Abstract
In addition to controlling food intake, the dorsomedial hypothalamus (DMH) plays an important role in thermoregulation. Within the DMH, a number of neuropeptides and receptors have been found and their roles in controlling energy balance are being investigated. We recently found that the orexigenic neuropeptide Y (NPY) in the DMH has specific actions on body adiposity and thermogenesis using a viral-mediated manipulation of NPY in the DMH. Knockdown of NPY in the DMH promotes the development of brown adipocytes in white adipose tissue and increases brown adipocyte activity. DMH NPY knockdown also causes increased thermogenesis and energy expenditure. Finally, DMH NPY knockdown prevents high-fat diet-induced obesity and improves glucose homeostasis. This review focuses on the role of DMH NPY in modulating body adiposity and thermogenesis.
Keywords: dorsomedial hypothalamus, neuropeptide Y, brown adipocyte development, brown adipose tissue, thermogenesis, energy expenditure
1. Introduction
The human body maintains energy homeostasis through regulating food intake and energy expenditure. Increases in food intake and/or decreases in energy expenditure lead to excessive energy accumulation in the form of fat stored in adipose tissue that over the time results in obesity. Obesity has increasingly become a global health problem. For instance, over the last thirty years, obesity rates in the United States (US) have doubled for adults and tripled for children. Now about one third of US adults (33.8%) and approximately 17% of children and adolescents aged 2–19 years are obese [1, 2]. Obesity has serious health consequences linked to various life-threatening diseases, such as diabetes, cardiovascular diseases and some types of cancer, resulting in its becoming a leading preventable cause of death [3, 4]. However, current approaches for tackling the obesity epidemic are inadequate.
The identification of brown adipose tissue (BAT) in adult humans [5–7] has promoted the potential of BAT activity for combatting obesity. Two types of fat, white adipose tissue (WAT) and BAT, exist in mammals. WAT consists of unilocular adipocytes that contain a large lipid droplet. WAT acts as an energy reservoir to store excess calories and supply the stored lipid in response to decreased caloric intake or increased energy needs. In contrast, BAT is comprised of multilocular and mitochondrial-rich adipocytes that contain multiple small lipid droplets. BAT dissipates lipid energy to produce heat via mitochondrial uncoupling protein 1 (UCP1)-mediated nonshivering thermogenesis as a defense against cold and the potential intervention for the prevention and treatment of obesity. We have recently reported that knockdown of the orexigenic peptide neuropeptide Y (NPY) in the dorsomedial hypothalamus (DMH) via the vector of adeno-associated virus (AAV)-mediated NPY-specific RNAi (AAVshNPY) promotes the development of brown adipocytes in inguinal WAT, elevates interscapular BAT activity, and increases body energy expenditure in addition to its feeding effects [8]. This manipulation causes reduced fat accumulation, prevents high-fat diet-induced obesity, and ameliorates high-fat diet-induced hyperinsulinemia and glucose intolerance [8]. This article will review these new findings and discuss the potential for manipulation of DMH NPY for the fight against obesity and its related metabolic disorders.
2. DMH in thermoregulation
Beginning with the work of Bernardis and colleagues [9], our understanding of the role for the DMH in the control of energy balance has significantly progressed. Lesions of the DMH have been well-known to cause hypophagia, hypodipsia, reduced body weight and reduced linear growth [10]. Recent studies have further shown that stimulation or disinhibition of neurons in the DMH results in significant increases in sympathetic nerve activity to interscapular BAT and elevates local BAT and core body temperature [11, 12]. These data indicate that the DMH not only affects food intake, but also plays an important role in thermoregulation although the nature of DMH neurons or the neural mechanism underlying these effects remains to be determined. Within the DMH, a number of neuropeptides such as NPY, cholecystokinin (CCK), corticotrophin-releasing factor (CRF), and peptide receptors such as CCK-1 (CCK1Rs), melanocortin (MC) 4 and leptin receptors have been found, and their roles in controlling food intake and energy balance are being investigated [13]. Among them, NPY appears to serve as an important orexigenic output of the DMH to affect food intake and body weight. While DMH NPY overexpression results in hyperphagia and obesity, knockdown of NPY in the DMH ameliorates these alterations in Otsuka Long Evans Tokushima Fatty (OLETF) rats [14] and prevents diet-induced obesity [8]. In addition, DMH NPY knockdown affects brown adipocyte development, interscapular BAT activity and body energy expenditure [8], suggesting that DMH NPY also serves as an important neuromodulator in thermogenesis or thermoregulation.
