Abstract
To understand the mechanisms leading to hydatidiform mole formation in patients with NLRP7 mutations, we used a combination of various approaches to characterize five products of conception, from two patients, shown by flow cytometry to contain non-diploid cells. We demonstrate that four of these conceptions are triploid and two of them originated from fertilization with more than one sperm. We show that three of these triploid conceptions fulfill the histopathological criteria of partial hydatidiform mole and one fulfills the histopathological criteria of spontaneous abortion. Our data demonstrate that oocytes from one patient with NLRP7 mutations are not able to prevent polyspermic fertilization and highlight the importance of using several approaches to characterize the genetic complexity of molar tissues and reproductive wastage. Altogether, our previous and current data show the association of NLRP7 mutations with several types of hydatidiform moles and with triploid spontaneous abortions.
Keywords: hydatidiform mole, NLRP7, polyspermic fertilization, triploid diandry, dispermy
1. Introduction
Hydatidiform mole (HM) is an abnormal human pregnancy characterized by absence of, or abnormal, embryonic development and hydropic degeneration of chorionic villi. NLRP7 (NOD-like receptor, pyrin domain containing 7) is a gene responsible for an autosomal recessive form of recurrent hydatidiform moles (RHMs) (1). Women with recurrent moles have usually two defective alleles. However, so far, seven patients have been found to have a single defective allele by sequencing the 11 NLRP7 exons (2, 3). To date, it is not clear how NLRP7 defects lead to this condition. NLRP7 is a cytoplasmic protein expressed in a wide range of normal human tissues including Fallopian tubes, endometrium, as well as oocytes at the germinal vesicle and metaphase II stages and early embryonic cleavage stages, (1, 4) (and unpublished data). We previously reported early cleavage abnormalities during in-vitro and in-vivo development of embryos from patients with NLRP7 mutations and variants (2). We have also shown the presence of NLRP7 mutations, not only in women with diploid biparental moles, as observed so far in all characterized molar tissues from familial cases of RHMs, but also in two patients with diploid androgenetic monospermic moles and in one patient with a triploid partial mole (2). Here, we show the recurrence of triploid conceptions in two patients with heterozygous NLRP7 mutations and document the diandric origin of two products of concepions (POCs) from one patient.
2. Cases
2.1. History and mutations
The reproductive outcomes of the two patients, 636 and 647, and their NLRP7 mutations were previously reported (2) and are summarized in Figs. 1 and 2. Patient 636 is heterozygous for c.2156C>T, p.Ala719Val (A719V), and patient 647 is heterozygous for c.467G>A, p.Arg156Gln (R156Q) (2). Both patients are of European ancestry and their two missense mutations, A719V and R156Q, were not found, respectively, in 100 and 150 women of European descent from the general population (2).
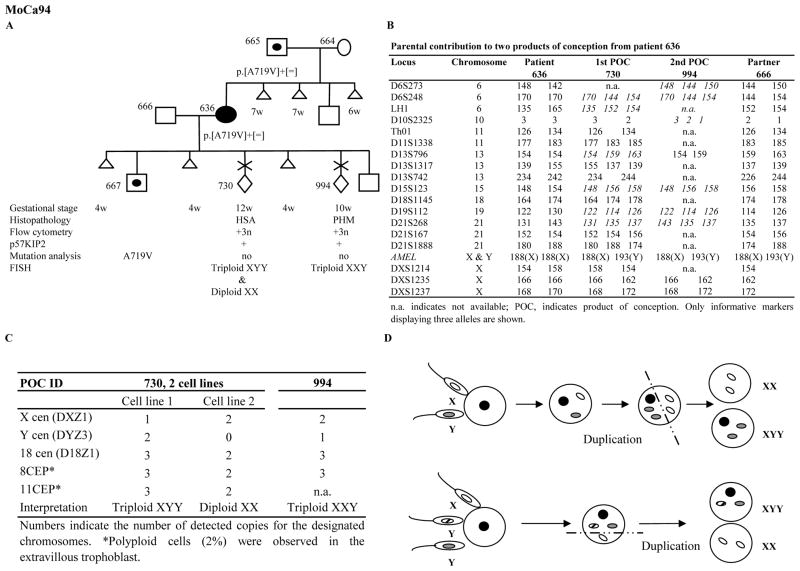
Fig. 1.
