Hydatidiform mole (HM) is an abnormal human pregnancy characterized by absence of, or abnormal, embryonic development and hydropic degeneration of chorionic villi. The common form of this condition occurs once in every 1000–1500 pregnancies in Western countries (1), but at higher frequencies in India. The incidence of HMs in India is 1 in every 83–500 pregnancies depending on regions and studies (2, 3). HMs are usually sporadic and not recurrent. Among women with sporadic moles, 1–6% will have a second mole (4–11), and 10–20% will have a second non-molar reproductive wastage, mostly as a spontaneous abortion (7, 11, 12). The frequency of familial recurrent HMs (RHMs) is not known, but such cases are believed to be very rare.
To date, 23 different mutations in NLRP7 have been reported in women with RHMs and reproductive wastage. Four of these mutations, p.Arg693Pro, p.Glu710Aspfs7X, p.Leu750Val, p.Leu825X, have been found in unrelated families from the same population indicating the presence of founder effects for these mutations (13–16). We previously reported two mutations in Indian families, p.Arg693Pro and p.Asn913Ser, and the former was found in two unrelated families (14).
In this study, we searched for mutations in NLRP7 in 10 new unrelated Indian patients with recurrent HMs and reproductive wastage. Eight of the cases originate from various states in Northwest India, Jammu and Kashmir, Punjab, Chandigarh, Haryana, and Uttar Pradesh, and two cases, MoIn105 and MoIn106, originate from West Pakistan and migrated to India about 50 years ago.
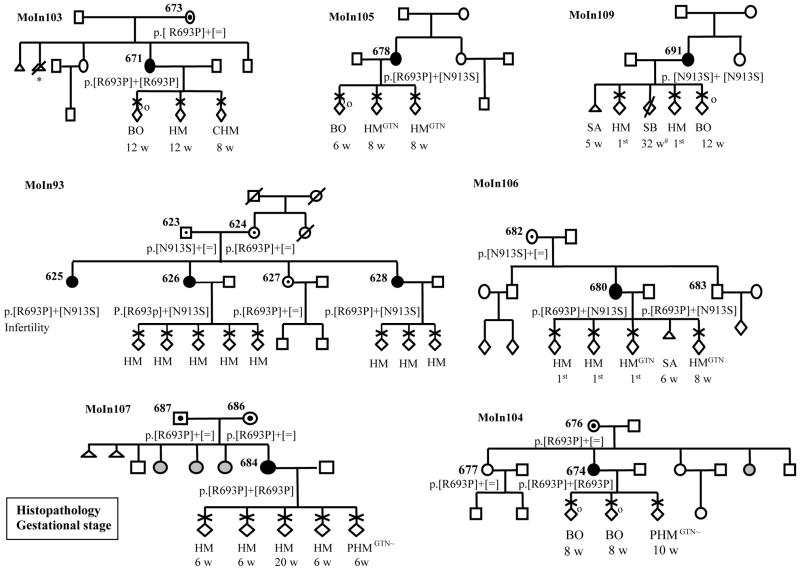
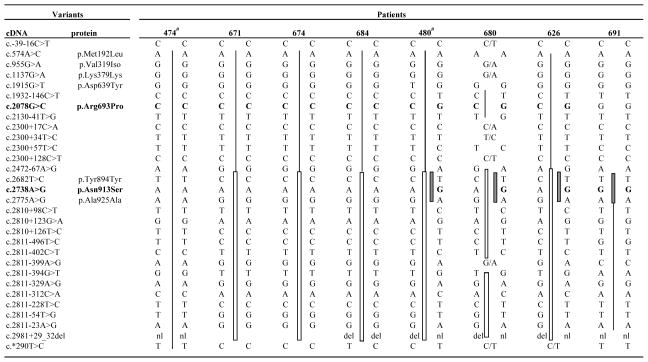
Mutation analysis was performed as previously described by genomic DNA sequencing of the 11 NLRP7 exons in the two directions and revealed NLRP7 mutations in seven patients. All the seven patients had two previously identified mutations in Indian patients, c.2078G>C, p.Arg693Pro and c.2738A>G, p.Asn913Ser. Three patients were compound heterozygous for the two mutations; three were homozygous for p.Arg693Pro; and one was homozygous for p.Asn913Ser. The analysis of available parents and relatives demonstrated the segregation of the mutations in their respective families (Figure 1). In family MoIn93, a woman with a history of infertility of unexplained clinical origin was found compound heterozygous for the two mutations. To date, we have analyzed 12 unrelated Indian patients, selected based only on their medical history and not on linkage to the locus spanning NLRP7, and found NLRP7 mutations in nine of them (75%). This result was unexpected since only half of patients with recurrent HM and no family history of moles referred to our laboratory (from various populations) have mutations/variants in NLRP7. The DNA change leading to p.Arg693Pro was found on 12 chromosomes (66.6%) and the allele leading to p.Asn913Ser on six chromosomes (33.4%). Haplotypes were established based on alleles found in five homozygous patients and on the analysis of three heterozygous patients and their available parents and siblings at 30 single nucleotide polymorphisms in NLRP7. Haplotype analysis shows a common