Abstract
Background
Since most clinical guidelines address single diseases, treatment of patients with multimorbidity, the co-occurrence of multiple (chronic) diseases within one person, can become complicated. Information on highly prevalent combinations of diseases can set the agenda for guideline development on multimorbidity. With this systematic review we aim to describe the prevalence of disease combinations (i.e. disease clusters) in older patients with multimorbidity, as assessed in available studies. In addition, we intend to acquire information that can be supportive in the process of multimorbidity guideline development.
Methods
We searched MEDLINE, Embase and the Cochrane Library for all types of studies published between January 2000 and September 2012. We included empirical studies focused on multimorbidity or comorbidity that reported prevalence rates of combinations of two or more diseases.
Results
Our search yielded 3070 potentially eligible articles, of which 19 articles, representing 23 observational studies, turned out to meet all our quality and inclusion criteria after full text review. These studies provided prevalence rates of 165 combinations of two diseases (i.e. disease pairs). Twenty disease pairs, concerning 12 different diseases, were described in at least 3 studies. Depression was found to be the disease that was most commonly clustered, and was paired with 8 different diseases, in the available studies. Hypertension and diabetes mellitus were found to be the second most clustered diseases, both with 6 different diseases. Prevalence rates for each disease combination varied considerably per study, but were highest for the pairs that included hypertension, coronary artery disease, and diabetes mellitus.
Conclusions
Twenty disease pairs were assessed most frequently in patients with multimorbidity. These disease combinations could serve as a first priority setting towards the development of multimorbidity guidelines, starting with the diseases with the highest observed prevalence rates and those with potential interacting treatment plans.
Introduction
The growing interest in the concept of multimorbidity, which refers to the co-occurrence of multiple (often chronic) diseases or medical conditions within one person[1], is motivated by the rising prevalence of multimorbidity, its negative health consequences, and the challenge to manage multimorbid patients in health care settings, often family medicine practice[2-11].
Managing patients with multimorbidity is much more complicated than managing patients with a single condition[10]. Clinical evidence-based guidelines have been developed to provide recommendations for patient management, to define standards of care, and focus efforts to improve quality. However, most clinical guidelines address single diseases, and do not always provide guidance for patients with multimorbidity. Simply combining the current disease oriented guidelines might result in a complex, inconvenient or even conflicting treatment regime, in terms of interactions between drugs and diseases, conflicting management strategies, and polypharmacy[10-12]. To support health care providers in daily practice, guidelines for combinations of diseases are thus warranted, especially for the most prevalent combinations with complex or incompatible regimes.
Despite the increasing body of research that has been conducted in the field of multimorbidity, there is still no clear, uniform operational definition for multimorbidity, and thus no clear picture of common multimorbidity combinations. Over the years, various methods have been developed and employed to measure multimorbidity. There are indices available that estimate a multimorbidity-score by weighting a range of diseases (e.g. Charlson Comorbidity Index[13] or Cumulative Illness Rating Scale[14]). Other applied multimorbidity measures are the Chronic Disease Score[15], RxRisk Model[16], or the Duke Severity of Illness Checklist[17]. Furthermore, multimorbidity can be assessed by simply counting the number of co-existing diseases within a person, using a predefined list of medical conditions. As disease counts are easy to use, it is presumably the most common approach to define multimorbidity.
Two recent systematic reviews described the available measures of multimorbidity in more detail and pointed out that the choice of a measure depends on the outcome of interest and the type of data available[18,19]. Overall, these methods are employed to predict health outcomes, for instance, disability, quality of life, health care utilization or mortality. Additionally, these methods are often applied to assess prevalence rates. Prevalence estimates vary widely depending on the study population, setting, data sources, the type of the diseases considered and the number of conditions included in the analysis[18,20-23].
Although evidence for the overall prevalence of multimorbidity is accumulating, insight into the prevalence of specific disease combinations (i.e. disease clusters) is limited. A few studies explored disease clusters of multimorbidity by conducting statistical cluster or factor analysis[24-26]. These studies identified several broad clusters of diseases, but it remained unclear which specific combinations of diseases were most frequently occurring, taken into account the variation in prevalence rates. To the best of our knowledge, there are no systematic reviews that have investigated multimorbidity clusters, and therefore, a complete overview is still lacking.
With this current systematic review we aim to describe the prevalence of disease clusters in older patients with multimorbidity, as found in published studies. In addition, we intend to acquire information that can be supportive in the process of developing multimorbidity guidelines that could assist patient management and improve quality of health care.
Methods
Search strategy
To find eligible studies we consulted the electronic databases MEDLINE/PubMed, Embase and Cochrane Library. A search strategy was developed for each database, using a combination of key words and Medical Subject Headings (MEDLINE) or Emtree terms (EMBASE and Cochrane Library). Since the term multimorbidity does not have an equivalent in the database’s thesaurus, it was only searched as a key word. Until recently, the term comorbidity was used interchangeably with multimorbidity, as it also refers to the co-existence of multiple conditions[1,27]. Hence, both terms and their spelling variations were included in our search algorithm. We combined search terms relating to multimorbidity (e.g. “multimorbid*”, “multiple chronic diseas*”, “multiple illness*”), comorbidity, chronic disease, and the definition or measurement (e.g. “index”, “definition”, “measurement”, “list”, “instrument”). The search strategy was developed iteratively to identify a combination of terms with an acceptable level of sensitivity and specificity. We restricted the search to articles with an available abstract, published in English or Dutch, and those published between January 2000 and September 2012. Before the year 2000, only a few articles had been published on the concept of multimorbidity. We did not restrict the search to a specific study type. To be complete, we also screened reference lists of all included articles. The final search strategy for MEDLINE is given in Appendix S1.
Study selection
The selection of studies followed several steps. First, different inclusion and exclusion criteria were specified for the selection of studies by title, abstract and full-text (Table 1 ). Second, a random sample of fifteen titles was screened by two authors (JS and JK) to control for unclear formulated inclusion and exclusion criteria, before screening all titles of the yielded articles; there was no disagreement or vagueness. Subsequently, one author (JS) screened all titles for relevancy, based on the defined inclusion and exclusion criteria (Table 1). Third, two authors (JK and JS) independently appraised a sample of twenty abstracts. There was no disagreement between the two authors, after which all remaining abstracts were screened for eligibility by one author (JS) and, when necessary, by a second author (JK or JB). Last, full-text articles were independently screened for eligibility by at least two authors (JS screened all the full texts, and JK and JB both screened half of the full texts). To evaluate the full text articles on the inclusion and exclusion criteria, both authors appointed to screen the full text article filled out a self-constructed checklist. Discrepancies and ambiguities were solved by discussion between the two authors and, when necessary, by a third author.
