Abstract
Hypothesis
Malignant mesotheliomas (MM) express VEGFR, PDGFR, and cKIT. Sorafenib is a potent inhibitor of the RAS/RAF/MEK pathway and also targets VEGFR and cKIT. We evaluated the activity of sorafenib in patients with unresectable mesothelioma.
Methods
MM patients who had received 0 to 1 prior chemotherapy regimens were treated with sorafenib 400mg orally twice daily continuously. The primary endpoint was objective response. ERK1/2 phosphorylation in archival tissues was correlated with response and survival.
Results
51 patients were enrolled, 50 were evaluable and included in analysis. Three patients had a partial response (6% (95% CI 1.3–16.6%)), and 27 (54%(95% CI 39.3–68.2%)) had stable disease. Median progression-free survival and median overall survival were 3.6 months and 9.7 months, respectively. Median survival was superior in epithelioid histology versus other types (10.7 months versus 3.7 months, p=0.0179). The difference in median overall survival between pre-treated and chemo-naive patients was not statistically significant (13.2 months versus 5 months, p=0.3117). Low/negative baseline tumor phospho-ERK1/2 levels were associated with improved overall survival (13.9 months versus 5.2 months; p=0.0066).
Conclusion
Sorafenib has limited activity in advanced MM patients, similar to that seen in with other VEFGR tyrosine kinase inhibitors. Additional studies of sorafenib in MM are not warranted.
Keywords: mesothelioma, sorafenib, vascular endothelial growth factor, tyrosine kinase inhibitor, clinical trial
INTRODUCTION
The median survival of patients with unresectable malignant mesothelioma is a year or less1. The only FDA approved treatment in this disease is the combination of cisplatin with pemetrexed, which achieves a response rate (RR) of 41%, time to progression (TTP) of 6 months, and median overall survival (OS) of 12 months1. There are no approved agents for patients who progress after first-line chemotherapy.
Vascular endothelial growth factor (VEGF), is a mitogen for vascular endothelial cells. Acting through its receptors VEGFR-1(Flt-1) and VEGFR-2 (KDR), VEGF is necessary for maintenance of tumor vasculature2. Treatment of mesothelioma cell lines with recombinant human VEGF results in phosphorylation of VEGFR-1 and KDR and induces proliferation of mesothelial cells3, while the addition of VEGF neutralizing antibodies inhibits this proliferative effect. Serum VEGF levels and tumor microvessel density correlate in patients with mesothelioma inversely with survival3, 4. VEGF also acts through Raf/Mek/Erk kinase pathways5. Multiple growth factor receptors that act through the Ras/Raf/Mek/Erk pathway such as EGFR, IGF-1R, PDGF BB and the receptor PDGFR β and VEGFR1/2 are over-expressed or aberrant in mesothelioma3, 6–8.
Sorafenib (BAY 43–9006) is a potent inhibitor of the RAF/MEK/ERK signaling pathway with additional activity against VEGFR 2, PDGFR β, and cKIT9. It is an approved agent in the treatment of renal cell carcinoma10 and hepatocellular carcinoma11. Given the selective inhibitory activity of sorafenib against Raf, VEGFR2, and PDGFR β, which are potential therapeutic targets in mesothelioma, a phase II study of sorafenib in unresectable mesothelioma was undertaken by the Cancer and Leukemia Group B (CALGB).
MATERIALS AND METHODS
Eligibility Criteria
Eligible patients had histologically confirmed malignant mesothelioma not amenable to curative surgery, including sarcomatoid, epitheloid, and mixed histologies of the pleura, peritoneum, pericardium and tunica vaginalis. Patients may have received no more than one pemetrexed-containing chemotherapy regimen. No prior tyrosine kinase/signal transduction/angiogenesis inhibitor therapy was allowed. Any prior chemotherapy or radiation must have been administered ≥4 weeks earlier. Other eligibility criteria included: ECOG PS 0 or 1, measurable disease, no therapeutic anticoagulation, no currently active second malignancy (completed treatment with <30% risk of relapse) other than non-melanoma skin cancers and carcinoma in situ of the cervix, and lab values reflective of adequate organ function (granulocytes ≥1500/mm3, platelets ≥100,000/mm3, total bilirubin ≤1.5 X upper limit of normal (ULN), AST ≤2.5 xULN, creatinine≤1.5 X ULN, INR <1.5). Availability of pathology blocks or slides from a core surgical biopsy was required for evaluation of biological correlates. The study was approved by the Institutional Review Board at each center and all patients were required to sign informed consent.
Study treatment and evaluation
Sorafenib was administered orally at a fixed dose of 400 mg twice daily. Twenty-eight days of treatment constituted once cycle. Treatment was continued until disease progression or unacceptable toxicity. Dose modifications included dose level-1 (200mg twice daily) and -2 (200mg once daily). Dose modification was recommended for grade ≥3 toxicities that were attributable to sorafenib. No dose re-escalation was allowed. Evaluations included weekly blood pressure measurements during the first treatment cycle and CT scans after every two cycles of treatment. Disease burden was measured by RECIST criteria, and in patients with solely a pleural rind, the modified RECIST criteria were used12.
