Abstract
The burgeoning obesity epidemic has placed enormous strains on individual and societal health mandating a careful search for pathogenic factors, including the contributions made by endocrine disrupting chemicals (EDCs). In addition to evidence that some exogenous chemicals have the capacity to modulate classical hormonal signaling axes, there is mounting evidence that several EDCs can also disrupt metabolic pathways and alter energy homeostasis. Adipose tissue appears to be a particularly important target of these metabolic disruptions. A diverse array of compounds has been shown to alter adipocyte differentiation, and several EDCs have been shown to modulate adipocyte physiology, including adipocytic insulin action and adipokine secretion. This rapidly emerging evidence demonstrating that environmental contaminants alter adipocyte function emphasizes the potential role that disruption of adipose physiology by EDCs may play in the global epidemic of metabolic disease. Further work is required to better characterize the molecular targets responsible for mediating the effects of EDCs on adipose tissue. Improved understanding of the precise signaling pathways altered by exposure to environmental contaminants will enhance our understanding of which chemicals pose a threat to metabolic health and how those compounds synergize with lifestyle factors to promote obesity and its associated complications. This knowledge may also improve our capacity to predict which synthetic compounds may alter energy homeostasis before they are released into the environment while also providing critical evidentiary support for efforts to restrict the production and use of chemicals that pose the greatest threat to human metabolic health.
1. The Obesity Epidemic and Endocrine Disruption
The last several decades have witnessed a dramatic deterioration in global metabolic health with the emergence of the obesity and diabetes epidemics. In the United States over two-thirds of the adult population is overweight, with more than a third characterized as obese [1]. Importantly, children are among the groups with the fastest rising rates of obesity [2,3]. The societal burden of obesity and its metabolic complications is significant, with annual obesity-related healthcare costs in the United States estimated at $147 to $210 billion [4]. It is expected that these costs will rise dramatically as the global burden of obesity-related illnesses such as diabetes increase from approximately 371 million individuals worldwide in 2012 to a staggering 552 million people by 2030 [5,6]. With healthcare costs rising unsustainably [7], addressing the myriad factors contributing to the burgeoning metabolic disease epidemic is critical for improving individual health as well as the financial viability of health care systems around the world.
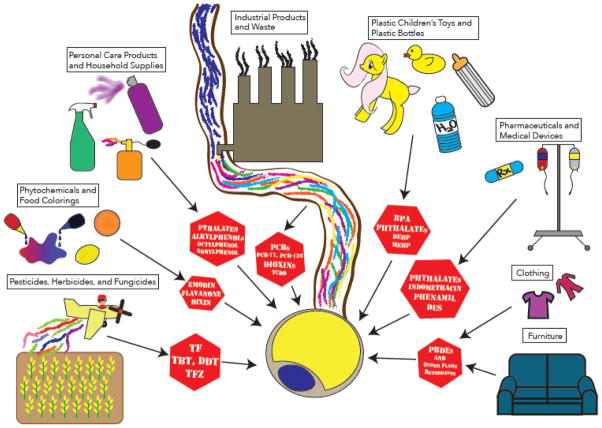
Increased consumption of highly palatable and calorically dense foods coupled with increased physical inactivity are undoubtedly central drivers of the metabolic disease epidemic; however, these factors alone fail to fully account for the rapidity and magnitude of the rise in obesity and diabetes rates. Likewise, shifts in population genetics are unlikely to occur on a time scale that explains the recent deterioration in human metabolic health. As such, increasing attention has turned to the identification of additional factors that may impact energy metabolism, including exposure to chemical pollutants [8]. First articulated in 1992, the theory of endocrine disruption postulates that exogenous chemicals can modulate homeostasis by interfering with the action of endogenous hormonal axes [9]. Since the theory’s inception, a host of endocrine disrupting chemicals (EDCs) have been identified, including industrial products and waste, flame retardants, phytochemicals, pharmaceuticals, pesticides, food additives, and personal care products (Figure 1). Humans are exposed to these compounds via multiple routes, including ingestion, inhalation, injection, transdermal contact, and transplacental carriage [10]. Initial studies of EDCs focused on their capacity to disrupt sex steroid signaling [11–13] through modulation of estrogenic [14,15] and/or androgenic pathways [16,17]. Later, compounds with the ability to modulate thyroid hormone signaling were identified [18–20]. More recently, attention has turned to the capacity of environmental contaminants to disrupt metabolic pathways.
Figure 1. Adipocytes Under Assault: endocrine disrupting chemicals and their sources.
BPA, bisphenol A; DDT, dichlorodiphenyltrichloroethane; DEHP, di-2-ethylhexyl phthalate; DES, diethylstilbestrol; MEHP, mono-ethylhexyl phthalate; 4-NP, 4-nonylphenol; PBDEs, polybrominated diphenyl ethers; PCB, polychlorinated biphenyl; TF, tolylfluanid; TBT, tributyltin; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TFZ, triflumizole.
The concept that EDCs might alter energy metabolism and contribute to the development of metabolic diseases was first proposed in 2002 by Paula Baillie-Hamilton who noted the coordinate increases in the rates of overweight/obesity and synthetic chemical production in the United States [21]. This ultimately led to the articulation of the “environmental obesogen hypothesis” by Grun and Blumberg in 2006, which postulated a causal link between “obesogens” in the environment and the obesity epidemic [22]. In support of this theory is an expanding body of epidemiological studies correlating various chemicals with elevated body weight in humans [reviewed in refs. [23–25]], as well as literature linking exposure to synthetic chemicals with diabetes [reviewed in ref. [26]]. Interestingly, increases in body weight have also been documented in domesticated, feral, and laboratory animals in industrialized countries [27]. These findings suggest that obesogenic agents in the environment may play an important role in promoting the accretion of fat mass beyond that induced by lifestyle factors alone.
While links between environmental contaminants and the epidemic of metabolic disease have generated significant interest and inclusion in national obesity research recommendations [28–30], many questions remain regarding the molecular mechanisms by which these compounds contribute to the pathogenesis of metabolic diseases. For several reasons, adipose tissue is likely a critical target for metabolic disruption by EDCs. First, adipose tissue is a dynamic organ centrally involved in the integrative network that maintains global energy homeostasis. Second, many classes of EDCs are highly lipophilic and predicted to bioaccumulate in the lipid droplet of mature adipocytes resulting in high local concentrations in the fat pad [31]. Third, bioaccumulation of EDCs in adipose tissue may result in sustained local exposure of adipocytes after acute enrichment through slow leaching from the lipid droplet. Fourth, adipocyte physiology is tightly regulated by multiple nuclear hormone receptors that have lipophilic compounds as their endogenous ligands, and several of these receptors have emerged as molecular targets of various EDCs [32,33]. Consequently, adipocytes are primed for metabolic disruption by EDCs. Furthermore, adipose tissue likely serves as a reservoir of bioactive EDCs that can be released into the systemic circulation or sequestered in the adipocyte lipid droplet in response to hormonal and neuronal signals regulating adipose physiology. Thus, alterations in adipose tissue function can modulate EDC flux from this storage depot, thereby influencing the function of other metabolic tissues. Understanding how these environmental pollutants modulate adipose development and function is critical for determining their potential role in the pathogenesis of metabolic diseases.