Table 1. Inclusion and exclusion criteria of the screening process of the yielded articles.
|
Inclusion criteria
|
Exclusion criteria | |
|---|---|---|
| Titles | - Included the words ‘multimorbidity’ or ‘comorbidity’ or related words (see step 1 and 2 in Appendix S2) | - No data of disease combinations (or impossible to calculate prevalence rates) |
| Titles not including these words were excluded | - Age of at least half of the study population was ≤ 55 years | |
| - Diagnosis of a disease was based on medication prescription (ATC codes) only | ||
| Abstracts | - Evidence that multimorbidity/comorbidity was the outcome variable, or the central independent variable | - Study size less than 500 persons† |
| - Availability of a list of diseases to account for multimorbidity/comorbidity, morbidity indices or measures. | - Study conducted in a hospital setting‡ | |
| Abstracts not meeting these criteria were excluded. | - Study examined solely two diseases§ | |
| - Study was focused on an index-disease with a prevalence < 0.5% in the total population in the Netherlands | ||
| Full-texts | - Availability of prevalence rates of specific disease clusters* | - Study with a non-empiric research type: ‘letter’, ‘(narrative) review’, ‘editorial’, ‘case-study’, ‘presentation’, ‘commentary’ |
* or results that allowed the calculation of a prevalence rate: Some studies reported odds ratios instead of prevalence rates. These data were converted into prevalence rates. If not possible, the article was excluded.
† to include studies with results based on solid, robust data
‡ our study is more focused on primary care as health professionals in primary care often see patients with multiple health conditions
§ we assumed that studies solely focusing on two diseases would provide insufficient disease clusters with applicable prevalence rates
Assessment of study quality
After titles and abstracts had been screened, all remaining articles had an observational design. Therefore, quality assessment of the articles was based on several items of the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) checklist[28], which we included in our checklist. The items that were required to be described in the articles were (1) the study design; (2) the setting; (3) the study size; (4) eligibility criteria of participants; (5) the type of diseases included to measure comorbidity or multimorbidity; (6) the data collection method; and (7) outcome data related to the prevalence of combinations of diseases. These items, with specific conditions, were also considered as inclusion and exclusion criteria (see also Table 1). In addition, to be retained in our review, only those articles that met our inclusion and exclusion criteria, and thus our specified quality standard, were selected.
Data extraction and synthesis
For each included study, the following data were extracted:
1 Study characteristics: First author, year of publication, country, study size, setting, population age;
2 Information relating to the number and types of diseases examined;
3 Information relating to (the prevalence of) the presented disease clusters.
The checklist was employed to gather data about the study characteristics. These data were tabulated and ordered according to the population setting and the presence or absence of a specific index-disease. A mean age was given or calculated, but when impossible the age range was given. Subsequently, all possible diseases, and disease combinations as described in the included studies, were gathered, counted, and tabulated. In addition, the accompanying prevalence rates for each combination were collected and presented. When necessary, odds ratios were converted into prevalence rates. All given prevalence rates concerned the total study sample, and if not, prevalence rates were converted to relate to the total sample.
Results
Included studies
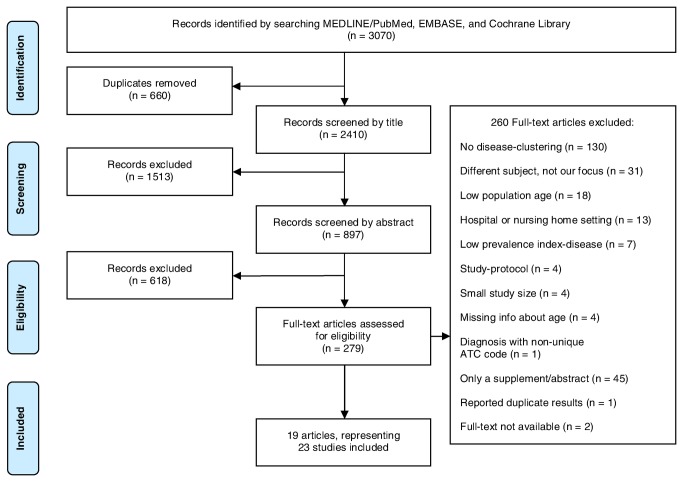
In total, 3070 potentially eligible articles were identified, of which 2410 remained after exclusion of duplicates, see Figure 1 . After screening of titles and abstracts, 279 articles remained to be read completely. Of these articles, 212 were excluded because they did not meet our inclusion criteria, as shown in Figure 1. Additionally, 45 articles were found to be an abstract or supplement for a congress and were excluded, 1 article was excluded because of double publication of part of the results of the same research project, and of 2 articles we had no access to the full-text. As a result, 19 articles remained. One of these articles focused on multimorbidity in different settings and described the data of these populations separately. These different settings were regarded as 5 individual studies and therefore, our final sample for analysis represented 23 studies. All 23 studies fulfilled our inclusion criteria and met our quality criteria.
Figure 1. PRISMA Flow chart outlining the study selection process.
Study characteristics
All 23 studies had an observational design and were conducted in either the general population (n =13)[23,29-38], primary care (n =7)[23,39-43] or ambulatory care setting (n =1)[44]. Two studies were based on data of the Veterans Health Administration system (VHA)[6,45] (Table 2 ). The population size of the studies varied from 599[23] to over one million[45] individuals. Except for two[44,45], all studies reported clusters of two diseases. In five studies[37,38,42,43,45] patients were only included when diagnosed with a specific disease (i.e. index-disease). In 8 studies[29,30,32-34,36,39,40] prevalence rates were converted to provide comparable prevalence rates of the disease clusters. In one study, odds ratios were converted into prevalence rates[35].
