Abstract
Background
The placebo effect often undermines efforts to determine treatment effectiveness in clinical trials. A significant placebo response occurs in alcohol trials, but it is not well understood. The purpose of this study was to characterize the placebo response across multiple naltrexone and acamprosate studies.
Methods
Fifty-one trials, 3 with a naltrexone and an acamprosate arm, 31 with at least 1 naltrexone arm, and 17 with at least 1 acamprosate arm, were identified from Cochrane reviews and PubMed search. To be included in this study, patients had to be at least 18 years old, abstinent from alcohol before randomization, and meet a diagnosis of alcohol dependence. Pearson correlation coefficients (rp) and simple linear regression were used to describe the strength of linear relationships between placebo response and treatment effect size. Spearman’s rank correlation coefficients (rs) were used to examine the strength of associations between study characteristics and placebo response.
Results
For the end-point measures of percent days abstinent and total abstinence, a negative relationship was evident between placebo response and treatment effect size in the naltrexone trials (rp = −0.55, p < 0.01 and rp = −0.20, p = 0.35, respectively) as well as in the acamprosate trials (rp = −0.45, p = 0.09 and rp = −0.56, p = 0.01, respectively). The placebo response for percent days abstinent was negatively correlated with mean age of participants (rs = −0.42, p = 0.05) across naltrexone trials and positively correlated with publication year (rs = 0.57, p = 0.03) across acamprosate trials. However, these two study characteristics were not significantly correlated with treatment effect size.
Conclusion
The placebo response varied considerably across trials and was negatively correlated with the treatment effect size. Additional studies are required to fully understand the complex nature of the placebo response and to evaluate approaches to minimize its effects.
Keywords: Alcohol Dependence, Placebo Effect, Naltrexone, Acamprosate, Meta-Analysis
Introduction
The phrase “placebo effect” refers to improvements in treatment outcomes that result from a complex interaction of patient, clinical trial staff, and treatment environment factors rather than from the treatment itself (Finniss et al., 2010). This phenomenon has been observed in clinical trials for a wide range of therapeutic areas, including depression, pain, Parkinson’s disease, irritable bowel syndrome, overactive bladder, anxiety, and addiction (Finniss et al., 2010; Kaptchuk et al., 2008; Lee et al., 2009; Lidstone et al., 2010). Psychological mechanisms that contribute to placebo response include positive expectations, conditioning, learning, memory, motivation, reward, and anxiety reduction (Finniss et al., 2010).
The placebo effect makes it especially difficult to detect a treatment effect in clinical trials for psychiatric medications. For example, higher placebo responses have been associated with smaller effect sizes (i.e., differences in treatment response between the experimental medication and placebo groups) in meta-analysis studies of several psychiatric diseases, such as depression and schizophrenia (Khan et al., 2003; Khin et al., 2011; Kirsch et al., 2008; Mallinckrodt et al., 2010; Nunes and Levin, 2004). Moreover, the placebo response often varies according to both patient and study characteristics. For example, in depression trials, those patients with more severe depression at baseline had smaller placebo responses and greater effect sizes in relieving depression than did those with less severe depression (Khin et al., 2011; Kirsch et al., 2008; Papakostas and Fava, 2009). Other study characteristics associated with the placebo effect in depression and schizophrenia studies include the year of publication, number of sites and subjects, number of treatment arms, number of study visits, the use of placebo lead-ins, and centralized versus site-based rating (Faries et al., 2001; Kobak et al., 2010; Mallinckrodt et al., 2010; Mallinckrodt et al., 2011; Papakostas and Fava, 2009; Sinyor et al., 2010; Undurraga and Baldessarini, 2012; Walsh et al., 2002).
The placebo response in alcohol trials appears to be even greater than that observed in depression and schizophrenic trials (Anton et al., 2006; Fertig et al., 2012; Khan et al., 2003; Kirsch et al., 2008; Litten et al., 2012; Mallinckrodt et al., 2010). Nonetheless, few studies have been conducted to characterize the placebo effect in alcohol clinical trials. Weiss et al. (2008) reported that factors such as pill taking, meeting with a health care professional, and optimism about a medication’s effect contributed to a significant placebo response in the large alcohol trial—Combined Pharmacotherapies and Behavioral Interventions (COMBINE). The purpose of the current study is to determine how differences in placebo response influence an experimental medication’s treatment effect and to characterize the placebo response across multiple alcohol clinical studies. In this analysis, we selected alcohol clinical trials that tested the efficacy of naltrexone and acamprosate. Both medications are approved by the U.S. Food and Drug Administration (FDA) to treat alcohol dependence and have been studied in numerous international clinical trials. Previous meta-analyses of these studies, however, have yielded only a small effect size for both medications (Kranzler and Gage, 2008; Kranzler and Van Kirk, 2001; Maisel et al., 2013; Mann et al., 2004; Mason and Lehert, 2012; Rösner et al., 2010a; Rösner et al., 2010b; Srisurapanont and Jarusuraisin, 2005; Streeton and Whelan, 2001). In this study, we used common treatment endpoint measures to assess the relationship between the placebo response and treatment effect size. We also examined various patient and study characteristics to determine their associations with the placebo response.
Methods
Data sources and Study Selection
Naltrexone and acamprosate trials were identified from two recent Cochrane reviews – Opioid Antagonists for Alcohol Dependence (2010) and Acamprosate for Alcohol Dependence (2010) (Rösner et al., 2010a, Rösner et al., 2010b). The search dates of the Cochrane reviews were 1966 to January 2010 for naltrexone trials and 1966 to January 2009 for acamprosate trials. Trials published after the ending dates of the Cochrane reviews to December 2011 were obtained via a PubMed search using the search terms “alcohol dependence AND naltrexone” and “alcohol dependence AND acamprosate.” The search was further refined to include clinical trials in human subjects and those published in the English language. Data were extracted from the newly identified studies using methods specified in the Cochrane reviews. Study authors were contacted directly to obtain specific data on endpoint measures that were not included in the original study publications.
To be included in this analysis, the naltrexone and acamprosate trials had to meet the following criteria: 1) studies were randomized, double-blinded, and placebo-controlled; 2) studies primarily included adults (aged ≥ 18 years) who were diagnosed with alcohol dependence according to research diagnostic criteria, such as the Diagnostic and Statistical Manual (DSM) (American Psychiatric Association, 1980, American Psychiatric Association, 1987, American Psychiatric Association, 1994) and the International Classification of Diseases (ICD) (World Health Organization, 1992); 3) participants reported being abstinent from alcohol before randomization; 4) participants received a minimum of 4 weeks of treatment; and, 5) studies included one or more of the following endpoints —percent days abstinent, total abstinence, percent days without heavy drinking, or abstinence from heavy drinking. Trials of participants with comorbid psychiatric or substance use (other than nicotine) disorders and trials of injectable naltrexone were included. In all, our analysis included 51 studies, of which 31 had at least 1 naltrexone arm (Ahmadi and Ahmadi, 2002; Anton et al., 1999; Anton et al., 2005; Anton et al., 2011; Balldin et al., 2003; Baltieri et al., 2008; Chick et al., 2000a; de Goes e Castro, 2004; Gastpar et al., 2002; Guardia et al., 2002; Johnson et al., 2004; Kranzler et al., 1998; Kranzler et al., 2000; Kranzler et al., 2004; Krystal et al., 2001; Latt et al., 2002; Lee et al., 2001; Monti et al., 2001; Morris et al., 2001; O’Malley et al., 1992; O’Malley et al., 2007; O’Malley et al., 2008; Oslin et al., 1997; Oslin, 2005; Oslin et al., 2008; Petrakis et al., 2004; Petrakis et al., 2005; Pettinati et al., 2008; Pettinati et al., 2010; Volpicelli et al., 1992; Volpicelli et al., 1997), 17 had at least 1 acamprosate arm (Baltieri and De Andrade, 2004; Barrias et al., 1997; Besson et al., 1998; Chick et al., 2000b; Geerlings et al., 1997; Gual and Lehert, 2001; Ladewig et al., 1993; Lhuintre et al., 1985; Lhuintre et al., 1990; Paille et al., 1995; Pelc et al., 1992; Pelc et al., 1997; Poldrugo, 1997; Sass et al., 1996; Tempesta et al., 2000; Whitworth et al., 1996; Wölwer et al., 2011), and 3 had both a naltrexone arm and an acamprosate arm (Anton et al., 2006; Kiefer et al., 2003; Morley et al., 2006). The 3 trials with both arms were treated as separate trials, resulting in a total of 34 naltrexone trials and 20 acamprosate trials for the analyses. Because not all endpoints were measured in every trial, the number of trials available for each analysis varied by the availability of the endpoint.
