Abstract
Interferon-gamma release assays (IGRAs) have proven to be useful to accurately detect Mycobacterium tuberculosis (Mtb) infection, but they cannot reliably discriminate between active tuberculosis (TB) and latent tuberculosis infection (LTBI). This study aims to test whether Mtb-specific tumor necrosis factor-alpha (TNF-α) could be used as a new tool for the rapid diagnosis of active TB disease. The secretion of TNF-α by Mtb-specific antigen-stimulated peripheral blood mononuclear cells (PBMCs) of sixty seven participants was investigated in the study. Our results showed that the total measurement of TNF-α secretion by Mtb-specific antigen-stimulated PBMCs is not a good biomarker for active TB diagnosis. However, we found that calculation of Mtb-specific TNF-α not only distinguish between active and latent TB infection, but also can differentiate active TB from non-TB patients. Using the cutoff value of 136.9 pg/ml for Mtb-specific TNF-α, we were able to differentiate active TB from LTBI. Sensitivity and specificity were 72% and 90.91%. These data suggest that Mtb-specific TNF-α could be a potential biomarker for the diagnosis of active TB disease.
Introduction
Tuberculosis (TB) is one of the leading causes of death by infectious diseases worldwide. Approximately one-third of population in the world is infected with Mycobacterium tuberculosis (Mtb). Of those exposed, eight million develop symptoms and approximately two million die from the infection each year [1]. TB has the special characteristic that most of the infected individuals remain latent. Most individuals with latent TB are asymptomatic for their lifetime, with only approximately 15% ever developing active disease [2]. However, the global problem of TB is worsening with emergence of drug resistant Mtb strains and the increased susceptibility of HIV-infected individuals to developing TB [3], [4]. Early diagnosis and treatment of active TB disease are the key points to prevent epidemic of this disease.
There are many methods for diagnosis of Mtb infection. Microscopy of acid-fast staining (AFS) and culture for Mtb are gold standards for the diagnosis of active TB. However, some patients are unable to produce suitable sputum and culture time is longer than two weeks, leading to unsatisfied demand for clinicians. Although direct smear microscopy is rapid and inexpensive, its sensitivity is limited [5]. The tuberculin skin test (TST) used as a classic way to diagnose active TB disease or latent tuberculosis infection (LTBI) is unreliable, for the reason that it has low sensitivity and specificity for TB infection in clinical use [6]. Molecular approaches such as the Xpert MTB/RIF assay [7], [8] reduce the detection time and have been endorsed by the WHO. Although they are sensitive and relatively easy to use, these tests are costly and cannot completely substitute for classic methods especially in developing countries.
Interferon-gamma release assays (IGRAs) are based on interferon-gamma (IFN-γ) secretion by lymphocytes exposed to Mtb-specific antigens such as early secreted antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10). IGRAs have been shown to be very sensitive and specific for LTBI especially in comparison to the TST [9]–[11]. While IGRAs are useful in the diagnosis of Mtb infection, an important limitation of these assays is their inability to discriminate between active TB and LTBI [12]. Thus, IGRAs are of little value in high TB incidence areas with a very high LTBI burden like China.
Discovery of biomarkers that can rapidly distinguish active TB from LTBI would be a major breakthrough in high-TB-prevalence countries. In this study, we demonstrate that Mtb-specific tumor necrosis factor-alpha (TNF-α) is a new tool for the rapid diagnosis of active TB disease.
Materials and Methods
Study groups
This study was carried out from January to May 2013 at Tongji Hospital (TJH), the largest hospital in central region of China. Participants were selected based on Mtb-specific IFN-γ ELISPOT responses routinely performed for the diagnosis of Mtb infection at TJH. Subjects were classified into the following four categories: (1) active TB patients; (2) LTBI individuals; (3) non-TB control subjects; (4) healthy volunteer subjects.