2. EDCs Promote Adiposity and Weight Gain in Animal Models
Supporting the emerging epidemiological evidence linking chemical exposures with obesity and its metabolic consequences in humans [reviewed in refs. [23–26]], animal studies have identified a variety of compounds with the capacity to alter adipose mass and energy homeostasis. Tributyltin (TBT) is a persistent organic pollutant (POP) that has been used as a fungicide and, until recently banned, as an antifouling agent in marine paints. Male C57BL/6 mice acutely exposed to TBT exhibited augmented gene expression of the adipogenic markers CCAAT enhancer binding protein-β (C/EBPβ) and sterol regulatory element-binding protein-1 (Srebp1), with in utero exposure resulting in increased adipose mass in 10-week old males [34]. Interestingly, the obesogenic effect of TBT was species-independent as developmental exposure of Xenopus laevis resulted in an increase in ectopic adipocyte formation as well [34]. The obesity-promoting action of TBT has been confirmed in male KM mice [35], making TBT the prototypical environmental obesogen. The ability of TBT to promote the accretion of adiposity has been linked to its ability to function as a nanomolar agonist of peroxisome proliferator-activated receptors (PPARs) and retinoid X receptor α (RXRα) [36].
Polychlorinated biphenyls (PCBs) are a family of congeners that found wide industrial use as dielectric and coolant fluids. Despite being banned in the United States in 1979, the extensive use and chemical stability of these compounds have resulted in their persistence in the environment. Adult male C57BL/6 mice exposed to PCB-77 showed increased body weight gain, an effect that was dependent on the aryl hydrocarbon receptor (AhR), as AhR-null mice did not exhibit the same PCB-induced increase in body weight [37]. In a recent series of papers, it was shown that one-time exposure of adult male C57BL/6J mice to the compounds diethylstilbestrol (DES) [38], 4-nonylphenol (4-NP) [39], and mono-2-ethylhexyl phthalate (MEHP) [40] resulted in increased adipose gene expression of PPARγ, adipocyte protein 2 (aP2), and lipoprotein lipase (LPL), markers of adipocyte development. Interestingly, developmental exposure to MEHP increased body weight and adiposity in males, while similar exposure to DES or 4-NP augmented weight gain and adiposity only in females. In contrast, exposure to MEHP’s parent compound di(2-ethylhexyl)phthalate (DEHP) in adult female C3H/N mice resulted in augmented food consumption, body weight gain, and visceral adiposity, a phenotype that was also observed in the offspring of exposed females [41]. Because DEHP is metabolized to MEHP in vivo, the obesogenic effect of DEHP in these studies may result, wholly or partly, from MEHP exposure. Extending the existence of obesogens into other model organisms, exposure of zebrafish to an environmentally relevant mixture of POPs consisting of multiple flame retardant polybrominated diphenyl ethers (PBDEs), the organochlorine insecticide dichlorodiphenyltrichloroethane (DDT), and PCBs resulted in significantly increased mean body weight [42]. Other EDCs have also been implicated in the promotion of weight gain and adiposity in animal models at some levels of exposure, including lead [43], bisphenol A (BPA) [44,45], PCB-126 [46], organophosphate insecticides [47,48], the widely used herbicide atrazine [49], and the fungicide triflumizole [50].
In contrast to these studies, several compounds have also been shown to decrease body weight and adiposity. For example, adult male C57BL/6 mice exposed to perfluorooctanoic acid (PFOA), a POP used industrially as a surfactant, exhibited reduced adipose mass [51], while the fungicide triphenyltin (fentin, TPT) attenuated weight gain in golden hamsters [52]. Likewise, other models examining the metabolic effects of DEHP have shown protection from diet-induced obesity in C57BL/6J mice [53], weight loss in male Crlj:CD1 mice [54], and reduced weight gain and adipose mass in male Wistar rats [55,56]. Collectively, these studies suggest that a structurally and functionally diverse array of environmental contaminants have the capacity to modulate body weight and fat mass; however, much less is known about the mechanisms by which these compounds act to promote alterations in adipose mass or function.
3. Exogenous Modulation of Adipocyte Development
Under conditions of caloric excess, adipose mass is expanded via both adipocyte hypertrophy and hyperplasia, with new adipocytes recruited from adipose stromal mesenchymal stem cells (MSCs) to the adipocyte lineage (preadipocytes) and subsequently differentiated into mature adipocytes [57,58]. The development of adipocytes from MSCs is tightly regulated through the coordinated action of multiple signaling pathways [reviewed in ref. [59]] (Figure 2). Because expansion of the adipocyte pool is critical for safely storing excess lipid, understanding how these signaling axes can be altered by EDCs is critical for appreciating how environmental contaminants might contribute to the development of metabolic diseases.
Figure 2. Regulation of adipocytic differentiation.
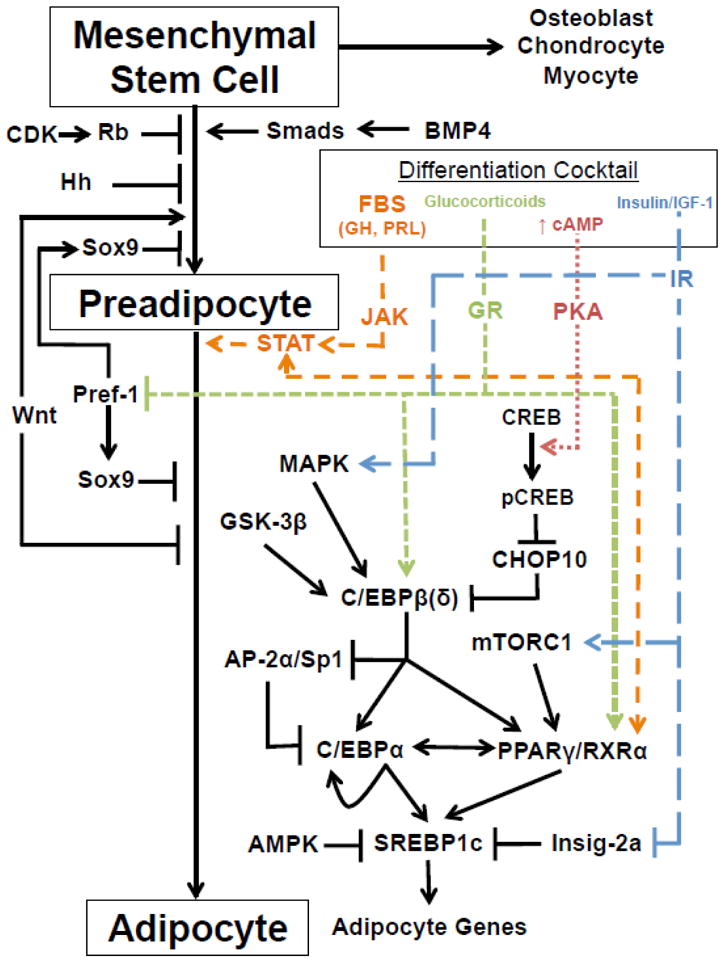
AMPK, 5′-adenosine monophosphate-activated protein kinase; AP-2, adipocyte protein-2; BMP4, bone morphogenetic protein-4; CDK, cyclin-dependent kinase; C/EBP, CCAAT-enhancer-binding protein; CHOP10, C/EBP homologous protein-10; CREB, cAMP response element-binding protein; FBS, fetal bovine serum; GH, growth hormone; GR, glucocorticoid receptor; GSK-3β, glycogen synthase kinase-3β; Hh, hedgehog; IGF-1, insulin-like growth factor-1; Insig-2a, insulin induced gene-2a; IR, insulin receptor; JAK, Janus kinase; MAPK, mitogen-activated protein kinase; mTORC1, mammalian target of rapamycin complex-1; PKA, protein kinase A; PPAR, peroxisome proliferator-activated receptor; Pref-1, preadipocyte factor-1; PRL, prolactin; Rb, retinoblastoma; RXR, retinoid X receptor; Sp1, specificity protein-1; SREBP1c, sterol regulatory element-binding transcription factor-1c; STAT, signal transducer and activator of transcription.
