Abstract
Metastasis remains the major cause of therapeutic failure, poor prognosis and high mortality in breast and prostate cancer patients. Aberrant microenvironments including hypoxia and acidic pH are common features of most solid tumors that have been long associated with enhanced metastasis and poor patient outcomes. Novel approaches to reduce metastatic incidences and improve overall survival of cancer patients clearly are needed. The crucial role of Cathepsin L (CTSL) in the dissemination of tumor cells has led to the development of novel cathepsin L inhibition strategies. The present study evaluated the ability of KGP94, a small molecule inhibitor of CTSL, to impair the metastatic phenotype of prostate (PC-3ML) and breast (MDAMB- 231) cancer cells both under normal and aberrant microenvironmental conditions. To assess the role of CTSL in hypoxia and acidosis triggered metastasis associated cell functions, secreted CTSL levels were determined under conditions pertinent to the tumor microenvironment. Acute exposures to hypoxic or acidic conditions significantly elevated secreted CTSL levels either through an increase in intracellular CTSL levels or through activation of lysosomal exocytosis or both, depending on the tumor type. Increases in CTSL secretion closely paralleled enhanced tumor cell migration and invasion suggesting that CTSL could be an essential factor in tumor microenvironment triggered metastasis. Importantly, KGP94 treatment led to marked attenuation of tumor cell invasion and migration under both normal and aberrant microenvironmental conditions suggesting that it may have significant utility as an anti-metastatic agent.
Keywords: Metastasis, Cathepsin L, KGP94, Hypoxia, Acidic pH
Introduction
Prostate and breast cancer are the leading causes of cancer-related death in men and women and metastasis is the primary factor underlying the high mortality rates [1]. Proteolytic enzymes that promote metastasis such as the lysosomal cysteine protease cathepsin L (CTSL) may offer a promising therapeutic target [2–4]. Expression of CTSL is up regulated in a wide range of human cancers including glioma, melanoma, pancreatic, breast and prostate carcinoma [5]. Under normal physiological conditions, CTSL is sequestered within the lysosomes. However, in tumors, alteration in expression level and translocation pathway results in secretion of CTSL [6– 8]. The observed increase in CTSL secretion is however not paralleled by a commensurate increase in levels of endogenous inhibitors of CTSL such as cystatin C, which ultimately results in unregulated CTSL activation [9]. Secreted CTSL enhances the metastatic potential of cancer cells through direct degradative proteolysis of several components of the extracellular matrix, basement membrane and E-Cadherin. In the presence of surface glycosaminoglycans, secreted CTSL degrades extracellular matrix components such as laminin, Type I and IV collagen, fibronectin, elastin, etc [10, 11]. In addition, secreted CTSL plays a critical role in the amplification of the proteolytic cascade by activating latent pro-forms of other key metastasis associated proteases such as proheparanase, urokinase plasminogen activator, cathepsin D and members of the matrix metalloproteinase family [12–14].
Though numerous clinical observations have associated CTSL upregulation with metastatic aggressiveness, very few have investigated its activity and function under physiological conditions pertinent to the tumor microenvironment. The tumor microenvironment is acidic and hypoxic in nature [15, 16]. Increased tumor hypoxia and acidosis correlate with increased metastatic occurrence [16–18]. Tumor hypoxia can be broadly classified into chronic and acute hypoxia [19]. Chronic hypoxia occurs in regions that are beyond the diffusion limit of oxygen from the existing vasculature. Acute hypoxia can result from transient collapse of blood vessels leading to tumor cells that consequently experience periods of hypoxia and reoxygenation. Studies in experimental metastatic models suggest that the correlation between tumor hypoxia and metastatic incidence is primarily attributable to acute rather than chronic hypoxia in the primary tumor [20, 21].
Elevated CTSL secretion is not accompanied by corresponding increases in the levels of its endogenous inhibitors. Secreted CTSL thereby engages in unregulated activation of migratory and invasive cascades [22]. Thus, molecules capable of inactivating CTSL could potentially serve as effective anti-metastatic treatments. Recently, the reversibly binding small molecule CTSL inhibitor KGP94 (3-bromophenyl-3-hydroxyphenyl-ketone thiosemicarbazone) was shown to abolish CTSL function by blocking its active site [23] which significantly delayed the growth of primary tumors [24]. In this study, we investigated the ability of KGP94 to inhibit CTSL activity and decrease prostate and breast cancer cell migration and invasion under normal as well as hypoxic and acidic microenvironmental conditions.
Materials and Methods
Cell culture
RWPE-1, PC-3, MCF-7, SKBR-3, T47D and MDA-MB-231 were purchased from American Type Culture Collection. PC-3ML and PC-3N cells were gifts from Dr. Alessandro Fatatis (Drexel University). PC-3ML and PC-3N are highly and poorly metastatic sublines isolated from PC-3 cells [25]. MDA-MB-435 cells were received from Dr. Jianrong Lu (University of Florida). Although there has been controversy that the MDA-MB435 cell line may have been derived from M14 melanoma [26], a subsequent review by Chambers (2009) [27] concluded that rather than both lines being of M14 melanoma origin, evidence is consistent with both cell lines being of MDA-MB-435 breast cancer origin. M-4A4 and NM-2C5 are highly and poorly invasive sublines isolated from MDA-MB-435 cells [28] provided by Dr. Steve Goodison (MD Anderson Cancer Center). All cell lines were cultured in appropriate media (RWPE-1 in Keratinocyte serum free medium supplemented with 0.05 mg/ml bovine pituitary extract and 5 ng/ml epidermal growth factor; PC-3 and PC-3ML in HAM’s F12 nutrient mixture; MCF-7, SKBR-3, T47D, MDA-MB435, M-4A4, NM-2C5 and MDA-MB-231 in DMEM media) supplemented with 10% FBS at 37°C in a humidified atmosphere of 5% CO2 in air.
Drug preparation
KGP94 was dissolved in sterile DMSO to obtain 10 and 25 mM stock solutions. For treatment, stocks were diluted 1000 fold in cell culture media to achieve final concentration of 10 and 25 µM respectively.
Enzyme linked Immunosorbent Assay
Prostate and breast cell lines were cultured in 100 mm dishes. When the cells reached ~ 60– 70% confluency, media were replaced and collected 24 h later. Cell conditioned media were centrifuged (1000 rpm, 10 min) and stored at −80°C until further analysis. Cells from each sample were trypsinized and counted for normalization of secreted levels with total cell number. Cathepsin L ELISA was performed using human Cathepsin L DuoSet kit (DY952) from R&D systems as per manufacturer’s insructions. Cystatin C ELISA was performed using Quantikine human Cystatin C Immunoassay kit (DSCTC0) from R&D systems. Conditioned media from each sample were evaluated in triplicate and secreted levels of CTSL and cystatin C were normalized to 106 cells.
Clonogenic cell survival assay
Cells were treated with desired concentration of KGP94 for 24 h. Subsequently, cells were harvested and seeded into 60 mm dishes (200, 100 or 50 cells/dish) and incubated for 14 days. 2 weeks later, cells were stained with crystal violet and the number of colonies (>50 cells) were counted. Plating efficiency was calculated from the ratio of the number of colonies formed divided by the number of cells seeded.
CTSL activity measurement assay
PC-3ML and MDA-MB-231 cells were cultured in 100 mm dishes. When the cells reached ~ 60–70% confluency, media were replaced. Twenty-four h later cell conditioned media were harvested, centrifuged (1000 rpm, 10 min), concentrated 20× using Amicon centrifugal filter units (Millipore) stored at −80°C until further analysis. Cells were trypsinized and counted for normalization of CTSL activity with total cell number. Cathepsin L activity assays were performed using the InnoZyme Cathepsin L Activity Kit (CBA023) as per manufacturer’s instructions. Conditioned media from each sample were evaluated in triplicate and secreted CTSL activity was normalized to fold concentration and cell number.
Cell migration assay
Transwell migration assays were performed using BD falcon cell culture inserts (353097). Cell migration assay was performed as described previously [29]. The bottom of the insert is a polyethylene terephthalate membrane with 8 µm pores. Cells (PC-3ML, 2×104; MDA-MB-231, 1×104) suspended in complete media were seeded into the inserts and incubated for 24 h to allow them to migrate through 8 µm pores, to the other side of the membrane. For experiments determining the effect of CTSL inhibition, KGP94 was added to both the top and bottom chambers at equal concentration. 24 h later, non-migrated cells on the upper surface of the membrane were scraped off using a cotton swab. Cells that had migrated to the underside of the membrane were stained with crystal violet and the total number of migrated cells was enumerated under a microscope.