Characterization of two POCs from patient 636 heterozygous for one NLRP7 mutation illustrating the importance of using various approaches. A. The reproductive outcomes are shown by chronological order from left to right. B. Italicized genotypes are informative and display two paternal alleles. C. Summary of the FISH analysis with probes from five chromosomes on the two POCs from patient 636. D. Illustration of the two scenarios that may have led to the mosaicism in POC 730.
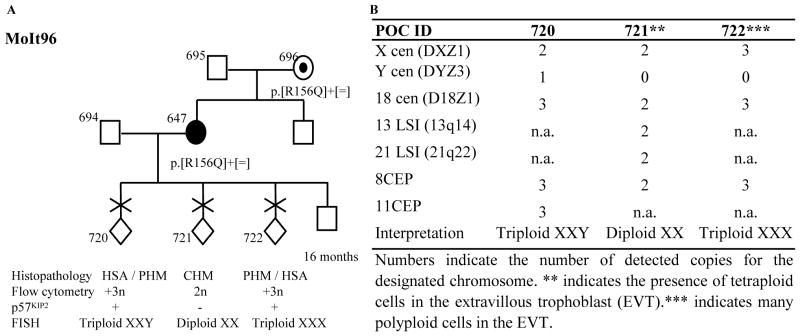
Fig. 2.
A. Characterization of three POCs from patient 647 heterozygous for one NLRP7 mutation. The reproductive outcomes are shown by chronological order from left to right. Histopathological diagnoses of pathologists 1 and 2 are separated by a “/”. B. Summary of the FISH analysis with probes from 7 chromosomes on the three POCs from patient 647.
2.2. Parental contribution to the products of conception
Flow cytometry on five of their POCs revealed that four contain triploid cells and one is diploid (Figs. 1 and 2). Tissue sections from the available paraffin blocks were examined independently by two pathologists who agreed on the diagnosis of three out of five POCs, two triploid partial HMs (PHMs) and one diploid complete HM (CHM). For one of the remaining POCs, both pathologists did not reach a conclusion on whether to classify it as hydropic spontaneous abortion (HSA) or PHM. For the other, one pathologist diagnosed it as PHM, while the other as HSA (Figs. 1 and 2) (Supplementary materials).
p57KIP2 immunohistochemistry demonstrated its nuclear expression in the cytotrophoblast and mesenchyme stromal cells of the villi of the four triploid POCs, 730, 994, 720 and 722 and the absence of its expression in the villi of the diploid POC 721 (Figs. 1 and 2), which is in agreement with their histopathological diagnoses.
Fluorescent microsatellite genotyping was performed on DNA extracted from chorionic villi using Pinpoint solution (Zymo Research, Orange CA) as previously described (5). This analysis revealed the dispermic contribution to the two POCs from patient 636 (Fig. 1B). In patient 647, genotyping data showed paternal contributions to the three POCs (Supplementary materials). However, because of the absence of the paternal DNA and the limited amount of tissues from the three POCs, we could not reach a conclusion as to whether the diploid POC 721 is biparental or androgenetic dispermic and whether the two triploid POCs, 720 and 722 are diandric or digynic.