Definition of Endpoints
Percent days abstinent and percent days without heavy drinking were continuous outcomes. They were defined as the sum of days a trial participant remained completely abstinent and remained abstinent from heavy drinking, respectively, during the course of a trial divided by the duration of treatment. Most trials defined heavy drinking as ≥ 5 standard drinks for men or ≥ 4 drinks for women, per drinking day. Several studies alternatively defined heavy drinking as >6 for men and >5 for women (Monti et al., 2001), or ≥ 6 for men and ≥ 4 for women (Krystal et al., 2001; Morley et al., 2006) per drinking day. A few studies also considered > or ≥5 drinking days per week (Ahmadi and Ahmadi, 2002; Guardia et al., 2002; Kiefer et al., 2003; Morris et al., 2001; Oslin et al., 1997; Volpicelli et al., 1992) or a blood alcohol concentration > or ≥100 mg/dL (Morris et al., 2001; Oslin et al., 1997; Volpicelli et al., 1992; Volpicelli et al., 1997) as returning to heavy drinking. In addition, the definition used by Baltieri et al. (2008) and Latt et al. (2002) was drinking >90 grams of alcohol per week and drinking to previous heavy levels, respectively. Total abstinence and abstinence from heavy drinking were binary outcomes and were defined as remaining completely abstinent and remaining abstinent from heavy drinking, respectively, throughout the duration of treatment.
For the studies that used continuous outcomes (25 naltrexone and 15 acamprosate trials), we used the means and standard deviations reported in the study publications or provided by the authors, regardless of their statistical approaches to handling missing data. These approaches included assuming missing cases to have relapsed to any drinking or to heavy drinking (in 7 naltrexone and 13 acamprosate trials), imputing missing data by other statistical methods (in 4 naltrexone trials), and excluding missing cases from the analysis (in 5 naltrexone and 1 acamprosate trials). In addition, there was no report on how missing data were handled in 9 naltrexone and 1 acamprosate trials. For the studies that used binary outcomes (30 naltrexone and 20 acamprosate), missing data associated with dropout or lost to follow-up events were treated as relapses. All estimates reported in the Cochrane reviews were checked against those presented in the original articles. When discrepancies occurred, proper corrections were made wherever possible.
Statistical Analysis
All statistical analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC). Pearson correlation coefficients (rp) and simple linear regression were used to describe the strength of linear relationships between the placebo response and the treatment effect size. For continuous outcomes, the treatment effect size was the difference in means between the medication group and the placebo group, and for binary outcomes, it was the natural log of the risk ratio (RR) between the medication and placebo groups (or equivalently, the difference between the natural log of the proportions). The RR here refers to the ratio of the proportion of total abstinence or abstinence from heavy drinking in the treatment group to the proportion in the placebo group. The natural log transformation provides a symmetric distribution for the analysis. As an example, a 0.5 difference in the natural log of the proportions of total abstinence between the medication and placebo groups yields a e0.5=1.65 RR, indicating that the medication group had an abstinence rate 1.65 times higher than the placebo group. In contrast, a −0.5 difference yields a e−0.5=0.61 RR, indicating that the medication group had an abstinence rate 39% lower than the placebo group For the outcomes total abstinence and percent days abstinent, analyses were conducted separately for the naltrexone and acamprosate trials as well as combined. In studies with both a naltrexone and an acamprosate arm, the results of the two medication types were treated as from two separate trials. Because few acamprosate trials had primary outcomes related to heavy drinking, the analyses for abstinence from heavy drinking and percent days without heavy drinking were conducted only for the naltrexone trials. In addition to linear regression, we used a SAS macro (Wilson, 2010) to conduct random-effects meta-regression with the method-of-moments approach to examine if the linear relationships remained when taking into account within-trial variance of effect size and between-trial heterogeneity (Baker et al., 2009; Sutton et al., 2000; Thompson and Higgins, 2002). Similar linear relationships were derived from both simple linear regression (unweighted) and meta-regression models (weighted) (results can be found in Table S1 online); so, for simplicity, we presented only the unweighted results. Because the placebo response was included in the calculation of the effect size, it was expected that the placebo response and effect size would be negatively correlated. However, the magnitude of the observed correlation reflected not only this mathematical component but also the aggregate relationship across trials.
Spearman’s rank correlation coefficients (rs) were used to examine the strength of associations between study characteristics and the placebo response, treatment response, and effect size. Study characteristics selected in this analysis were those commonly and consistently reported in the study publications. These included duration of treatment (weeks), publication year, mean age of participants at baseline, percentage of male participants, and number of sites.
Results
Characteristics of Selected Studies
Table 1 shows the characteristics of the 34 naltrexone and 20 acamprosate trials included in the analysis. Compared with the acamprosate trials, the naltrexone trials had shorter treatment durations, were mostly conducted in the United States and at a single site, and were more likely to use high intensity behavioral platforms. The 10,392 participants in these trials were predominately men (78.3%) and had a mean age of 43.7 years. Across all trials, percent days abstinent and total abstinence were the two most commonly reported outcome measures. In the naltrexone trials, abstinence from heavy drinking was another common outcome measure.
Table 1.
Characteristics of Trials Included in the Meta-analysis and Demographic Summary of Participants in the Trials.