For wound healing assays, cells were allowed to grow to confluence before ~1 mm wide scratches were made in the cell monolayers using sterilized 200 µl pipette tips. 24 h later, the plates were photographed and cell migration into the denuded areas was determined.
Cell invasion assay
Invasion assays were performed using matrigel coated invasion inserts from BD Biosciences (354480) as described previously [29]. Cells (PC-3ML, 4×104; MDA-MB-231, 2×104) suspended in serum free media were seeded into the inserts. Media supplemented with 10% FBS was added to the bottom chamber to serve as a chemoattractant. For experiments determining the effect of CTSL inhibition, equal concentrations of KGP94 were added to both the top and the bottom chamber. The cells were incubated under the desired conditions and 24 h later, cells that invaded to the underside of the membrane were stained and the total number of invaded cells was determined by counting under a microscope.
CTSL knockdown
1.25×105 PC-3 cells were seeded in 6-well plates in complete media. 48 h later, media were replaced with antibiotic free media and the cells were transfected with CTSL shRNA plasmids (Origene TG305172) using Lipofectamine LTX reagent (Invitrogen) as per manufacturer’s instructions. Transfected cells were selected using puromycin followed by isolation and expansion of clonal populations. Western blot was performed on stably transfected cells to determine the extent of CTSL knockdown. Clones exhibiting greater than 85% reduction in CTSL expression levels were used to perform transwell migration experiments.
Hypoxic exposure
Hypoxia experiments were performed as described previously [30]. Cells were plated in notched glass dishes to facilitate easy gas exchange. When the cells reached a confluency of 60–70%, hypoxia experiments were initiated. To induce hypoxia, cells were incubated in special aluminum chamber designed by Dr. Cameron Koch (University of Pennsylvania). These chambers were flushed with a gas mixture containing 1% O2, 5% CO2 and the remainder balanced with N2 and incubated at 37°C for the desired duration. For reoxygenation, cells were removed from hypoxia chambers and placed back in incubator gassed with 5%CO2 and air.
Low pH exposure
Low pH experiments were performed as described by Cuvier et al. 1997 [31] with some modifications. Acidic HAM’s F12 nutrient mixture and DMEM were prepared by replacing NaHCO3 with 25 mM HEPES and 25 mM MES. The pH was set to 6.8 using 10N NaOH solution. Cells were plated under neutral pH conditions in 60 mm dishes. When the cells became ~60% confluent, the media was replaced with low pH HAM’s or DMEM depending on the cell line.
Western blotting
Cells were lysed using radioimmunoprecipitation assay lysis buffer (50 mMTris–HCl, pH 8.0, 150 mM NaCl, 0.1% SDS, 1% NP-40, 0.25% Sodium deoxycholate and 1 mM EDTA) supplemented with protease inhibitor cocktail (Sigma), 1 mM NaF and 1mM Na3VO4. Protein concentration was estimated by Bradford method. 35 µg of whole cell lysates were fractioned on a 12% SDS-PAGE gel. Subsequently, the proteins were electroblotted onto a nitrocellulose membrane. The membranes were blocked with 5% non-fat dry milk for 1 h at RT. The membranes were probed with relevant primary antibodies [mouse α-human CTSL antibody (Abcam Ab6314), 1:1000 dilution; mouse anti β-actin antibody (Sigma A1978), 1:10,000 dilution] overnight at 4°C. Subsequently, the membranes were incubated with horseradish peroxidase conjugated goat α-mouse secondary antibody (Jackson ImmunoResearch) (1:10,000 dilution), and detected with an enhanced chemiluminescence substrate (Amersham).
Immunofluorescence staining and microscopy
Cells were grown to 60–70% confluency on coverslips housed within 60 mm tissue culture dishes (BD Falcon) and were then incubated under appropriate conditions [normoxia and pH 7.4 (control) or 1% O2 or pH 6.8] for desired durations. Following incubation, the cells were rinsed with PBS and fixed with 4% (w/v) paraformaldehyde (Fisher Scientific) in PBS for 20 min at RT. Cells were then washed on a rocker for 20 min with ice cold PBS, replacing the PBS every 5 min. The fixed cells were incubated with permeabilization buffer (0.2% Triton-X 100 and 50 mM NH4Cl in PBS) for 15 min at RT. Following permeabilization, cells were washed on a rocker for 20 min with ice cold PBST (0.05% Triton-X 100 in PBS), replacing the PBST every 5 min. Cells were blocked with 10% (v/v) goat serum in PBS for 1 h at RT and were then washed with PBST as described above. Following the washes, the cells were incubated with mouse α-human H4A3 monoclonal antibody (1:200 dilution; Developmental Studies Hybridoma Bank) diluted in blocking solution for 2 h at RT. Incubation in primary antibody was followed by four washes with PBST. Cells were then incubated with Alexa Fluor 594 secondary antibody (1:800 dilution; Invitrogen, Molecular probes) diluted in blocking solution for 1 h in the dark at RT. Finally, cells were washed with PBS for 20 min, allowed to air dry, and mounted with Vectashield mounting medium containing DAPI (Vector laboratories). Cells were visualized and photographed using a Zeiss Axiophot microscope (Carl Zeiss Meditec, Dublin, CA).
β-Hexosaminidase assay
Because β-Hexosaminidase is present within the endosomal lysosomal compartment, exocytosis of lysosomal contents can be determined by measuring glucosaminidase activity in the conditioned media. Conditioned media were harvested from cells exposed to hypoxia or acidic pH for desired durations. 500 µl sodium citrate buffer (100 mM sodium citrate, 0.2% BSA, 0.2% Triton X-100, 0.04% NaN3, pH 4.5) with 10 mM p-nitrophenyl-N-acetyl β-D glucosaminide were added to 200 µl of conditioned media. The reaction mixture was incubated at 37°C for 1 h. Subsequently, the reaction was stopped by adding 400 µl of stop solution (0.4 M Glycine at pH 10.7). Absorbance was measured at 405 nm using the Spectramax M5 (Molecular Devices) spectrophotometer. 1 unit β-Hexosaminidase corresponds to the amount of enzyme that can hydrolyze 1 µmole of 4-Nitrophenyl N-acetyl-β-D glucosaminide per hour at 37°C.
Statistical analysis
Two tailed Student’s t test was used for analyzing data by GraphPad Prism 5.0. A threshold of P<0.05 was defined as statistically significant.