Besides positive Mtb-specific IFN-γ ELISPOT responses, patients with active TB had a diagnosis based on laboratory isolation of Mtb in mycobacterial culture from sputum, broncho alveolar lavage fluid or plerual effusion, and/or AFS, and/or PCR. The final diagnosis was given by a clinician after validation of these criteria associated with clinical symptoms. Individuals who had a positive Mtb-specific IFN-γ ELISPOT responses but lacked clinical or radiographic evidence of active TB were diagnosed as LTBI. Patients with clinical symptoms (fever, night sweats, weight-loss) but without evidence of active TB were recruited as non-TB control subjects. Volunteers who had a negative Mtb-specific IFN-γ ELISPOT responses and without any pulmonary symptoms or active disease were recruited as healthy control subjects. This study was approved by the ethical committee of Tongji hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. All participants gave written consent to the study.
IFN-γ ELISPOT assay
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood of four groups of participants by using Ficoll-Hypaque density gradients (Sigma-Aldrich, St Louis, MO). Mtb-specific IFN-γ ELISPOT assay was performed according to the instruction of T-SPOT.TB kit (Oxford Immunotec, Abingdon, UK).
ELISA for determination of Mtb-specific TNF-α
PBMCs isolated as above were also used for determination of Mtb-specific TNF-α. In order to determinate Mtb-specific TNF-α, 100 µl of fresh PBMCs (2.5×105/well) were seeded in 96-well plates and were stimulated with 50 µl of ESAT-6, CFP-10 or medium ( ESAT-6 and CFP-10 reagents were the same as T-SPOT.TB kit). The plates were incubated at 37°C for 16–20 h. After incubation, the supernatant was collected after centrifugation. Concentration of TNF-α in culture supernatant was measured by a standard sandwich cytokine ELISA procedure according to the instruction of human TNF-α detection kit (R&D Systems, Minneapolis, MN). Mtb-specific TNF-α was defined by the following rule: Mtb-specific TNF-α = TNF-αESAT-6 or CFP-10 stimulation - TNF-αbackground. TNF-αbackground is the secretion of TNF-α by medium-stimulated PBMCs.
Analysis of ESAT-6-induced cytotoxicity
PBMCs were isolated from three non-TB patients. One hundred microliters of PBMCs (2.5 × 105/well) were seeded in 96-well plates and were stimulated with different concentrations (10, 20, 30 µg/ml) of phytohemagglutinin (PHA) (Sigma-Aldrich, St Louis, MO). In some experiments, 50 µl of ESAT-6 was added to culture medium. The plates were incubated at 37°C for 16–20 h. After incubation, the supernatant was collected and concentration of TNF-α in culture supernatant was measured by sandwich ELISA, as above. To further determine ESAT-6-induced cytotoxicity, PBMCs isolated from another five non-TB patients were stimulated with 30 µg/ml PHA for 24 h in the presence or absence of ESAT-6. After culture, the Annexin V-APC Apoptosis Detection Kit (17-8007, eBioscience) was used for detection of apoptosis in PBMCs. All procedures were performed according to the manufacturer’s instructions. Stained cells were then analyzed on FACScalibur using Cell Quest software (Becton Dickinson, San Jose, CA).
Statistical analysis
The statistical significance of differences in Mtb-specific TNF-α concentration among the four groups of participants was evaluated with the Mann-Whitney U test. Differences in percentages of apoptotic cells between nonstimulated and ESAT-6-stimulated PBMCs were assessed using the paired Student’s t-test. Receiver operating characteristic (ROC) analysis was performed to determine cutoff levels of Mtb-specific TNF-α in discriminating between active TB and LTBI. Statistical significance was determined as p< 0.05 (*p< 0.05, **p< 0.001).
Results
Participants
Sixty seven participants were included in the study. Ten subjects were classified as non-TB patients, 10 as healthy volunteers, 22 as LTBI individuals and 25 as active TB patients. Active TB disease was diagnosed in 25 subjects on the basis of positive Mtb-specific IFN-γ ELISPOT responses, clinical signs (for example, cough, fever and weight loss), AFS, culture or PCR for Mtb, and chest radiography (Table S1 contains a full clinical description of each subject).