| Naltrexone | Acamprosate | Total | |
|---|---|---|---|
| Trial characteristics | |||
| Number of trialsa | 34 | 20 | 54 |
| Duration of treatment, weeks, median (range) | 12 (4 – 24) | 24 (12 – 48) | 12 (4 – 48) |
| Publication year, median (range) | 2003 (1992–2011) | 1997 (1985–2011) | 2001.5 (1985–2011) |
| Geographic location, no. (%) | |||
| USA | 21 (61.8) | 1 (5.0) | 22 (40.7) |
| Europe | 5 (14.7) | 16 (80.0) | 21 (38.9) |
| USA and Europe | 1 (2.9) | 0 (0.0) | 1 (1.9) |
| Australia | 3 (8.8) | 2 (10.0) | 5 (9.3) |
| Brazil | 2 (5.9) | 1 (5.0) | 3 (5.6) |
| Singapore | 1 (2.9) | 0 (0.0) | 1 (1.9) |
| Iran | 1 (2.9) | 0 (0.0) | 1 (1.9) |
| Diagnostic criteria, no. (%) | |||
| DSM-IV | 23 (67.6) | 4 (20.0) | 27 (50.0) |
| DSM-III-R | 9 (26.5) | 6 (30.0) | 15 (27.8) |
| DSM-III | 0 (0.0) | 7 (35.0) | 7 (13.0) |
| ICD-10 | 1 (2.9) | 1 (5.0) | 2 (3.7) |
| Not specified | 1 (2.9) | 2 (10.0) | 3 (5.6) |
| Outpatient settingb | 33 (97.1) | 20 (100.0) | 53 (98.1) |
| Single site | 22 (64.7) | 3 (15.0) | 25 (46.3) |
| All participants with comorbid conditions | 5 (14.7) | 0 (0.0) | 5 (9.3) |
| Behavioral platformc | |||
| High intensity | 22 (64.7) | 4 (20.0) | 26 (48.1) |
| Low intensity | 12 (35.3) | 11 (55.0) | 23 (42.6) |
| Not specified | 0 (0.0) | 5 (25.0) | 5 (9.3) |
| Endpoints reported, no. (%) | |||
| Percent days abstinent | 23 (67.6) | 15 (75.0) | 38 (70.4) |
| Total abstinence | 24 (70.6) | 20 (100.0) | 44 (81.5) |
| Percent days without heavy drinking | 16 (47.1) | -- | -- |
| Abstinence from heavy drinking | 27 (79.4) | -- | -- |
| Participant characteristics | |||
| Total participants | 4878 | 5514 | 10392 |
| Age, year, mean (SD) | 44.4 (9.1) | 43.0 (9.5) | 43.7 (9.3) |
| % Male, mean (SD) | 77.7 (16.9) | 79.0 (6.6) | 78.3 (12.5) |
| % Married, mean (SD)d | 42.8 (13.8) | 56.9 (11.2) | 49.9 (14.4) |
| % Employed, mean (SD)e | 67.6 (17.3) | 64.6 (7.9) | 66.1 (13.5) |
no., number; SD, standard deviation.
Each of three trials involving both naltrexone and acamprosate treatment arms was treated as two separate trials in the analysis.
1 naltrexone trial and 3 acamprosate trials indicated that the medication treatment was initiated before the patients were discharged from the inpatient care.
High intensity behavioral platform included cognition/skills therapies and 12-step facilitation, and low intensity behavioral platform included motivational therapies, compliance based therapies, and treatment as usual.
Data missing from 7 naltrexone trials and 7 acamprosate trials.
Data missing from 12 naltrexone trials and 12 acamprosate trials.
Placebo Response and Treatment Effect Size
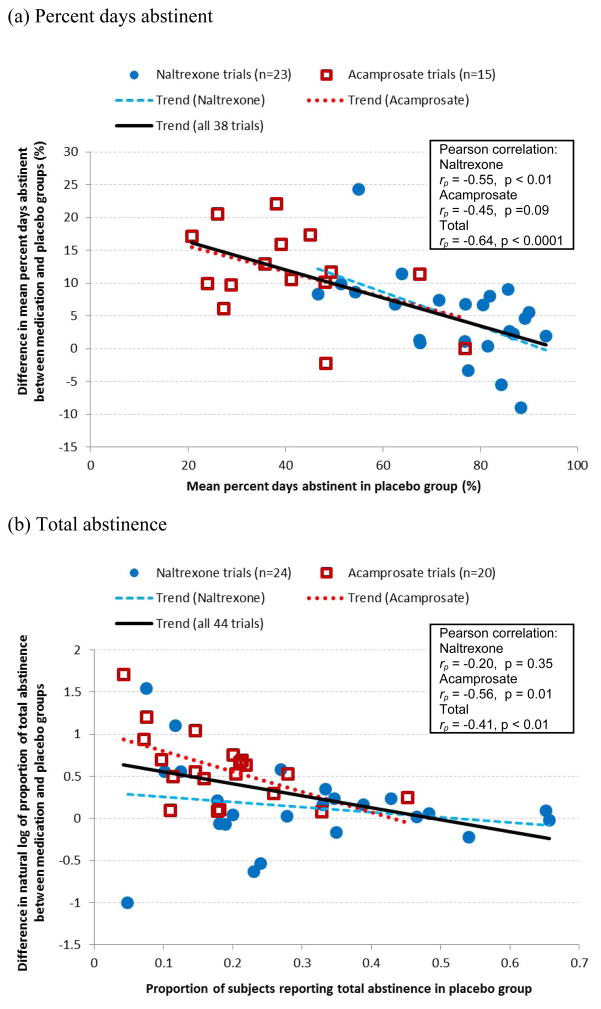
For both endpoint measures—percent days abstinent and total abstinence—a significant negative relationship was found between placebo response and the treatment effect size (Figures 1a and 1b). For percent days abstinent, the negative correlation was strong (rp = −0.64) and highly significant (p < 0.0001) when averaged across the 38 trials that used this endpoint (Figure 1a). Of these trials, the negative correlation remained strong and significant for the 23 trials that looked at naltrexone alone (rp = −0.55, p < 0.01) but was not significant for the 15 trials that examined acamprosate alone (rp = −0.45, p = 0.09). Similarly, a significant negative correlation was observed for total abstinence across the 44 naltrexone and acamprosate trials (rp = −0.41, p < 0.01) (Figure 1b). The negative correlation was not significant for the 24 naltrexone trials alone (rp = −0.20, p = 0.35) but was strong and significant for the 20 acamprosate trials (rp = −0.56, p = 0.01). Upon visual inspection of Figure 1b, one point at the bottom left (i.e., a naltrexone study with a small placebo response and small effect size) appeared to be an outlier and may have weakened the relationship observed in the naltrexone trials. Exclusion of this study (Balldin et al., 2003) substantially increased the magnitude and significance of the correlation from −0.20 (p = 0.35) to −0.41 (p = 0.05).
Figure 1.
Relationships between placebo response (x–axis) and treatment effect size (y-axis) for endpoints (a) percent days abstinent and (b) total abstinence in naltrexone and acamprosate trials.
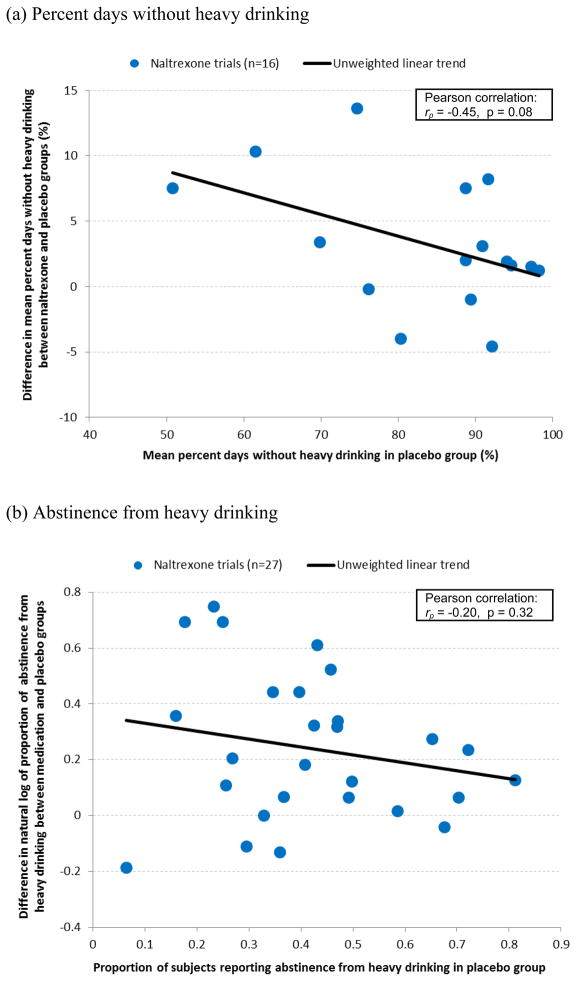
Two other commonly used drinking endpoints in the naltrexone trials—percent days without heavy drinking and abstinence from heavy drinking—were examined to determine the relationship between the placebo response and treatment effect size. For percent days without heavy drinking, the correlation was negative with a nonsignificant trend (rp = −0.45, p = 0.08) across the 16 naltrexone trials (Figure 2a). For abstinence from heavy drinking, the correlation was smaller (rp = −0.20) and not significant (p = 0.32) across the 27 naltrexone trials (Figure 2b). Upon visual inspection of Figure 2b, one point at the bottom left, i.e., the Balldin et al. (2003) study, again appeared to be an outlier and weakened the relationship. Excluding this study increased the magnitude of the correlation, which then nearly reached statistical significance (rp = −0.37, p = 0.06).