Results
Highly invasive prostate and breast cancer cells secrete high levels of cathepsin L
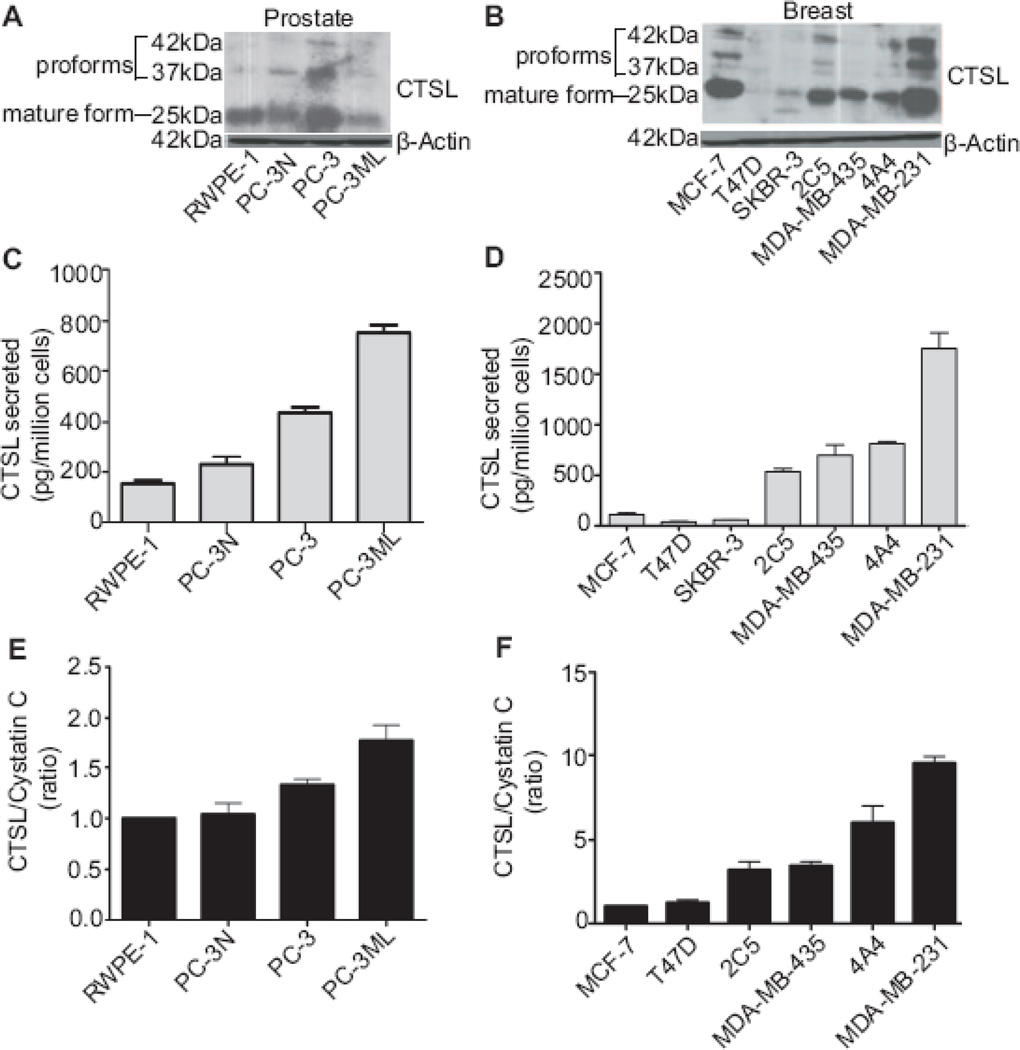
Both experimental and clinical investigations have implicated cathepsin L as an important factor in the metastatic process [32, 33]. Since invasiveness is a critical attribute of metastatic cells, we compared the cellular CTSL levels in prostate and breast cancer cells of varying invasive capacities (Fig 1A and B) [25, 34] but found no correlation between the intracellular expression of CTSL and the invasive capacities of these cell lines. In addition to intracellular CTSL (mainly present within the lysosomes where it is committed to housekeeping functions such as proteolytic turnover of intracellular and endocytosed extracellular proteins [35]) tumor cells secrete CTSL to facilitate invasion. This is achieved by over-expressing CTSL and altering its trafficking mechanism [6–8]. Highly metastatic PC-3ML and MDA-MB-231 cells were found to secrete significantly higher levels of CTSL compared to non-invasive and poorly invasive prostate and breast cancer cells respectively (Fig 1C and D) suggesting a correlation between CTSL secretion and the tumor cells’ invasive potential. Since metastatic aggressiveness reflects an imbalance between CTSL and its endogenous inhibitors, we also explored the balance between secreted CTSL and extracellular cystatin C levels in the various prostate and breast cancer cell lines. CTSL/cystatin C ratios are shown as relative levels, with RWPE-1 and MCF-7 being the respective reference cell lines (Fig 1E and F). The results showed the ratio of CTSL to cystatin C to increase with increasing invasive potential indicating that an imbalance between the extracellular CTSL and cystatin C level might be a key attribute of aggressively metastatic prostate and breast cancer cells.
Fig. 1. Cathepsin L secretion and activity positively correlates with invasive potential.
A and B Intracellular CTSL levels in prostate and breast cancer cells were determined by performing Western blots using whole cell lysates. C and D, CTSL secretion levels of prostate and breast cancer cells were determined by performing ELISA on cell conditioned media. Means and standard errors of three independent experiments are shown. E and F, Ratios of secreted CTSL to cystatin C levels for prostate and breast cancer cells were determined by performing ELISA on cell conditioned media. Ratios shown are relative levels normalized to least invasive prostate and breast cancer cell lines, respectively.
KGP94 treatment significantly attenuates migration and invasion of prostate and breast cancer cells
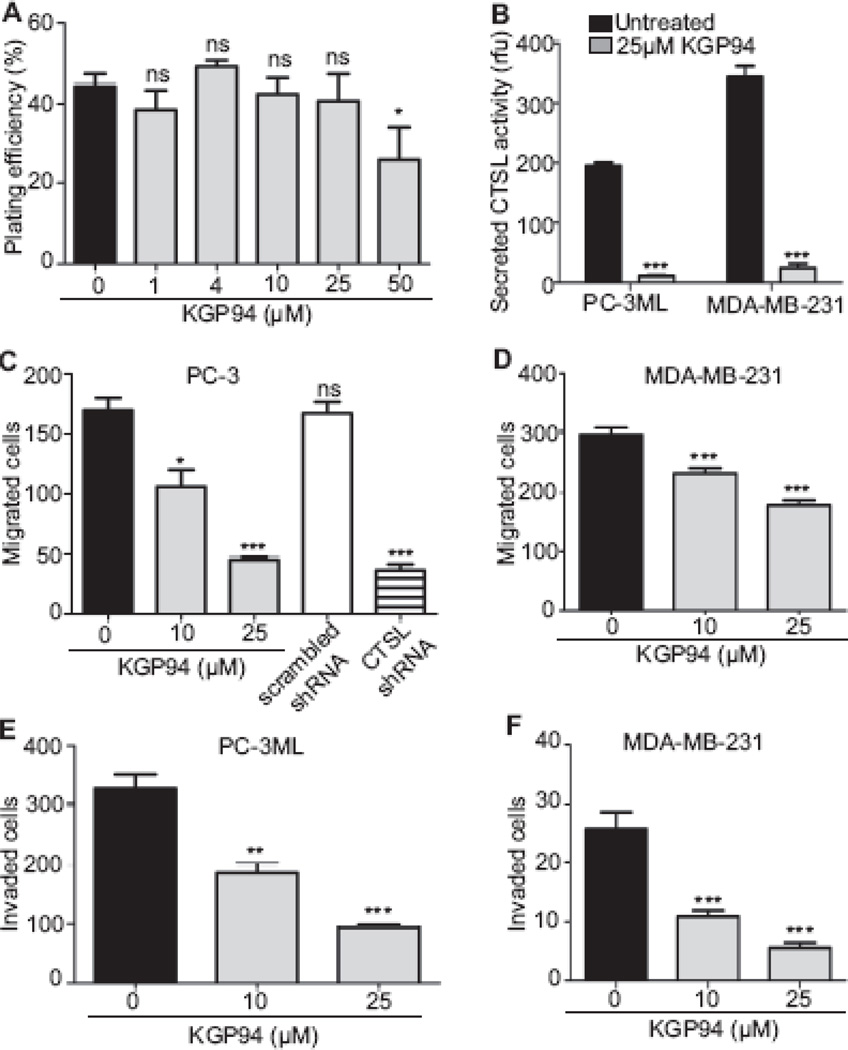
Since CTSL is involved not only in unregulated proteolysis of extracellular matrix and basement membrane components but also the activation of other key metastasis associated proteases [10–14], specifically abolishing CTSL activity using synthetic inhibitors should impair the metastatic phenotype. The impact of one such inhibitor, KGP94 [23], on CTSL activity and prostate and breast cancer cell migration and invasion is shown in Fig 2. To separate anti-tumorigenic from anti-metastatic effects, non-cytotoxic doses of 10 and 25 µM (Fig 2A) were used. The results showed the CTSL activity in PC-3ML and MDA-MB-231 conditioned media to be significantly reduced (by 94 and 92% respectively (p<0.0001)) in the presence of 25 µM KGP94 (Fig 2B). Treatment with 10 and 25 µM KGP94 also decreased the migratory potential of prostate cancer cells by 38 and 74% respectively (Fig 2C) in a manner comparable to what could be achieved by CTSL knockdown (Fig 2C). Similarly, migratory capacity of MDA-MB-231 cells was reduced by 22 and 40% upon treatment with 10 and 25 µM KGP94 respectively (Fig 2D). Furthermore, treatment with KGP94 significantly impaired the invasive capacities of both prostate and breast cancer cells by 44 and 72% at 10 µM and 53 and 88% at 25 µM respectively (Fig 2E and F).
Fig. 2. KGP94 abrogates CTSL activity and metastatic phenotype of prostate and breast cancer cells.
A, The effect of 24 h treatment with KGP94 on the clonogenicity of PC-3 cells was evaluated 2 weeks later. (*) p<0.01. B The effect of KGP94 treatment on the activity of CTSL secreted byPC-3ML and MDA-MB-231 cells was assessed by incubating cell conditioned media with a fluorogenic CTSL substrate Z-Phe-Arg-AMC in the absence (black bars) or presence (grey bars) of 25 µM KGP94. Activity is expressed as relative fluorescence units and results of three independent experiments are shown. (***) p<0.0001. C and D, PC-3, MDA-MB-231 and CTSL knock down PC-3 cells were seeded into transwell migration chambers in the presence or absence of KGP94 and the number of migrated cells was enumerated 24 h later. Mean and standard error values of three independent experiments are shown. (*) p<0.05, (***) p<0.0005. E and F, The impact of KGP treatment on PC-3ML and MDA-MB-231 cell transwell invasion is shown. Results are mean and standard error values of three independent experiments. (**) p<0.01, (***) p< 0.001.