MSCs have the capacity to differentiate into myocytes, osteoblasts, chondrocytes, and preadipocytes. Recently, studies have begun investigating the capacity of EDCs to promote MSC differentiation into the adipocyte lineage (Table 1). For example, TBT was shown to induce adipogenesis from human and mouse adipose-derived MSCs [60]. Using the murine C3H/10T1/2 MSC cell line, DEHP and TBT were also shown to enhance adipogenesis while BPA impaired adipocyte differentiation [61]. Current evidence suggests that MSC-to-preadipocyte development is stimulated by bone morphogenic proteins (BMPs) operating through Smad transcription factors, with a potential role for Wnt signaling in promoting preadipocyte commitment; in contrast, hedgehog (Hh) signaling and potentially retinoblastoma (Rb) inhibit MSC-to-preadipocyte differentiation [59]. Whether these or other EDCs promote MSC differentiation to the adipocyte lineage through an augmentation of BMP/Smad or Wnt signaling or through an inhibition of Hh or Rb pathways remains unknown.
Table 1.
EDC effects on adipocyte development.
| EDC and Model System | Dose | MSC → Preadipocyte | Preadipocyte → Adipocyte |
|---|---|---|---|
| PCB-77 | |||
| 3T3-L1 [37] | 3.4 μM | ↑ (Reduced at higher dose) | |
| TCDD | |||
| 3T3-L1 [101] | .1 – 30 nM | ↓ | |
| MEHP (phthalate) | |||
| 3T3-L1 [68] | 10 – 100 μM | ↑ (Dose responsive) | |
| 3T3-L1 [40] | 1 – 100 μM | ↑ (Dose responsive) | |
| 3T3-L1 [165] | 50 μM | ↑ | |
| DCHP (phthalate) | |||
| 3T3-L1 [32] | 100 nM | ↑ | |
| MBzP (phthalate) | |||
| 3T3-L1 [165] | 100 – 300 μM | ↑ (Dose responsive) | |
| MBuP (phthalate) | |||
| 3T3-L1 [165] | 100 – 300 μM | ↑ (Dose responsive) | |
| DEHP (phthalate) | |||
| C3H/10T1/2 [61] | 100 μM | ↑ (Not seen at 100 nM) | |
| Nonylphenol | |||
| 3T3-L1 [39] | 100 nM – 10 μM | ↑ (Dose responsive, not seen at lower doses) | |
| Tributyltin | |||
| 3T3-L1 [34] | 10 – 100 μM | ↑ (Dose responsive) | |
| 3T3-L1 [166] | 50 – 100 nM | ↑ | |
| mADSC, hADSC [60] | 50 nM | ↑ (Not seen at 5 nM) | |
| C3H/10T1/2 [61] | 100 nM | ↑ (Not seen at 1 nM) | |
| hBMC [167] | 1 – 10 nM | ↑ | |
| Tolylfluanid | |||
| 3T3-L1 [32] | 100 nM | ↑ | |
| Triflumizole | |||
| 3T3-L1 [50] | 10 nm – 10 μM | ↑ (Dose responsive) | |
| hADSC [50] | 100 nM | ↑ | |
| Endrin | |||
| 3T3-L1 [32] | 100 nM | ↑ | |
| Bisphenol A | |||
| 3T3-L1 [32] | 100 nM | ↑ | |
| 3T3-L1 [168] | 20 – 80 μM | ↑ (Dose responsive) | |
| 3T3-L1 [169] | 10 – 100 nM | ↑ | |
| mMSC, hMSC [169] | 1 – 1000 nM | No significant effect | ↑ |
| C3H/10T1/2 [61] | 10 μM | ↓ (Not seen at 10 nM) | |
| HgCl2 | |||
| 3T3-L1 [170] | 5 – 10 μM | ↓ (Dose responsive, not seen at lower doses) | |
| C3H/10T1/2 [170] | 5 – 10 μM | ↓ (Dose responsive, not seen at lower doses) | |
| BADGE | |||
| 3T3-L1 [169] | 10 nM | ↑ | |
| mMSC [169] | 10 – 100 nM | ↑ (Dose responsive, no effect at higher/lower dose) | |
| hMSC [169] | 10 – 1000 nM | ↑ (Dose responsive, no effect at lower dose) | |
| DES | |||
| 3T3-L1 [38] | 1 – 10 μM | ↑ (Dose responsive) | |
| Phenamil (Amiloride derivative) | |||
| 3T3-L1, 3T3-F442A [171] | 10 μM | ↑ | |
| Flavanone | |||
| 3T3-L1 [70] | 10 – 100 μM | ↑ (Dose responsive, maximal at 30 μM) | |
| Indomethacin | |||
| C3H/10T1/2, mdMSCs [172] | 10 – 50 μM | ↑ (Dose responsive) | |
| Bixin (natural food coloring) | |||
| 3T3-L1 [71] | 30 – 70 μM | ↑ (Dose responsive) | |
| Emodin (rhubarb derivative) | |||
| 3T3-L1 [173] | 25 – 50 μM | ↑ (Dose responsive, no effect at higher/lower dose) | |
| Sildenafil | |||
| 3T3-L1 [174] | 10 – 40 μM | ↑ (Dose responsive) |
Upward-facing arrows indicate that the study demonstrated an augmentation of adipogenesis in the specified period of adipocyte development; a downward-facing arrow indicates attenuation of adipocyte differentiation.
BADGE, bisphenol A diglycidyl ether; DEHP, di-2-ethylhexyl phthalate; DES, diethylstilbestrol; hADSC, human adipose derived stem cells; hBMC, human bone marrow cells; hMSC, human mesenchymal stem cells; mADSC, murine adipose derived stem cells; MBuP, monobutyl phthalate; MBzP, monobenzyl phthalate; mdMSC, murine marrow derived mesenchymal stem cells; MEHP, mono-ethylhexyl phthalate; mMSC, murine mesenchymal stem cells; PCB, polychlorinated biphenyl; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
Model systems such as the 3T3-L1 preadipocyte cell line [62] have greatly advanced our understanding of the signaling events regulating preadipocyte-to-adipocyte differentiation. Confluent 3T3-L1 preadipocytes can be induced to differentiate into mature adipocytes with an induction cocktail that includes glucocorticoids, insulin, an agent to raise intracellular cAMP, and fetal bovine serum (FBS) [63]. This stimulation results in the development of mature adipocytes through a carefully choreographed cascade of transcription factor activation, including the glucocorticoid receptor (GR), PPARγ, RXRα, cAMP response element-binding protein (CREB), C/EBPs, and SREBP1c (Figure 2) [64]. More recent evidence suggests a critical role for the JAK-STAT pathway in promoting adipogenesis [reviewed in ref. [65]]. Negative regulation of preadipocyte-to-adipocyte differentiation is thought to occur principally through Pref-1 activation of Sox9 signaling [66].