Immunohistochemical analysis of tumor phospho-ERK 1/2 (p-ERK1/2) was performed on archival tissue with primary antibody p-ERK 1/2 (Thr202/Tyr204-Cell Signaling Technology). Staining of p-ERK 1/2 was based on intensity and degree of tumor staining. The results were subsequently divided into two categories: high/medium (group 1) and low/negative (group 2). BRAF mutation status was determined by amplification of exons 11 and 15 with flanking intronic primers followed by direct sequencing. Specific primer sequences are available upon request.
Statistical Methods
The primary endpoint of the study was objective response rate. Secondary endpoints included i) overall survival and progression free survival, ii) toxicities, and iii) correlation of BRAF mutations and p-ERK1/2 expression with anti-tumor activity. A one stage phase II design was used. A forty-four patient sample size was designed to differentiate response rates of 5% and 20% with 95% power by a two-sided test at 0.10 level of significance. It was assumed that two-thirds (n=29) of the patients would be treated on the study as second line therapy and would provide sufficient power for a subgroup analysis of outcome by line of treatment. Specifically 29 patients would provide an 86% power to differentiate between 5% and 20% response rate by a two-sided test at 0.10 level of significance. Response rate (complete/partial response) was calculated as well as its 95% confidence interval. Overall survival and progression free survival curve were estimated by Kaplan-Meier product limit method. The difference in OS and PFS between pre-treated vs. chemo naive patients was compared by log rank test, so as that between histological type (epitheloid vs. others). A step-wise multivariate cox regression with stay level of 0.15 and entry level of 0.20 was also performed adjusting for baseline covariates such as histological type (epitheloid vs. others), gender (female vs. male), performance status and age. For exploratory analysis of biological markers, Fisher’s exact tests were performed to evaluate association between p-ERK1/2 expression and response. Cox models were fit to test the correlations between progression-free survival / overall survival and biomarker p-ERK 1/2 while adjusting for other baseline covariates such as histological type (epitheloid vs. others), gender (female vs. male), and age (continuous variable). A step-wise method was used with the same entry and stay criteria. Patient registration and data collection were managed by the CALGB Statistical Center. Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chairperson. Statistical analyses were performed by CALGB statisticians.
RESULTS
Patient Characteristics
Fifty-one patients were enrolled between October 2004 and August 2005. One patient did not receive any treatment due to hospitalization for pain. All other patients are eligible and included in the analysis. Baseline characteristics of the patients are described in table 1. As expected for this disease, most patients were male. The most frequent histology was epithelioid, and the pleura was the predominant site of involvement. Sixty percent (60%) of the patients had been previously-treated with pemetrexed-based chemotherapy.
Table 1.
Patient Demographic and Clinical Characteristics (N=50)
| Characteristics | N | (%) | |
|---|---|---|---|
| Gender | Male | 35 | (70%) |
| Female | 15 | (30%) | |
| Age | < 50 | 3 | (6%) |
| Median: 69 | 50 – 59 | 8 | (16%) |
| Range (36,88) | 60 – 69 | 17 | (34%) |
| 70+ | 22 | (44%) | |
| Mesothelioma | Epithelioid | 37 | (74%) |
| Histology | Sarcomatoid | 4 | (8%) |
| Mixed | 7 | (14%) | |
| Subtype | |||
| Unknown | 2 | (4%) | |
| Site of Origin | Pleura | 45 | (90%) |
| Peritoneum | 5 | (10%) | |
| Previous | Yes | 30 | (60%) |
| Chemo | No | 20 | (40%) |
| Performance | 0 | 11 | (22%) |
| Status | 1 | 39 | (78%) |
A total of 252 cycles of sorafenib were administered to the 50 patients. The median number of treatment cycles administered was 3 (range 1–32). Patients who received prior chemotherapy underwent a median of 3.5 cycles (range 1–32) of chemotherapy while chemo-naïve patients received a median of 2 cycles (range 1–31) of chemotherapy (p=0.66)
Toxicity
Grade 3 and 4 toxicities are displayed in table 2. The most common grade 3 toxicity was fatigue followed by hand-foot syndrome. Grade 3 hypertension was uncommon occurring only in <5% of patients, and there were no incidences of grade 4 hypertension. The only grade 4 event was fatigue in one patient. There were no grade 5 toxicities. Five patients (10%) discontinued treatment due to toxicities. Sixteen patients (32 %) had dose reduction due to toxicity. Skin toxicity in ten patients and fatigue in eight patients were the most common reasons for dose reductions.
Table 2.