Acute hypoxia elevates extracellular CTSL levels
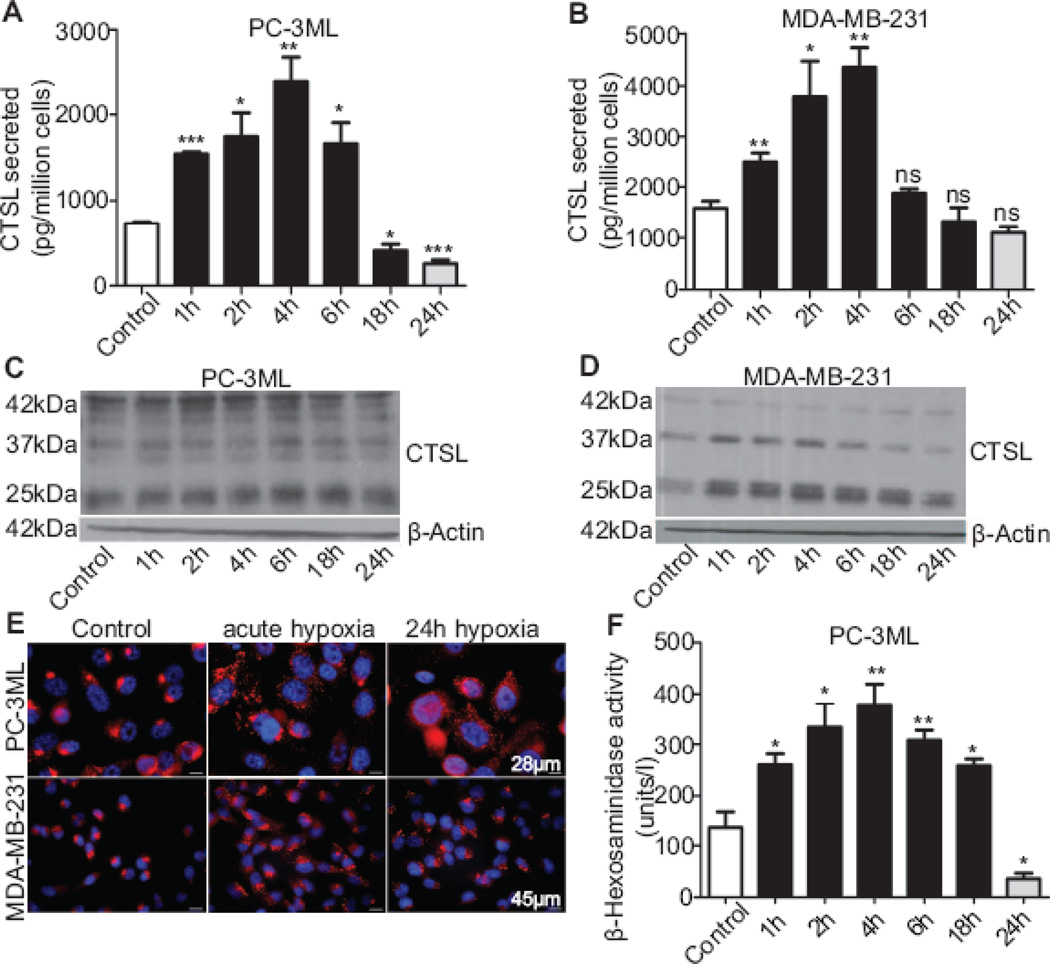
To examine the effect of hypoxic exposures on CTSL secretion PC-3ML and MDA-MB-231 cells were exposed to1% O2 for 1–24 h followed by reoxygenation for the remaining time such that the total length of the cycle was 24 h. Compared to normoxic control, CTSL secretion in response to hypoxia peaked at about 4 h in both PC-3ML and MDA-MB-231 cells and then tapered off to near normoxic levels after about 18 h of hypoxia (Fig 3A and B). CTSL secretion in cells chronically exposed to hypoxia (24 h) was significantly lower. To elucidate whether increased CTSL synthesis was the mechanism through which hypoxia upregulated CTSL secretion, intracellular levels of CTSL were determined following exposure to hypoxia. In MDA-MB-231 cells, intracellular CTSL levels increased in response to brief exposures to hypoxia followed by reoxygenation (Fig 3D). The close association between the synthesis and CTSL secretion patterns observed suggests that in these cells the increase in secreted CTSL could be due to increased synthesis. However, in contrast, PC-3ML cells showed no increase in intracellular CTSL levels in response to hypoxic exposures (Fig 3C). In fact, upon chronic (24 h) hypoxic exposure, intracellular CTSL levels were found to be lower than those observed under normoxic conditions.
Fig. 3. Acute exposure to hypoxia enhances CTSL secretion.
A and B, CTSL secreted levels in PC-3ML and MDA-MB-231 cells exposed to hypoxic conditions (1% O2) for the indicated durations followed by reoxygenation for a total time of 24 h. Secreted CTSL levels were determined by ELISA on cell conditioned media and normalized to cell numbers. Shown are mean and standard error values calculated from three independent experiments. (*) p<0.05, (**) p<0.005, (***) p<0.001 C and D, Western blot analysis of PC-3ML and MDA-MB-231 cells exposed to hypoxia for various durations. E, Lysosomal localization in response to exposure to hypoxia was determined by immunostaining for lysosomal marker protein LAMP-1. F, The effect of hypoxia and reoxygenation on exocytosis of lysosomal content was determined by quantifying the lysosomal marker enzyme β-Hexosaminidase activity in the media. Results from three independent experiments are shown. (*) p<0.05, (**) p<0.01.
The increase in CTSL secretion by PC-3ML cells which occurred in the absence of an increase in de-novo CTSL synthesis, suggested that hypoxia prompted the release of pre-synthesized CTSL from their repositories. Studies in hepatic and myocardial ischemia - reperfusion models have reported that hypoxia-reoxygenation can trigger the lysosomes to undergo exocytosis [36]. We therefore determined whether hypoxia affects the subcellular location of lysosomes in PC-3ML and MDA-MB-231 cells using the endosomal / lysosomal marker protein LAMP-1. The results (Fig 3E) showed that in contrast to their mainly peri-nuclear localization observed in normoxic cells, lysosomes in cells exposed to 1% O2, trafficked towards the plasma membrane. These observations suggest that hypoxia activates the lysosomes to redistribute, fuse with the plasma membrane, and release their content (including CTSL) into the extracellular milieu. To biochemically validate the release of lysosomal content in response to hypoxia-reoxygenation exposure, we measured the activity of lysosomal enzyme β-hexosaminidase in media of cells exposed to hypoxia for 1–18 h and reoxygenated for 24 h (black bars) (Fig 3F). The results showed that exposure to hypoxia followed by reoxygenation led to a significant and time-dependent increase in β-hexosaminidase enzyme release. In concert with CTSL secretion pattern, lysosomal exocytosis peaked in cells exposed to hypoxia for 4 h and reoxygenated for 24 h (~3 fold). Cells that were chronically (24 h) exposed to hypoxia and not reoxygenated (grey bar) showed a significant drop in lysosomal exocytosis despite lysosomal re-distribution.