Total TNF-α level cannot distinguish active TB disease from LTBI or non-TB infection
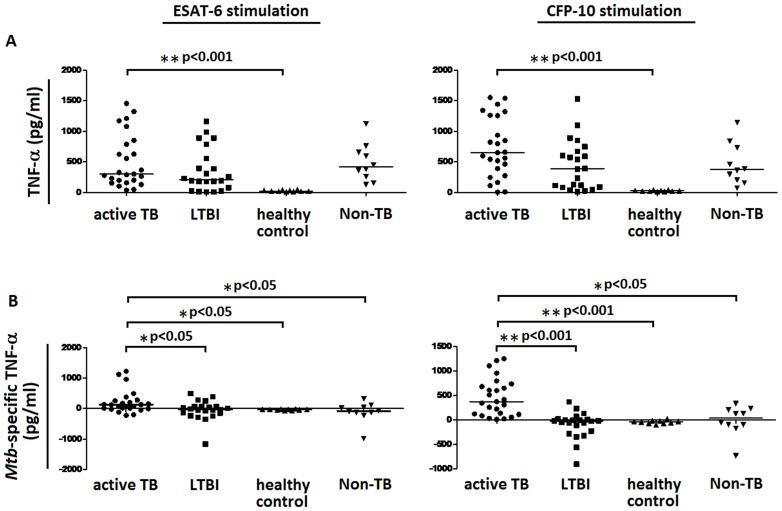
The secretion of TNF-α by ESAT-6 or CFP-10-stimulated PBMCs from different groups of participants was detected in this study. After stimulation with ESAT-6, the secretion of TNF-α was significantly increased by PBMCs of active TB patients in comparison to healthy volunteer subjects. However, TNF-α secretion had no statistical difference among active TB patients, LTBI individuals, and non-TB patients. Similar results were obtained in CFP-10 stimulation experiment. TNF-α secretion was also significantly increased by PBMCs of active TB patients, as compared with healthy control subjects, and still had no statistical difference as compared with LTBI individuals or non-TB patients (Fig. 1A). These data suggest that although the secretion of TNF-α by ESAT-6 or CFP-10-stimulated PBMCs is significantly increased in active TB group, TNF-α levels do not distinguish active TB from LTBI or non-TB control. Thus, the secretion of TNF-α is not a good biomarker for the diagnosis of active TB disease.
Figure 1. The secretions of TNF-α and Mtb-specific TNF-α by ESAT-6 or CFP-10-stimulated PBMCs.
PBMCs obtained from active TB patients (n = 25), LTBI individuals (n = 22), healthy control subjects (n = 10) and non-TB patients (n = 10) were stimulated with ESAT-6 or CFP-10. PBMCs stimulated with medium alone were used as a background control. (A) After 16–20 h of incubation, the supernatant was collected and tested for concentrations of secreted TNF-α by ELISA. (B) Mtb-specific TNF-α was calculated by subtracting background TNF-α secreted by medium-stimulated PBMCs from TNF-α secreted by ESAT-6 or CFP-10-stimulated PBMCs. Median values for each group of participants are represented by a horizontal bar. *p < 0.05, **p < 0.001.
Mtb-specific TNF-α is a potential biomarker for the diagnosis of active TB disease
Given that high background secretion of TNF-α was found in ESAT-6 or CFP-10-stimulated PBMCs from both TB and non-TB patients, we further proposed a new calculation method: Mtb-specific TNF-α. Mtb-specific TNF-α was defined by the following rule: Mtb-specific TNF-α = TNF-αESAT-6 or CFP-10 stimulation - TNF-αbackground. Interestingly, after stimulation with ESAT-6 or CFP-10, Mtb-specific TNF-α was secreted at significantly higher concentration by PBMCs of active TB patients, as compared with LTBI individuals, healthy control subjects or non-TB control subjects (Fig. 1B). Mean values and standard error of the mean (SEM) of TNF-α and Mtb-specific TNF-α secreted by PBMCs of four groups of participants were shown in Table 1. These data suggest that calculation of Mtb-specific TNF-α not only distinguish active TB from LTBI, but also can differentiate between active TB and non-TB. Thus, Mtb-specific TNF-α is a potential biomarker for the diagnosis of active TB disease.