3. DMH NPY modulates adiposity and thermogenesis
NPY is a 36-amino acid neuropeptide, initially discovered by Tatemoto and colleagues [15], that belongs to the pancreatic polypeptide family including peptide tyrosine-tyrosine (PYY) and pancreatic polypeptide (PP) [15, 16]. NPY is ubiquitously distributed in the central nervous systems [17, 18] and has a variety of biological and physiological actions [13, 19, 20]. Within the hypothalamus, NPY plays a pivotal role in the controls of food intake and energy balance. Intracerebroventricular (icv) or intrahypothalamic injection of NPY causes robust increases in food intake and body weight [21–24] and, with chronic administration, can eventually produce obesity [25]. In addition, icv administration of NPY promotes WAT lipid storage and decreases BAT thermogenesis [26]. Central administration of NPY suppresses sympathetic activity in interscapular BAT in rats [27]. Thus, hypothalamic NPY not only acts as an orexigenic peptide, but also affects adipocyte lipid mobilization and thermogenesis.
3.1 Differential regulation of NPY in the ARC and the DMH
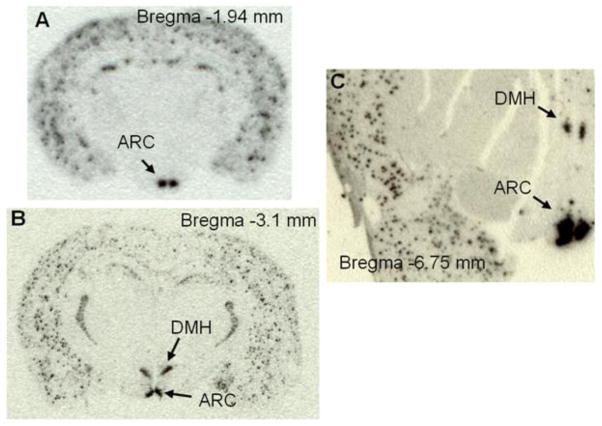
Within the hypothalamus, NPY-expressing neurons have been identified in the arcuate nucleus (ARC) and the DMH in both rat and non-human primate brains [28–30] (Figure 1), but the regulation of NPY in the ARC and the DMH differs. While ARC NPY serves as one of the downstream mediators of leptin’s actions [31–36], DMH NPY is not under the control of leptin [28]. DMH NPY neurons do not co-express the long form of the leptin receptors (LepRbs) although abundant LepRbs are present in the DMH [28]. In fact, DMH NPY neurons contain CCK1Rs in the rat [37] and parenchymal injection of CCK into the DMH of rats inhibits Npy mRNA expression and decreases food intake [38]. Consistent with this DMH CCK-NPY signaling, lack of CCK1Rs in OLETF rats has been proposed to contribute to increased Npy expression in the DMH that plays a causal role in the development of their hyperphagia and obesity [14, 39]. Moreover, although Npy gene expression in the DMH is undetectable in normally growing mice [37](Figure 1), an induction of Npy expression in the DMH has been found in a number of mouse models of obesity including the lethal yellow agouti (Ay) [40], MC4R knockout [40], diet-induced obese [41], tubby [42], and brown adipose tissue-deficient obese mice [43]. However, such induction of Npy gene expression is not evident in leptin deficient ob/ob mice [40], supporting the view that DMH NPY acts independently of leptin, but the detailed neural circuits remain to be characterized.