Acute acidosis increases extracellular CTSL levels in both prostate and breast cancer cells
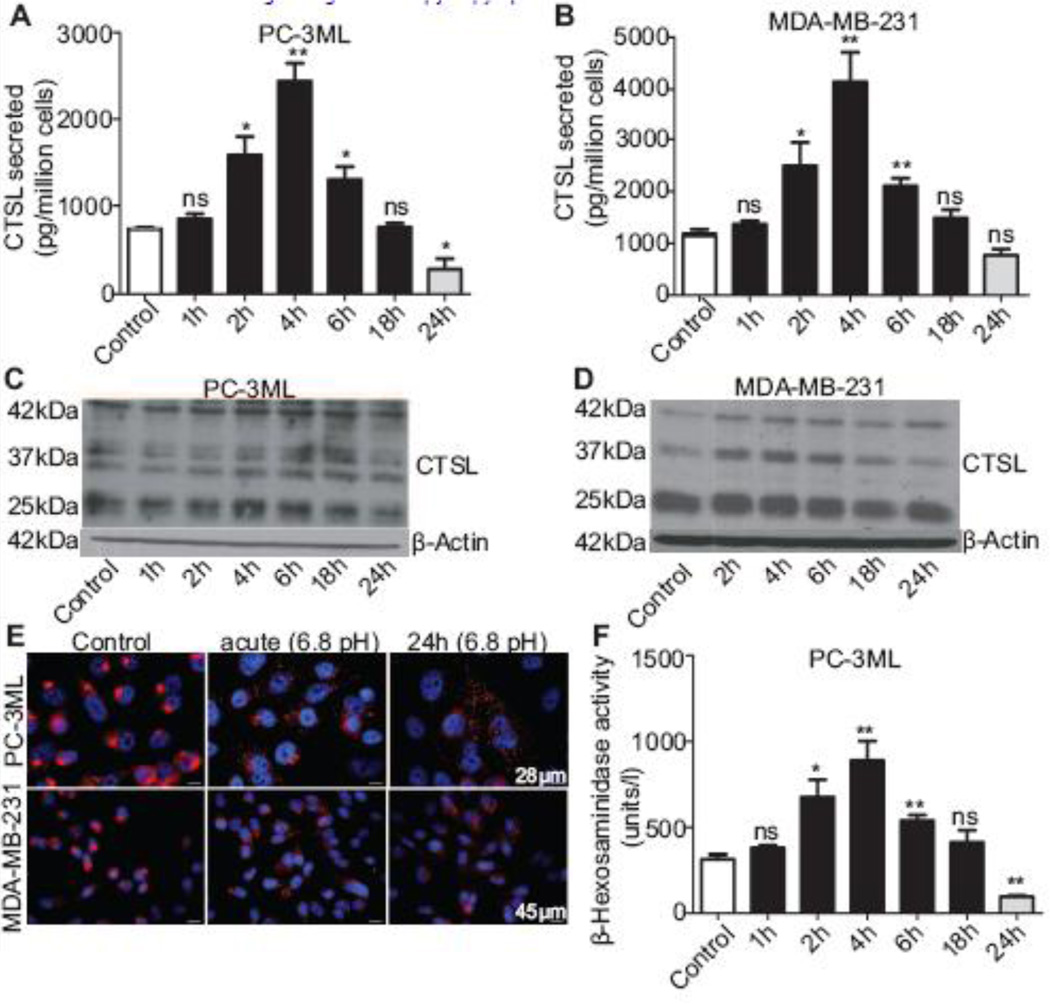
Since cells in solid tumors commonly experience an acidic extracellular condition, we also evaluated the impact of acidic culture medium (pH 6.8) on CTSL secretion in PC-3ML and MDA-MB-231 cells. In comparison to cells maintained at pH 7.4, tumor cells exposed acutely to an acidic extracellular environment, secreted increased amounts of CTSL (Fig 4A and B). This increase peaked at 4 h, dropped to control cell levels at 18 h exposure, and fell below control levels in cells chronically exposed to acidosis (24 h). Mechanistically, Western blot analysis revealed that in response to acute exposures to pH 6.8, intracellular CTSL levels increased in MDA-MB-231 cells (Fig 4D). Furthermore, the close association between CTSL secretion and synthesis patterns in MDA-MB-231 cells suggested that the observed increase in secreted levels could at least in part be due to increased synthesis. Acidosis did not however upregulate CTSL synthesis in PC-3ML cells.
Fig. 4. Acute exposure to an acidic extracellular environment enhances CTSL secretion.
A and B, CTSL secretion levels in PC-3ML and MDA-MB-231 cells pre-exposed to acidic conditions for the indicated durations followed by incubation under neutral pH conditions for 24 h. Secreted CTSL levels were determined by performing ELISA on cell conditioned media and normalized to cell numbers. Results from three independent experiments are shown. (*) p<0.05, (**) p<0.01. C and D, Western blot analysis of PC-3ML and MDA-MB-231 cells exposed to 6.8pH for various durations. E, Effect of acidic exposure on lysosomal localization in response to acidic extracellular condition was determined by immunostaining for lysosomal marker protein LAMP-1. F, Exocytosis of lysosomal content was determined by quantifying of β- Hexosaminidase activity in conditioned media. Results are mean and standard errors from three independent experiments. (*) p<0.05, (**) p<0.01.
Like hypoxia, acidosis also triggered the lysosomal redistribution and trafficking toward the cell periphery (Fig 4E). PC-3ML and MDA-MB-231 cells that were acutely exposed to acidic conditions and then restored to neutral pH, showed a significant increase in the release of β-hexosaminidase enzyme (Fig 4F and G) while cells that were chronically exposed to acidic conditions, showed decreased exocytosis. Consistent with CTSL secretion results, lysosomal exocytosis peaked in cells acutely exposed to acidic pH for 4 h and re-incubated under neutral conditions for 24 h (2.9-fold increase).
Acute hypoxia and acidosis increase tumor cell aggressiveness
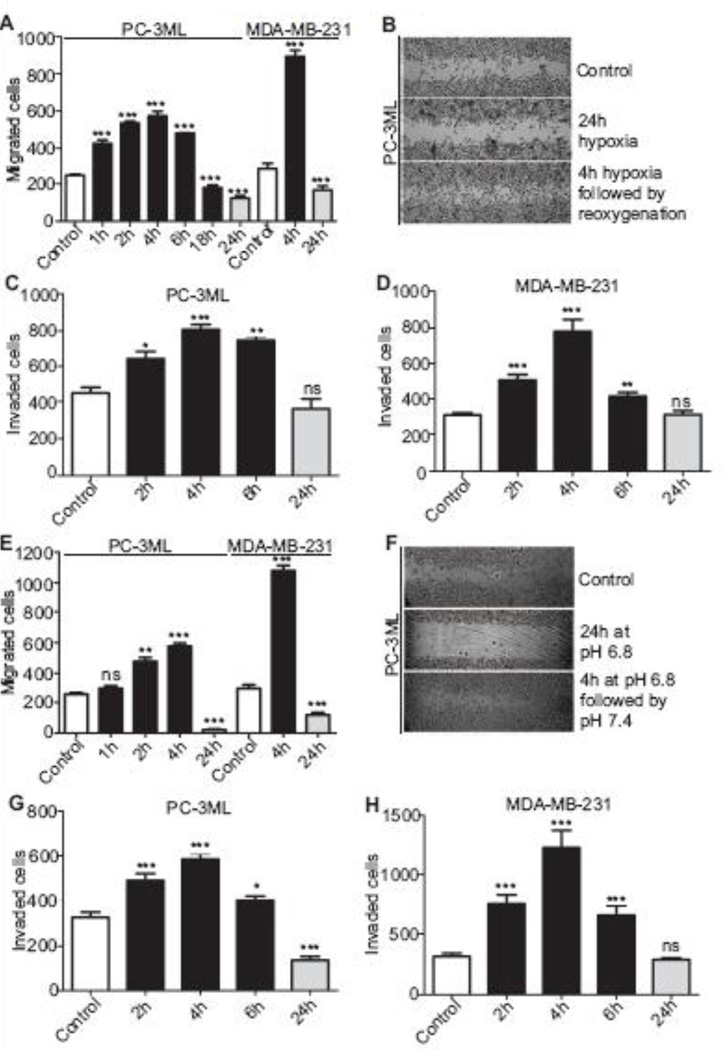
To assess whether the enhanced secretion of CTSL in response to acute exposures to hypoxia and acidic environments affects metastatic phenotype, their impact on PC-3ML and MDA-MB- 231 cell migration and invasion was determined. Acute hypoxic exposures of 1 to 6 h significantly increased the migratory potential of PC-3ML cells (Fig 5A); peaking at 4 h (~3-fold increase). Similarly, MDA-MB-231 cells that were acutely exposed to hypoxia also showed a significantly enhanced migratory capacity (~3-fold). Longer exposures to hypoxia reduced the migratory potential in both cell lines. Similar results were obtained using a wound healing assay to assess migration of cells into a denuded area (Fig 5B). Short term hypoxic exposures (2–6 h) also significantly enhanced (1.9- and 2.5-fold, respectively at 4 h) the invasive capacities of PC-3ML and MDA-MB-231 cells (5C and D).
Fig. 5. Acute hypoxia and acidosis augments metastatic phenotype.