Table 1. Levels of TNF-α and Mtb-specific TNF-α secreted by ESAT-6 or CFP-10-stimulated PBMCs of four groups of participants.
 Active TB (n = 25) Active TB (n = 25) |
 LTBI (n = 22) LTBI (n = 22) |
 Healthy control (n = 10) Healthy control (n = 10) |
 Non-TB control (n = 10) Non-TB control (n = 10) |
p vs vs
|
p vs vs
|
p vs vs
|
|
| TNF-α result | |||||||
| ESAT-6 | 511.2±87.40 | 359.6±76.19 | 24.61±3.742 | 487.2±96.68 | 0.144 | <0.001 | 0.770 |
| CFP-10 | 733.1±97.74 | 443.5±87.14 | 25.17±3.269 | 464.9±107.3 | 0.052 | <0.001 | 0.116 |
| Mtb -specific TNF-α result | |||||||
| ESAT-6 | 209.4±74.80 | –57.27±69.26 | –39.62±6.555 | –114.8±108.0 | 0.010 | 0.002 | 0.014 |
| CFP-10 | 465.3±76.75 | –97.20±57.26 | –41.70±10.89 | –11.83±96.01 | <0.001 | <0.001 | 0.002 |
Note. Data are expressed as means±SEM; TB: tuberculosis; LTBI: latent tuberculosis infection.
Mtb-specific TNF-α is a prominent biomarker in differentiating between active TB and LTBI
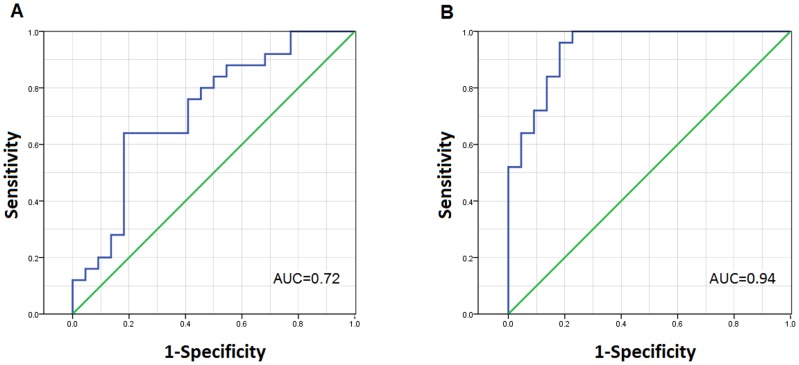
To differentiate between active TB and LTBI, ROC analysis was then performed to determine the exact cutoff level for Mtb-specific TNF-α (Fig. 2). Statistical data of ROC curve for Mtb-specific TNF-α were shown in Table 2, using the cutoff value of 71.1 pg/ml for Mtb-specific TNF-α in ESAT-6 stimulation experiment, we were able to differentiate active TB from LTBI. Sensitivity and specificity were 64% and 81.82%, respectively. Furthermore, better results were obtained with CFP-10 stimulation. ROC analysis demonstrated sensitivity of 72% and specificity of 90.91%, in discriminating between active TB and LTBI, if using a cutoff value of 136.9 pg/ml. These data suggest that Mtb-specific TNF-α is a prominent biomarker in differentiating between active TB and LTBI.
Figure 2. ROC for Mtb-specific TNF-α as a classifier to distinguish between active TB and LTBI.
With ESAT-6 (A) or CFP-10 (B) stimulation, Mtb-specific TNF-α was calculated, then ROC analysis was performed for Mtb-specific TNF-α to determine cutoff levels in discriminating between active TB (n = 25) and LTBI (n = 22). AUC = Area under the curve.