A host of chemicals have been shown to induce preadipocyte-to-adipocyte differentiation (Table 1). Interestingly, EDCs shown to promote adipogenesis are structurally diverse, suggesting that they may promote adipocyte differentiation through distinct pathways. PPARγ, the widely regarded “master regulator” of adipogenesis [67], is a ligand-activated nuclear hormone receptor with a large ligand-binding domain that accommodates structurally diverse ligands, making it a prime target for endocrine disruption [33]. The prototypical obesogen TBT was identified as a high affinity, nanomolar agonist for PPARα, PPARδ, and PPARγ as well as their shared heteromeric partner RXRα [34,36]; obesogenic phthalates such as DEHP and MEHP are also known to activate PPARγ [41,68]. Several other EDCs including some flame retardants [69], triflumizole [50], the phytochemical flavanone [70], and bixin, a natural compound commonly used in food coloring [71], have also been shown to function as PPARγ agonists, raising the possibility that they may alter adipose development and function through modulation of this critical transcription factor.
It is important to note that PPARγ activation results in heterogeneous patterns of gene/protein expression that are ligand-dependent [72]. This has been observed with pharmacological ligands as well as with EDCs. For example, compared to the pharmacological PPARγ agonist rosiglitazone, exposure of adipocytes to the phthalate MEHP induces expression of only a subset of PPARγ-dependent genes, primarily those related to adipogenic differentiation [68]. Because the functional state of the adipocyte is critical for regulating global energy metabolism, it is vital that studies examining putative obesogens functioning as PPARγ ligands broadly assess the metabolic phenotype of those adipocytes, particularly since pharmacological PPARγ agonists improve insulin sensitivity. Thus, EDCs with PPARγ activity may have salutary effects on metabolism depending on the ultimate phenotype of the adipocyte, which will be at least partially dictated by the pattern of adipocyte gene expression induced by the specific compound.
Another ligand-activated nuclear hormone receptor that plays a critical role in adipogenesis is the GR. Using a low potency differentiation cocktail, BPA, dicyclohexyl phthalate (DCHP), endrin, and tolylfluanid (TF) were shown to promote 3T3-L1 differentiation, likely through GR activation [32]. This mechanism of BPA-induced adipogenesis is supported by in silico modeling suggesting that BPA can bind the GR as an agonist [73]. TF-mediated alterations in GR signaling was suggested by data showing that this fungicide had the capacity to displace radiolabeled glucocorticoid from the GR [74], and more recent evidence has shown that TF can stimulate GR signaling in primary murine adipocytes [75]. In addition to direct stimulation of the receptor, glucocorticoid signaling is also regulated by the interconversion of glucocorticoids between active and inactive states through the enzymatic action of 11β-hydroxysteroid dehydrogenase type 1 and 2 (11β-HSD-1/2). The 11β-HSD-2 enzyme primarily inactivates glucocorticoids in the kidney to help regulate blood pressure, and several EDCs have been shown to inhibit this enzyme, including organotins [76], dithiocarbamates [77], phthalates [78], perfluoroalkylated compounds [79], silanes [80], and some antibiotics [80]. While EDC-mediated inhibition of 11β-HSD-2 may contribute to the development of hypertension, the effect of these compounds in adipose tissue is less clear given the low levels of this enzyme found in fat. In contrast, 11β-HSD-1 is a bidirectional enzyme that is receiving increasing attention as a contributor to metabolic disease and, therefore, as a potential therapeutic target [81]. Whether EDCs modulate the activity or directionality of 11β-HSD-1 is an important question necessitating further study.
Increased fat mass has clear deleterious effects on metabolism; however, failure to increase adipose tissue mass in the face of caloric excess also adversely impacts metabolic homeostasis, as is seen clinically in lipodystrophic states [82,83]. While a central focus of EDC research has been on identifying pro-adipogenic compounds, chemicals that antagonize endogenous signals promoting adipocyte differentiation may also be harmful, particularly when coupled with the metabolic stress of caloric excess. To date, several EDCs have been shown to impair adipogenesis (Table 1), with some compounds exerting differential effects depending on the experimental context. Further studies are required to characterize the metabolic consequences of anti-adipogenic EDCs, especially in the context of caloric excess in which these two metabolic stressors may synergize to disrupt energy homeostasis by promoting adipocyte hypertrophy and ectopic fat deposition.
While an increasing body of evidence implicates EDC-mediated activation of PPARγ and the GR in promoting adipogenesis, less is known about compounds that activate other signaling pathways regulating adipocyte development. The role of sex steroids in body fat distribution has been recognized since 1956 [84], with android and gynoid forms of obesity associated with more visceral or subcutaneous fat, respectively [reviewed in ref. [85]]. Because visceral fat is particularly deleterious to metabolic health, EDCs that modulate fat distribution may have unique effects on metabolism that could be mediated through disruption of sex steroid signaling, a common target of EDCs [reviewed in ref. [10]]. Likewise, modulation of estrogen and androgen signaling, which is likely dependent on the endogenous hormonal milieu, may explain sexually dimorphic metabolic effects observed with some EDCs [38–40,86,87]. Furthermore, identification of compounds that modulate the adipogenic pathway through different mechanisms might help predict which interactions among EDCs are antagonistic, additive, or synergistic with respect to body weight gain and metabolic disturbances. This is an important consideration given the approximately 100,000 unique chemicals to which humans are exposed [88]. Finally, emerging evidence suggests that white adipose tissue has the capacity to “brown” and increase energy utilization [89–92], a process that may improve energy metabolism; whether and by which mechanisms EDCs affect this process remains largely unresolved.
4. Modulation of Adipocyte Metabolism
Adipocytes play a central role in regulating metabolic homeostasis, storing energy during periods of caloric surfeit and mobilizing those stores during periods of caloric deficit. The transition between these states is governed by circulating hormones, principally insulin during periods of energy excess and catecholamines when energy mobilization is required [93,94]. EDCs that disrupt this carefully coordinated regulation of adipocyte metabolism may result in disturbances in global energy metabolism that promote the development of diabetes, dyslipidemia and cardiovascular disease (Table 2). Because absolute or relative insulin deficiency is central to the development of type 1 and type 2 diabetes, respectively, EDCs that modulate the action of this anabolic hormone in adipose tissue may be of paramount importance in environmentally-induced metabolic diseases [reviewed in ref. [95]]
Table 2.
EDCs modulate adipocyte metabolism and endocrine function.