A, PC-3ML and MDA-MB-231 cells were exposed to 1% O2 for indicated durations and then allowed to migrated under normoxic conditions. The number of migrated cells was quantified 24 h later. Mean and standard error from three independent experiments are shown. (***) p<0.0001. B, PC-3ML monolayers were exposed to hypoxic conditions following which a scratch was made on the monolayers. Cells were incubated under normoxic conditions and imaged at 5× magnification 24 h later. C and D, PC-3ML and MDA-MB-231 cells were seeded into invasion inserts and exposed to hypoxia for indicated durations. 24 h later, invaded cells were stained and counted. Mean and standard error are shown. (*) p<0.05, (**) p<0.005, (***) p<0.001. E, PC-3ML and MDA-MB-231 cells were briefly exposed to 6.8pH, maintained under neutral conditions for 24 h and the number of migrated cells was quantified. Mean and standard errors for three independent experiments are shown. (**) p<0.005, (***), p<0.0001. F, Wound healing in response to acidic extracellular condition was assessed as mentioned in 5B. G and H, PC-3ML and MDA-MB-231 cells were exposed to pH 6.8 for indicated durations and seeded into invasion inserts. 24 h later, invaded cells were stained and counted. Mean and standard error are shown. (*) p<0.05, (***) p<0.001.
In terms of acidic extracellular microenvironments, both transwell and wound healing assays (Fig 5E and F) showed the migratory capacities of PC-3ML and MDA-MB-231 cells to be significantly enhanced by acute (4 h) acidic exposure and hampered by prolonged (24 h) acidic exposures. Consistent with the cell migration results, acute exposure to pH 6.8, specifically 4 h, enhanced the invasive potential of PC-3ML and MDA-MB-231 cells 1.8- and 4-fold respectively.
KGP94 abrogates hypoxia and acidosis triggered invasiveness in prostate and breast cancer cells
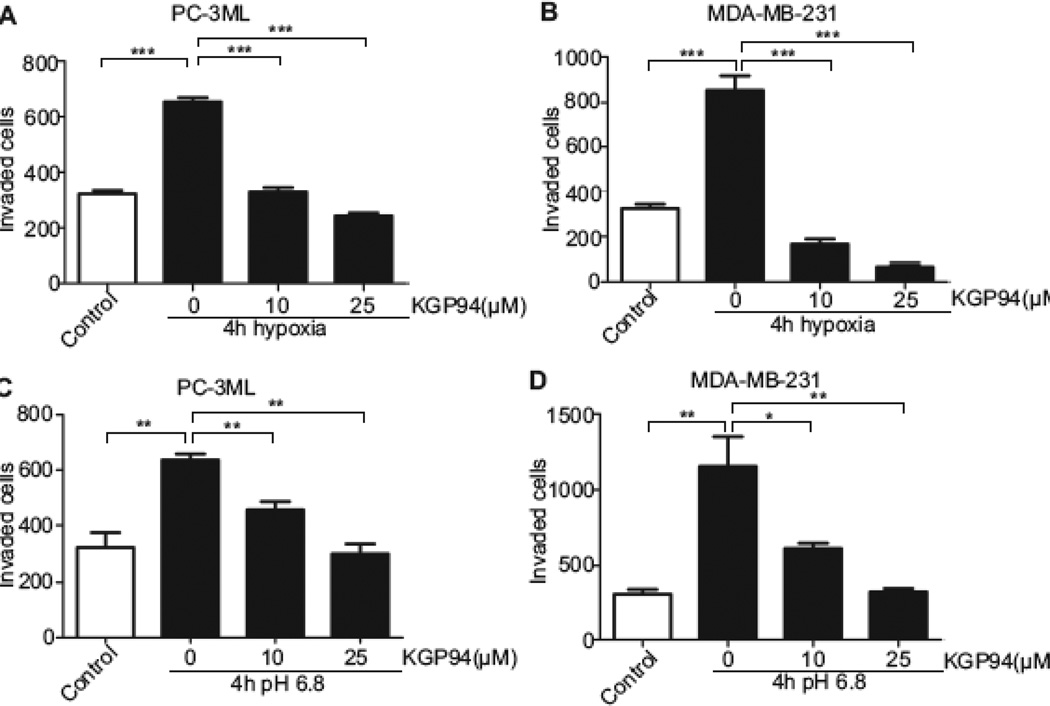
To determine whether KGP94 treatment could impair the enhanced tumor cell aggressiveness conferred by acute hypoxic and acidic exposures, the impact of this agent on the invasive capacity of PC-3ML and MDA-MB-231 cells pre-exposed to 1% O2 or pH 6.8 was determined (Fig 6). The results showed that in the presence KGP94, the enhanced invasiveness of both PC-3ML and MDA-MB-231 cells was reduced below basal invasion levels observed under normoxic conditions (PC-3ML, 50% reduction at 10 µM; 63% reduction at 25 µM; MDA-MB-231, 80% reduction at 10 µM; 92% reduction at 25 µM). Similarly the enhanced invasive potential of tumor cells exposed to an acidic environment was significantly attenuated in the presence of KGP94 (PC-3ML, 20% reduction at 10 µM and 50% reduction at 25 µM; MDA-MB-231, 47% reduction at 10 µM and 72% reduction at 25 µM).
Fig. 6. KGP94 obliterates acidosis and hypoxia triggered invasiveness.
A and B, PC-3ML and MDA-MB-231 cells were exposed to hypoxic conditions for 4 h. Cells were seeded into invasion inserts and allowed to invade for 24 h in the presence of indicated doses of KGP94. Results from three independent experiments are shown. (***) p<0.0001. C and D, PC-3ML and MDA-MB-231 cells were exposed to pH 6.8 for 4 h, seeded into invasion inserts and allowed to invade for 24 h in the presence of indicated doses of KGP94. (*) p<0.05, (**) p< 0.01.
Discussion
Tumor cells rely primarily on secreted extracellular proteases to facilitate invasion through extracellular matrix [37, 38]. One such enzyme, cathepsin L (CTSL) has been strongly implicated in the metastatic spread of tumor cells [3, 39]. It is overexpressed in a wide range of human cancers including carcinomas of the pancreas, brain, skin, breast and prostate [5] and has prognostic value in predicting relapse free and overall survival [40, 41]. CTSL also has been linked with skeletal morbidities of bone metastases; common occurrences in both prostate and breast cancer patients [33]. The association of CTSL with metastasis, disease relapse, and skeletal adversities, provides a compelling rationale for targeting this proteolytic enzyme to improve treatment outcomes and quality of life of advanced prostate and breast cancer patients [32, 33].
Although our studies suggest a strong correlation between secreted CTSL levels and invasive capacities of prostate and breast cancer cells (Fig 1C and D), it should be noted that the extracellular matrix is safeguarded against the proteolytic activity of cathepsins by endogenous inhibitors of the cystatin superfamily comprised of the intracellular stefins and kininogens and extracellular cystatins. Studies that correlate metastatic aggressiveness to an imbalance between CTSL and its endogenous inhibitors have either measured the expression levels of stefins and kininogens, which are the intracellular inhibitors of cathepsins, or have measured intracellular levels of cystatins [22, 42]. We show that highly invasive cells exhibit a striking imbalance between their extracellular CTSL and cystatin C levels when compared to poorly invasive prostate and breast cancer cells (Fig 1); a finding consistent with the previously reported correlation between secreted CTSL to cathepsin inhibitor ratio and experimental metastatic potential in Ras transfected NIH 3T3 cells [9]. While clinical reports have documented either CTSL level alone or serum cystatin C level alone as important prognosticators of disease outcome [40, 41, 43], there are discrepancies as to whether cystatin C is positively or inversely correlated with disease outcome [43–45]. To this point, we observed that highly invasive cells secreted somewhat more cystatin C compared to their non- or poorlyinvasive counterparts, but the ratio of secreted CTSL to cystatin C increased with increasing invasiveness. Taken together these findings suggest that the CTSL/cystatin C ratio may serve as a better prognosticator of disease outcome than absolute levels of CTSL or cystatin C.
The significance of CTSL in tumor aggression and the metastatic process is further supported by studies seeking to abrogate this enzyme by gene deletion, anti-sense RNA or cystatin C over-expression [39, 46, 47]. The strategy pursued in the present investigation was to inactive CTSL using the selective small molecule inhibitor of CTSL KGP94 [23]. The results showed that CTSL inhibition by non-cytotoxic doses of KGP94 could significantly impair the invasive and migratory ability of the highly metastatic prostate (PC3-ML) and breast (MDA-MB-231) cell lines (Fig 2). These findings are consistent with a recent overview of the KGP94 molecule [24].
Given its impact on proteolytic enzymes, the mechanism responsible for KGP94 suppression of tumor cell invasion is readily apparent. How CTSL inhibition affects tumor cell migration is not nearly as clear. As was the case in our investigations (Fig 2), studies examining the effect of CTSL abrogation by RNA interference also have shown that CTSL inhibition can affect tumor cell migration [48, 49]. Although the mechanism by which CTSL promotes tumor cell migration has not been delineated, Reiser et al. 2004 [50]; have shown that in nephrotic syndromes such as glomerular proteinuria, CTSL can enhance podocyte migration by promoting cell detachment and remodeling of extracellular matrix components while migration of normal podocytes was found to be independent of CTSL activity. Similarly, in a recent study on gene therapy for ischemic diseases Chung et al. 2011 [51] showed that exogenous CTSL can promote endothelial cell migration through activation of the JNK pathway.