haplotype carrying each of the mutations and demonstrates the presence of a strong founder effect for these two mutations in the Indian population (Table 1). This is further supported by the fact that four of the analyzed patients are homozygous despite the absence of known consanguinity between their parents. The two mutations modify recognition sites for restriction enzymes and can be easily tested in Indian patients with recurrent HMs and reproductive wastage. A number of synonymous and non-synonymous variants were also found in the patients. c.574A>C, p.Met192Leu was found in a heterozygous state in patient 678 and in 2 out 38 controls of Indo-Pakistani origin. All the other variants are known polymorphisms frequently found in several populations.
Figure 1.
Pedigree structures and reproductive outcomes of seven Indian families with recurrent reproductive wastage and NLRP7 mutations. The reproductive outcomes are listed by chronological order from left to right. Gestational age is from the last menstrual period. “1st, indicates first trimester when the number of weeks for gestational stage is not available; CHM, complete HM; PHM, partial HM; HM, is used when no histo-pathological data or tissues were available to review the diagnosis; GTN, indicates gestational trophoblastic neoplasia; BO, blighted ovum; SB, stillbirth; SA, spontaneous abortion, *, indicates an elective abortion; ~, indicates low risk GTN requiring chemotherapy. Gray circles indicate women with unknown reproductive outcomes. Patient 625 has infertility of unexplained clinical origin; male factors were excluded in her partner.
Table 1.
Haplotype analysis at all NLRP7 variants found in 8 unrelated Indian patients
|
indicates previously reported patients by Murdoch et al., 2006; missing genotypes are not available; nl/del indicates heterozygous for the deletion. Mutations are in bold character. Shared haplotypes are indicated with similar vertical bars. “/” is used when parental phase could not be established.
In family MoIn106, one male with one child and no reproductive problems was found compound heterozygous for the two mutations. This is the third reported male with two NLRP7 mutations and no reproductive problems (13). Our data demonstrate the presence of two founder mutations in the Indian population, expand further the involvement of NLRP7 mutations to a wider spectrum of reproductive wastage, and provide further indications that NLRP7 mutations have no consequences on male reproduction. The presence in several populations of founder effects for a condition that prevents the reproduction of homozygous females is by itself intriguing and may reveal in the future male and/or heterozygous females advantage.
Acknowledgments
We thank the patients, their families, and control subjects for their cooperation. Funding for this study was provided by the McGill India Strategic Research Initiative (number 120070) and by the Canadian Institute of Health research (MOP 86546). R.S. is supported by a Fraser, Monat and McPherson Scholarship salary award. We thank the Indian Council of Medical Research (ICMR) for approving this collaborative study.
