (t, J = 8.9 Hz, 1H), 3.53–3.45 (m, 1H), 3.48 (dd, J = 9.1, 5.9 Hz, 2H), 2.24–2.11 (m, 2H), 1.78 (ddd, J = 14.5, 3.9, 1.8 Hz, 1H), 1.19 (d, J = 7.2 Hz, 3H), 1.01 (d, J = 6.6 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 159.1, 136.0, 135.4 (d, JCP = 10.5 Hz), 130.7, 129.2, 128.1, 117.1 (d, JCP = 1.3 Hz), 113.7, 83.6 (d, JCP = 7.4 Hz), 77.8 (d, JCP = 7.2 Hz), 76.1 (d, JCP = 6.3 Hz), 72.9, 72.2, 55.2, 36.5 (d, JCP = 6.2 Hz), 35.1, 32.6 (d, JCP = 1.3 Hz), 17.4, 9.4; 31P NMR (162 MHz, CDCl3) δ −10.71; FTIR (neat): 2982, 2934, 1605, 1507, 1268, 1241, 1003, 831, 741 cm−1; HRMS: cald. for C21H29O6PNa (M+Na)+ 431.1599; found 431.1575 (TOF MS ES+). For all other experimental data and spectra, see supporting information.
Supplementary Material
Scheme 5.
Kinetic control to bicyclo[4.3.1]phosphate.
Acknowledgments
This investigation was generously supported by funds provided by the National Institute of General Medical Sciences (NIH RO1 GM077309). We thank Dr. Justin Douglas and Sarah Neuenswander for assistance with NMR measurements and Dr. Todd Williams for HRMS analysis. We kindly acknowledge Dr. Victor Day of the Molecular Structure Group (MSG) at the University of Kansas for X-ray analysis (NSF-MRI grant CHE-0923449). We also thank Materia, Inc. for supplying metathesis catalyst.
Footnotes
Supporting information for this article is available on the WWW under http://www.chemeurj.org/ or from the author.
References
- 1.a) Trost BM. Science. 1991;254:1471–1477. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]; b) Trost BM. Angew Chem, Int Ed. 1995;34:259–281. [Google Scholar]
- 2.Wender PA, Verma VA, Paxton TJ, Pillow TH. Acc Chem Res. 2008;41:40–49. doi: 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]
- 3.a) Young IS, Baran PS. Nat Chem. 2009;1:193–205. doi: 10.1038/nchem.216. [DOI] [PubMed] [Google Scholar]; b) Hoffmann RW. Synthesis. 2006:3531–3541. [Google Scholar]
- 4.Cusak A. Chem Eur J. 2012;18:5800–5824. doi: 10.1002/chem.201103186. [DOI] [PubMed] [Google Scholar]
- 5.a) Whitehead A, McReynolds MD, Moore JD, Hanson PR. Org Lett. 2005;7:3375–3378. doi: 10.1021/ol0512886. [DOI] [PubMed] [Google Scholar]; b) Thomas CD, McParland JP, Hanson PR. Eur J Org Chem. 2009:5487–5500. doi: 10.1002/ejoc.200900560. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Venukadasula PKM, Chegondi R, Suryn GM, Hanson PR. Org Lett. 2012;14:2634–2637. doi: 10.1021/ol301007h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Venukadasula PKM, Chegondi R, Maitra S, Hanson PR. Org Lett. 2010;12:1556–1559. doi: 10.1021/ol1002913. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chegondi R, Tan MML, Hanson PR. J Org Chem. 2011;76:3909–3916. doi: 10.1021/jo200337v. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hanson PR, Chegondi R, Nguyen J, Thomas CD, Waetzig JD, Whitehead A. J Org Chem. 2011;76:4358–4370. doi: 10.1021/jo2003506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Evans PA, Cui J, Buffone GP. Angew Chem Int Ed. 2003;42:1734–1737. doi: 10.1002/anie.200250486. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Matsui R, Seto K, Fujita K, Suzuki T, Nakazaki A, Kobayashi S. Angew Chem Int Ed. 2010;49:10068–10073. doi: 10.1002/anie.201004746. [DOI] [PubMed] [Google Scholar]; c) Eiseman JL, Bai L, Jung WH, Moura-Letts G, Day BW, Curran DP. J Med Chem. 2008;51:6650–6653. doi: 10.1021/jm800979v. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Hoye TR, Jeon J, Kopel LC, Ryba TD, Tennakoon MA, Wang Y. Angew Chem Int Ed. 2010;49:6151–6155. doi: 10.1002/anie.201002293. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Evans PA. In: Metathesis in Natural Product Synthesis: Strategies, Substrates and Catalysis. Cossy J, Arseniyadis S, Meyer C, editors. Wiley-VCH; Weinheim: 2010. pp. 225–259. [Google Scholar]
- 8.a) Pettit GR, Chicacz ZA, Gao F, Boyd MR, Schmidt JM. J Chem Soc Chem Commun. 1994:1111–1112. [Google Scholar]; b) Paterson I, Britton R, Delgado O, Gardner NM, Meyer A, Naylor GJ, Poullennec KG. Tetrahedron. 2010;66:6534–6545. [Google Scholar]; c) Shin Y, Fournier JH, Fukui Y, Brückner AM, Curran DP. Angew Chem. 2004;116:4734–4737. doi: 10.1002/anie.200460593. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2004;43:4634–4637. doi: 10.1002/anie.200460593. [DOI] [PubMed] [Google Scholar]; d) Zhu W, Jiménez M, Jung WH, Camarco DP, Balachandran R, Vogt A, Day BW, Curran DP. J Am Chem Soc. 2010;132:9175–9187. doi: 10.1021/ja103537u. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) O’Neil GW, Phillips AJ. J Am Chem Soc. 2006;128:5340–5341. doi: 10.1021/ja0609708. [DOI] [PubMed] [Google Scholar]; f) Ramachandran PV, Srivastava A, Hazra D. Org Lett. 2007;9:157–160. doi: 10.1021/ol062737k. [DOI] [PubMed] [Google Scholar]; g) Jogalekar AS, Damodaran K, Kriel FH, Jung WH, Alcaraz AA, Zhong S, Curran DP, Snyder JP. J Am Chem Soc. 2011;133:2427–2436. doi: 10.1021/ja1023817. [DOI] [PubMed] [Google Scholar]; h) Gallon J, Esteban J, Bouzbouz S, Campbell M, Reymond S, Cossy J. Chem Eur J. 2012;18:11788–11797. doi: 10.1002/chem.201201001. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 9.This result is in contrast to the elegant RCM studies by Eustache and Curran to derive Si-tethered 8-membered monocyclic subunits en route to attenol A and C6-epi-dictyostatin, see Van de Weghe P, Aoun D, Boiteau JG, Eustache J. Org Lett. 2002;4:4105–4108. doi: 10.1021/ol0268438. and reference 7c, respectively.
- 10.For a similar observation in all carbon-based RCM, see: Liu J, Lotesta SD, Sorensen EJ. Chem Commun. 2011;47:1500–1502. doi: 10.1039/c0cc04077k.
- 11.See Supporting Information for 13C NMR analysis of45aand45b.
- 12.CCDC 905668 (trans-19) and 905667 (46) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif
- 13.All other trienes are synthesized following the same procedure.
- 14.Scholl M, Ding S, Lee CW, Grubbs RH. Org Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 15.All the ring-closing metathesis reactions are carried out following the same procedure with appropriate solvent, temperature and catalyst loading mentioned in the schemes.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.