Unlike the well-oxygenated and near neutral pH conditions maintained in cell culture systems, the microenvironment within most solid tumors is hypoxic and acidic in nature [15, 16]. These very aberrant physiological conditions have been known to confer and further augment the metastatic capacities of tumor cells [16–18]. In light of the possible role of CTSL in the metastatic process, we investigated whether hypoxic and acidic microenvironmental conditions might influence CTSL function in prostate and breast cancer cells. The results showed that CTSL secretion is significantly upregulated by acute but not chronic exposures to hypoxia and acidosis (Fig 3 and 4). In concert with the enhanced CTSL secretion, brief exposures to hypoxia or acidosis also led to significant enhancement of the metastatic attributes such as migration and invasion (Fig 5 and 6). The relative importance of acute versus chronic hypoxic effects on the metastatic phenotype noted here is consistent with observations made in experimental metastasis models which showed transient exposures to hypoxia or acidosis to promote metastasis to a greater extent than chronic exposures [20, 21, 52].
Mechanistically we postulated that in response to hypoxia and acidosis, lysosomes undergo exocytosis and release their contents, including CTSL, into the extracellular space. Indeed, prior studies had shown that tumor cell lysosomes undergo exocytosis during acidic exposure [53]. In our investigations we observed that hypoxic and acidic exposures led to anterograde trafficking of lysosomes, but this alone did not result in a remarkable increase in the release of lysosomal content. It was the restoration of normoxia or neutral pH that triggered lysosomal exocytosis. Moreover, while the migratory and invasive capacities of PC-3ML and MDA-MB-231 cells were significantly hampered under hypoxic or acidic conditions, restoration of normoxic or neutral pH conditions dramatically increased their metastatic phenotype. These findings concur with prior studies of Cuvier et al. 1997 [31] who reported that rodent KHT-LP1 and SSC-VII tumor cells showed increased CTS L+B activity and increased invasiveness only upon reoxygenation and no enhanced invasiveness when exposed to prolonged (24 h) acidosis. Furthermore, studies in hepatic ischemia reperfusion models reported that while brief hypoxia followed by reoxygenation confers cytoprotection by inducing lysosomal exocytosis in hepatocytes, hepatocytes subjected to prolonged hypoxia without reoxygenation lost viability because their lysosomes failed to exocytose [36].
The close association between CTSL secretion patterns and invasive and migratory tumor cell behavior noted in the present investigations (Fig 5) suggests that CTSL may be a key player in hypoxia and acidosis triggered enhanced metastatic aggressiveness. Selective targeting with the small molecule CTSL inhibitor KGP94 effectively impaired the hypoxia and acidosis potentiated pro-metastasis behavior in both breast and prostate cancer cells (Fig 6).Taken together, the ability of KGP94 to attenuate the metastatic phenotype of tumor cells experiencing normal physiological or aberrant microenvironmental conditions lends further credence to the pursuit of CTSL targeting strategies as means to impede the dissemination of tumor cells. Although clearly encouraging, the in-vitro findings reported here await in-vivo validation studies of KGP94 for the advancement of CTSL intervention strategies to the treatment of metastatic prostate and breast cancers.
Acknowledgements
The authors thank Dr. Kevin Pinney of Baylor University for providing KGP94 and Drs. Yao Dai and Kyung-Mi Bae as well as Sharon Lepler for scientific input. The H4A3 mouse LAMP1 antibody developed by J. Thomas August and James E.K. Hildreth was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. These studies were supported in part by a grant from the National Cancer Institute (US Public Health Service grant R01 CA169300).
Footnotes
Disclosure of potential conflicts of interest: The authors have no conflict of interest to disclose.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Duffy MJ. The role of proteolytic enzymes in cancer invasion and metastasis. Clin Exp Metastasis. 1992;10:145–155. doi: 10.1007/BF00132746. [DOI] [PubMed] [Google Scholar]
- 3.Gocheva V, Joyce JA. Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle. 2007;6:60–64. doi: 10.4161/cc.6.1.3669. [DOI] [PubMed] [Google Scholar]
- 4.Laufs S, Schumacher J, Allgayer H. Urokinase-receptor (u-PAR): an essential player in multiple games of cancer: a review on its role in tumor progression, invasion, metastasis, proliferation/dormancy, clinical outcome and minimal residual disease. Cell Cycle. 2006;5:1760–1771. doi: 10.4161/cc.5.16.2994. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan SS, Goldstein LJ, Gottesman MM. Expression of cathepsin L in human tumors. Cancer Res. 1991;51:1478–1481. [PubMed] [Google Scholar]
- 6.Gottesman MM, Sobel ME. Tumor promoters and Kirsten sarcoma virus increase synthesis of a secreted glycoprotein by regulating levels of translatable mRNA. Cell. 1980;19:449–455. doi: 10.1016/0092-8674(80)90519-x. [DOI] [PubMed] [Google Scholar]
- 7.Prence EM, Dong JM, Sahagian GG. Modulation of the transport of a lysosomal enzyme by PDGF. J Cell Biol. 1990;110:319–326. doi: 10.1083/jcb.110.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stearns NA, Dong JM, Pan JX, Brenner DA, Sahagian GG. Comparison of cathepsin L synthesized by normal and transformed cells at the gene, message, protein, and oligosaccharide levels. Arch Biochem Biophys. 1990;283:447–457. doi: 10.1016/0003-9861(90)90666-m. [DOI] [PubMed] [Google Scholar]
- 9.Chambers AF, Colella R, Denhardt DT, Wilson SM. Increased expression of cathepsins L and B and decreased activity of their inhibitors in metastatic, ras-transformed NIH 3T3 cells. Mol Carcinog. 1992;5:238–245. doi: 10.1002/mc.2940050311. [DOI] [PubMed] [Google Scholar]
- 10.Ishidoh K, Kominami E. Procathepsin L degrades extracellular matrix proteins in the presence of glycosaminoglycans in vitro. Biochem Biophys Res Commun. 1995;217:624–631. doi: 10.1006/bbrc.1995.2820. [DOI] [PubMed] [Google Scholar]
- 11.Mason RW, Johnson DA, Barrett AJ, Chapman HA. Elastinolytic activity of human cathepsin L. Biochem J. 1986;233:925–927. doi: 10.1042/bj2330925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abboud-Jarrous G, Atzmon R, Peretz T, et al. Cathepsin L is responsible for processing and activation of proheparanase through multiple cleavages of a linker segment. J Biol Chem. 2008;283:18167–18176. doi: 10.1074/jbc.M801327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everts V, Korper W, Hoeben KA, et al. Osteoclastic bone degradation and the role of different cysteine proteinases and matrix metalloproteinases: differences between calvaria and long bone. J Bone Miner Res. 2006;21:1399–1408. doi: 10.1359/jbmr.060614. [DOI] [PubMed] [Google Scholar]
- 14.Laurent-Matha V, Derocq D, Prebois C, Katunuma N, Liaudet-Coopman E. Processing of human cathepsin D is independent of its catalytic function and auto-activation: involvement of cathepsins L and B. J Biochem. 2006;139:363–371. doi: 10.1093/jb/mvj037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillies RJ, Schornack PA, Secomb TW, Raghunand N. Causes and effects of heterogeneous perfusion in tumors. Neoplasia. 1999;1:197–207. doi: 10.1038/sj.neo.7900037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lunt SJ, Chaudary N, Hill RP. The tumor microenvironment and metastatic disease. Clin Exp Metastasis. 2009;26:19–34. doi: 10.1007/s10585-008-9182-2. [DOI] [PubMed] [Google Scholar]
- 17.Schwickert G, Walenta S, Sundfor K, Rofstad EK, Mueller-Klieser W. Correlation of high lactate levels in human cervical cancer with incidence of metastasis. Cancer Res. 1995;55:4757–4759. [PubMed] [Google Scholar]
- 18.Walenta S, Salameh A, Lyng H, et al. Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am J Pathol. 1997;150:409–415. [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudary N, Hill RP. Hypoxia and metastasis. Clin Cancer Res. 2007;13:1947–1949. doi: 10.1158/1078-0432.CCR-06-2971. [DOI] [PubMed] [Google Scholar]