Figure 2.
Relationships between placebo response (x-axis) and treatment effect size (y-axis) for endpoints (a) percent days without heavy drinking and (b) abstinence from heavy drinking in naltrexone trials.
Study Characteristics and Placebo Response
The correlation of different study characteristics with the placebo response was examined using percent days abstinent as the endpoint because it was commonly measured across both the naltrexone and acamprosate studies and had the strongest correlation between the placebo response and the treatment effect size among reported endpoints. Because the naltrexone and acamprosate trials differed substantially in terms of their placebo response (naltrexone trials: median [range] = 77.5% [46.7% – 93.5%]; acamprosate trials: 39.1% [20.8% – 76.1%]), study designs, and patient populations (as indicated in Table 1), we examined the correlations separately by medication type in an attempt to avoid the confounding effects between medication type and study characteristics.
For the 23 naltrexone trials, greater placebo response was significantly associated with younger mean age of participants (rs = −0.42, p = 0.05) (Table 2). For the 15 acamprosate trials, greater placebo response was significantly associated with later publication years (rs = 0.57, p = 0.03) (Table 2). Duration of treatment, percentage of male participants, and number of sites were not significantly correlated with placebo response in either naltrexone or acamprosate studies. None of the study characteristics assessed were significantly associated with effect size (Table 3).
Table 2.
Spearman’s Rank Correlation Between Study Characteristics and Placebo Response Using the Endpoint Percent Days Abstinent.
| Naltrexone (n = 23) | Acamprosate (n = 15) | |||
|---|---|---|---|---|
|
| ||||
| Characteristics | rs | p-value | rs | p-value |
| Duration of treatment (weeks) | −0.12 | 0.59 | −0.37 | 0.17 |
| Publication year | −0.18 | 0.41 | 0.57 | 0.03 |
| Mean age of participants | −0.42 | 0.05 | 0.39 | 0.15 |
| % Male | −0.04 | 0.85 | −0.12 | 0.67 |
| Number of sites | −0.22 | 0.31 | 0.35 | 0.20 |
Note: each of two trials involving both naltrexone and acamprosate treatment arms was treated as two separate trials in the analysis.
Table 3.
Spearman’s Rank Correlation Between Study Characteristics and Treatment Effect Size Using the Endpoint Percent Days Abstinent.
| Naltrexone (n = 23)
|
Acamprosate (n = 15)
|
|||
|---|---|---|---|---|
| Characteristics | rs | p-value | rs | p-value |
| Duration of treatment (weeks) | 0.01 | 0.96 | 0.06 | 0.83 |
| Publication year | −0.17 | 0.44 | −0.20 | 0.47 |
| Mean age of participants | 0.20 | 0.36 | −0.05 | 0.85 |
| % Male | 0.29 | 0.18 | 0.25 | 0.37 |
| Number of sites | 0.11 | 0.60 | −0.33 | 0.23 |
Note: each of two trials involving both naltrexone and acamprosate treatment arms was treated as two separate trials in the analysis.
Sensitivity analyses were conducted to assess the relationships between placebo response and a) effect size and b) study characteristics, while excluding the 5 naltrexone trials where all patients had comorbid conditions. The results of the sensitivity analyses were generally similar to the original results. In other words, the results are not sensitive to the inclusion of the studies with comorbid patients.
Discussion
The placebo effect often is evident in alcohol trials yet, to date, it has not been well characterized (Weiss et al., 2008). In this paper, we took a first step to better understand this effect by conducting a systematic review of clinical trials examining the use of naltrexone and acamprosate for the treatment of alcohol dependence. We found a negative correlation between the placebo response and the treatment effect size. This finding is similar to that observed across depression and schizophrenia medication trials (Kemp et al., 2010; Khan et al., 2003; Khin et al., 2011; Kirsch et al., 2008; Mallinckrodt et al., 2010; Nunes and Levin, 2004). Moreover, in this study, the magnitude of the correlation varied according to the specific endpoint used. For example, among the naltrexone studies, the correlations (rp) were −0.55, −0.45, −0.20, and −0.20 for percent days abstinent, percent days without heavy drinking, total abstinence, and abstinence from heavy drinking endpoints, respectively. Differences in the magnitude of correlations among the endpoints may be explained, in part, by the different range or variability of the endpoint measures.
These results suggest that it may be more difficult to demonstrate an experimental medication’s efficacy in studies that have higher placebo responses. What are some of the reasons that may explain this observation? First, for the outcomes assessed in this study, there is a ceiling (e.g., 100% being the maximum value of percent days abstinent) that limits the maximal treatment effect that can be observed for any given placebo response level, thereby limiting the degree to which the experimental medication can show an improvement over the placebo. This was most pronounced with very high placebo responses (e.g., percent days abstinent close to 100%) where little room exists for a medication to cause additional improvement. Second, the placebo may be utilizing the same or similar biological mechanism of action as the experimental medication. This phenomenon has been observed for numerous medical disorders (Finniss et al., 2010). For example, in analgesia studies, the placebo was shown to activate the opioid receptors, a mechanism of action similar to that used by analgesia medicines to reduce pain (Finniss et al., 2010). Essentially, the greater the placebo response, the less likely the medication will show significant efficacy.
The differences found in the placebo response may be attributed to numerous factors. In examining study characteristics, we found that as the publication year of the acamprosate studies increased from 1985 to 2011, the placebo response also significantly increased. This pattern was similar to that reported in analyses of depression and schizophrenia studies (Kemp et al., 2010; Khin et al., 2011; Papakostas and Fava, 2009; Walsh et al., 2002). For example, across 81 depression trials, Khin et al. (2011) reported a modest increase in placebo response from 1983 to 2008. Similarly, Kemp et al. (2010) reported higher placebo response across 10 schizophrenia trials from 1993 to 2006. One possible underlying explanation for this trend, at least in the depression trials, is that the severity of depression in patients being recruited for these trials has decreased over the past 20 years and baseline depression scores have been shown to be inversely correlated with placebo response in several meta-analyses (Bridge et al., 2009; Khin et al., 2011; Kirsch et al., 2008; Papakostas and Fava, 2009). It is unclear if a similar trend exists in clinical trials for alcohol dependence. In our preliminary analyses, we attempted to capture severity using different measures of pre-baseline drinking (e.g., percent days drinking and number of drinks per drinking day) and level of dependence (e.g., the Alcohol Dependence Scale [Skinner and Allen, 1982] and Michigan Alcohol Screening Test [Selzer, 1971]) and to correlate these with the placebo response found in the naltrexone and acamprosate studies. Unfortunately, the resulting number of studies that reported these measures was too small to produce reliable estimates. More studies reporting common measures of severity are needed for addressing the influence of pre-baseline severity on placebo response across alcohol trials.