Table 2. Characteristics of included studies examining clusters of comorbidity or multimorbidity.
|
Type of diseases/ disease categories examined in the study
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author (year) | Country | Setting, (no. of participants used in analyses), Mean age/ percentage | Data collection multi/co-morbidity | No. of diagnoses examined incl. index-disease | Index-disease | CVD | Diabetes | COPD/ asthma | Cancer | Musculo-skeletal | Depression/anxiety | Dementia | Neurological | Eye/ ear | Digestive | Urinary | |
| 1 | Fiest29 (2011) | Canada | Gen. pop., (n= 15 591), 64 years | Interview with participants | 12 (out of 19 diagnoses) | - | yes | yes | yes | yes | yes | yes | |||||
| 2 | Niti30 (2007) | Singapore | Gen. pop., (n= 2 611), 66 years | Interview with participants | 12 | - | yes | yes | yes | yes | yes | yes | yes | ||||
| 3 | Marengoni31 (2009) | Sweden | Gen. pop, (n= 1 099), 85 years | Physician’s examination, hospital records, drug use and clinical examination | 11 (out of 15 diagnoses) | - | yes | yes | yes | yes | yes | yes | |||||
| 4 | Kriegsman32 (2004) | The Netherlands | Gen. pop., (n= 2 497), 69 years | Interview with participants | 7 | - | yes | yes | yes | yes | yes | ||||||
| 5 | Fuchs33 (2012) | Germany | Gen. pop., (n= 9 155), 56% 55-64 years, 31% 65-74 years, 13% ≥ 75 years | Interview with participants | 6 | - | yes | yes | yes | yes | yes | yes | |||||
| 6 | Lee P34 (2009) | United States | Gen. pop., (n= 11 113), 55% 65-75 years, 45% ≥ 76 years | Interview with participants | 3 diseases and 2 syndromes | - | yes | yes | yes | ||||||||
| 7 | Fillenbaum35 (2000) | United States | Gen. pop., (n= 4 034), 73 years | Interview with participants | 5 | - | yes | yes | yes | ||||||||
| 8a | Schram23 LASA* (2008) | The Netherlands | Gen. pop., (n= 2 463), 55‑94 years | Interview with participants, validated by family physician | 5 (out of 10 diagnoses) | - | yes | yes | yes | yes | |||||||
| 8b | Schram23 The Rotterdam Study† (2008) | The Netherlands | Gen. pop., (n= 3 550), 65‑99 years | Interview with participants, validated by family physician, physical examination | 4 (out of 15 diagnoses | - | yes | yes | |||||||||
| 8c | Schram23 Leiden 85-plus Study‡ (2008) | The Netherlands | Gen. pop., (n= 599), 85 years | Interview with family physician, electronic medical records | 5 (out of 12 diagnoses) | - | yes | yes | yes | yes | |||||||
| 9 | Mannino36 (2008) | United States | Gen. pop., (n= 20 296), 60% ≥ 55 years | Interview with participants, clinical examination | 4 | - | yes | yes | yes | ||||||||
| 10 | Wesseling37 (2013)§ | The Netherlands | Gen. pop., (n= 979), 56 years | Survey with participants | 19 (out of 25 diagnoses) | Osteoarthritis | yes | yes | yes | yes | yes | yes | yes | ||||
| 11 | Lyketsos38 (2005) | United States | Gen. pop., (n= 695), 82 years | Interview with participants | 12 (out of 26 diagnoses) | Dementia or Other cognitive impairment | yes | yes | yes | yes | yes | yes | |||||
| 12 | Pfaff39 (2009) | Australia | Primary care, (n= 20 183) 72 years | Survey with participants | 15 | - | yes | yes | yes | yes | yes | yes | yes | yes | |||
| 13 | Schubert40 (2006) | United States | Primary care, (n= 3 013), 71 years | Electronic medical records | 11 | - | yes | yes | yes | yes | yes | yes | yes | yes | |||
| 14 | Van Oostrom41 (2012) | The Netherlands | Primary care, (n= 52 014) 43% 55-64 years 34% 65-74 years 23% ≥ 75 years | Electronic medical records | 10 (out of 29 diagnoses) | - | yes | yes | yes | yes | yes | yes | |||||
| 8d | Schram23 CMR Nijmegen|| (2008) | The Netherlands | Primary care, (n= 2 895) 100% ≥ 55 years | Electronic medical records | 6 (out of a total of 68 diagnoses) | - | yes¶ | yes | yes | yes | |||||||
| 8e | Schram23 RNGP** (2008) | The Netherlands | Primary care, (n= 5 610) 100% ≥ 55 years | Electronic medical records | 6 (out of a total of 83 diagnoses) | - | yes | yes | yes | yes | |||||||
| 15 | Noël42 (2004) | United States | Primary care, (n= 1 801) 77% ≥ 65 years | Interview with participants | 11 | Major depression or dysthymia | yes | yes | yes | yes | yes | yes | yes | yes | yes | ||
| 16 | Struijs43 (2006) | The Netherlands | Primary care, (n= 7 499) 65 years | Electronic medical records | 11 | Diabetes mellitus | yes | yes | yes | yes | yes | yes | yes | yes | yes | ||
| 17 | Findley45 (2011) | United States | VHA clinical services users (veterans), (n= 1 383 950) 90% ≥ 50 years | VHA electronic medical records and Medicare claims data | 4 | Diabetes mellitus, heart disease, hypertension | yes | yes | yes | ||||||||
| 18 | Lee T6 (2007) | United States | VHA clinical services users (veterans), (n= 741 847) 100% 55‑64 years | VHA electronic medical records | 6 (out of 11 diagnoses) | - | yes | yes | yes | yes | yes | ||||||
| 19 | Van den Bussche44 (2011) | Germany | Ambulatory care, (n= 123 224) 74 years | Claims data | 19 (out of 46 diagnoses) | - | yes | yes | yes | yes | yes | yes | yes | yes | |||
Gen. pop.: General population; CVD: cardiovascular diseases; VHA: Veterans Health Administration system
* Schram et al. analyzed data from seven registries, these are presented separately. This is data from a population-based registry, LASA.
† Schram et al. analyzed data from seven registries, these are presented separately. This is data from a population-based registry, The Rotterdam Study.
‡ Schram et al. analyzed data from seven registries, these are presented separately. This is data from a population-based registry, Leiden 85-plus Study.