- 20.Cairns RA, Kalliomaki T, Hill RP. Acute (cyclic) hypoxia enhances spontaneous metastasis of KHT murine tumors. Cancer Res. 2001;61:8903–8908. [PubMed] [Google Scholar]
- 21.Rofstad EK, Galappathi K, Mathiesen B, Ruud EB. Fluctuating and diffusion-limited hypoxia in hypoxia-induced metastasis. Clin Cancer Res. 2007;13:1971–1978. doi: 10.1158/1078-0432.CCR-06-1967. [DOI] [PubMed] [Google Scholar]
- 22.Zajc I, Sever N, Bervar A, Lah TT. Expression of cysteine peptidase cathepsin L and its inhibitors stefins A and B in relation to tumorigenicity of breast cancer cell lines. Cancer Lett. 2002;187:185–190. doi: 10.1016/s0304-3835(02)00452-4. [DOI] [PubMed] [Google Scholar]
- 23.Kishore Kumar GD, Chavarria GE, Charlton-Sevcik AK, et al. Design, synthesis, and biological evaluation of potent thiosemicarbazone based cathepsin L inhibitors. Bioorg Med Chem Lett. 2010;20:1415–1419. doi: 10.1016/j.bmcl.2009.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chavarria GE, Horsman MR, Arispe WM. Initial evaluation of the antitumour activity of KGP94, a functionalized benzophenone thiosemicarbazone inhibitor of cathepsin L. Eur J Med Chem. 2012;58:568–572. doi: 10.1016/j.ejmech.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 25.Wang M, Stearns ME. Isolation and characterization of PC-3 human prostatic tumor sublines which preferentially metastasize to select organs in S.C.I.D. mice. Differentiation. 1991;48:115–125. doi: 10.1111/j.1432-0436.1991.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 26.Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD. MDA-MB-435 cells are derived from M14 melanoma cells--a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res Treat. 2007;104:13–19. doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- 27.Chambers AF. MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? Cancer Res. 2009;69:5292–5293. doi: 10.1158/0008-5472.CAN-09-1528. [DOI] [PubMed] [Google Scholar]
- 28.Urquidi V, Sloan D, Kawai K, et al. Contrasting expression of thrombospondin-1 and osteopontin correlates with absence or presence of metastatic phenotype in an isogenic model of spontaneous human breast cancer metastasis. Clin Cancer Res. 2002;8:61–74. [PubMed] [Google Scholar]
- 29.Dai Y, Siemann DW. Constitutively active c-Met kinase in PC-3 cells is autocrine-independent and can be blocked by the Met kinase inhibitor BMS-777607. BMC Cancer. 2012;12:198. doi: 10.1186/1471-2407-12-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai Y, Bae K, Siemann DW. Impact of hypoxia on the metastatic potential of human prostate cancer cells. Int J Radiat Oncol Biol Phys. 2011;81:521–528. doi: 10.1016/j.ijrobp.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuvier C, Jang A, Hill RP. Exposure to hypoxia, glucose starvation and acidosis: effect on invasive capacity of murine tumor cells and correlation with cathepsin (L + B) secretion. Clin Exp Metastasis. 1997;15:19–25. doi: 10.1023/a:1018428105463. [DOI] [PubMed] [Google Scholar]
- 32.Lankelma JM, Voorend DM, Barwari T, et al. Cathepsin L, target in cancer treatment? Life Sci. 2010;86:225–233. doi: 10.1016/j.lfs.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Leto G, Sepporta MV, Crescimanno M, Flandina C, Tumminello FM. Cathepsin L in metastatic bone disease: therapeutic implications. Biol Chem. 2010;391:655–664. doi: 10.1515/BC.2010.069. [DOI] [PubMed] [Google Scholar]
- 34.Sommers CL, Byers SW, Thompson EW, Torri JA, Gelmann EP. Differentiation state and invasiveness of human breast cancer cell lines. Breast Cancer Res Treat. 1994;31:325–335. doi: 10.1007/BF00666165. [DOI] [PubMed] [Google Scholar]
- 35.Turk B, Turk D, Turk V. Lysosomal cysteine proteases: more than scavengers. Biochim Biophys Acta. 2000;1477:98–111. doi: 10.1016/s0167-4838(99)00263-0. [DOI] [PubMed] [Google Scholar]
- 36.Carini R, Castino R, De Cesaris MG, et al. Preconditioning-induced cytoprotection in hepatocytes requires Ca(2+)-dependent exocytosis of lysosomes. J Cell Sci. 2004;117:1065–1077. doi: 10.1242/jcs.00923. [DOI] [PubMed] [Google Scholar]
- 37.Lee M, Fridman R, Mobashery S. Extracellular proteases as targets for treatment of cancer metastases. Chem Soc Rev. 2004;33:401–409. doi: 10.1039/b209224g. [DOI] [PubMed] [Google Scholar]
- 38.Dong JM, Prence EM, Sahagian GG. Mechanism for selective secretion of a lysosomal protease by transformed mouse fibroblasts. J Biol Chem. 1989;264:7377–7383. [PubMed] [Google Scholar]
- 39.Gocheva V, Zeng W, Ke D, et al. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 2006;20:543–556. doi: 10.1101/gad.1407406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foekens JA, Kos J, Peters HA, et al. Prognostic significance of cathepsins B and L in primary human breast cancer. J Clin Oncol. 1998;16:1013–1021. doi: 10.1200/JCO.1998.16.3.1013. [DOI] [PubMed] [Google Scholar]
- 41.Thomssen C, Schmitt M, Goretzki L, et al. Prognostic value of the cysteine proteases cathepsins B and cathepsin L in human breast cancer. Clin Cancer Res. 1995;1:741–746. [PubMed] [Google Scholar]
- 42.Friedrich B, Jung K, Lein M, et al. Cathepsins B, H, L and cysteine protease inhibitors in malignant prostate cell lines, primary cultured prostatic cells and prostatic tissue. Eur J Cancer. 1999;35:138–144. doi: 10.1016/s0959-8049(98)00273-1. [DOI] [PubMed] [Google Scholar]
- 43.Kos J, Krasovec M, Cimerman N, et al. Cysteine proteinase inhibitors stefin A, stefin B, and cystatin C in sera from patients with colorectal cancer: relation to prognosis. Clin Cancer Res. 2000;6:505–511. [PubMed] [Google Scholar]
- 44.Strojan P, Oblak I, Svetic B, Smid L, Kos J. Cysteine proteinase inhibitor cystatin C in squamous cell carcinoma of the head and neck: relation to prognosis. Br J Cancer. 2004;90:1961–1968. doi: 10.1038/sj.bjc.6601830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolwijck E, Massuger LF, Thomas CM, et al. Cathepsins B, L and cystatin C in cyst fluid of ovarian tumors. J Cancer Res Clin Oncol. 2010;136:771–778. doi: 10.1007/s00432-009-0716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ervin H, Cox JL. Late stage inhibition of hematogenous melanoma metastasis by cystatin C over-expression. Cancer Cell Int. 2005;5:14. doi: 10.1186/1475-2867-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirschke H, Eerola R, Hopsu-Havu VK, Bromme D, Vuorio E. Antisense RNA inhibition of cathepsin L expression reduces tumorigenicity of malignant cells. Eur J Cancer. 2000;36:787–795. doi: 10.1016/s0959-8049(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 48.Yang Z, Cox JL. Cathepsin L increases invasion and migration of B16 melanoma. Cancer Cell Int. 2007;7:8. doi: 10.1186/1475-2867-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fortenberry YM, Brandal S, Bialas RC, Church FC. Protein C inhibitor regulates both cathepsin L activity and cell-mediated tumor cell migration. Biochim Biophys Acta. 2010;1800:580–590. doi: 10.1016/j.bbagen.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Reiser J, Oh J, Shirato I, et al. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem. 2004;279:34827–34832. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- 51.Chung JH, Im EK, Jin TW, et al. Cathepsin L derived from skeletal muscle cells transfected with bFGF promotes endothelial cell migration. Exp Mol Med. 2011;43:179–188. doi: 10.3858/emm.2011.43.4.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schlappack OK, Zimmermann A, Hill RP. Glucose starvation and acidosis: effect on experimental metastatic potential, DNA content and MTX resistance of murine tumour cells. Br J Cancer. 1991;64:663–670. doi: 10.1038/bjc.1991.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steffan JJ, Snider JL, Skalli O, Welbourne T, Cardelli JA. Na+/H+ exchangers and RhoA regulate acidic extracellular pH-induced lysosome trafficking in prostate cancer cells. Traffic. 2009;10:737–753. doi: 10.1111/j.1600-0854.2009.00904.x. [DOI] [PubMed] [Google Scholar]