Figure 1.
In situ hybridization with [35S]-labeled antisense riboprobe of rodent or human Npy shows Npy gene expression in the arcuate nucleus (ARC) and/or the dorsomedial hypothalamus (DMH) in adult mouse (A), rat (B), and rhesus monkey brain (C).
3.2. DMH NPY knockdown promotes brown adipocyte development
To determine a functional role for DMH NPY in energy balance control, we have developed an AAVshNPY vector for knocking down NPY expression in the DMH and examined the effects of DMH NPY knockdown on food intake, energy expenditure and adiposity. We found that knockdown of NPY in the DMH affects body weight through affecting food intake, specifically showing a nocturnal and meal size-specific feeding effect [14], physical activity, thermogenesis, energy expenditure, and body adiposity [8]. DMH NPY knockdown decreases subcutaneous inguinal fat mass in rats on regular chow and ameliorates high-fat diet-induced increases in fat accumulation in the inguinal and epididymal fat and in the interscapular BAT mass [8]. Intriguingly, DMH NPY knockdown promotes the development of brown adipocytes in inguinal WAT. This white-into-brown adipocyte transformation has been confirmed by histochemistry, showing that inguinal adipocytes of NPY knockdown rats form new large clusters containing multilocular adipocytes (brown-fat-like adipocytes) [8]. In support of this view, the BAT-specific marker UCP1 is highly expressed in these new formed cells and also in a number of unilocular adipocytes around these clusters [8]. UCP1 expression in this inguinal fat is further confirmed by using both real-time RT-PCR (reverse transcriptase-polymerase chain reaction) and Western blot analyses [8]. But, whether these browning adipocytes in WAT are directly derived from specific precursor cells such as beige (or brite) cells, or transdifferentiated from unilocular white adipocytes remains to be determined.
Since previous evidence has shown that the sympathetic nervous system (SNS) mediates WAT into BAT transformation [44–46], we next determined whether the sympathetic innervation is necessary for DMH NPY knockdown-induced transformation of white to brown adipocytes in inguinal WAT. In this experiment, we injected the neurotoxin 6-hydroxydopamine (6-OHDA) unilaterally into the inguinal fat area for regional sympathetic denervation 2 weeks prior to bilateral DMH injections of the vector AAVshNPY. The contralateral site received a saline injection as an internal control. We found that norepinephrine (NE) levels were significantly increased in inguinal fat of NPY knockdown rats compared to levels in the animals receiving DMH injections of control vectors, indicating that DMH NPY knockdown causes increased sympathetic activity to the inguinal fat area [8]. Injection of 6-OHDA into the inguinal fat area significantly lowered the NE levels, i.e., caused a sympathetic denervation [8]. As a result, within the rats with DMH NPY knockdown, while the site of inguinal fat receiving saline injection became brown-like fat, the color of inguinal fat receiving 6-OHDA injection showed significantly less browning [8]. This observation was further confirmed by the histochemical study showing that the number of brown-fat-like adipocytes (multilocular adipocytes) was dramatically reduced on the side with 6-OHDA treatment as compared to the contralateral side where brown-fat-like adipocytes remained. 6-OHDA treatment also prevented UCPl expression in inguinal adipocytes at both the protein and mRNA levels [8]. Together, these results indicate that DMH NPY modulates sympathetic nervous activity in inguinal fat and knockdown of NPY in the DMH results in increased sympathetic activity in this fat, leading to white to brown adipocyte transformation.