§ During the search, this was still a provisional publication
|| Schram et al. analyzed data from seven registries, these are presented separately. This is data from a primary care registry, CMR Nijmegen.
¶ hypertension only
** Schram et al. analyzed data from seven registries, these are presented separately. This is data from a primary care registry, RNGP.
Type of diseases
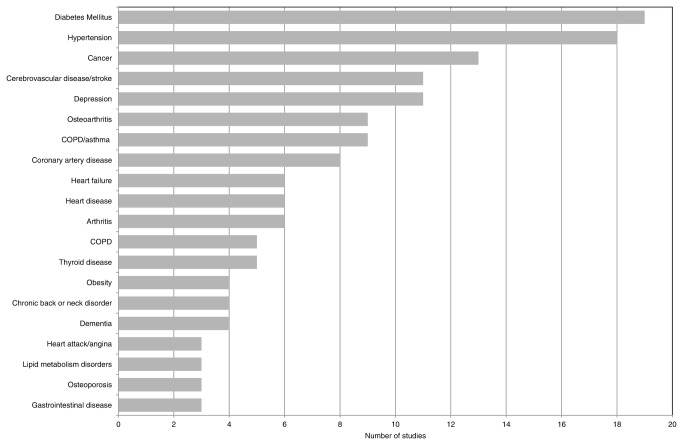
Sixty-three different diseases were found, of which some were defined rather broadly (e.g. heart disease, gastrointestinal disease), while others were described in more detail (e.g. cataract, atrial fibrillation). Diabetes mellitus was the most frequently measured disease (described in 19 out of 23 studies). Other commonly assessed diseases were hypertension, cancer, stroke, and depression (Figure 2 ). Besides the 63 diseases, 165 combinations of two diseases (i.e. disease pairs) and 50 combinations of three diseases (i.e. disease triplets) were reported in the studies. Of the disease pairs, 20 were described rather frequently (≥ 3 studies), see Table 3 . The disease triplets could not be replicated in any of the other studies and were therefore not further analyzed.
Figure 2. Type of diseases examined in the included studies (top 20).
Table 3. Prevalence of clusters of two diseases.
| Disease | Clustered with | Prevalence per study (%; %; %), data gathered by an interview/survey* | Prevalence per study (%; %; %), data collected by patients’ EMRs* | No. of study* |
|---|---|---|---|---|
| Depression | Hypertension | 1.2; 3.9; 7.6; 12.9 | 1, 12, 2, 8c | |
| Arthritis | 1.7; 2.8; 4.9 | 1, 2, 12 | ||
| Diabetes Mellitus | 1.7; 2.8 | 1.4 | 12, 2, 14 | |
| COPD/Asthma | 0.9; 1.8 | 2, 12 | ||
| Stroke | 0.2; 0.9; 1.0 | 0.8; 1.1 | 1, 2, 12, 14, 3 | |
| Cancer | 1.1 | 0.9 | 12, 14 | |
| Heart failure | 0.7; 0.8 | 0.7 | 12, 2, 14 | |
| Heart disease | 0.6 | 1 | ||
| Hypertension | Osteoarthritis | 18.7; 20.1 | 3.2; 4.1; 9.1 | 8c, 8a, 18, 8e, 8d |
| Coronary artery disease | 9.8; 14.9 | 7.6 | 7, 8a, 3 | |
| Diabetes Mellitus | 12.0; 14.0 | 2.5; 6.2; 6.4; 7.4 | 8b, 7, 3, 8e, 18, 8d | |
| Cancer | 5.5; 10.6 | 1.0; 3.4 | 7, 8c, 18, 8e | |
| Depression | 1.2; 3.9; 7.6; 12.9 | 1, 12, 2, 8c | ||
| Dementia | 2.9; 5.5 | 13, 3 | ||
| Diabetes Mellitus | Hypertension | 12.0; 14.0 | 2.5; 6.2; 6.4; 7.4 | 8b, 7, 3, 8e, 18, 8d |
| Coronary artery disease | 4.1; 4.5 | 3.6 | 7, 6, 14 | |
| Stroke | 0.6; 2.9 | 1.9 | 4, 7, 14 | |
| Depression | 1.7; 2.8 | 1.4 | 12, 2, 14 | |
| Heart failure | 1.8 | 1.8; 2.2 | 6, 3,14 | |
| Cancer | 0.8; 2.2 | 1.9 | 4, 7, 14 | |
| Cancer | Hypertension | 5.5; 10.6 | 1.0; 3.4 | 7, 8c, 18, 8e |
| Diabetes Mellitus | 0.8; 2.2 | 1.9 | 4, 7, 14 | |
| Depression | 1.1 | 0.9 | 12, 14 | |
| Stroke | 0.5; 0.9 | 0.9 | 4, 7, 14 | |
| Stroke | Diabetes Mellitus | 0.6; 2.9 | 1.9 | 4, 7, 14 |
| Dementia | 0.4; 2.7 | 13,3 | ||
| Depression | 0.2; 0.9; 1.0 | 0.8; 1.1 | 1, 2, 12, 14, 3 | |
| Cancer | 0.5; 0.9 | 0.9 | 4, 7, 14 | |
| Coronary artery disease | Hypertension | 9.8; 14.9 | 7.6 | 7, 8a, 3 |
| Heart failure | 2.8 | 2.8; 5.6 | 6, 14, 3 | |
| Diabetes Mellitus | 4.1; 4.5 | 3.6 | 7, 6, 14 | |
| Heart failure | Coronary artery disease | 2.8 | 2.8; 5.6 | 6, 14, 3 |
| Diabetes Mellitus | 1.8 | 1.8; 2.2 | 6, 3,14 | |
| Depression | 0.7; 0.8 | 0.7 | 12, 2, 14 | |
| Dementia | Hypertension | 2.9; 5.5 | 13, 3 | |
| Stroke | 0.4; 2.7 | 13, 3 | ||
| Osteoarthritis | Hypertension | 18.7; 20.1 | 3.2; 4.1; 9.1 | 8c, 8a, 18, 8e, 8d |
| Arthritis | Depression | 1.7; 2.8; 4.9 | 1, 2, 12 | |
| COPD/Asthma | Depression | 0.9; 1.8 | 2, 12 | |
| Heart disease | Depression | 0.6 | 1 |
Prevalence of disease clusters found in at least three studies
EMR: Electronic medical record
* Not bold: studies conducted in a primary care setting, bold: studies conducted in the general population, italic: study based on VHA (Veterans Health Administration system) data.