References
- 1.Grimes DA. Epidemiology of gestational trophoblastic disease. American journal of obstetrics and gynecology. 1984;150:309–318. doi: 10.1016/s0002-9378(84)90370-3. [DOI] [PubMed] [Google Scholar]
- 2.Balaram P, Alex S, Panikkar B, et al. Adhesion-related proteins E-cadherin, P-cadherin, CD44, and CD44v6, and antimetastatic protein nm23H1 in complete hydatidiform moles in relation to invasion potential. Int J Gynecol Cancer. 2004;14:532–539. doi: 10.1111/j.1048-891x.2004.014316.x. [DOI] [PubMed] [Google Scholar]
- 3.Balagopal P, Pandey M, Chandramohan K, et al. Unusual presentation of choriocarcinoma. World J Surg Oncol. 2003;1:4. doi: 10.1186/1477-7819-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao J, Moss J, Sebire NJ, et al. Analysis of the chromosomal region 19q13. 4 in two Chinese families with recurrent hydatidiform mole. Human reproduction (Oxford, England) 2006;21:536–541. doi: 10.1093/humrep/dei357. [DOI] [PubMed] [Google Scholar]
- 5.Sebire NJ, Fisher RA, Foskett M, et al. Risk of recurrent hydatidiform mole and subsequent pregnancy outcome following complete or partial hydatidiform molar pregnancy. BJOG: An International Journal of Obstetrics and Gynaecology. 2003;110:22–26. [PubMed] [Google Scholar]
- 6.Sand PK, Lurain JR, Brewer JI. Repeat gestational trophoblastic disease. Obstet Gynecol. 1984;63:140–144. [PubMed] [Google Scholar]
- 7.Lan Z, Hongzhao S, Xiuyu Y, et al. Pregnancy outcomes of patients who conceived within 1 year after chemotherapy for gestational trophoblastic tumor: a clinical report of 22 patients. Gynecol Oncol. 2001;83:146–148. doi: 10.1006/gyno.2001.6170. [DOI] [PubMed] [Google Scholar]
- 8.Kronfol NM, Iliya FA, Hajj SN. Recurrent hydatidiform mole: a report of five cases with review of the literature. J Med Liban. 1969;22:507–520. [PubMed] [Google Scholar]
- 9.Kim JH, Park DC, Bae SN, et al. Subsequent reproductive experience after treatment for gestational trophoblastic disease. Gynecol Oncol. 1998;71:108–112. doi: 10.1006/gyno.1998.5167. [DOI] [PubMed] [Google Scholar]
- 10.Horn LC, Kowalzik J, Bilek K, et al. Clinicopathologic characteristics and subsequent pregnancy outcome in 139 complete hydatidiform moles. Eur J Obstet Gynecol Reprod Biol. 2006;128:10–14. doi: 10.1016/j.ejogrb.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Berkowitz RS, Im SS, Bernstein MR, et al. Gestational trophoblastic disease. Subsequent pregnancy outcome, including repeat molar pregnancy. The Journal of reproductive medicine. 1998;43:81–86. [PubMed] [Google Scholar]
- 12.Flici O, Tadjerouni A, Robyn C. The reproductive function following a hydatidiform mole. J Gynecol Obstet Biol Reprod (Paris) 1984;13:67–76. [PubMed] [Google Scholar]
- 13.Qian J, Deveault C, Bagga R, et al. Women heterozygous for NALP7/NLRP7 mutations are at risk for reproductive wastage: report of two novel mutations. Hum Mutat. 2007;28:741. doi: 10.1002/humu.9498. [DOI] [PubMed] [Google Scholar]
- 14.Murdoch S, Djuric U, Mazhar B, et al. Mutations in NALP7 cause recurrent hydatidiform moles and reproductive wastage in humans. Nature genetics. 2006;38:300–302. doi: 10.1038/ng1740. [DOI] [PubMed] [Google Scholar]
- 15.Kou YC, Shao L, Peng HH, et al. A recurrent intragenic genomic duplication, other novel mutations in NLRP7 and imprinting defects in recurrent biparental hydatidiform moles. Mol Hum Reprod. 2008;14:33–40. doi: 10.1093/molehr/gam079. [DOI] [PubMed] [Google Scholar]
- 16.Deveault C, Qian J, Chebaro W, et al. NLRP7 mutations in women with diploid androgenetic and triploid moles: a proposed mechanism for mole formation. Human molecular genetics. 2008 doi: 10.1093/hmg/ddn418. [DOI] [PubMed] [Google Scholar]