A number of molecules have been identified as contributing to brown adipocyte development. These include peroxisome proliferator-activated receptor-γ (PPAR-γ) [47], PPAR-γ coactivator-1α (PGC-1α) [48], PRD1-BF-1-RIZ1 homologous domain containing protein-16 (PRDM16) [49], CCAAT/enhancer-binding protein β (C/EBP-β) [50], bone morphogenetic protein 7 (BMP7) [51], and forkhead box C2 (FOXC2) [52]. PPAR-γ is an essential factor for the development of both white and brown fat cells [47]. Chronic treatment with the PPAR-γ agonist rosiglitazone promotes brown adipogenesis in WAT characterized with distinct adipocytes, namely, “brite” adipocytes [53]. The rosiglitazone treatment causes not only the expression of PGC-1α and mitochondriogenesis, but also NE-augmentable expression of UCP1 in these brite cells [53]. PGC-1a is another key factor involved in brown adipocyte development and thermogenesis [48]. Ectopic expression of PGC-1α in white fat cells induces a number of mitochondrial genes and thermogenic genes, such as Ucp1, whereas genetic ablation of PGC-1α results in reduced capacity for cold-induced thermogenesis in vivo [54]. PRDM16, the other transcription factor that interacts with the active form of C/EBPβ, acts as a critical determinant of the brown fat lineage from myoblast progenitors during the embryonic development [55]. Based on the actions of these molecules, we initially examined whether PPAR-γ and PGC-1α were involved in DMH NPY knockdown-induced transformation of white to brown adipocytes. We found that DMH NPY knockdown promotes both Ppar-γ and Pgc-1α gene expression in inguinal fat [8], suggesting that these key transcription factors are contributing to the browning effects of DMH NPY knockdown on inguinal WAT. A detailed examination of the molecular control of this white to brown adipocyte transformation is ongoing in our laboratory.
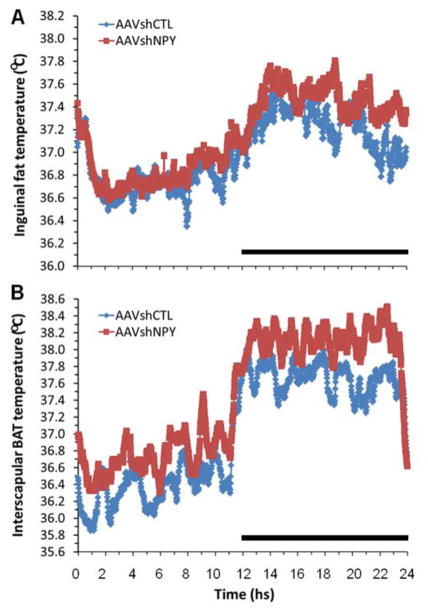
In the BAT, fatty acids serve as the thermogenic substrate and the β-oxidation of the fatty acids (or lipolysis) is coupled with thermogenesis through UCP1. In other words, evoking thermogenesis depends upon evoking lipolysis [56]. We examined whether DMH NPY knockdown affects fat metabolism in the transformed inguinal fat in addition to its resulting in increased Ucp1 and Pgc-1α gene expression [8]. Fatty acid synthase (FAS) and carnitine palmitoyltransferase 1 (CPT1) are two important enzymes involved in fat metabolism. FAS plays a key role in fatty acid synthesis, whereas CPT1 is the rate-limiting enzyme controlling fatty acid oxidation. Compared to control rats, Cpt1 gene expression is significantly increased in the inguinal fat of NPY knockdown rats with a trend toward a decrease in Fas gene expression [8], suggesting a transformation away from fat synthesis and toward fat oxidation in this tissue. Consistent with this view, a follow-up study has revealed increased thermogenesis in the inguinal fat of NPY knockdown rats. In the study, the inguinal fat temperature was directly examined using a radio transmitter device (E-mitter, Mini-Mitter) that was buried under the inguinal fat. At a normal ambient room temperature condition (23±1°C), the inguinal fat temperature is significantly increased by an average of 0.3°C during the dark period in rats with DMH NPY knockdown compared to control animals (Figure 2A). Thus, these results demonstrate that the inguinal browning fat of NPY knockdown rats exhibits the functional features of the BAT.