The rank in frequency of diseases examined in the included studies depended on the definition of the diseases. As displayed in Figure 2, various diseases of the circulatory tract were examined frequently (6 diseases in the top 20). However, the definition of these diseases differed in level of detail. If heart failure, coronary artery disease and heart attack/angina were defined as heart disease (this broad definition could comprise the separate diseases), heart disease was examined in 17 studies instead of in 6 (in some studies coronary artery disease and heart failure were both examined), making it the third most commonly assessed disease. This also applied the category COPD/asthma and the separate diseases asthma and COPD. If the specific diseases were grouped into the broad combined category, then COPD/asthma was investigated in 14 studies, instead of in 9 studies.
Disease clusters
The most frequently assessed combinations concerned 12 different diseases (Table 3). Regarding these diseases, several clusters were identified. Of the assessed diseases, depression was most frequently clustered, and was paired with 8 other diseases. Additionally, hypertension and diabetes mellitus were also found to be commonly clustered in the available studies (with 6 different diseases). Although depression was the disease most frequently assessed in pairs, the highest prevalence rates were found for disease pairs including hypertension, highest for its combination with osteoarthritis (20%). The top ten disease combinations with the highest prevalence rates all included the diseases hypertension, coronary artery disease, and diabetes mellitus. In the studies that focused on a specific index-disease, mainly studies concerning depression, even higher prevalence rates were identified; 57% of the patients with a major depression were also diagnosed with hypertension (see Table 4 ).
Table 4. Prevalence of clusters of two diseases, including an index-disease.
| Index-disease | Clustered with | Prevalence per study (%; %; %), data gathered by an interview/survey* | Prevalence per study (%; %; %), data collected by patients’ EMRs* | No. of study* |
|---|---|---|---|---|
| Depression | Hypertension | 57.9 | 15 | |
| Arthritis | 55.6 | 15 | ||
| Diabetes Mellitus | 23.2 | 15 | ||
| COPD/Asthma | 23.3 | 15 | ||
| Cancer | 10.9 | 15 | ||
| Heart disease | 27.6 | 15 | ||
| Hypertension | Depression | 16.7 | 17 | |
| Diabetes Mellitus | Stroke | 2.9 | 16 | |
| Depression | 3.9; 17.6 | 16, 17 | ||
| Cancer | 2.7 | 16 | ||
| Dementia | Hypertension | 37.1 | 11 | |
| Stroke | 16.4 | 11 | ||
| Osteoarthritis | Hypertension | 19.8 | 10 | |
| Heart disease | Depression | 16.6 | 17 |
Prevalence of disease clusters found in at least three studies
EMR: Electronic medical record
* Not bold: studies conducted in a primary care setting, bold: studies conducted in the general population, italics: study based on VHA (Veterans Health Administration system) data.
Per study, varying prevalence rates for each disease combination were found. Especially for depression with hypertension (from 1.2% to 12.9%), and for cancer with hypertension (from 1.0% to 10.6%). Further, the highest prevalence values were often found in studies in which the morbidity data were collected via interviews or surveys. These studies almost always concerned the general population. Nearly all studies that applied electronic medical records (EMRs) to collect morbidity data were executed in a primary care setting.
Discussion
While multimorbidity in older people seems to be the rule rather than the exception, evidence on the prevalence of specific disease clusters in patients with multimorbidity is limited. In this systematic review 19 articles were included, representing 23 studies, that described 63 diseases and 165 disease pairs. Twenty disease pairs, comprising 12 different diseases, were examined rather frequently. Of the assessed diseases, depression was the disease most frequently clustered, and was paired with 8 different diseases. Hypertension and diabetes mellitus were found to be the second most commonly clustered diseases, and were combined with 6 different diseases. The combinations with the highest prevalence rates included hypertension, coronary artery disease and diabetes mellitus.
The prevalence estimates of disease clusters differed widely among studies, a result that is in line with findings reported in other reviews[20,46]. We will discuss two main possible explanations. First, differences in the population under study may affect the prevalence of multimorbidity and related disease clusters, like age, income, or ethnicity[47-52]. Multimorbidity is strongly associated with age[47-50]. Although we focused on older adults, the population’s mean age still varied considerably (from 56 years to 85 years). Further, multimorbidity seems more common among people living in socioeconomically deprived areas or people with a low income[47,49,50]. Second, variation in prevalence rates might be due to the applied definition of the diseases, the applied data collection method and the study setting[18-21,53,54]. In our review, some diseases were defined very broadly (e.g. cancer, heart disease) while other diseases were defined in more detail (e.g. osteoarthritis, atrial fibrillation). Studies executed in a primary care setting often applied medical records with information on a detailed level, yet they applied different classification codes with different definitions or based on different diagnostic methods (e.g. depression). In contrast, studies applied in the general population often used surveys or interviews, all inquiring about diseases differently. Other diseases, like obesity, are not always considered as a disease and therefore not included. As a consequence, few disease combinations and accompanying prevalence rates were identical.
With our current results we have identified combinations of diseases that are likely to co-occur and thus, a suitable treatment plan needs to be developed. Existing clinical practice guidelines, however, do not often address multimorbidity, and following all guidelines for all individual diseases may lead to a considerable treatment burden and to contradictory drug and self-care regimes[10,11,55]. Indeed, Boyd et al.[10] reported that several potential medication interactions were found for a pattern that consisted of the diseases hypertension, diabetes mellitus, osteoarthritis, osteoporosis, and COPD. Contradicting life-style recommendations were found for osteoporosis and diabetes mellitus.. As it is reasonable that our identified disease pairs are highly common in (elderly) adults, it would be useful if guidelines address potential drug interactions and contradicting treatment recommendations (drug-disease interactions, and disease-disease interactions) for these disease pairs.