| EDC and Model System | Leptin | Adiponectin | Inflammatory Mediators | Resistin | Lipogenesis/Lipolysis | Adipocyte Morphology | Glucose Homeostasis |
|---|---|---|---|---|---|---|---|
| Bisphenol A | |||||||
| OF-1 pregnant mice | [87] ↑ plasma conc. | [87] Impaired GTT; IR | |||||
| ICR mice (perinatal exposure) | [117] ↑ plasma conc. (correlated w/adipose mass) | ||||||
| 3T3-L1 adipocytes | [119] ↑ mRNA expression | [32] ↑ protein levels | [106] ↑ basal and insulin-stimulated glucose uptake | ||||
| Human adipose explants | [121,126]↓ secretion | [126] ↑ secretion (TNFα, IL-6) | |||||
| Sprague-Dawley rat (perinatal exposure) | [45] ↑ lipolytic gene expression | [45] Hypertrophy | |||||
| OF1 male mice | [138] Impaired GTT; IR | ||||||
| Wistar rat (perinatal exposure) | [175] Impaired GTT; IR | ||||||
| PCB-77 | |||||||
| 3T3-L1 adipocytes | [37] ↓ mRNA and secretion | [37] ↑ mRNA (TNFα, CD-36) | |||||
| ApoE−/− mice | [37] Hypertrophy | ||||||
| Benzo[a]pyrene | |||||||
| C57Bl/6 male mice | [108] ↓ leptin:body weight ratio | [108] ↓ β-adrenergic stimulated lipolysis | |||||
| Tributyltin | |||||||
| KM male mice | [35] ↑ plasma conc. | [35] ↓ plasma conc. | [35] ↑ plasma conc. | ||||
| Trimethyltin | |||||||
| SW872 human adipose cell line | [176] ↓ mRNA at 3 hours; ↑ mRNA at 48 hours; ↓ secretion at 24 and 48 hours | [176] ↑ mRNA and secretion (TNFα, IL-6) | |||||
| PFOA | |||||||
| CD-1 female mice (in utero exposure) | [118] ↑ plasma conc. | [118] ↑ plasma insulin conc. | |||||
| DES | |||||||
| CD-1 mice (in utero exposure) | [177] ↑ plasma conc. | [177] ↑ plasma conc. | [177] ↑ plasma conc. (IL-6) | [177] ↓ plasma insulin conc. | |||
| CD57Bl/6J female mice (perinatal exposure) | [38] ↑ lipogenic gene expression | [38] ↑ plasma glucose conc. | |||||
| Nonylphenol | |||||||
| C57BL/6J female mice (perinatal exposure) | [39] ↑ plasma glucose conc. | ||||||
| 3T3-L1 adipocytes | [125] ↑ mRNA | [125] mRNA levels exhibit inverse dose response relationship | [125] ↑ mRNA | ||||
| Octylphenol | |||||||
| 3T3-L1 adipocytes | [125] ↑ mRNA | [125] ↓ mRNA | [125] ↑ mRNA and protein expression | ||||
| C57BL/6J male mice | [125] ↑ adipose mRNA and plasma conc. | [125] ↓ plasma conc. | [125] ↑ adipose mRNA and plasma conc. | [125] ↑ plasma glucose conc. | |||
| TCDD | |||||||
| Human multipotent adipose-derived stem cells | [124] ↑ mRNA (IL-8, MCP-1); ↑ secretion (IL-8) | ||||||
| C57BL/6 male mice | [124] ↑ macrophage infiltration of adipose | [178] ↓ plasma insulin conc. | |||||
| English shorthair male guinea pigs | [179] ↓ glucose uptake in adipose, pancreas | ||||||
| Nicotine | |||||||
| Sprague-Dawley rats (in utero exposure) | [180] Hypertrophy | [180] Impaired GTT/ITT; IR | |||||
| PBDE | |||||||
| Sprague-Dawley male rats | [100] ↑ isoproterenol stimulated lipolysis | [100] ↓ insulin-stimulated glucose oxidation in adipose | |||||
| Atrazine | |||||||
| Sprague-Dawley male rats | [49] ↑ plasma glucose conc.; impaired GTT; IR | ||||||
| Tolylfluanid | |||||||
| C57Bl/6 male mice | [102] ↓ secretion | [75] ↓ mRNA in adipose | [75] ↑ insulin-stimulated lipogenesis | [102] ↓ insulin-stimulated Akt phosphorylation | |||
| CD-1 male mice | [102] ↓ insulin-stimulated Akt phosphorylation | ||||||
| Sprague-Dawley male rats | [102] ↓ insulin-stimulated Akt phosphorylation | ||||||
| Wistar-Kyoto male rats | [102] ↓ insulin-stimulated Akt phosphorylation | ||||||
| Human adipose explants | [102] ↓ insulin-stimulated Akt phosphorylation | ||||||
| 3T3-L1 adipocytes | [32] ↑ protein levels | ||||||
| Endrin | |||||||
| 3T3-L1 adipocytes | [32] ↑ protein levels | ||||||
| Chlorpyrifos (OP) | |||||||
| Long-Evans rats (perinatal exposure) | [86] Altered leptin:body weight ratio | ||||||
| Dimethoate (OP) | |||||||
| Wistar-CFT male rats | [181] Impaired GTT | ||||||
| Diazinon (OP) | |||||||
| Goto-Kakizaki male rats | [182] Impaired GTT | ||||||
| Malathion (OP) | |||||||
| Wistar male rats | [183,184]↑ plasma glucose conc. and insulin conc. | ||||||
| Parathion (OP) | |||||||
| Sprague-Dawley male rats (neonatal exposure) | [185] ↑ plasma conc. | [185] ↑ plasma conc. at low dose (chow); ↓ plasma conc. at high dose (HFD) | [185] ↑ conc. in inguinal adipose depot | ||||
| Sprague-Dawley female rats (neonatal exposure) | [185] ↓ plasma conc. (HFD) | [185] ↑ conc. in mesenteric adipose depot | |||||
| DiBP (phthalate) | |||||||
| Wistar fetal rats (in utero exposure) | [186] ↓ plasma conc. | [186] ↓ plasma insulin conc. | |||||
| DEHP (phthalate) | |||||||
| C3H/N female mice | [41] ↑ adipose mRNA; ↑ plasma conc. | [41] ↓ adipose mRNA | [41] Hypertrophy | ||||
| Wistar male rats | [56] Atrophy | [187] Impaired GTT; ↑ plasma glucose conc. | |||||
| Wistar male rats (in utero exposure) | [98] 27 weeks: ↑ plasma insulin conc.; IR on GTT | ||||||
| Wistar female rats (in utero exposure) | [98] 27 weeks: ↑ plasma glucose conc.; ↓ plasma insulin conc.; impaired GTT | ||||||
| Wistar albino male rats | [99] ↑ plasma glucose conc.; ↓ insulin-stimulated glucose uptake in adipose | ||||||
| MEHP (phthalate) | |||||||
| C57Bl/6 male mice (perinatal exposure) | [40] ↑ plasma glucose conc. | ||||||
| DCHP (phthalate) | |||||||
| 3T3-L1 adipocytes | [32] ↑ protein levels |
CD-36, cluster of differentiation 36; DCHP, dicyclohexyl phthalate; DEHP, di-2-ethylhexyl phthalate; DES, diethylstilbestrol; DiBP, diisobutyl phthalate; GTT, glucose tolerance test; HFD, high fat diet; IL-6, interleukin-6; IL-8, interleukin 8; IR, insulin resistance; ITT, insulin tolerance test; MCP-1, monocyte chemoattractant protein-1; MEHP, mono-ethylhexyl phthalate; OP, organophosphate; PBDE, Polybrominated diphenyl ether; PFOA, perfluorooctanoic acid; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TNFα, tumor necrosis factor-α.
POPs are found at high concentrations in the oils from fatty fish, providing an important source of human exposure to these compounds. Compared to a high fat diet containing purified fish oil, consumption of crude fish oil naturally enriched in lipophilic POPs induced insulin resistance, abdominal obesity, and hepatic steatosis in male Sprague-Dawley rats [96]. Similar effects were observed in adult male C57BL/6J mice fed a diet including farmed salmon high in POPs, which also induced cellular insulin resistance [97]. Exposure of female Wistar rats to DEHP during development resulted in insulin resistance in adulthood [98], and exposure of adult male Wistar rats resulted in insulin resistance in adipose tissue, potentially through a reduction in AS160 and GLUT4 protein levels [99]. Adipocytes from Sprague-Dawley rats exposed to PBDEs for four weeks exhibited a reduction in insulin-stimulated glucose oxidation [100], while studies using 3T3-L1 adipocytes have demonstrated that exposure to POPs including organochlorine pesticides [96] and the prototypical dioxin 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) [101] attenuate insulin-stimulated glucose uptake. While these studies provide important insight into the functional outcomes of EDC exposure, further studies are required to identify the molecular mechanisms responsible for the insulin resistance induced by these compounds.