NCI CTC grade 3 and 4 toxicities following sorafenib treatment
| Toxicity | N = 50 | |
|---|---|---|
| Grade 3 N (%) |
Grade 4 N (%) |
|
| HEMATOLOGICAL | ||
| Lymphopenia | 2 (4%) | 0 |
| Hemoglobin | 1 (2%) | 0 |
| Hemolysis | 1 (2%) | 0 |
| NON-HEMATOLOGICAL | ||
| Fatigue | 12 (24%) | 1 (2%) |
| Rash: hand-foot skin reaction | 6 (12%) | 0 |
| Dyspnea (shortness of breath) | 4 (8%) | 0 |
| Pain | 3 (6%) | 0 |
| Anorexia | 2 (4%) | 0 |
| Neuropathy: sensory | 2 (4%) | 0 |
| Hypertension | 2 (4%) | 0 |
| Hypotension | 1 (2%) | 0 |
Efficacy outcomes
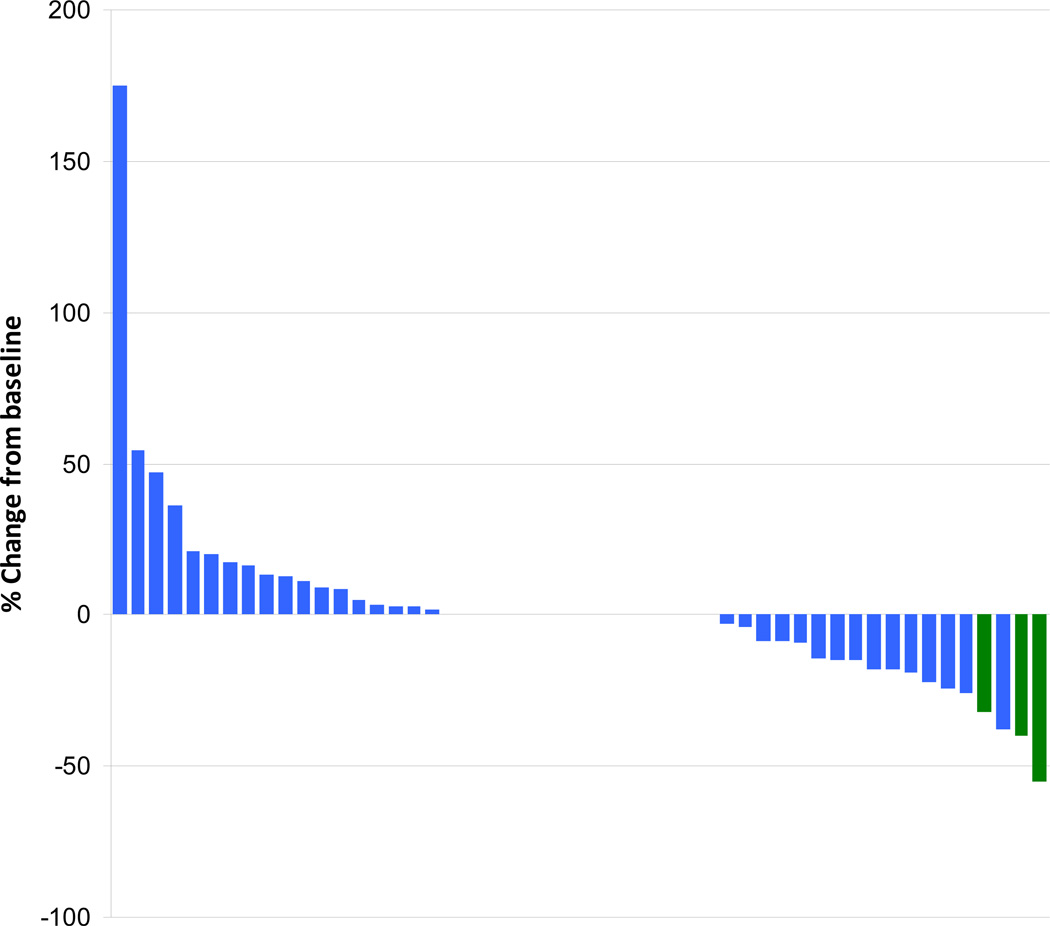
Response data are available for 50 patients. Three patients (6%, 95% CI 1.3–16.6%) had partial responses which lasted three, six, and six months. Stable disease occurred in 27 (54%, 95% CI 39.3, 68.2%) of patients. We failed to reject the null hypothesis that the response rate is 5% or less. The responses are illustrated as a waterfall plot in Figure 1, which shows that tumor shrinkage and growth was about equally distributed. The median duration of stable disease was 7.95 months (95% CI 3.58, 18.63). For patients with prior chemo, the response rate is (3.33%, 95% CI 0, 9.76%). For patients who are chemo naive, the response rate is (10%, 95% CI 0, 23%). At the time of this analysis three of the treated patients are still alive for whom the follow up times are 32, 37, and 40 months. Two of the three patients who demonstrated a response had not received prior chemotherapy.
Figure 1. Waterfall plot of Tumor response.
Three patients (3%; labeled in green) achieved a partial response with sorafenib treatment.