This systematic review has some limitations. We used the term multimorbidity in our search process. This term is not well indexed in literature databases, and we might have missed some studies. To compensate for this constraint, we combined an extended list of text words referring to the term multimorbidity and we included the term comorbidity (with its possible spelling variations) to our search strategy. Next, we developed a scoring method based on several items of the STROBE checklist[28], and added these items to our strict inclusion and exclusion criteria, in order to obtain a minimal quality standard of all included studies. As a result, we could not differentiate further between levels of quality. Last, with this type of study we were restricted to merely describe the most frequently explored disease pairs in patients with multimorbidity, and not necessarily the most occurring disease pairs. Yet, the 12 identified diseases do represent highly prevalent diseases internationally[56,57], and the accompanying combinations of these diseases are also likely to be highly prevalent.
Reflecting on our findings and limitations, more effort should be made to establish a multimorbidity disease list with uniformly defined diseases. Only by doing so, heterogeneity between study results can be diminished, and information about the prevalence and burden of multimorbidity will be more genuine and comparable. It seems also important to have a better understanding of specific treatment conflicts concerning certain disease clusters, and not merely by scrutinizing the existing guidelines, but by actually assessing daily practice according to guideline recommendations. In this regard, it seems practical to start with the most frequently occurring diseases. Furthermore, it is still valuable to gain more insight into (the prevalence of) specific co-occurring disease clusters, especially of clusters of three, and four diseases, as a large proportion of the elderly population is diagnosed with more than two chronic conditions[50]. For the development of a multimorbidity guideline, however, it might be easier to take into account rather small disease clusters instead of broad, comprehensive disease clusters[25,26].
Conclusion
Management of care for (older) patients with multimorbidity can be challenging, or even burdensome. To be more concrete, health professionals need to strike a balance between the various disease-specific guidelines before one can develop an appropriate treatment plan with feasible recommendations and advices, taking the patient’s personal abilities into account. The disease clusters that we have distinguished, could serve as a first priority setting towards the development of multimorbidity guidelines. A likely option is to start with the most frequently occurring disease combinations, as regards the evaluation of potential treatment conflicts, the adjustment of existing clinical guidelines, or even the development of new guidelines.
Supporting Information
Electronic literature search of PubMed/MEDLINE, September 2012.
(DOC)
PRISMA checklist.
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1. Van den Akker M, Buntinx F, Knottnerus JA (1996) Comorbidity or multimorbidity: what's in a name? A review of literature. Eur J Gen Pract 2: 65-70. doi: 10.3109/13814789609162146. [DOI] [Google Scholar]
- 2. Uijen AA, Van de Lisdonk EH (2008) Multimorbidity in primary care: Prevalence and trend over the last 20 years. Eur J Gen Pract 14: 28-32. doi: 10.1080/13814780802436093. PubMed: 18949641. [DOI] [PubMed] [Google Scholar]
- 3. Agborsangaya CB, Lau D, Lahtinen M, Cooke T, Johnson JA (2013) Health-related quality of life and healthcare utilization in multimorbidity: results of a cross-sectional survey. Qual Life Res 22: 791-799. doi: 10.1007/s11136-012-0214-7. PubMed: 22684529. [DOI] [PubMed] [Google Scholar]
- 4. Kadam UT, Croft PR, Staffordshire North, GP Consortium Group (2007) Clinical multimorbidity and physical function in older adults: a record and health status linkage study in general practice. Fam Pract 24: 412-419. doi: 10.1093/fampra/cmm049. PubMed: 17698977. [DOI] [PubMed] [Google Scholar]
- 5. Fortin M, Lapointe L, Hudon C, Vanasse A, Ntetu AL et al. (2004) Multimorbidity and quality of life in primary care: a systematic review. Health Qual Life Outcomes 2: 51. doi: 10.1186/1477-7525-2-51. PubMed: 15380021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee TA, Shields AE, Vogeli C, Gibson TB, Woong-Sohn M et al. (2007) Mortality rate in veterans with multiple chronic conditions. J Gen Intern Med 22: 403-407. doi: 10.1007/s11606-007-0277-2. PubMed: 18026809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vyas A, Pan X, Sambamoorthi U (2012) Chronic condition clusters and polypharmacy among adults. Int J Family Med, 2012: 2012: 193168. PubMed: 22900173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calderón-Larrañaga A, Poblador-Plou B, Gozález-Rubio F, Gimeno-Feliu LA, Abad-Díez JM et al. (2012) Multimorbidity, polypharmcay, referrals, and adverse drug events: are we doing things well? Br J Gen Pract 62: e821-e826. doi: 10.3399/bjgp12X659295. PubMed: 23211262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolff JL, Starfield B, Anderson G (2002) Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med 162: 2269-2276. doi: 10.1001/archinte.162.20.2269. PubMed: 12418941. [DOI] [PubMed] [Google Scholar]
- 10. Boyd CM, Darer J, Boult C, Fried LP, Boult L et al. (2005) Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 294: 716-724. doi: 10.1001/jama.294.6.716. PubMed: 16091574. [DOI] [PubMed] [Google Scholar]
- 11. Hughes LD, McMurdo MET, Guthrie B (2013) Guidelines for people not for diseases: the challenges of applying UK clinical guidelines to people with multimorbidity. Age Ageing 42: 62-69. doi: 10.1093/ageing/afs100. PubMed: 22910303. [DOI] [PubMed] [Google Scholar]
- 12. Van Weel C, Schellevis FG (2006) Comorbidity and guidelines: conflicting interests. Lancet 367: 550-551. doi: 10.1016/S0140-6736(06)68198-1. PubMed: 16488782. [DOI] [PubMed] [Google Scholar]