Another possible explanation for the increased placebo response over the years is that more recently published acamprosate trials had a shorter duration of treatment (mean = 35 weeks for the studies published before 2000 vs. 20 weeks for the studies published later). The correlation observed between placebo response and publication year can be partly driven by the negative correlation between placebo response and duration of treatment (rs = −0.37, p = 0.17) and the inter-correlation between publication year and duration of treatment (rs = −0.47, p = 0.07). The reason for the decreased placebo response in longer trials may be because the novelty of participating in the trial begins to subside or simply due to an increased risk of relapse with longer study times. Still, this association was not as clearly observed across depression trials and appears to be unique for alcohol medication studies. It may be due to the fact that the longest depression trials tended to be much shorter in duration than the longest alcohol trials (eight weeks for depression trials versus one year for alcohol trials [Khin et al., 2011; Papakostas and Fava, 2009]).
In addition to the year of publication, we found that studies with older participants tended to have decreased placebo response among naltrexone trials (Table 2). In contrast, studies of older participants in depression studies, displayed a slightly increased placebo response (Walsh and Sysko, 2005). Overall, in our study, the year of publication and age of participants did not significantly affect the effect size because the correlation patterns observed in the placebo group were also seen in the treatment group. This indicates that although these two factors influenced the placebo response, they did not have a significant impact on the effect size.
Interestingly, we did not find a significant relationship between placebo response and the number of sites, although opposite directions of correlations—negative in naltrexone studies and positive in acamprosate studies—were observed (Table 2). In addition, there was no significant correlation between the number of sites and the effect size (Table 3). In an earlier study, Feinn and Kranzler (2005) reported a larger naltrexone effect size in single site versus multisite alcohol trials, whereas Del Re et al. (2013) recently reported no difference. In depression trials, the placebo response increased and the medication-placebo difference decreased with the addition of more sites (Bridge et al., 2009; Undurraga and Baldessarini, 2012).
Other study characteristics shown to be related to the placebo response across depression trials were, unfortunately, not consistently measured or did not have sufficient variability across the alcohol trials and therefore could not be analyzed in the present study. Some study characteristics previously reported to influence the placebo response across depression and schizophrenic studies include severity of the disease, centralized versus site-based assessments, and the number of treatment arms included in a trial (Kirsch et al., 2008; Kobak et al., 2010; Mallinckrodt et al., 2010; Mallinckrodt et al., 2011; Sinyor et al., 2010).
The current study had several limitations. First, there may be any number of unmeasured or unanalyzed factors that could have affected placebo responses across the studies. These include variations in sampled subjects (e.g., race/ethnicity, socioeconomic status, culture), in physician and research staff characteristics, or in study design (e.g., number of assessments, type of behavioral platform, and number of study visits/contact). Second, the relationship between individual study characteristics and the placebo response is confounded by the potential interrelatedness among the study characteristics. Unfortunately, the limited sample size precluded the use of multivariate models to analyze the unique effect of each of these variables on placebo response after taking into account their inter-correlations. Third, our analysis was restricted to studies that required abstinence before randomization because we wanted to know the exact change of placebo response from randomization to treatment. As a result, 6 naltrexone and 2 acamprosate trials that allowed drinking up to randomization were not included in our analysis. Those trials did not report their measurements of drinking at randomization and thus the change in placebo response could not be accurately determined. Unfortunately, there were not enough studies that allowed drinking up to randomization for further examination. Finally, our analysis did not take into account the various methods used in the individual studies for handling missing outcome data, a factor that presumably has a direct effect on placebo response and effect size. For example, if missing data on drinking are treated as a relapse to heavy drinking, this would result in a decreased placebo response with regard to abstinence from heavy drinking. However, few studies handled missing data in the same way, making it impossible to evaluate this effect. This might be better addressed by analyses of simulated incomplete data for individual patients pooled from different studies (Lane, 2008; Siddiqui, 2011; Siddiqui et al., 2009). Then different handling methods could be applied to examine the magnitude of changes in the results.
A number of strategies have been developed to control or minimize the placebo response in clinical trials to better detect the efficacy of drug treatment. These include the use of placebo lead-ins, in which subjects are provided with the placebo in advance of study period; sequential parallel comparison design, which involves two phases of treatment, with non-responders to the placebo during Phase 1 included in the efficacy analysis of Phase 2; and elimination of extreme placebo groups, that is, those responders at both the very high and low ends in the placebo groups (Re et al., 2013; Faries et al., 2001; Fava et al., 2003; Merlo-Pich et al., 2010). To date, few of these strategies have been adopted in today’s alcohol trials. These strategies may help to enhance the medication-placebo difference by minimizing the placebo response. Still, given the complex nature of the placebo, it is unlikely that any single approach can completely reduce untoward placebo effects. In this analysis, we have only begun to understand the placebo response in alcohol trials. Additional studies are needed to comprehensively evaluate different approaches to minimize the placebo effect and thereby improve our ability to detect the true treatment effects in alcohol trials.
Supplementary Material
Acknowledgments
This research was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institutes of Health, Department of Health and Human Services, Bethesda, MD and by the Medications Development Program Analytic Contract funded by NIAAA Contract No. HHSN275201100002G to CSR, Incorporated, Arlington, VA. The authors thank Barbara Vann (CSR, Incorporated) for her excellent editorial comments, Robin A. LaVallee (CSR, Incorporated) for data entry, and Drs. Patrick K. Randall and Raymond F. Anton (Medical University of South Carolina, Charleston, SC, USA), Drs. William D. Dundon and Helen M. Pettinati (University of Pennsylvania School of Medicine, Philadelphia, PA, USA), and Dr. Wolfgang Wölwer (Heinrich Heine University, Düsseldorf, Germany) for providing their unpublished results.
Footnotes
Disclaimers
The views and opinions expressed in this paper are those of the authors and should not be construed to represent the views of the sponsoring agency or the Federal government.