Figure 2.
Effects of DMH NPY knockdown on inguinal fat (A) and interscapular brown fat temperature (B) in rats receiving bilateral DMH injections of the vector adeno-associated virus (AAV)-mediated RNAi (AAVshNPY) compared with the rats receiving bilateral DMH injections of the control vector containing scrambled shRNA (AAVshCTL). Rats were housed individually at room temperature condition (23±1°C) on a 12:12 hr light-dark cycle. Black bar indicates a dark period.
3.3. DMH NPY modulates interscapular BAT activity
Recent evidence has indicated the importance of the DMH in regulating sympathetic nerve activity to interscapular BAT and thermogenesis. Stimulation or disinhibition of neurons in the DMH by parenchymal microinjection of glutamate or γ-aminobutyric acid (GABA)-A receptor antagonist results in great increases in sympathetic nerve activity to interscapular BAT and increases in local BAT and core body temperature [11, 12]. Collectively, the DMH has been proposed as an intermediate relay receiving the inputs from the hypothalamic preoptic area (POA), a center of integrating central and peripheral thermal signals, and sending outputs to the rostral raphe pallidus (rRP) in the medulla, the area containing premotor neurons that regulate sympathetic nerve activity to interscapular BAT [11, 12]. However, the nature of DMH neurons mediating these effects has not yet been fully characterized. Using a model of LepRbEGFP reporter mice, Zhang and colleagues [57] have identified LepRb-expressing neurons in the DMH and dorsal hypothalamic area (DHA) as being involved in these sympathetic BAT circuits. Our recent findings provide support for the view that DMH NPY also serves as one of the important mediators of these DMH actions. DMH NPY knockdown causes increased expression of Ucp1 in the interscapular BAT [8]. This suggests that DMH NPY affects interscapular BAT activity or thermogenesis. In support of this view, DMH NPY knockdown results in increased interscapular BAT thermogenesis as directly determined by increased BAT temperature. At room temperature condition (23±1°C), interscapular BAT temperature is significantly increased by an average of 0.43°C (p <.05) during the dark and there is a trend for increases (p >.05) during the light compared to control animals (Figure 2B). These data demonstrate that DMH NPY also modulates interscapular BAT thermogenesis or activity to affect energy expenditure, but it is unclear whether DMH NPY is involved in the POA-DMH-rRP-BAT circuits.
3.4. DMH NPY affects energy expenditure
Consistent with the actions of DMH NPY on activity of both inguinal fat and interscapular BAT, manipulation of NPY expression in the DMH affects energy expenditure and thermogenesis. DMH NPY knockdown causes increased oxygen consumption during both dark and light phases of the circadian cycle at an ambient room temperature condition (23±1°C). NPY knockdown rats have also decreased respiratory exchange rates during the dark and total 24 h periods. As a result, their energy expenditure is significantly increased over the 24 hour period [8]. Furthermore, although core body temperature does not differ between NPY knockdown and control rats at room temperature (23±1°C), NPY knockdown rats have a greater increase in thermogenic response to 6 hours of cold exposure (6°C) compared to their control counterparts [8]. In addition, DMH NPY knockdown increases spontaneous physical activity measured as increased locomotor activity during the dark period [8]. Overall, DMH NPY plays a functional role in the regulation of thermogenesis and energy expenditure likely through affecting activity of inguinal fat and interscapular BAT as well as physical activity.