Mtb-specific antigen shows obvious cytotoxicity to PHA-stimulated PBMCs of non-TB patients
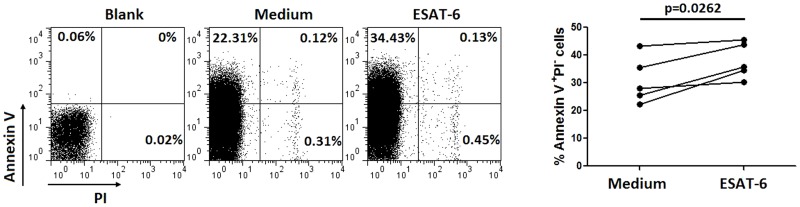
We confirmed in this study that high background secretion of TNF-α, even without any stimulation, was found in PBMCs of both LTBI individuals and non-TB patients. According to this, large negative values of Mtb-specific TNF-α which was calculated by subtracting background TNF-α was obtained. We thus investigated why TNF-α secretion by ESAT-6-stimulated PBMCs of non-TB patients was mostly lower than background secretion by these cells. We found that the secretion of TNF-α by PHA-stimulated PBMCs was obvious decreased when adding ESAT-6 to the culture medium (Table 3). This was observed in three non-TB patients. To further determine ESAT-6-induced cytotoxicity, we used flow cytometric analysis with annexin V staining. As shown in Figure 3, the percentage of Annexin V+PI− apoptotic cells increased significantly after adding ESAT-6 to the culture medium. These data suggest that Mtb-specific antigen like ESAT-6 has obvious cytotoxicity to PHA-stimulated PBMCs of non-TB patients.
Table 2. Statistical analysis of ROC curve for Mtb-specific TNF-α to distinguish between active TB and LTBI.
| AUC (95% CI) | P-value | Cut-off | Likelihood ratio | Sensitivity% (95% CI) | Specificity% (95% CI) | |
| ESAT-6 stimulation | 0.72 (0.57–0.86) | 0.009 | >71.10 | 3.52 | 64 (42.52–82.03) | 81.82 (59.72–94.81) |
| CFP-10 stimulation | 0.94 (0.87–1.00) | <0.0001 | >136.9 | 7.92 | 72 (50.61–87.93) | 90.91 (70.84–98.88) |
Note. TB: tuberculosis; LTBI: latent tuberculosis infection; AUC: area under the curve.
Figure 3. Annexin V staining of medium and ESAT-6-stimulated PBMCs.
PBMCs isolated from five non-TB patients were stimulated with 30 µg/ml PHA for 24 h in the presence of 50 µl of ESAT-6 or medium. After culture, PBMCs were collected and the percentages of apoptotic cells (Annexin V+PI−) were analyzed by flow cytometry. Shown are representative flow cytometry analyses of the apoptotic cells in non-TB patients.
Table 3. The cytotoxicity of ESAT-6 to PHA-stimulated PBMCs of three non-TB patients.
| non-TB patient 1 | non-TB patient 2 | non-TB patient 3 | |
| clinical signs | fever, cough, headache | fever, plerual effusion | fever, cough, headache |
| T-SPOT.TB | - | - | + |
| mycobacterial culture | - | - | - |
| AFS | - | - | - |
| PCR | - | - | - |
| TNF-α secretion by PBMCs (pg/ml) | |||
| 10 µg/ml PHA + 50 µl medium | 180±15.5 | 48.8±5.5 | 135±13.2 |
| 10 µg/ml PHA + 50 µl ESAT-6 | 95.9±11.2 | 14.1±3.5 | 31.8±5.9 |
| 20 µg/ml PHA + 50 µl medium | 810±34.5 | 204.1±15.6 | 650±31.8 |
| 20 µg/ml PHA + 50 µl ESAT-6 | 181.2±19.9 | 26.5±4.2 | 145.3±14.5 |
| 30 µg/ml PHA + 50 µl medium | 1317.6±56.7 | 300.6±28.4 | 1123±45.7 |
| 30 µg/ml PHA + 50 µl ESAT-6 | 374.1±23.6 | 61.8±10.9 | 305.9±21.4 |
Note. PHA: phytohemagglutinin; TB: tuberculosis; AFS: acid fast staining.