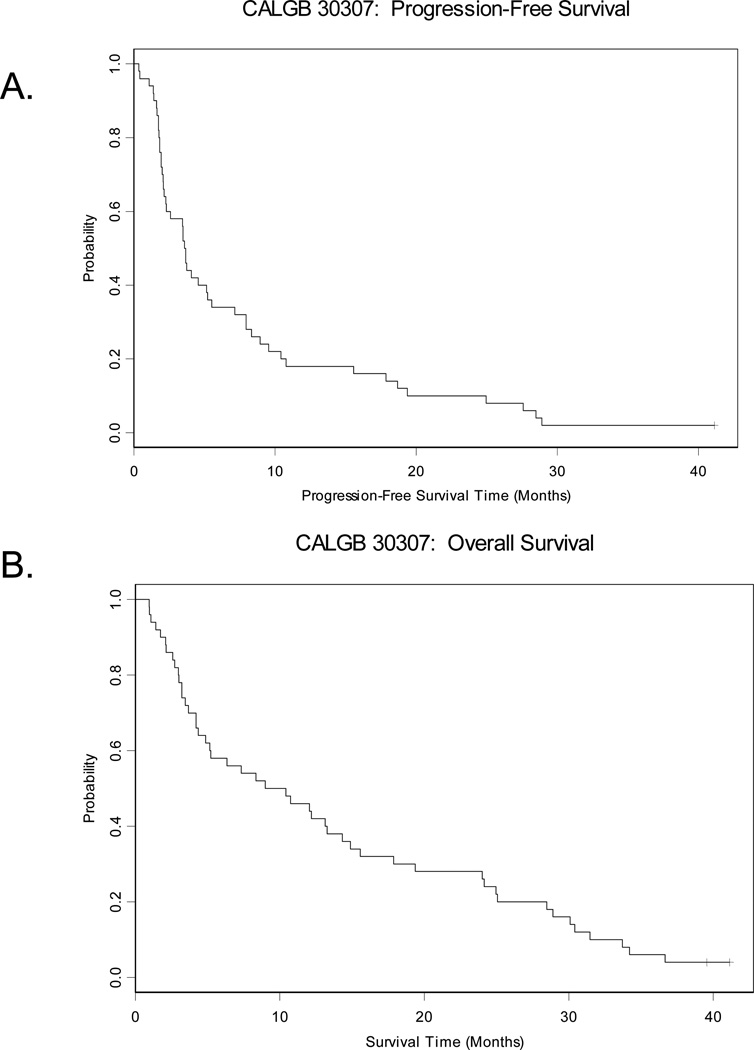
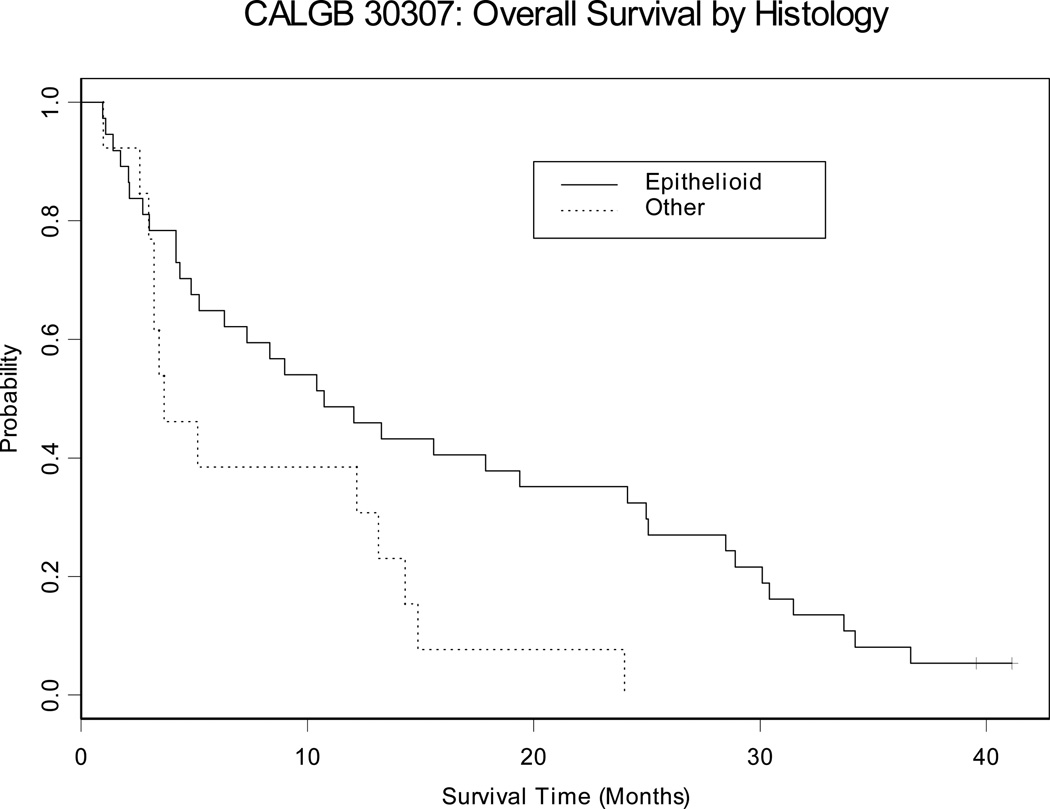
The median progression-free survival (PFS) and overall survival (OS) are 3.6 months and 9.7 months, respectively (Table 3) (Figure 2). Patients who had received previous chemotherapy survived for a median of 13.2 months, compared with 5.0 months for chemo-naïve patients; this difference was not statistically significant (p=0.3117). One year survival was also greater in the previously-treated patients compared with those who were chemo-naïve (57% vs. 30%). As expected, median survival in patients with epithelioid histology was significantly longer than in those with sarcomatoid or mixed histology (10.7 versus 3.7 months p =0.0179) (Table 3, Figure 3).