References
- Ahmadi J, Ahmadi N. A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol dependence. German J Psychiatry. 2002;5:85–89. [Google Scholar]
- American Psychiatric Association. Diagnotic and Statistical Manual of Mental Disorders. 3. American Psychiatric Association; Washington, DC: 1980. [Google Scholar]
- American Psychiatric Association. Diagnotic and Statistical Manual of Mental Disorders. 3. American Psychiatric Association; Washington, DC: 1987. revised. [Google Scholar]
- American Psychiatric Association. Diagnotic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington DC: 1994. [Google Scholar]
- Anton RF, Moak DH, Latham P, Waid LR, Myrick H, Voronin K, Thevos A, Wang W, Woolson R. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol. 2005;25:349–357. doi: 10.1097/01.jcp.0000172071.81258.04. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999;156:1758–1764. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- Anton RF, Myrick H, Wright TM, Latham PK, Baros AM, Waid LR, Randall PK. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168:709–717. doi: 10.1176/appi.ajp.2011.10101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA: the journal of the American Medical Association. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Baker WL, White CM, Cappelleri JC, Kluger J, Coleman CI. Understanding heterogeneity in meta-analysis: the role of meta-regression. Int J Clin Pract. 2009;63:1426–1434. doi: 10.1111/j.1742-1241.2009.02168.x. [DOI] [PubMed] [Google Scholar]
- Balldin J, Berglund M, Borg S, Mansson M, Bendtsen P, Franck J, Gustafsson L, Halldin J, Nilsson LH, Stolt G, Willander A. A 6-month controlled naltrexone study: combined effect with cognitive behavioral therapy in outpatient treatment of alcohol dependence. Alcohol Clin Exp Res. 2003;27:1142–1149. doi: 10.1097/01.ALC.0000075548.83053.A9. [DOI] [PubMed] [Google Scholar]
- Baltieri DA, Daro FR, Ribeiro PL, de Andrade AG. Comparing topiramate with naltrexone in the treatment of alcohol dependence. Addiction. 2008;103:2035–2044. doi: 10.1111/j.1360-0443.2008.02355.x. [DOI] [PubMed] [Google Scholar]
- Baltieri DA, De Andrade AG. Acamprosate in alcohol dependence: a randomized controlled efficacy study in a standard clinical setting. J Stud Alcohol. 2004;65:136–139. doi: 10.15288/jsa.2004.65.136. [DOI] [PubMed] [Google Scholar]
- Barrias J, Chabac S, Ferreira L, Fonte A, Potgieter AS, Teixeira de Sousa E. Acamprosate: multicenter Portuguese efficacy and tolerance evaluation study. Psiquiatria Clinica. 1997;18:149–160. [Google Scholar]
- Besson J, Aeby F, Kasas A, Lehert P, Potgieter A. Combined efficacy of acamprosate and disulfiram in the treatment of alcoholism: a controlled study. Alcohol Clin Exp Res. 1998;22:573–579. doi: 10.1111/j.1530-0277.1998.tb04295.x. [DOI] [PubMed] [Google Scholar]
- Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA. Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry. 2009;166:42–49. doi: 10.1176/appi.ajp.2008.08020247. [DOI] [PubMed] [Google Scholar]
- Chick J, Anton R, Checinski K, Croop R, Drummond DC, Farmer R, Labriola D, Marshall J, Moncrieff J, Morgan MY, Peters T, Ritson B. A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcohol. 2000a;35:587–593. doi: 10.1093/alcalc/35.6.587. [DOI] [PubMed] [Google Scholar]
- Chick J, Howlett H, Morgan MY, Ritson B. United Kingdom Multicentre Acamprosate Study (UKMAS): a 6-month prospective study of acamprosate versus placebo in preventing relapse after withdrawal from alcohol. Alcohol Alcohol. 2000b;35:176–187. doi: 10.1093/alcalc/35.2.176. [DOI] [PubMed] [Google Scholar]
- de Goes e Castro LA. Unpublished dissertation. Federal University of Sao Paulo; 2004. Randomized, double-blind clinical trial with naltrexone and brief therapy for the in-patient treatment of alcohol dependence [Ensaio Clinico duplo-cego randomizado e placebo-controlado com naltrexona associado a intervencao breve no treatmento ambulatorial da dependencia alcohol] [Google Scholar]
- Del Re AC, Maisel N, Blodgett J, Finney J. The declining efficacy of naltrexone pharmacotherapy for alcohol use disorders over time: A multivariate meta-analysis. Alcohol Clin Exp Res. 2013 doi: 10.1111/acer.12067. on line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faries DE, Heiligenstein JH, Tollefson GD, Potter WZ. The double-blind variable placebo lead-in period: results from two antidepressant clinical trials. J Clin Psychopharmacol. 2001;21:561–568. doi: 10.1097/00004714-200112000-00004. [DOI] [PubMed] [Google Scholar]
- Fava M, Evins AE, Dorer DJ, Schoenfeld DA. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother Psychosom. 2003;72:115–127. doi: 10.1159/000069738. [DOI] [PubMed] [Google Scholar]
- Feinn R, Kranzler HR. Does effect size in naltrexone trials for alcohol dependence differ for single-site vs multi-center studies? Alcohol Clin Exp Res. 2005;29:983–988. doi: 10.1097/01.alc.0000171061.03686.bc. [DOI] [PubMed] [Google Scholar]
- Fertig JB, Ryan ML, Falk DE, Litten RZ, Mattson ME, Ransom J, Rickman WJ, Scott C, Ciraulo D, Green AI, Tioririne NA, Johnson BA, Pettinati H, Strain EC, Devine E, Brunette MF, Kampman K, Tompkins DA, Stout R. A double-blind, placebo-controlled trial assessing the effiacy of levetiracetam extended-release in very heavy drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36:1421–1430. doi: 10.1111/j.1530-0277.2011.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375:686–695. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastpar M, Bonnet U, Boning J, Mann K, Schmidt LG, Soyka M, Wetterling T, Kielstein V, Labriola D, Croop R. Lack of efficacy of naltrexone in the prevention of alcohol relapse: results from a German multicenter study. J Clin Psychopharmacol. 2002;22:592–598. doi: 10.1097/00004714-200212000-00009. [DOI] [PubMed] [Google Scholar]
- Geerlings PJ, Ansoms C, van den Brink W. Acamprosate and prevention of relapse in alcoholics. Results of a randomised, placebo-controlled, double-blind study in out-patient alcoholics in the Netherlands, Belgium and Luxembourg. Eur Addict Res. 1997;3:129–137. [Google Scholar]
- Gual A, Lehert P. Acamprosate during and after acute alcohol withdrawal: a double-blind placebo-controlled study in Spain. Alcohol Alcohol. 2001;36:413–418. doi: 10.1093/alcalc/36.5.413. [DOI] [PubMed] [Google Scholar]
- Guardia J, Caso C, Arias F, Gual A, Sanahuja J, Ramirez M, Mengual I, Gonzalvo B, Segura L, Trujols J, Casas M. A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol-dependence disorder: results from a multicenter clinical trial. Alcohol Clin Exp Res. 2002;26:1381–1387. doi: 10.1097/01.ALC.0000030561.15921.A9. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Aubin HJ, Van Den Brink W, Guzzetta R, Loewy J, Silverman B, Ehrich E. A pilot evaluation of the safety and tolerability of repeat dose administration of long-acting injectable naltrexone (Vivitrex) in patients with alcohol dependence. Alcohol Clin Exp Res. 2004;28:1356–1361. doi: 10.1097/01.alc.0000139823.30096.52. [DOI] [PubMed] [Google Scholar]