4. DMH NPY as a target for combatting obesity and diabetes
Although a variety of pharmacological experiments have demonstrated a pivotal role for NPY in the controls of food intake and body weight [21–25] and the actions of ARC NPY in the control of energy balance have been well studied and its underlying neural circuits have also been well documented [31–36], NPY knockout has little or no effect on food intake and body weight [58], implying that the lack of effect in the knockout is likely due to developmental compensations. In support of this view, neonatal ablation of NPY neurons in the ARC has minimal effects on feeding, whereas their ablation in adults causes rapid starvation [59]. Moreover, AAV-mediated expression of antisense NPY cRNA in the ARC of adult rats decreases ARC NPY expression and results in decreases in food intake and weight gain [60], and knockdown of NPY in the ARC of adult rats via AAV-mediated RNAi attenuates the feeding response to food deprivation [14]. These data further suggest that the system of AAV-mediated manipulation of gene expression could provide a unique and efficient approach for studying the function of the target gene as well as the potential for gene therapeutic intervention.
In contrast to ARC NPY, our understanding of the roles for DMH NPY in maintaining energy homeostasis is just beginning. The limits on our understanding are likely due to the fact that Npy gene expression in the DMH (particularly in the compact region) is normally undetectable in normally growing mice (Figure 1A) but only detected in rats (Figure 1B), i.e. arguing that NPY in the compact region of the DMH is rat-specific. Using the radioactively labeled riboprobe of human Npy antisense, we have identified Npy expression in the DMH of non-human primate (Figure 1C), providing support for a potential role for DMH NPY in human energy balance. Using the rat model of high-fat diet-induced obesity and disordered glucose homeostasis, mimicking human obesity and diabetes, we found that knockdown of NPY in the DMH prevents high-fat diet-induced hyperphagia and obesity, and ameliorates diet-induced glucose intolerance, hyperinsulinemia and insulin insensitivity [8]. Since BAT plays an enormous role in glucose and triglyceride clearance [61], the effects of DMH NPY knockdown on brown adipocyte development and BAT activity may contribute to its actions in the prevention of diet-induced obesity and disordered glucose homeostasis, providing a potential target for combating human obesity and diabetes or related metabolic disorders.
Highlights.
Knockdown of dorsomedial hypothalamic (DMH) NPY affects body adiposity
DMH NPY knockdown promotes development of brown adipocytes in white fat depots
DMH NPY knockdown promotes interscapular BAT activity
DMH NPY knockdown increases thermogenesis and energy expenditure
Acknowledgments
This article is based on a presentation made during the 2012 Annual Meeting of the Society for the Study of Ingestive Behavior, July 10-14, 2012. The SSIB meeting was made possible in part by generous unrestricted donations from Novo Nordisk A/S, Research Diets, Inc., Sanofi, Inc., and TSE, Inc. This work was supported by US National Institute of Diabetes and Digestive and Kidney Diseases Grants DK074269 and DK087888.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 4.Jia H, Lubetkin EI. Trends in quality-adjusted life-years lost contributed by smoking and obesity. American Journal of Preventive Medicine. 2010;38:138–144. doi: 10.1016/j.amepre.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. The New England Journal of Medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. The New England Journal of Medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 7.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. The New England Journal of Medicine. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 8.Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metabolism. 2011;13:573–583. doi: 10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernardis LL, Box BM, Stevenson JA. Growth following hypothalamic lesions in the weanling rat. Endocrinology. 1963;72:684–692. doi: 10.1210/endo-72-5-684. [DOI] [PubMed] [Google Scholar]