Table 3.
Progression free and overall survivals based on prior chemotherapy treatment and histology.
| Overall Survival | Progression Free survival | ||||||
|---|---|---|---|---|---|---|---|
| Median in months (95% CI) |
1-year Survival (95% CI) |
p value | Median in months (95% CI) |
3-month Survival (95% CI) |
p value | ||
| Overall Population | N=50 | 9.7 (4.4, 14.3) | 46% (31.9, 59) | 3.6 (2.3, 5.5) | 58.0 % (43.2, 70.2) | ||
| Prior Chemotherapy | Chemo naive N=20 | 5.0 (3.0, 12.2) | 30% (12.3, 50) | 0.3117 | 2.9 (1.9, 5.5) | 50.0 (27.1, 69.2) | 0.3181 |
| Prior chemo N=30 | 13.2 (5.2, 19.4) | 56.7% (37.3, 72.1) | 3.7 (2.3, 8.9) | 63.3 % (43.6, 77.8) | |||
| Histology | Epithelioid N= 37 | 10.7 (5.2,24.1) | 48.6 % (32.0, 63.4) | 0.0179 | 4.1 (2.1, 8.0) | 59.5 % (42.0, 73.2) | 0.1034 |
| Other N=13 | 3.7 (3.0, 13.1) | 38.5 % (14.1, 62.8) | 3.4 (1.9, 3.7) | 53.9 % (24.8, 76.0) | |||
Figure 2. Kaplan-Meier survival curves for progression free and overall survivals.
A. Median progression-free survival was 3.6 months with 18% 1-year progression free survival. B. Median overall survival was 9.7 months with 46% 1-year survival.
Figure 3. Kaplan-Meier Survival Curves for Overall Survival by histology.
Median overall survival was higher with epithelioid histology than others (10.7 vs 3.7 months months, p=0.0179).
Biological outcomes
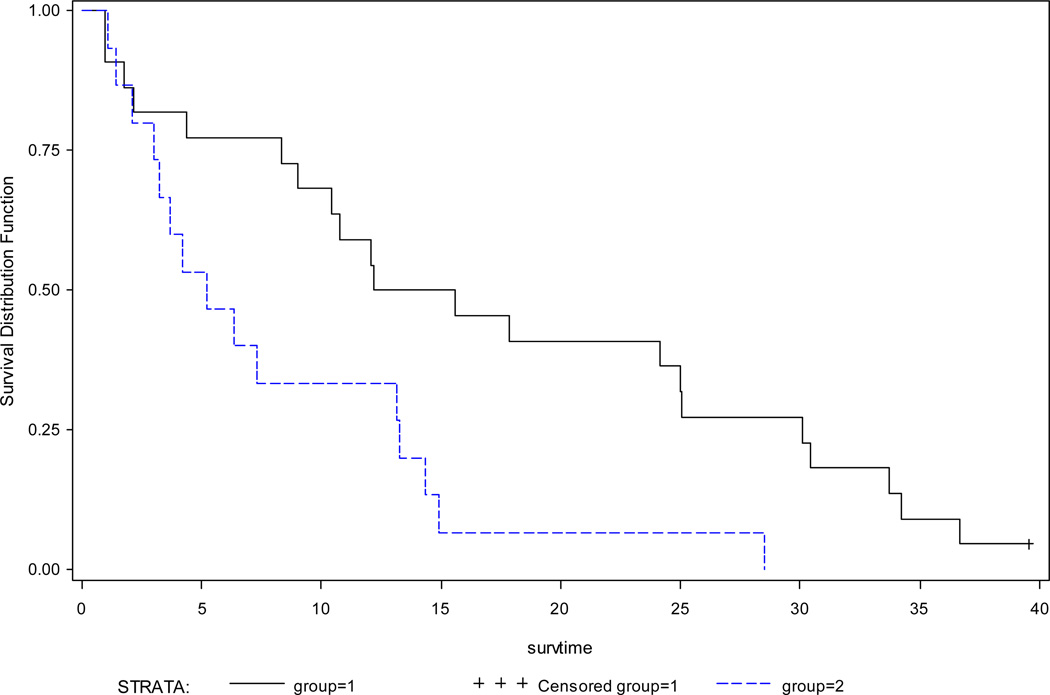
Archival tissue for analysis was available on 42 patients. There were no BRAF mutations detected among these samples. Of the 42 samples, 37 were evaluable for expression of p-ERK 1/2. ERK1/2 phosphorylation could not be evaluated due to insufficient samples in the 3 patients who developed a partial response. Thirty patients were p-ERK 1/2 positive and 7 patients were p-ERK 1/2 negative. Response to treatment (progressive disease, stable disease, inadequately assessed) neither correlated with presence or absence of p-ERK 1/2 (p=0.6745) nor with level of expression of p-ERK1/2 (low versus high) (p=0.1071). Similarly, p-ERK1/2 expression (positive versus negative) had no bearing on either PFS (HR 1.51 95% CI 0.65–3.50, p =0.342) or OS (HR 1.34 95% CI 0.55–3.23, p =0.5211). However, patients with medium or high levels (group 1) had poorer overall survival than those with low or negative (group 2) expression (HR 3.41 95% CI 1.41–8.25, p=0.0066) (Table 4, Figure 4). Because of the small sample size, other factors such as previous treatments, and performance status were unable to be incorporated into the Cox models.