Studies examining the effects of the phenylsulfamide fungicide and antifouling agent TF identified this EDC as an insulin disruptor in adipose tissue [102]. The impairment in insulin action was likely a result of activation of glucocorticoid signaling [75], a pathway of interest in metabolic disruption [103]. Interestingly, TF-mediated attenuation of insulin signal transduction resulted from a specific down-regulation in insulin receptor substrate-1 (IRS-1) levels [102]. IRS proteins are an attractive target for EDC-mediated disruption of insulin action. While activated by tyrosine phosphorylation mediated by the insulin receptor, these proteins contain multiple serine/threonine residues whose phosphorylation attenuates transmission of the insulin signal [104]. Multiple signaling cascades, including those induced by tumor necrosis factor-α (TNFα), resistin, free fatty acids, and diacylglycerol promote phosphorylation of these serine/threonine sites, suggesting that IRS proteins integrate multiple cellular signals to set the insulin responsiveness of the cell [105]. From an endocrine disruption standpoint, this suggests that structurally unique EDCs modulating distinct cell signaling cascades may be able to promote the induction of insulin resistance through convergent effects on IRS proteins.
In contrast to data demonstrating EDC-mediated insulin resistance, one study has shown that BPA enhances insulin-independent and -dependent glucose uptake in 3T3-L1 adipocytes [106]. It is not entirely clear what significance this holds given that the concentration used in this study was in the high micromolar range, a concentration unlikely to be relevant for human exposures. The interpretation of these findings is further complicated by a study showing that BPA exposure led to insulin resistance in a male mouse model [107]. However, these findings illustrate the fact that, while most focus is on the deleterious effects of synthetic chemicals on energy metabolism, it is possible that some compounds may exert beneficial effects. Interestingly, characterization of such compounds could provide serendipitous evidence of pathways that might serve as novel targets for pharmaceutical agents.
The effects of EDCs on other hormones regulating adipocyte metabolism are less well-studied. Adipose tissue from Sprague-Dawley rats exposed to PBDEs exhibited enhanced isoproterenol-induced lipolysis [100], while exposure of C57BL/6J mice to the polyaromatic hydrocarbon benzo[a]pyrene resulted in adipose tissue that was resistant to catecholamine-induced lipolysis [108]. Because of insulin’s prominent role in modulating catecholamine action in adipose tissue, these findings may reflect effects on adipocytic insulin action; however, specific alterations in catecholamine signal transduction should be explored in further mechanistic detail. Because catecholamines signal through cAMP, it is interesting that dioxin-like PCBs were shown to attenuate hepatocyte up-regulation of phosphoenolpyruvate carboxykinase gene expression in response to forskolin-mediated activation of cAMP signaling, an effect that was proportional to AhR agonism [109]. Whether EDCs similarly alter genes/proteins regulating intermediary metabolism in adipocytes requires further investigation.
5. Alterations in Adipocyte Endocrine Function
In addition to storing and mobilizing energy, adipose tissue plays an integral role in maintaining global energy homeostasis through its function as an endocrine organ releasing a number of secreted factors (adipokines) that regulate global insulin sensitivity, satiety, energy expenditure, and inflammation [110]. Chief among these adipokines are adiponectin and leptin, as well as additional secreted factors such as resistin, TNFα, and interleukin-6 (IL-6). Dysregulation of these adipokines has been implicated in the development of obesity and diabetes [110,111]. Recently, studies have examined how EDCs modulate synthesis and release of these key metabolic hormones (Table 2).
Leptin is a hormone secreted by adipocytes in proportion to fat mass that signals the status of peripheral energy stores to the arcuate nucleus of the hypothalamus, prompting changes in energy expenditure and satiety [112–114]. Deficiencies in leptin secretion or signaling promote the development of metabolic dysfunction, and leptin resistance appears to develop in obesity [115]. While the extent to which leptin resistance drives fat accretion remains controversial [116], disruption of this metabolic circuit is an attractive molecular target for studies of obesogenic EDCs. Several EDCs have been shown to alter leptin levels in animal models, including BPA [117], benzo[a]pyrene [108], and chlorpyrifos [86]. Prenatal exposure of female mice to low doses of PFOA resulted in significantly elevated leptin levels in adulthood, an effect not observed in animals exposed to high doses [118]. Because leptin is secreted in proportion to fat mass, increased levels of this adipokine may merely reflect increased adiposity rather than the causative mechanism for the increase in adipose mass. However, exposure of Long-Evans rats to the organophosphate insecticide chlorpyrifos resulted in an increase in body weight coupled with an alteration in the relationship between body weight and leptin levels, suggesting that impaired leptin release and/or signaling may have contributed to the weight gain in this model system [86]. In 3T3-L1 preadipocytes, incorporation of BPA into the differentiation cocktail augmented leptin gene expression [119]; however, whether this reflects a change in the physiological state of the adipocyte or is merely a marker of adipocyte differentiation remains unclear. In primary murine fat, exposure to TF has been shown to attenuate insulin-stimulated leptin release, suggesting that disruption of adipocyte function by this EDC could affect systemic energy homeostasis [102].
Adiponectin is an adipokine that acts both locally and globally as an anti-inflammatory, anti-apoptotic, and insulin-sensitizing hormone [reviewed in ref. [120]]. Thus, EDCs with the capacity to reduce adiponectin release from adipose tissue and/or attenuate adiponectin signal transduction have the capacity to adversely impact local and systemic energy homeostasis. When incorporated into a low-potency induction cocktail, BPA, DCHP, endrin, and TF all increased adiponectin expression in 3T3-L1 adipocytes [32]; however, this finding likely reflects increased adipogenesis rather than a physiological shift in the functional state of those adipocytes. In contrast, PCB-77 augmented 3T3-L1 adipogenesis but reduced adiponectin gene expression and basal release [37]. Likewise, TBT exposure reduced plasma adiponectin levels in male KM mice despite an increase in body weight [35]. In human tissue, environmentally-relevant BPA treatment of primary breast, subcutaneous, and omental adipose tissue reduced basal adiponectin release, suggesting one mechanism by which BPA can influence systemic physiology [121].
Other adipocyte-secreted proteins also modulate local and systemic energy metabolism. Resistin is an adipokine that correlates with insulin resistance and has been found to be elevated in obese and diabetic subjects [122], while IL-6, TNFα, and monocyte chemoattractant protein-1 (MCP-1) are secreted factors that stimulate the immune response in adipose tissue [123]. These and other inflammatory mediators provide an important link between obesity and the development of diabetes [124]. Because EDCs are synthetic compounds, it is likely that some activate cell stress pathways, which may trigger the release of inflammatory mediators. Treatment of 3T3-L1 adipocytes with octylphenol augmented resistin expression and release in an estrogen receptor α-(ERα) and ERK-dependent manner [125]. Similarly, chronic TBT exposure in male KM mice elevated plasma resistin levels [35]. In parallel with the decrease in adiponectin release, BPA exposure increased release of IL-6 and TNFα from primary human adipose tissue [126]. When 3T3-L1 adipocytes were differentiated in the presence of PCB-77, they exhibited augmented expression of the proinflammatory cytokines angiotensinogen, TNFα, and CD36 [37]. Exposure of preadipocytes and mature adipocytes to the AhR ligands TCDD and PCB-126 increased expression of the proinflammatory genes plasminogen activator inhibitor-1, MCP-1, and interleukin-8 [124]. Because infiltration by inflammatory cells appears to play an important role in the induction of adipose insulin resistance, it is important that the increased MCP-1 gene expression in male C57BL/6 mice exposed to TCDD was accompanied by enhanced macrophage infiltration of fat [124]. Further work is required to fully elucidate how these inflammatory cascades are triggered and whether these effects can be predicted for other EDCs.