- Kaptchuk TJ, Kelley JM, Conboy LA, Davis RB, Kerr CE, Jacobson EE, Kirsch I, Schyner RN, Nam BH, Nguyen LT, Park M, Rivers AL, McManus C, Kokkotou E, Drossman DA, Goldman P, Lembo AJ. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336:999–1003. doi: 10.1136/bmj.39524.439618.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AS, Schooler NR, Kalali AH, Alphs L, Anand R, Awad G, Davidson M, Dube S, Ereshefsky L, Gharabawi G, Leon AC, Lepine JP, Potkin SG, Vermeulen A. What is causing the reduced drug-placebo difference in recent schizophrenia clinical trials and what can be done about it? Schizophr Bull. 2010;36:504–509. doi: 10.1093/schbul/sbn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Detke M, Khan SR, Mallinckrodt C. Placebo response and antidepressant clinical trial outcome. J Nerv Ment Dis. 2003;191:211–218. doi: 10.1097/01.NMD.0000061144.16176.38. [DOI] [PubMed] [Google Scholar]
- Khin NA, Chen YF, Yang Y, Yang P, Laughren TP. Exploratory analyses of efficacy data from major depressive disorder trials submitted to the US Food and Drug Administration in support of new drug applications. J Clin Psy. 2011;72:464–472. doi: 10.4088/JCP.10m06191. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Tarnaske T, Helwig H, Briken P, Holzbach R, Kampf P, Stracke R, Baehr M, Naber D, Wiedemann K. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2003;60:92–99. doi: 10.1001/archpsyc.60.1.92. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobak KA, Leuchter A, DeBrota D, Engelhardt N, Williams JB, Cook IA, Leon AC, Alpert J. Site versus centralized raters in a clinical depression trial: impact on patient selection and placebo response. J Clin Psychopharmacol. 2010;30:193–197. doi: 10.1097/JCP.0b013e3181d20912. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Gage A. Acamprosate efficacy in alcohol-dependent patients: summary of results from three pivotal trials. Am J Addict. 2008;17:70–76. doi: 10.1080/10550490701756120. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Modesto-Lowe V, Nuwayser ES. Sustained-release naltrexone for alcoholism treatment: a preliminary study. Alcohol Clin Exp Res. 1998;22:1074–1079. [PubMed] [Google Scholar]
- Kranzler HR, Modesto-Lowe V, Van Kirk J. Naltrexone vs. nefazodone for treatment of alcohol dependence. A placebo-controlled trial. Neuropsychopharmacology. 2000;22:493–503. doi: 10.1016/S0893-133X(99)00135-9. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25:1335–1341. [PubMed] [Google Scholar]
- Kranzler HR, Wesson DR, Billot L. Naltrexone depot for treatment of alcohol dependence: a multicenter, randomized, placebo-controlled clinical trial. Alcohol Clin Exp Res. 2004;28:1051–1059. doi: 10.1097/01.alc.0000130804.08397.29. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Ladewig D, Knecht T, Leher P, Fendl A. Acamprosate--a stabilizing factor in long-term withdrawal of alcoholic patients. Ther Umsch. 1993;50:182–188. [PubMed] [Google Scholar]
- Lane P. Handling drop-out in longitudinal clinical trials: A comparison of the LOCF and MMRM approaches. Pharm Stat. 2008;7:93–106. doi: 10.1002/pst.267. [DOI] [PubMed] [Google Scholar]
- Latt NC, Jurd S, Houseman J, Wutzke SE. Naltrexone in alcohol dependence: a randomised controlled trial of effectiveness in a standard clinical setting. Med J Aust. 2002;176:530–534. doi: 10.5694/j.1326-5377.2002.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Lee A, Tan S, Lim D, Winslow RM, Wong KE, Allen J, Hall W, Parker G. Naltrexone in the treatment of male alcoholics—an effectiveness study in Singapore. Drug Alcohol Rev. 2001;20:193–199. [Google Scholar]
- Lee S, Malhotra B, Creanga D, Carlsson M, Glue P. A meta-analysis of the placebo response in antimuscarinic drug trials for overactive bladder. BMC Med Res Methodol. 2009;9:55. doi: 10.1186/1471-2288-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhuintre JP, Daoust M, Moore ND, Chretien P, Saligaut C, Tran G, Bosimare F, Hillemand B. Ability of calcium bis acetyl homotaurine, a GABA agonist, to prevent relapse in weaned alcoholics. Lancet. 1985;1:1014–1016. doi: 10.1016/s0140-6736(85)91615-0. [DOI] [PubMed] [Google Scholar]
- Lhuintre JP, Moore N, Tran G, Steru L, Langrenon S, Daoust M, Parot P, Ladure P, Libert C, Boismare F, et al. Acamprosate appears to decrease alcohol intake in weaned alcoholics. Alcohol Alcohol. 1990;25:613–622. doi: 10.1093/oxfordjournals.alcalc.a045057. [DOI] [PubMed] [Google Scholar]
- Lidstone SC, Schulzer M, Dinelle K, Mak E, Sossi V, Ruth TJ, de la Fuente-Fernandez R, Phillips AG, Stoessl AJ. Effects of expectation on placebo-induced dopamine release in Parkinson disease. Arch Gen Psychiatry. 2010;67:857–865. doi: 10.1001/archgenpsychiatry.2010.88. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Fertig JB, Falk DE, Ryan ML, Mattson ME, Collins JF, Murtaugh C, Ciraulo D, Green AI, Johnson B, Pettinati H, Swift R, Afshar M, Brunette MF, Tiouririne NAD, Kampman K, Stout R. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36:406–416. doi: 10.1111/j.1530-0277.2011.01649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275–93. doi: 10.1111/j.1360-0443.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallinckrodt CH, Tamura RN, Tanaka Y. Recent developments in improving signal detection and reducing placebo response in psychiatric clinical trials. J Psychi Res. 2011;45:1202–1207. doi: 10.1016/j.jpsychires.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Mallinckrodt CH, Zhang L, Prucka WR, Millen BA. Signal detection and placebo response in schizophrenia: parallels with depression. Psychopharmacol Bull. 2010;43:53–72. [PubMed] [Google Scholar]
- Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28:51–63. doi: 10.1097/01.ALC.0000108656.81563.05. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Lehert P. Acamprosate for alcohol dependence: a sex-specific meta-analysis based on individual patient data. Alcohol Clin Exp Res. 2012;36:497–508. doi: 10.1111/j.1530-0277.2011.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo-Pich E, Alexander RC, Fava M, Gomeni R. A new population-enrichment strategy to improve efficiency of placebo-controlled clinical trials of antidepressant drugs. Clin Pharmaco Ther. 2010;88:634–642. doi: 10.1038/clpt.2010.159. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Swift RM, Gulliver SB, Colby SM, Mueller TI, Brown RA, Gordon A, Abrams DB, Niaura RS, Asher MK. Naltrexone and cue exposure with coping and communication skills training for alcoholics: treatment process and 1-year outcomes. Alcohol Clin Exp Res. 2001;25:1634–1647. [PubMed] [Google Scholar]
- Morley KC, Teesson M, Reid SC, Sannibale C, Thomson C, Phung N, Weltman M, Bell JR, Richardson K, Haber PS. Naltrexone versus acamprosate in the treatment of alcohol dependence: A multi-centre, randomized, double-blind, placebo-controlled trial. Addiction. 2006;101:1451–1462. doi: 10.1111/j.1360-0443.2006.01555.x. [DOI] [PubMed] [Google Scholar]
- Morris PL, Hopwood M, Whelan G, Gardiner J, Drummond E. Naltrexone for alcohol dependence: a randomized controlled trial. Addiction. 2001;96:1565–1573. doi: 10.1046/j.1360-0443.2001.961115654.x. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. 2004;291:1887–1896. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Robin RW, Levenson AL, GreyWolf I, Chance LE, Hodgkinson CA, Romano D, Robinson J, Meandzija B, Stillner V, Wu R, Goldman D. Naltrexone alone and with sertraline for the treatment of alcohol dependence in Alaska natives and non-natives residing in rural settings: a randomized controlled trial. Alcohol Clin Exp Res. 2008;32:1271–1283. doi: 10.1111/j.1530-0277.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Sinha R, Grilo CM, Capone C, Farren CK, McKee SA, Rounsaville BJ, Wu R. Naltrexone and cognitive behavioral coping skills therapy for the treatment of alcohol drinking and eating disorder features in alcohol-dependent women: a randomized controlled trial. Alcohol Clin Exp Res. 2007;31:625–634. doi: 10.1111/j.1530-0277.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Oslin D, Liberto JG, O’Brien J, Krois S, Norbeck J. Naltrexone as an adjunctive treatment for older patients with alcohol dependence. Am J Geriatr Psychiatry. 1997;5:324–332. doi: 10.1097/00019442-199700540-00007. [DOI] [PubMed] [Google Scholar]