Table 4.
Progression free and overall survivals based on p-ERK 1/2 expression
| N | Median (months) |
HR (95% CI) | P value | |
|---|---|---|---|---|
| Progression Free Survival | ||||
| pERK (negative/low) | 22 | 6.7 | ||
| pERK (medium/high) | 15 | 2.1 | 2.15 (0.94–4.90) | 0.0701 |
| Overall Survival | ||||
| pERK (negative /low) | 22 | 13.9 | ||
| pERK (medium/high) | 15 | 5.2 | 3.41 (1.41–8.25) | 0.0066 |
Figure 4. Kaplan Meier survival curves for overall survival time by p-ERK expression.
High baseline pERK 1/2 expression is associated with poorer overall survival. Median survivals were 5.2 months for medium/high expression (blue; group 1) and 13.9 months negative / low expression groups (black; group 2); p=0.0066.
DISCUSSION
Malignant mesothelioma continues to be a therapeutically challenging disease. Outcomes with cytotoxic agents have produced median survivals of about a year or less1, 13–16. Given these findings there has been an interest to evaluate novel therapeutic agents in patients with malignant mesothelioma. Angiogenesis is a key event in carcinogenesis and angiogenesis inhibitors have previously been evaluated in mesothelioma. Targeting angiogenesis with a multitude of agents with varied mechanism of action has resulted in response rates of 0–23% and overall survival of 5.9–12.4 months (Table 5). Our current study with single agent sorafenib produced a median survival of 9.7 months and is similar to prior agents targeting this pathway. However, the study failed to meet its primary endpoint. Furthermore, although outcomes appeared to be better in previously treated patients, this likely reflects patient selection as opposed to true clinical activity of sorafenib in this patient population.
Table 5.
Phase II studies of anti-angiogenic agents in mesothelioma.
| Drug | Line of therapy |
Response | SD | TTP / PFS months |
Median OS months |
|---|---|---|---|---|---|
| Vatalanib24 | 1st line | 8% | 72% | 10 | |
| Thalidomide25 | 1st and 2nd line | 0% | 28% | 7.5 | |
| SU541626 | 2nd line | 11% | 38% | 2 (TTP) | 12.4 |
| Thalidomide27 | 2nd line | 6% | 50% | 8 weeks (TTP) | 11 |
| Sunitinib28 | 2nd line | 23% | 3.5 (TTP) | 5.9 | |
| AZD217129 | 2nd line | 9% | 33% | 3 (PFS) | 10 |
| Sorafenib (Current study) | 1st and 2nd line | 6% | 54% | 3.6(PFS) | 9.7 |
SD; stable disease, TTP; Time To Progression, OS; overall survival, PFS; Progression free survival
The outcome of mesothelioma patients treated with sorafenib was similar to other multi-targeted kinase inhibitors (Table 5). As expected, patients with epitheloid histology fared better than the rest. In a phase II study of sorafenib in hepatocellular carcinoma, high p-ERK 1/2 was associated with improved time to progression than patients with low expression (p=0.00034)17. These results were mirrored in our study with the finding of improved overall survival in patients with low levels of baseline p-ERK 1/2. This improvement was seen as more than a doubling in overall survival in comparison to those with medium or high levels of expression. Low levels of p-ERK 1/2 may be a reflection of the proliferative state of the cancer. Hence cancers with low levels of p-ERK 1/2 may in general be less aggressive and thus be associated with a better outcome. Although sorafenib inhibits RAF signaling, the current study did not specifically evaluate the effects on p-ERK 1/2 following sorfenib treatment. Furthermore, as sorafenib inhibits angiogenesis, biomarkers such as soluble vascular endothelial growth factor receptor (sVEGFR) or CD31 expression may help define patients likely to benefit from treatment. Additional studies are needed to help further clarify the role of these biomarkers, if any, with sorafenib efficacy.