Fluorescent in situ hybridization (FISH) was performed using probes (Vysis Inc) from seven chromosomes and confirmed the triploid nature of the four POCs, 730, 994, 720 (Supplementary materials) and 722 and the diploid nature of POC 721 (Figs 1 & 2). In POC 730, two cell lines one, triploid XYY, and another, diploid XX, were observed (Fig. 1C). XYY and XX cells were both present within the same chorionic villous with XX cells being less frequent and only in the villous stroma. Taking into consideration the presence of two paternal alleles at several autosomes in this POC and the presence of the paternal X chromosome alleles, two scenarios are possible (Fig. 1D). The first one involves dispermic fertilization with two spermatozoa, one carrying an X and one carrying a Y chromosome, followed by the endoduplication of the two paternal pronuclei and asymmetric first cell division leading to the generation of two cell lines, diploid androgenetic monospermic (XX) and triploid diandric monospermic (XYY). The second scenario involves the fertilization with three spermatozoa with one carrying an X and two, each carrying a Y chromosome, followed by asymmetric first cell division and the generation of two cell lines, diploid androgenetic monospermic (XX) and triploid diandric dispermic (XYY) (Fig. 1D). In POCs 730, 722, and 721, some cells confined to the extravillous trophoblast displayed polyploidy (Figs 1 and 2). Polyploid cells in the extravillous trophoblast are known to occur in normal as well as molar conceptions.
3. Discussion
In the last 30 years, the accepted model of androgenetic complete mole formation has been the fertilization of an empty oocyte by one or two haploid spermatozoa. Consequently, most scientists in this field, including us, were characterizing the parental contribution to moles using only one, and sometimes two, methods. However, based on various observations and mainly on data from assisted reproductive technologies, Golubovsky (6) rejected the empty oocyte model and proposed a nice model implying dispermic fertilization and the formation of tripronuclear zygotes at the origin of various types of triploidies, aneuploidies, heteroploidies, and some genotypic types of moles. In line with Golubovsky’s proposal are our observations that oocytes from patients with heterozygous NLRP7 mutations were not empty, but abnormalities in their embryos started at the first zygotic cell and during early cleavage stages (2). In a different independent study, Niemann et al (7) reached a similar conclusion and proposed that fertilization of a single nucleated oocyte by one, two, or three spermatozoa followed by abnormal first zygotic cell division are at the origin of several twin pregnancies of diploid moles (monospermic or dispermic) and a co-existing normal fetus. Therefore, data from various groups looking at the pathology of moles from different perspectives are now converging toward several mechanisms leading to different types of moles, but all involving a single nucleated oocyte and some occurring around the time of fertilization and/or during early embryonic cleavage. The latter would be expected to generate very complex aneuploidies and mosaicisms that cannot be detected by any single method and would require a combination of several methods.
Our histopathologial analysis confirms interobserver variability in the classification of some moles into CHM and PHM and in distinguishing PHMs from hydropic spontaneous abortions (8) and indicates that in the same patient, under the same genetic background (for NLRP7 mutation and for other genes), the histopathological type of the mole is greatly influenced by its genotype. Recently, we have shown that embryos from patients with NLRP7 mutations and variants have early cleavage abnormalities during in vivo and in vitro post-zygotic development (2). Now, we show the occurrence of triploid diandric conceptions in a patient with one NLRP7 mutation, which suggests a pre-existing defect in her oocytes at the time of fertilization. Altogether, these data suggest that some oocytes from patients with heterozygous NLRP7 mutations are defective at several levels.
To date, three cases of recurrent triploidies have been described (9–11), but a clear conclusion on the parental contribution to the POCs was reached in only one of these cases where recurrent triploid digyny was demonstrated in two POCs by karyotype and DNA genotyping analyses (10). Our report is the first demonstrating the recurrence of triploid diandry in a patient with a heterozygous NLRP7 mutation. Altogether, our previous and current data suggest that NLRP7 may be a susceptibility gene for several types of hydatidiform moles and triploid spontaneous abortions. Since triploid conceptions are common and represent 1–2 % of all recognized pregnancies in the first trimester, additional data are needed to further delineate the extent of NLRP7 involvement in triploid conceptions.