- 10.Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiology & Behavior. 2002;76:431–442. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- 11.Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2007;292:R47–63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- 12.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Frontiers in Bioscience. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bi S. Role of dorsomedial hypothalamic neuropeptide Y in energy homeostasis. Peptides. 2007;28:352–356. doi: 10.1016/j.peptides.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, Bi S. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. The Journal of Neuroscience. 2009;29:179–190. doi: 10.1523/JNEUROSCI.4379-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y--a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- 16.Tatemoto K. Neuropeptide Y: complete amino acid sequence of the brain peptide. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:5485–5489. doi: 10.1073/pnas.79.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adrian TE, Allen JM, Bloom SR, Ghatei MA, Rossor MN, Roberts GW, Crow TJ, Tatemoto K, Polak JM. Neuropeptide Y distribution in human brain. Nature. 1983;306:584–586. doi: 10.1038/306584a0. [DOI] [PubMed] [Google Scholar]
- 18.Allen YS, Adrian TE, Allen JM, Tatemoto K, Crow TJ, Bloom SR, Polak JM. Neuropeptide Y distribution in the rat brain. Science. 1983;221:877–879. doi: 10.1126/science.6136091. [DOI] [PubMed] [Google Scholar]
- 19.Gray TS, Morley JE. Neuropeptide Y: anatomical distribution and possible function in mammalian nervous system. Life sciences. 1986;38:389–401. doi: 10.1016/0024-3205(86)90061-5. [DOI] [PubMed] [Google Scholar]
- 20.Colmers WF, Wahlestedt C. The biology of neuropeptide Y and related peptides. New Jersey: Humana Press; 1993. [Google Scholar]
- 21.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 22.Levine AS, Morley JE. Neuropeptide Y: a potent inducer of consummatory behavior in rats. Peptides. 1984;5:1025–1029. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- 23.Stanley BG, Leibowitz SF. Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:3940–3943. doi: 10.1073/pnas.82.11.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanley BG, Kyrkouli SE, Lampert S, Leibowitz SF. Neuropeptide Y chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides. 1986;7:1189–1192. doi: 10.1016/0196-9781(86)90149-x. [DOI] [PubMed] [Google Scholar]
- 25.Zarjevski N, Cusin I, Vettor R, Rohner-Jeanrenaud F, Jeanrenaud B. Chronic intracerebroventricular neuropeptide-Y administration to normal rats mimics hormonal and metabolic changes of obesity. Endocrinology. 1993;133:1753–1758. doi: 10.1210/endo.133.4.8404618. [DOI] [PubMed] [Google Scholar]
- 26.Billington CJ, Briggs JE, Grace M, Levine AS. Effects of intracerebroventricular injection of neuropeptide Y on energy metabolism. The American Journal of Physiology. 1991;260:R321–327. doi: 10.1152/ajpregu.1991.260.2.R321. [DOI] [PubMed] [Google Scholar]
- 27.Egawa M, Yoshimatsu H, Bray GA. Neuropeptide Y suppresses sympathetic activity to interscapular brown adipose tissue in rats. The American Journal of Physiology. 1991;260:R328–334. doi: 10.1152/ajpregu.1991.260.2.R328. [DOI] [PubMed] [Google Scholar]
- 28.Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2003;285:R1030–1036. doi: 10.1152/ajpregu.00734.2002. [DOI] [PubMed] [Google Scholar]
- 29.White JD, Kershaw M. Increased hypothalamic neuropeptide Y expression following food deprivation. Molecular and Cellular Neurosciences. 1990;1:41–48. doi: 10.1016/1044-7431(90)90040-b. [DOI] [PubMed] [Google Scholar]
- 30.Bi S, Kim YJ, Zheng F. Dorsomedial hypothalamic NPY and energy balance control. Neuropeptides. 2012;46:309–314. doi: 10.1016/j.npep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 32.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 34.Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 35.Cone RD. Studies on the physiological functions of the melanocortin system. Endocrine Reviews. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 36.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 37.Bi S, Scott KA, Kopin AS, Moran TH. Differential roles for cholecystokinin a receptors in energy balance in rats and mice. Endocrinology. 2004;145:3873–3880. doi: 10.1210/en.2004-0284. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Scott KA, Zhao Z, Moran TH, Bi S. Characterization of the feeding inhibition and neural activation produced by dorsomedial hypothalamic cholecystokinin administration. Neuroscience. 2008;152:178–188. doi: 10.1016/j.neuroscience.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bi S, Ladenheim EE, Schwartz GJ, Moran TH. A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2001;281:R254–260. doi: 10.1152/ajpregu.2001.281.1.R254. [DOI] [PubMed] [Google Scholar]