While several studies with targeted agents in mesothelioma have been to date negative, many important lessons have been learned. First is the issue of patient selection. In most malignancies, response rate and survival decrease with advancing lines therapy. However, such a consistent association in mesothelioma trials is lacking, and median survival of 11–12 months in pretreated patients are seen. This raises the possibility that patients ineligible for cytotoxic chemotherapy due to performance status or other factors, and hence with poorer prognoses are enrolled on front line non-cytotoxic chemotherapy trials thus blunting the effects of targeted therapy in the front line setting. Another important consideration in addition to performance status is the age of patients on mesothelioma trials. Almost half the patients in the current study were ≥ 70 years of age, which is consistent with the median age of diagnosis of mesothelioma (74 years)18. This feature has to be accounted for in the design of future trials and mesothelioma trials will need to cater to the elderly.
The heterogeneity within mesothelioma may also confound the results of an investigational agent if evaluated in a single arm study. For example, in a randomized study of cisplatin/gemcitabine with or without bevacizumab, while survival on both arms exceeded the survival in other mesothelioma studies, long term follow up failed to show a benefit with the addition of bevacizumab to chemotherapy (median survival 14.7 months chemotherapy arm, 15.6 months bevacizumab arm, p=0.91)19. In the absence of the chemotherapy control arm, this study would have led to the spurious conclusion that bevacizumab improves survival with chemotherapy. Hence future studies must strongly consider a randomized phase II design for drug selection. There has been no clear association between response rates and survival; most studies have shown low response rates and often targeted agents do not typically results in radiologic responses. Recently, multivariate cox regression analyses of 523 patients treated on EORTC (European Organization of Research and Treatment of Cancer) mesothelioma trials led to the development of a performance status, stage, and histology based prognosis index nomogram20. This nomogram separated patients into four risk categories with graded progression free survival. Perhaps it is time for a paradigm shift in mesothelioma trials to adopt survival endpoints such as progression free or overall survival as opposed to responses rates.
In conclusion, the current study is another example of an angiogenesis inhibitor with limited activity in mesothelioma. For this class of agents, future trials should include a chemotherapy backbone. Such chemotherapy should be tailored to the elderly and decreased functional status, with endpoints and study design that can adequately reflect the heterogeneity of this disease. Needless to say, therapy for mesothelioma will need to move toward targets beyond angiogenesis. Other likely targets include the proteasome, histone deacetylase, Src, Mesothelin, insulin-like growth factor, and MEK, which are currently being investigated in clinical trials21–23.
Acknowledgements
The authors would like to thank Ms. Alison Holmes-Tisch for performing the BRAF mutational studies and Dr. Neal Lindeman for the p-ERK 1/2 immunohistochemical analyses.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Sources of Funding
The research for CALGB 30307 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Richard L. Schilsky, MD, Chairman) and to the CALGB Statistical Center (Stephen George, PhD, CA33601). Support for the correlative science studies was provided through the NCI CTEP Translational Research Initiative administered by SAIC-Frederick (25XS051).
The following institutions participated in this study:
Dana Farber Cancer Institute, Boston, MA – Harold J. Burstein, M.D.,Ph.D., supported by CA32291; Duke University Medical Center, Durham, NC – Jeffrey Crawford, M.D., supported by CA47577; Hematology-Oncology Associates of Central New York CCOP, Syracuse, NY – Jeffrey Kirshner, M.D., supported by CA45389; Illinois Oncology Research Association, Peoria, IL – John W. Kugler, M.D., supported by CA35113; Kansas City Community Clinical Oncology Program CCOP, Kansas City, MO - Rakesh Gaur, M.D.; Memorial Sloan-Kettering Cancer Center, New York, NY – Clifford A. Hudis, M.D., supported by CA77651; Southeast Cancer Control Consortium Inc. CCOP, Goldsboro, NC – James N. Atkins, M.D., supported by CA45808; The Ohio State University Medical Center, Columbus, OH – Clara D. Bloomfield, M.D., supported by CA77658; University of California at San Diego, San Diego, CA – Barbara A. Parker, M.D., supported by CA11789; University of Chicago, Chicago, IL – Hedy L. Kindler, M.D., supported by CA41287; University of Minnesota, Minneapolis, MN – Bruce A. Peterson, M.D., supported by CA16450; University of North Carolina at Chapel Hill, Chapel Hill, NC – Thomas C. Shea, M.D., supported by CA47559; Washington University School of Medicine, St. Louis, MO – Nancy Bartlett, M.D., supported by CA77440
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 2.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 3.Strizzi L, Catalano A, Vianale G, et al. Vascular endothelial growth factor is an autocrine growth factor in human malignant mesothelioma. J Pathol. 2001;193:468–475. doi: 10.1002/path.824. [DOI] [PubMed] [Google Scholar]