Supplementary Material
Acknowledgments
We thank the patients and their families for their cooperation, Dr. Kevin Watters for providing paraffin blocks from one patient, and Dr. Lucy Gilbert for referring one patient. This study was supported by the Canadian Institute of Health Research [grant number MOP 86546]. R.S. is a recipient of a «Chercheur Boursier Senior» from the «Fonds de Recherche en Santé du Québec»
Glossary
- NLRP7
NOD-like receptor, pyrin domain containing 7
- POC
product of conception
References
- 1.Murdoch S, Djuric U, Mazhar B, Seoud M, Khan R, Kuick R, et al. Mutations in NALP7 cause recurrent hydatidiform moles and reproductive wastage in humans. Nat Genet. 2006 Mar;38(3):300–2. doi: 10.1038/ng1740. [DOI] [PubMed] [Google Scholar]
- 2.Deveault C, Qian JH, Chebaro W, Ao A, Gilbert L, Mehio A, et al. NLRP7 mutations in women with diploid androgenetic and triploid moles: a proposed mechanism for mole formation. Hum Mol Genet. 2009 Mar 1;18(5):888–97. doi: 10.1093/hmg/ddn418. [DOI] [PubMed] [Google Scholar]
- 3.Hayward BE, De Vos M, Talati N, Abdollahi MR, Taylor GR, Meyer E, et al. Genetic and Epigenetic Analysis of Recurrent Hydatidiform Mole. Hum Mutat. 2009 Mar 23; doi: 10.1002/humu.20993. [DOI] [PubMed] [Google Scholar]
- 4.Zhang P, Dixon M, Zucchelli M, Hambiliki F, Levkov L, Hovatta O, et al. Expression analysis of the NLRP gene family suggests a role in human preimplantation development. PLoS ONE. 2008;3(7):e2755. doi: 10.1371/journal.pone.0002755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy KM, McConnell TG, Hafez MJ, Vang R, Ronnett BM. Molecular genotyping of hydatidiform moles: analytic validation of a multiplex short tandem repeat assay. J Mol Diagn. 2009 Nov;11(6):598–605. doi: 10.2353/jmoldx.2009.090039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golubovsky MD. Postzygotic diploidization of triploids as a source of unusual cases of mosaicism, chimerism and twinning. Hum Reprod. 2003 Feb 1;18(2):236–42. doi: 10.1093/humrep/deg060. [DOI] [PubMed] [Google Scholar]
- 7.Niemann I, Bolund L, Sunde L. Twin pregnancies with diploid hydatidiform mole and coexisting normal fetus may originate from one oocyte. Hum Reprod. 2008 Jun 12; doi: 10.1093/humrep/den226. [DOI] [PubMed] [Google Scholar]
- 8.Fukunaga M, Katabuchi H, Nagasaka T, Mikami Y, Minamiguchi S, Lage JM. Interobserver and intraobserver variability in the diagnosis of hydatidiform mole. Am J Surg Pathol. 2005 Jul;29(7):942–7. doi: 10.1097/01.pas.0000157996.23059.c1. [DOI] [PubMed] [Google Scholar]
- 9.Bar-Ami S, Seibel MM, Pierce KE, Zilberstein M. Preimplantation genetic diagnosis for a couple with recurrent pregnancy loss and triploidy. Birth Defects Res A Clin Mol Teratol. 2003 Nov;67(11):946–50. doi: 10.1002/bdra.10099. [DOI] [PubMed] [Google Scholar]
- 10.Brancati F, Mingarelli R, Dallapiccola B. Recurrent triploidy of maternal origin. Eur J Hum Genet. 2003 Dec;11(12):972–4. doi: 10.1038/sj.ejhg.5201076. [DOI] [PubMed] [Google Scholar]
- 11.Pergament E, Confino E, Zhang JX, Roscetti L, Xien Chen P, Wellman D. Recurrent triploidy of maternal origin. Prenat Diagn. 2000 Jul;20(7):561–3. doi: 10.1002/1097-0223(200007)20:7<561::aid-pd875>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.