- Oslin DW. Treatment of late-life depression complicated by alcohol dependence. Am J Geriatr Psychiatry. 2005;13:491–500. doi: 10.1176/appi.ajgp.13.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin DW, Lynch KG, Pettinati HM, Kampman KM, Gariti P, Gelfand L, Ten Have T, Wortman S, Dundon W, Dackis C, Volpicelli JR, O’Brien CP. A placebo-controlled randomized clinical trial of naltrexone in the context of different levels of psychosocial intervention. Alcohol Clin Exp Res. 2008;32:1299–1308. doi: 10.1111/j.1530-0277.2008.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paille FM, Guelfi JD, Perkins AC, Royer RJ, Steru L, Parot P. Double-blind randomized multicentre trial of acamprosate in maintaining abstinence from alcohol. Alcohol Alcohol. 1995;30:239–247. [PubMed] [Google Scholar]
- Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol. 2009;19:34–40. doi: 10.1016/j.euroneuro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Pelc I, Le Bon O, Verbanck P, Lehert P, Opsomer L. Calcium-acetylhomotaurinate for maintaining abstinence in weaned alcoholic patients: A placebo-controlled double-blind multicenter study. In: Naranjo C, Sellers E, editors. Novel Pharmacological Interventions for Alcoholism. Springer; New York: 1992. pp. 348–352. [Google Scholar]
- Pelc I, Verbanck P, Le Bon O, Gavrilovic M, Lion K, Lehert P. Efficacy and safety of acamprosate in the treatment of detoxified alcohol-dependent patients. A 90-day placebo-controlled dose-finding study. Br J Psychiatry. 1997;171:73–77. doi: 10.1192/bjp.171.1.73. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, O’Malley S, Rounsaville B, Poling J, McHugh-Strong C, Krystal JH. Naltrexone augmentation of neuroleptic treatment in alcohol abusing patients with schizophrenia. Psychopharmacology (Berl) 2004;172:291–297. doi: 10.1007/s00213-003-1658-9. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Rounsaville B. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biol Psychiatry. 2005;57:1128–1137. doi: 10.1016/j.biopsych.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Kampman KM, Lynch KG, Suh JJ, Dackis CA, Oslin DW, O’Brien CP. Gender differences with high-dose naltrexone in patients with co-occurring cocaine and alcohol dependence. J Subst Abuse Treat. 2008;34:378–390. doi: 10.1016/j.jsat.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, Oslin DW, Kampman KM, Dundon WD, Xie H, Gallis TL, Dackis CA, O’Brien CP. A double-blind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. Am J Psychiatry. 2010;167:668–675. doi: 10.1176/appi.ajp.2009.08060852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrugo F. Acamprosate treatment in a long-term community-based alcohol rehabilitation programme. Addiction. 1997;92:1537–1546. [PubMed] [Google Scholar]
- Rösner S, Hackl-Herrwerth A, Leucht S, Lehert P, Vecchi S, Soyka M. Acamprosate for alcohol dependence. Cochrane Database Syst Rev. 2010a:CD004332. doi: 10.1002/14651858.CD004332.pub2. [DOI] [PubMed] [Google Scholar]
- Rösner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2010b:CD001867. doi: 10.1002/14651858.CD001867.pub3. [DOI] [PubMed] [Google Scholar]
- Sass H, Soyka M, Mann K, Zieglgansberger W. Relapse prevention by acamprosate. Results from a placebo-controlled study on alcohol dependence. Arch Gen Psychiatry. 1996;53:673–680. doi: 10.1001/archpsyc.1996.01830080023006. [DOI] [PubMed] [Google Scholar]
- Selzer ML. The Michigan Alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Siddiqui O. MMRM versus MI in Dealing with Missing Data—A Comparison Based on 25 NDA Data Sets. J Biopharm Stat. 2011;21:423–436. doi: 10.1080/10543401003777995. [DOI] [PubMed] [Google Scholar]
- Siddiqui O, Hung HMJ, O’Neill R. MMRM vs. LOCF: A Comprehensive Comparison Based on Simulation Study and 25 NDA Datasets. J Biopharm Stat. 2009;19:227–246. doi: 10.1080/10543400802609797. [DOI] [PubMed] [Google Scholar]
- Sinyor M, Levitt AJ, Cheung AH, Schaffer A, Kiss A, Dowlati Y, Lanctot KL. Does inclusion of a placebo arm influence response to active antidepressant treatment in randomized controlled trials? Results from pooled and meta-analyses. J Clin Psychiatry. 2010;71:270–279. doi: 10.4088/JCP.08r04516blu. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psych. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8:267–280. doi: 10.1017/S1461145704004997. [DOI] [PubMed] [Google Scholar]
- Streeton C, Whelan G. Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: a meta-analysis of randomized controlled trials. Alcohol Alcohol. 2001;36:544–552. doi: 10.1093/alcalc/36.6.544. [DOI] [PubMed] [Google Scholar]
- Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for Meta-Analysis in Medical Research. John Wiley & Sons, Ltd; West Sussex, England: 2000. [Google Scholar]
- Tempesta E, Janiri L, Bignamini A, Chabac S, Potgieter A. Acamprosate and relapse prevention in the treatment of alcohol dependence: a placebo-controlled study. Alcohol Alcohol. 2000;35:202–209. doi: 10.1093/alcalc/35.2.202. [DOI] [PubMed] [Google Scholar]
- Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- Undurraga J, Baldessarini RJ. Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology. 2012;37:851–864. doi: 10.1038/npp.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O’Brien CP. Naltrexone and alcohol dependence. Role of subject compliance. Arch Gen Psychiatry. 1997;54:737–742. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Sysko R. Placebo control groups in trials of major depressive disorder among older patients. J Clin Psychopharmacol. 2005;25:S29–33. doi: 10.1097/01.jcp.0000162810.76947.bf. [DOI] [PubMed] [Google Scholar]
- Weiss RD, O’Malley SS, Hosking JD, Locastro JS, Swift R. Do patients with alcohol dependence respond to placebo? Results from the COMBINE Study. J Stud Alcohol Drugs. 2008;69:878–884. doi: 10.15288/jsad.2008.69.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth AB, Fischer F, Lesch OM, Nimmerrichter A, Oberbauer H, Platz T, Potgieter A, Walter H, Fleischhacker WW. Comparison of acamprosate and placebo in long-term treatment of alcohol dependence. Lancet. 1996;347:1438–1442. doi: 10.1016/s0140-6736(96)91682-7. [DOI] [PubMed] [Google Scholar]
- Wilson DB. [Accessed November 9, 2011];Meta-analysis macros for SAS, SPSS, and Stata. 2010 Available at: http://mason.gmu.edu/~dwilsonb/ma.html.
- Wölwer W, Frommann N, Janner M, Franke PE, Scherbaum N, Lieb B, Falkai P, Wobrock T, Kuhlmann T, Radermacher M, Maier W, Schutz C, Ohmann C, Burtscheidt W, Gaebel W. The effects of combined acamprosate and integrative behaviour therapy in the outpatient treatment of alcohol dependence: a randomized controlled trial. Drug Alcohol Depend. 2011;118:417–422. doi: 10.1016/j.drugalcdep.2011.05.001. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.