6. Disruptions in Metabolic Cross-Talk
Energy homeostasis is maintained through a network of coordinated metabolic tissues, and adipose tissue dysfunction can adversely affect energy homeostasis in other tissues. For example, impaired lipid storage in adipose can result in increased lipid accumulation and cellular disruption in liver and muscle [reviewed in refs. [82,127]]. EDCs that facilitate lipolysis such as PBDEs [100] or that antagonize adipocytic insulin action like TF [102] and some POPs [96] could promote fatty acid efflux leading to metabolic dysfunction in liver and muscle. In fact, several EDCs appear to induce hepatic steatosis, a state arising from increased delivery of free fatty acids from adipose; implicated compounds include TBT [35,128], BPA [129], TCDD [130], and other POPs [reviewed in ref. [131]]. Because adiponectin improves energy utilization in muscle [132,133], sensitizes hepatocytes to insulin action [134], and exerts multiple beneficial effects on β-cell physiology [135,136], compounds that specifically modulate adipokine secretion could therefore exert multiple indirect effects on metabolism. Given the central role of adipose tissue in energy homeostasis, EDCs that induce changes in the global metabolic state of the organism should be examined for initiating defects in adipose tissue.
Conversely, primary disruption of other metabolic tissues by EDCs can result in adipose dysfunction. Because insulin deficiency can result in disturbances in adipose metabolism, compounds that alter pancreatic β-cell physiology are of particular interest. Several EDCs have been shown to modulate β-cell function or induce β-cell failure. For example, the banned rodenticide pyrinuron (Vacor) is directly toxic to β-cells and results in type 1 diabetes in those accidently or intentionally exposed [137]. More recently, extensive work has shown that BPA modulates β-cell function [107,138]. Additionally, heavy metals like arsenic [139], cadmium [140], and others are known to impair β-cell function [reviewed in ref. [141]]. Whether these compounds induce the predicted indirect effects on adipocyte physiology requires further exploration.
The liver also represents an important site of EDC-metabolism cross-talk by virtue of its dual role in energy metabolism and detoxification. Whether EDC detoxification generates chemical metabolites with effects on hepatic energy metabolism that are distinct from the parent compound is particularly important since the liver will likely be enriched in these compounds. Such a paradigm of differential effects has been shown in adipose tissue where the compound DEHP [56] and its primary metabolite MEHP [68] exert unique effects. Similarly, generation of reactive oxygen species (or other by-products) brought about by an up-regulation of detoxification pathways to eliminate EDCs from the body may also have spillover effects on hepatic energy metabolism. Because the liver delivers growth factors and nutrients back to adipose tissue, understanding how EDC-mediated disruption of hepatic function impacts adipose function must be explored.
7. Modeling Metabolism: Intrinsic Complexities of Experimental Systems
Diverse experimental models have been used to study the effects of EDCs on metabolism (Tables 1 and 2). It is critical to recognize that the model system employed may influence the phenotype observed. For example, the CD-1 strain of mice appears to be resistant to augmented body weight induced by BPA exposure [142]. As discussed above, the divergent effects of DEHP versus its metabolite MEHP on body weight may reflect differences in the metabolic effects of those compounds; however, these divergent responses may also reflect differential susceptibility of the C57BL/6J and C3H/N strains [40,41]. Interestingly, the protection against diet-induced obesity conferred by exposure to DEHP in C57BL/6J was abolished in PPARα humanized mice. These observations underscore the possibility of model-dependent effects on metabolic perturbations elicited by EDC exposure as well as potential differences between those models and effects on human physiology.
In addition to potential differences in susceptibility to EDCs among animal models, there is also differential sensitivity across the lifespan. Supported by an increasing body of experimental evidence, the Developmental Origins of Health and Disease (DOHaD) hypothesis posits that many chronic diseases have their origins during development, including insulin resistance and obesity [143,144]. Because adipogenesis is particularly active during sensitive developmental windows (i.e. in utero, postnatal, and peripubertal [145]), adipose tissue may be particularly susceptible to EDCs during these periods. The effects of EDCs on developmental programming has recently received a great deal of attention [reviewed in refs. [146,147]]. For example, female Sprague-Dawley rats exposed to BPA perinatally (during gestation and lactation) exhibited augmented body weight in adulthood [44]; neonatal exposure of male Long Evans rats to BPA also resulted in increased adult body weight [148]. The importance of developmental exposure studies is highlighted by resultant phenotypes not observed following equivalent adult exposures. For instance, in utero exposure of CD-1 mice to PFOA resulted in increased adiposity and hyperleptinemia, while adult exposure did not elicit such effects [118]. Similarly, C57BL/6 mice exposed to lead in utero, but not postnatally, showed increased body weight [43]. Notably, the metabolic effects of developmental exposure may not manifest until late adulthood. In male rats perinatally exposed to PCB-126, an increase in body weight was not evident until 6 months of age [46], while the increased body weight in mice following in utero lead exposure was not evident until 12 months of age [43]. In contrast to these studies, some compounds may exert effects only during adulthood. For example, chronic exposure of adult female rats to the organophosphate insecticide chlorpyrifos led to increased body weight while perinatal exposure to female offspring had no effect on body weight [47]. This suggests that sensitivity is a complex function of both timing of exposure and the precise nature of that exposure.
The mechanisms by which EDCs exert persistent effects following developmental exposure remain incompletely understood; however, emerging evidence suggests that some of these effects are mediated by changes in the epigenome [reviewed in ref. [149]]. These mechanisms may have broader implications for metabolic health as some epigenetic changes induced by EDC exposure may be passed transgenerationally. Recently, the offspring of female C57BL/6J exposed to TBT during pregnancy were shown to have expanded adipose depots, enhanced adipogenic potential of MSCs, and augmented lipid accumulation in the liver [128]. This phenotype continued through the F3 generation that had never been directly exposed to the EDC. Importantly, multiple genes regulating adipocyte development are regulated epigenetically via differential methylation patterns of CpG islands within gene promoter regions, including BMP4 [150], FABP4 and PPARγ2 [151]. Additionally, the C/EBP family of transcription factors bind to response elements that are highly methylated [59], suggesting one mechanism by which EDCs that modify the epigenome might promote or inhibit adipocyte development. Further studies are required to more completely elucidate the mechanisms, epigenetic or otherwise, underlying the metabolic perturbations elicited by developmental EDC exposure.