- 40.Kesterson RA, Huszar D, Lynch CA, Simerly RB, Cone RD. Induction of neuropeptide Y gene expression in the dorsal medial hypothalamic nucleus in two models of the agouti obesity syndrome. Molecular Endocrinology. 1997;11:630–637. doi: 10.1210/mend.11.5.9921. [DOI] [PubMed] [Google Scholar]
- 41.Guan XM, Yu H, Trumbauer M, Frazier E, Van der Ploeg LH, Chen H. Induction of neuropeptide Y expression in dorsomedial hypothalamus of diet-induced obese mice. Neuroreport. 1998;9:3415–3419. doi: 10.1097/00001756-199810260-00015. [DOI] [PubMed] [Google Scholar]
- 42.Guan XM, Yu H, Van der Ploeg LH. Evidence of altered hypothalamic pro-opiomelanocortin/neuropeptide Y mRNA expression in tubby mice. Brain research Molecular Brain Research. 1998;59:273–279. doi: 10.1016/s0169-328x(98)00150-8. [DOI] [PubMed] [Google Scholar]
- 43.Tritos NA, Elmquist JK, Mastaitis JW, Flier JS, Maratos-Flier E. Characterization of expression of hypothalamic appetite-regulating peptides in obese hyperleptinemic brown adipose tissue-deficient (uncoupling protein-promoter-driven diphtheria toxin A) mice. Endocrinology. 1998;139:4634–4641. doi: 10.1210/endo.139.11.6308. [DOI] [PubMed] [Google Scholar]
- 44.Himms-Hagen J, Cui J, Danforth E, Jr, Taatjes DJ, Lang SS, Waters BL, Claus TH. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. The American Journal of Physiology. 1994;266:R1371–1382. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- 45.Jimenez M, Barbatelli G, Allevi R, Cinti S, Seydoux J, Giacobino JP, Muzzin P, Preitner F. Beta 3-adrenoceptor knockout in C57BL/6J mice depresses the occurrence of brown adipocytes in white fat. European Journal of Biochemistry/FEBS. 2003;270:699–705. doi: 10.1046/j.1432-1033.2003.03422.x. [DOI] [PubMed] [Google Scholar]
- 46.Nagase I, Yoshida T, Kumamoto K, Umekawa T, Sakane N, Nikami H, Kawada T, Saito M. Expression of uncoupling protein in skeletal muscle and white fat of obese mice treated with thermogenic beta 3-adrenergic agonist. The Journal of Clinical Investigation. 1996;97:2898–2904. doi: 10.1172/JCI118748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Molecular Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 48.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocrine Reviews. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 49.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manchado C, Yubero P, Vinas O, Iglesias R, Villarroya F, Mampel T, Giralt M. CCAAT/enhancer-binding proteins alpha and beta in brown adipose tissue: evidence for a tissue-specific pattern of expression during development. The Biochemical Journal. 1994;302 ( Pt 3):695–700. doi: 10.1042/bj3020695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–573. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 53.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. The Journal of Biological Chemistry. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 55.Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, Spiegelman BM. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, Jones JC, Rhodes C, Munzberg H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. The Journal of Neuroscience. 2011;31:1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- 59.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 60.Gardiner JV, Kong WM, Ward H, Murphy KG, Dhillo WS, Bloom SR. AAV mediated expression of anti-sense neuropeptide Y cRNA in the arcuate nucleus of rats results in decreased weight gain and food intake. Biochemical and Biophysical Research Communications. 2005;327:1088–1093. doi: 10.1016/j.bbrc.2004.12.113. [DOI] [PubMed] [Google Scholar]
- 61.Bartelt A, Heeren J. The holy grail of metabolic disease: brown adipose tissue. Current Opinion in Lipidology. 2012;23:190–195. doi: 10.1097/MOL.0b013e328352dcef. [DOI] [PubMed] [Google Scholar]