Discussion
Commercial IGRAs such as the ELISA-based Quantiferon TB Gold In-Tube assay (QFT-IT) and the ELISPOT-based T-SPOT.TB, are widely used especially in high income, low burden settings for the diagnosis of Mtb infection. These assays have proven to be useful to accurately detect Mtb infection [13], ; however, they cannot reliably discriminate between active TB and LTBI [15]. Previous studies have shown that multi-cytokine analysis after stimulation of PBMCs from patients with latent or active TB infection indicates the possibility of successfully discerning these two disease states [16], [17]. However, no biomarker is currently ready for routine clinical use in distinguishing active TB from LTBI.
TNF-α is a cytokine with a long history in TB research and plays important roles in immune and pathological responses of TB patients [18]. Kupeli et al. showed that bronchoalveolar lavage fluid TNF-α level is found to be significantly increased in TB patients compared with both other pulmonary disease patients and healthy controls [19]. Pleural fluid TNF-α level also proved to be useful in prognosticating tuberculous pleurisy with high sensitivity and specificity rates [20]. Recently, Harari et al. proposed that the proportion of single-positive TNF-α Mtb-specific CD4+ T cells might be used as a signature of active TB disease [21]. Until now, however, the soluble Mtb-specific TNF-α secretion has not been fully evaluated in TB and non-TB patients.
In this study, we used Mtb-specific antigens such as ESAT-6 and CFP-10 to stimulate PBMCs isolated from active TB patients, LTBI individuals, non-TB patients and healthy volunteer subjects. We found that TNF-α secretion is not a good biomarker for the diagnosis of active TB disease because it cannot distinguish active TB from LTBI or non-TB. Interestingly, we found that although TNF-α secretion by PBMCs of LTBI individuals and non-TB patients was increased, the background secretion of TNF-α was also very high in these groups. Previous studies have shown that background level of TNF-α is observed in un-stimulated samples from patients whether infected with Mtb or not [22], [23]. We confirmed in this study that high background secretion of TNF-α was found in PBMCs of non-TB patients. We also investigated why TNF-α secretion by ESAT-6-stimulated PBMCs of non-TB patients was mostly lower than background secretion by these cells. We found that Mtb-specific antigen like ESAT-6 has obvious cytotoxicity to PHA-stimulated PBMCs of non-TB patients.
According to high level of background TNF-α secreted by PBMCs of LTBI individuals and non-TB patients, we proposed Mtb-specific TNF-α which is calculated by subtracting background level of TNF-α secreted by unstimulated PBMCs. Excitingly, we found that calculation of Mtb-specific TNF-α was substantially better than direct detection of TNF-α for the diagnosis of active TB disease.
Taken together, these data indicate that Mtb-specific TNF-α secreted by ESAT-6 or CFP-10-stimulated PBMCs is a new biomarker in distinguishing active TB from LTBI or non-TB. However, the sample size in this study was small, which may lead to inaccurate sensitivity and specificity. Moreover, the diagnosis of active TB was difficult sometimes. Some active TB patients may have no obvious clinical or radiographic evidence, leading to the wrong diagnosis and causing unmatched results. Thus even if intriguing, our findings should be confirmed in larger TB populations.
Supporting Information
Clinical description of patients diagnosed with active TB disease.