- 13. Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40: 373-383. doi: 10.1016/0021-9681(87)90171-8. PubMed: 3558716. [DOI] [PubMed] [Google Scholar]
- 14. Linn BS, Linn MW, Gurel L (1968) Cumulative illness rating scale. J Am Geriatr Soc 16: 622-626. PubMed: 5646906. [DOI] [PubMed] [Google Scholar]
- 15. Von Korff M, Wagner EH, Saunders K (1992) A chronic disease score from automated pharmacy data. J Clin Epidemiol 45: 197-203. doi: 10.1016/0895-4356(92)90016-G. PubMed: 1573438. [DOI] [PubMed] [Google Scholar]
- 16. Fishman PA, Goodman MJ, Hornbrook MC, Meenan RT, Bachman DJ et al. (2003) Risk adjustment using automated ambulatory pharmacy data: the RxRisk model. Med Care 41: 84-99. doi: 10.1097/00005650-200301000-00011. PubMed: 12544546. [DOI] [PubMed] [Google Scholar]
- 17. Parkerson GR Jr, Broadhead WE, Tse CK (1993) The Duke Severity of Illness Checklist (DUSOI) for measurement of severity and comorbidity. J Clin Epidemiol 46: 379-393. doi: 10.1016/0895-4356(93)90153-R. PubMed: 8483003. [DOI] [PubMed] [Google Scholar]
- 18. Diederichs C, Berger K, Bartels DB (2011) The measurement of multiple chronic diseases--A systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci 66A: 301-311. doi: 10.1093/gerona/glq208. PubMed: 21112963. [DOI] [PubMed] [Google Scholar]
- 19. Huntley AL, Johnson R, Purdy S, Valderas JM, Salisbury C (2012) Measures of multimorbidity and morbidity burden for use in primary care and community settings: A systematic review and guide. Ann Fam Med 10: 134-141. doi: 10.1370/afm.1363. PubMed: 22412005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fortin M, Stewart M, Poitras ME, Almirall J, Maddocks H (2012) A systematic review of prevalence studies on multimorbidity: Toward a more uniform methodology. Ann Fam Med 10: 142-151. doi: 10.1370/afm.1337. PubMed: 22412006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van den Akker M, Buntinx F, Roos S, Knottnerus JA (2001) Problems in determining occurrence rates of multimorbidity. J Clin Epidemiol 54: 675-679. doi: 10.1016/S0895-4356(00)00358-9. PubMed: 11438407. [DOI] [PubMed] [Google Scholar]
- 22. Taylor AW, Price K, Gill TK, Adams R, Pilkington R et al. (2010) Multimorbidity- not just an older person’s issue. Results from an Australian biomedical study. BMC Public Health 10: 718. doi: 10.1186/1471-2458-10-718. PubMed: 21092218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schram MT, Frijters D, Van de Lisdonk EH, Ploemacher J, de Craen AJM et al. (2008) Setting and registry characteristics affect the prevalence and nature of multimorbidity in the elderly. J Clin Epidemiol 61: 1104-1112. doi: 10.1016/j.jclinepi.2007.11.021. PubMed: 18538993. [DOI] [PubMed] [Google Scholar]
- 24. Cornell J, Pugh JA, Williams JW, Kazis L, Lee AFS et al. (2007) Multimorbidity clusters: Clustering binary data from multimorbidity clusters: clustering binary data from a large administrative medical database. Appl Multivariate Res 12: 163-182. [Google Scholar]
- 25. Holden L, Scuffham PA, Hilton MF, Muspratt A, Ng SK et al. (2011) Patterns of multimorbidity in working Australians. Popul Health Metr 9: 15. doi: 10.1186/1478-7954-9-15. PubMed: 21635787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schäfer I, von Leitner EC, Schön G, Koller D, Hansen H et al. (2010) Multimorbidity patterns in the elderly: A new approach of disease clustering identifies complex interrelations between chronic conditions. PLOS ONE 5: e15941. doi: 10.1371/journal.pone.0015941. PubMed: 21209965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fortin M, Lapointe L, Hudon C, Vanasse A (2005) Multimorbidity is common to family practice: Is it commonly researched? Can Fam Physician 51: 244-245. PubMed: 16926936. [PMC free article] [PubMed] [Google Scholar]
- 28. STROBE; STATEMENT.Strengthening the reporting of observational studies (2007) Checklist for cohort, case-control, and cross-sectional studies. Available: http://www.strobe.statement.org . Accessed January 2013 [Google Scholar]
- 29. Fiest KM, Currie SR, Williams JVA, Wang J (2011) Chronic conditions and major depression in community-dwelling older adults. J Affect Disord 131: 172-178. doi: 10.1016/j.jad.2010.11.028. PubMed: 21168918. [DOI] [PubMed] [Google Scholar]
- 30. Niti M, Ng TP, Kua EH, Ho RC, Tan CH (2007) Depression and chronic medical illnesses in Asian older adults: the role of subjective health and functional status. Int J Geriatr Psychiatry 22: 1087-1094. doi: 10.1002/gps.1789. PubMed: 17407107. [DOI] [PubMed] [Google Scholar]
- 31. Marengoni A, Rizzuto D, Wang HX, Winblad B, Fratiglioni L (2009) Patterns of chronic multimorbidity in the elderly population. J Am Geriatr Soc 57: 225-230. doi: 10.1111/j.1532-5415.2008.02109.x. PubMed: 19207138. [DOI] [PubMed] [Google Scholar]
- 32. Kriegsman DMW, Deeg DJH, Stalman WAB (2004) Comorbidity of somatic chronic diseases and decline in physical functioning: the Longitudinal Aging Study Amsterdam. J Clin Epidemiol 57: 55-65. doi: 10.1016/S0895-4356(03)00258-0. PubMed: 15019011. [DOI] [PubMed] [Google Scholar]
- 33. Fuchs J, Busch M, Lange C, Scheidt-Nave C (2012) Prevalence and patterns of morbidity among adults in Germany: Results of the German telephone health Interview survey German Health Update (GEDA) 2009. Bundesgesundheitsbl 55: 576-586. doi: 10.1007/s00103-012-1464-9. [DOI] [PubMed] [Google Scholar]
- 34. Lee PG, Cigolle C, Blaum C (2009) The co-occurrence of chronic diseases and geriatric syndromes: The health and retirement study. J Am Geriatr Soc 57: 511-516. doi: 10.1111/j.1532-5415.2008.02150.x. PubMed: 19187416. [DOI] [PubMed] [Google Scholar]
- 35. Fillenbaum GG, Pieper CF, Cohen HJ, Cornoni-Huntley JC, Guralnik JM (2000) Comorbidity of five chronic health conditions in elderly community residents: determinants and impact on mortality. J Gerontol A Biol Sci Med Sci 55A: M84-M89. PubMed: 10737690. [DOI] [PubMed] [Google Scholar]
- 36. Mannino DM, Thorn D, Swensen A, Holguin F (2008) Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 32: 962-969. doi: 10.1183/09031936.00012408. PubMed: 18579551. [DOI] [PubMed] [Google Scholar]