- 4.Ohta Y, Shridhar V, Bright RK, et al. VEGF and VEGF type C play an important role in angiogenesis and lymphangiogenesis in human malignant mesothelioma tumours. Br J Cancer. 1999;81:54–61. doi: 10.1038/sj.bjc.6690650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–2230. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- 6.Versnel MA, Claesson-Welsh L, Hammacher A, et al. Human malignant mesothelioma cell lines express PDGF beta-receptors whereas cultured normal mesothelial cells express predominantly PDGF alpha-receptors. Oncogene. 1991;6:2005–2011. [PubMed] [Google Scholar]
- 7.Whitson BA, Kratzke RA. Molecular pathways in malignant pleural mesothelioma. Cancer Lett. 2006;239:183–189. doi: 10.1016/j.canlet.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Klominek J, Baskin B, Hauzenberger D. Platelet-derived growth factor (PDGF) BB acts as a chemoattractant for human malignant mesothelioma cells via PDGF receptor beta-integrin alpha3beta1 interaction. Clin Exp Metastasis. 1998;16:529–539. doi: 10.1023/a:1006542301794. [DOI] [PubMed] [Google Scholar]
- 9.Wilhelm SM, Carter C, Tang L, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 10.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 12.Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15:257–260. doi: 10.1093/annonc/mdh059. [DOI] [PubMed] [Google Scholar]
- 13.Steele JP, Shamash J, Evans MT, et al. Phase II study of vinorelbine in patients with malignant pleural mesothelioma. J Clin Oncol. 2000;18:3912–3917. doi: 10.1200/JCO.2000.18.23.3912. [DOI] [PubMed] [Google Scholar]
- 14.Kindler HL, Millard F, Herndon JE, 2nd, et al. Gemcitabine for malignant mesothelioma: A phase II trial by the Cancer and Leukemia Group B. Lung Cancer. 2001;31:311–317. doi: 10.1016/s0169-5002(00)00166-5. [DOI] [PubMed] [Google Scholar]
- 15.Stebbing J, Powles T, McPherson K, et al. The efficacy and safety of weekly vinorelbine in relapsed malignant pleural mesothelioma. Lung Cancer. 2009;63:94–97. doi: 10.1016/j.lungcan.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 16.van Meerbeeck JP, Gaafar R, Manegold C, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol. 2005;23:6881–6889. doi: 10.1200/JCO.20005.14.589. [DOI] [PubMed] [Google Scholar]
- 17.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 18.Hayat MJ, Howlader N, Reichman ME, et al. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12:20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 19.Karrison T, Kindler HL, Gandara DR, et al. Final analysis of a multi-center, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin (GC) plus bevacizumab (B) or placebo (P) in patients (pts) with malignant mesothelioma (MM) Journal of Clinical Oncology. 2007;18s doi: 10.1200/JCO.2011.41.5869. Abstract 7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francart J, Vaes E, Henrard S, et al. A prognostic index for progression-free survival in malignant mesothelioma with application to the design of phase II trials: a combined analysis of 10 EORTC trials. Eur J Cancer. 2009;45:2304–2311. doi: 10.1016/j.ejca.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 21.Krug LM, Curley T, Schwartz L, et al. Potential role of histone deacetylase inhibitors in mesothelioma: clinical experience with suberoylanilide hydroxamic acid. Clin Lung Cancer. 2006;7:257–261. doi: 10.3816/CLC.2006.n.003. [DOI] [PubMed] [Google Scholar]
- 22.Tsao AS, Wistuba I, Roth JA, et al. Malignant pleural mesothelioma. J Clin Oncol. 2009;27:2081–2090. doi: 10.1200/JCO.2008.19.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsao AS, He D, Saigal B, et al. Inhibition of c-Src expression and activation in malignant pleural mesothelioma tissues leads to apoptosis, cell cycle arrest, and decreased migration and invasion. Mol Cancer Ther. 2007;6:1962–1972. doi: 10.1158/1535-7163.MCT-07-0052. [DOI] [PubMed] [Google Scholar]
- 24.Jahan T, Gu L, Wang X, et al. Valatinib in patients with previously untreated advanced malignant mesothelioma: Prelimninary analysis of a phase II study by the Cancer and Leukemia Group B. (CALGB 30107) Lung Cancer. 2005;49(S2) abstract P403. [Google Scholar]
- 25.Baas P, Boogerd W, Dalesio O, et al. Thalidomide in patients with malignant pleural mesothelioma. Lung Cancer. 2005;48:291–296. doi: 10.1016/j.lungcan.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Kindler HL, Vogelzang NJ, Chien K, et al. SU5416 in Malignant Mesothelioma: a University of Chicago Phase II Consortium Study. Proc Am Soc Clin Oncol. 2001;20 Abstract 1359. [Google Scholar]
- 27.Pavlakis N, Abraham R, Harvie R, et al. Thalidomide alone or in combination with Cisplatin/Gemcitabine in malignant pleural mesothelioma: interim results from two parallel non randomized phase II studies. Lung cancer. 2003;41(S2):S11. [Google Scholar]
- 28.Nowak AK, Milward MJ, Francis R, et al. Phase II study of sunitinib as second-line therapy in malignant pleural mesothelioma (MM) Journal of Clinical Oncology. 2008;26 Abstract 2063. [Google Scholar]
- 29.Garland LL, Redman M, Wozniak A, et al. A phase II study of novel oral antiangiogenic agent AZD2171 (NSC-732208) in malignant pleural mesothelioma. Journal of Clinical Oncology. 2009;27(15s) abstract 7511. [Google Scholar]