(TIF)
Funding Statement
This work was supported by the Infectious Diseases Control Project from Ministry of Health of China (2012zx10004-207). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kwan CK, Ernst JD (2011) HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev 24: 351–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Milburn H (2007) Key issues in the diagnosis and management of tuberculosis. J R Soc Med 100: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anandaiah A, Dheda K, Keane J, Koziel H, Moore DA, et al. (2011) Novel developments in the epidemic of human immunodeficiency virus and tuberculosis coinfection. Am J Respir Crit Care Med 183: 987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loddenkemper R, Hauer B (2010) Drug-resistant tuberculosis: a worldwide epidemic poses a new challenge. Dtsch Arztebl Int 107: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farnia P, Mohammadi F, Zarifi Z, Tabatabee DJ, Ganavi J, et al. (2002) Improving sensitivity of direct microscopy for detection of acid-fast bacilli in sputum: use of chitin in mucus digestion. J Clin Microbiol 40: 508–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barnes PF (2001) Diagnosing latent tuberculosis infection: the 100-year upgrade. Am J Respir Crit Care Med 163: 807–808. [DOI] [PubMed] [Google Scholar]
- 7. Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, et al. (2010) Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363: 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Theron G, Peter J, van Zyl-Smit R, Mishra H, Streicher E, et al. (2011) Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med 184: 132–140. [DOI] [PubMed] [Google Scholar]
- 9. Pai M, Riley LW, Colford JM Jr (2004) Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis 4: 761–776. [DOI] [PubMed] [Google Scholar]
- 10. Pai M, Kalantri S, Dheda K (2006) New tools and emerging technologies for the diagnosis of tuberculosis: part I. Latent tuberculosis. Expert Rev Mol Diagn 6: 413–422. [DOI] [PubMed] [Google Scholar]
- 11. Mandalakas AM, Hesseling AC, Chegou NN, Kirchner HL, Zhu X, et al. (2008) High level of discordant IGRA results in HIV-infected adults and children. Int J Tuberc Lung Dis 12: 417–423. [PubMed] [Google Scholar]
- 12. Amanatidou V, Syridou G, Mavrikou M, Tsolia MN (2012) Latent tuberculosis infection in children: diagnostic approaches. Eur J Clin Microbiol Infect Dis 31: 1285–1294. [DOI] [PubMed] [Google Scholar]
- 13. Hesseling AC, Mandalakas AM, Kirchner HL, Chegou NN, Marais BJ, et al. (2009) Highly discordant T cell responses in individuals with recent exposure to household tuberculosis. Thorax 64: 840–846. [DOI] [PubMed] [Google Scholar]
- 14. Diel R, Goletti D, Ferrara G, Bothamley G, Cirillo D, et al. (2011) Interferon-gamma release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur Respir J 37: 88–99. [DOI] [PubMed] [Google Scholar]
- 15. Sester M, Sotgiu G, Lange C, Giehl C, Girardi E, et al. (2011) Interferon-gamma release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J 37: 100–111. [DOI] [PubMed] [Google Scholar]
- 16. Tincati C, Cappione III AJ, Snyder-Cappione JE (2012) Distinguishing Latent from Active Mycobacterium tuberculosis Infection Using Elispot Assays: Looking Beyond Interferon-gamma. Cells 1: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chiappini E, Della Bella C, Bonsignori F, Sollai S, Amedei A, et al. (2012) Potential role of M. tuberculosis specific IFN-gamma and IL-2 ELISPOT assays in discriminating children with active or latent tuberculosis. PLoS One 7: e46041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mootoo A, Stylianou E, Arias MA, Reljic R (2009) TNF-alpha in tuberculosis: a cytokine with a split personality. Inflamm Allergy Drug Targets 8: 53–62. [DOI] [PubMed] [Google Scholar]
- 19. Kupeli E, Karnak D, Beder S, Kayacan O, Tutkak H (2008) Diagnostic accuracy of cytokine levels (TNF-alpha, IL-2 and IFN-gamma) in bronchoalveolar lavage fluid of smear-negative pulmonary tuberculosis patients. Respiration 75: 73–78. [DOI] [PubMed] [Google Scholar]
- 20. Tahhan M, Ugurman F, Gozu A, Akkalyoncu B, Samurkasoglu B (2003) Tumour necrosis factor-alpha in comparison to adenosine deaminase in tuberculous pleuritis. Respiration 70: 270–274. [DOI] [PubMed] [Google Scholar]
- 21. Harari A, Rozot V, Enders FB, Perreau M, Stalder JM, et al. (2011) Dominant TNF-alpha+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med 17: 372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lighter-Fisher J, Peng CH, Tse DB (2010) Cytokine responses to QuantiFERON(R) peptides, purified protein derivative and recombinant ESAT-6 in children with tuberculosis. Int J Tuberc Lung Dis 14: 1548–1555. [PubMed] [Google Scholar]
- 23. Chegou NN, Black GF, Kidd M, van Helden PD, Walzl G (2009) Host markers in QuantiFERON supernatants differentiate active TB from latent TB infection: preliminary report. BMC Pulm Med 9: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical description of patients diagnosed with active TB disease.
(TIF)