- 37. Wesseling J, Welsing PMJ, Bierma-Zeinstra SMA, Dekker J, Gorter KJ et al. (2013) Impact of self-reported comorbidity on physical and mental health status in early symptomatic osteoarthritis: the CHECK (Cohort Hip and Cohort Knee) study. Rheumatology 52: 180-188. doi: 10.1093/rheumatology/kes288. PubMed: 23093723. [DOI] [PubMed] [Google Scholar]
- 38. Lyketsos CG, Toone L, Tschanz J, Rabins PV, Steinberg M et al. (2005) Population-based study of medical comorbidity in early dementia and "Cognitive Impairment, No Dementia (CIND)": association with functional and cognitive impairment: The Cache County Study. Am J Geriatr Psychiatry 13: 656-664. doi: 10.1176/appi.ajgp.13.8.656. PubMed: 16085781. [DOI] [PubMed] [Google Scholar]
- 39. Pfaff JJ, Draper BM, Pirkis JE, Stocks NP, Snowdon JA et al. (2009) Medical morbidity and severity of depression in a large primary care sample of older Australians: the Deps-GP project. Med J Aust 190: S75-S80. PubMed: 19351298. [DOI] [PubMed] [Google Scholar]
- 40. Schubert CC, Boustani M, Callahan CM, Perkins AJ, Carney CP et al. (2006) Comorbidity profile of dementia patients in primary care: are they sicker? J Am Geriatr Soc 54: 104-109. doi: 10.1111/j.1532-5415.2005.00543.x. PubMed: 16420205. [DOI] [PubMed] [Google Scholar]
- 41. van Oostrom SH, Picavet HS, van Gelder BM, Lemmens LC, Hoeymans N et al. (2012) Multimorbidity and comorbidity in the Dutch population - data from general practices. BMC Public Health 12: 715. doi: 10.1186/1471-2458-12-715. PubMed: 22935268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Noël PH, Williams JW Jr, Unützer J, Worchel J, Lee S et al. (2004) Depression and comorbid illness in elderly primary care patients: Impact on multiple domains of health status and well-being. Ann Fam Med 2: 555-562. doi: 10.1370/afm.143. PubMed: 15576541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Struijs JN, Baan CA, Schellevis FG, Westert GP, van den Bos GAM (2006) Comorbidity in patients with diabetes mellitus: impact on medical health care utilization. BMC Health Serv Res 6: 84. doi: 10.1186/1472-6963-6-84. PubMed: 16820048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van den Bussche H, Koller D, Kolonko T, Hansen H, Wegscheider K et al. (2011) Which chronic diseases and disease combinations are specific to multimorbidity in the elderly? Results of a claims data based cross-sectional study in Germany. BMC Public Health 11: 101. doi: 10.1186/1471-2458-11-101. PubMed: 21320345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Findley P, Shen C, Sambamoorthi U (2011) Multimorbidity and persistent depression among veterans with diabetes, heart disease, and hypertension. Health Soc Work 36: 109-119. doi: 10.1093/hsw/36.2.109. PubMed: 21661300. [DOI] [PubMed] [Google Scholar]
- 46. Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A et al. (2011) Aging with multimorbidity: A systematic review of the literature. Ageing Res Rev 10: 430-439. doi: 10.1016/j.arr.2011.03.003. PubMed: 21402176. [DOI] [PubMed] [Google Scholar]
- 47. Agborsangaya CB, Lau D, Lahtinen M, Cooke T, Johnson JA (2012) Multimorbidity prevalence and patterns across socioeconomic determinants: a cross-sectional survey. BMC Public Health 12: 201. doi: 10.1186/1471-2458-12-201. PubMed: 22429338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L (2005) Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med 3: 223-228. doi: 10.1370/afm.272. PubMed: 15928225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Salisbury C, Johnson L, Purdy S, Valderas JM, Montgomery AA (2011) Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract 61: e12-e21. doi: 10.3399/bjgp11X548929. PubMed: 21401985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S et al. (2012) Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 380: 37-43. doi: 10.1016/S0140-6736(13)60393-1. PubMed: 22579043. [DOI] [PubMed] [Google Scholar]
- 51. Mathur R, Hull SA, Badrick E, Robson J (2011) Cardiovascular multimorbidity: the effect of ethnicity on prevalence and risk factor management. Br J Gen Pract 61: e262-e270. doi: 10.3399/bjgp11X572454. PubMed: 21619750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cabassa LJ, Humensky J, Druss B, Lewis-Fernández R, Gomes AP et al. (2013) Do race, ethnicity, and psychiatric diagnoses matter in the prevalence of multiple chronic medical conditions? Med Care 51: 540-547. doi: 10.1097/MLR.0b013e31828dbb19. PubMed: 23552429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Violán C, Foguet-Boreu Q, Hermosilla-Pérez E, Valderas JM, Bolíbar B et al. (2013) Comparison of the information provided by electronic health records data and a population health survey to estimate prevalence of selected health conditions and multimorbidity. BMC Public Health 13: 251. doi: 10.1186/1471-2458-13-251. PubMed: 23517342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fortin M, Hudon C, Haggerty J, Van den Akker M, Almirall J (2010) Prevalence estimates of multimorbididty: a comparative study of two sources. BMC Health Serv Res 10: 111. doi: 10.1186/1472-6963-10-111. PubMed: 20459621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lugtenberg M, Burgers JS, Clancy C, Westert GP, Schneider EC (2011) Current guidelines have limited applicability to patients with comorbid conditions: A systematic analysis of evidence-based guidelines. PLOS ONE 6: e25987. doi: 10.1371/journal.pone.0025987. PubMed: 22028802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gommer AM, Poos MJJC (2010) Welke ziekten hebben de hoogste prevalentie? In: Volksgezondheid Toekomst Verkenning, Nationaal Kompas Volksgezondheid. Bilthoven. Available: http://www.nationaalkompas.nl/. Accessed January 2013.
- 57. World Health Organization (2008) The global burden of disease 2004 update. Part 3: Disease incidence, prevalence and disability. Available: http://www.who.int/healthinfor/global_burden_disease/2004_report_update/en/index.html. Accessed January 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic literature search of PubMed/MEDLINE, September 2012.
(DOC)
PRISMA checklist.
(DOC)