8. The Dose May Not Always Make the Poison: The Challenge of Non-Monotonicity
The dictum of toxicology that “the dose makes poison” has recently been challenged by evidence that many EDCs exhibit non-monotonic dose-response relationships in which biological effects are not directly proportional to concentration [152]. For example, female mice exposed in utero to PFOA exhibited increased body weight and adiposity during adulthood with low dose exposure while higher doses did not alter body weight or composition [118]. Male Long-Evans offspring perinatally exposed to chlorpyrifos exhibited an inverted U-shaped dose-response relationship with respect to weight gain [86]. Similarly complex concentration-dependent effects are observed with in utero exposure to DES or DDT in both male and female CF-1 mice. Low dose DES exposure resulted in increased body weight in the postnatal period, while high dose exposure resulted in reduced body weight; in contrast, high dose exposure to DDT in utero led to elevated body weight, whereas lower dose exposure reduced body weight [153]. Likewise, TCDD has been shown to induce wasting at higher doses of exposure [154], while lower doses result in weight gain [155]. Whether these divergent effects result from alterations in the responsiveness of the same signal transduction cascade or represent activation/inhibition of different pathways at different concentrations requires further investigation.
As with the concentration-dependent effects, the duration of EDC exposure may also be critical for determining the ultimate metabolic phenotype. For example, TF up-regulated IRS-1 expression in adipocytes after acute exposure [75], while more prolonged exposure resulted in a reduction in IRS-1 levels and an attenuation in insulin action [102]. Thus, differences in the duration of exposure may lead to divergent metabolic phenotypes; this is particularly relevant if an EDC has a relatively short in vivo half-life or if exposure is short and/or episodic. Collectively, these findings underscore the importance of assessing biological effects across a wide range of concentrations, with specific emphasis on environmentally relevant exposures when the extent of those exposures is known. To date, many studies of EDCs have been conducted at concentrations that may not reflect relevant human exposure. While studies at pharmacological doses can provide important insights into the mechanistic action of EDCs, the complexities associated with non-monotonic dose-response relationships may result in true metabolic effects that are either over- or understated at levels of human exposure.
9. The Challenges of Coordinate Stressors and Mixtures
In addition to the challenges of dose, duration, and timing of exposure to EDCs as well as nuances of the experimental system, studying metabolic disruption is also complicated by potential interactions. Because humans are exposed to a plethora of chemicals, the effects of mixtures on the ultimate phenotype have received increasing attention [88]. This issue is immensely challenging as the list of chemicals to which humans are exposed continues to grow and is now somewhere near 100,000 distinct compounds. Importantly, each individual is likely to be exposed to a unique chemical cocktail, and the consequences of exposure to any specific combination may be dictated by antagonistic, additive, or synergistic actions among those compounds. For example, a study examining puberty in male rats demonstrated that exposure to TBT resulted in significantly increased weight gain; however, concurrent exposure to the DDT metabolite dichlorodiphenyldichloroethylene (DDE) abolished these effects [156]. How DDE antagonizes TBT-induced weight gain is not clear, but underscores the importance of characterizing the mechanisms by which these compounds work in order to better predict how EDC interactions might alter the ultimate metabolic phenotype of the exposed individual. The complexities arising in even binary systems underscore the magnitude of the scientific challenge of predicting metabolic effects arising from the seemingly infinite combinations of potential exposures to which any given individual might be subject. Reductionist methodologies will remain critical for identifying and characterizing molecular targets of EDCs; however, alternative approaches are needed to fully demonstrate the true threat to human health. Such efforts must include the identification of common EDC mixtures that have broad relevance to human populations while incorporating the use of high throughput technologies to study the metabolic consequences of those common EDC cocktails. Expanding our scientific approaches is fundamentally important for achieving a better understanding of the vast complexities introduced by mixtures.
In addition to the challenge of multiple exposures, the metabolic consequences of a particular chemical mixture may also be influenced by diet [157]. The connection between dietary fat and chemical exposure was first shown in 1974 when male rats exposed to the plasticizer DEHP only exhibited augmented body weight gain and adiposity when fat was present in the diet [158]. Interestingly, exposure of mice to TCDD, most often associated with anorexigenic and wasting effects [154], results in significantly increased body weight if the animals are also fed a high-fat diet [155]. Because of the clear role that the “Western” diet plays in the obesity epidemic, understanding how EDCs interact with caloric excess to promote metabolic disruption is critical for characterizing potential synergy among the various factors that comprise an obesogenic environment. Furthermore, studies examining the mechanisms by which EDCs interact with specific macronutrients in the diet, especially carbohydrates and fat, may suggest potential molecular pathways disrupted by these compounds.
10. Potential Benefits of Expanding Fat Mass
Lipid storage in adipose tissue protects against the accumulation of ectopic lipid in other metabolic tissues that can adversely impact global energy metabolism [82,127]. As discussed, EDCs that impair expansion of adipose tissue mass may also be metabolically deleterious, as in clinical states of lipodystrophy. Interestingly, even excess adiposity is not always deleterious. For example, overexpression of adiponectin in ob/ob mice promotes greater weight gain but improved metabolism [159]. Thus, it appears that the appropriate expansion of fat mass is critical for maintaining normal energy homeostasis. Importantly, the deposition of triglycerides in adipose tissue is likely accompanied by a similar enrichment of other lipophilic compounds, including EDCs. While this bioaccumulation may adversely affect adipose physiology, fat may also serve as an EDC sink that protects against the deleterious effects of these compounds. In a study examining the effects of the organophosphates diazinon and parathion on neurodevelopment, the adverse effects were ameliorated by consumption of a high fat diet, suggesting that sequestration in fat may play a protective role in limiting EDC toxicity [48]. Similarly, adverse metabolic effects of PCB-77 were not seen in obese mice but were induced upon weight loss [160], and diet-induced obesity in female Long-Evans rats significantly increased survival time following exposure to a lethal dose of TCDD [154]. Coupled with studies showing that weight loss promotes the systemic release of EDCs from adipose stores [161–164], these findings suggest that efforts at weight loss may elicit adverse effects of EDCs brought about by their mobilization from adipose stores. Whether mobilization of obesogenic EDCs contributes to the difficulty that many patients have losing weight is an interesting question that necessitates further study.
11. The Future of Adipose Disruption: The Need for More Mechanistic Studies
Understanding the impact of environmental contaminants in the etiology of obesity and its attendant metabolic disorders requires a greater appreciation for the molecular mechanisms by which pollutants modulate adipose physiology and global energy metabolism. This is critical given the nearly 100,000 chemicals to which humans are exposed since knowledge of mechanisms offers the best chance at improving predictive models to identify chemicals that threaten metabolic health. Advances in our understanding of adipocyte physiology will help identify new targets that may be subject to endocrine disruption, thus providing insights into potential modes of interactions among EDCs and between EDCs and other metabolic stressors. Conversely, examination of EDC effects on adipocyte physiology may also provide new insights into the role of classical toxin targets (e.g. aryl hydrocarbon receptor, cytochrome P450 enzymes, etc.) in adipocyte energy homeostasis. Of critical interest to the field is the identification of common mechanisms that integrate signals from diverse classes of EDCs to alter adipose development and function. Identification of such common effectors will not only provide insights into the pathways by which EDCs alter metabolism but may also identify pharmaceutical targets to mitigate the deleterious impact of EDCs on metabolic homeostasis.
The failure of genetic studies to fully account for the predisposition to obesity has led some to propose that susceptibility to lifestyle factors likely reflects the cumulative effects of small risks, perhaps across multiple metabolic pathways [116]. Similarly, the contribution of EDCs to the pathogenesis of metabolic diseases may be small for an individual chemical working on a single signaling pathway; however, when summated over thousands of chemicals operating through distinct pathways, the effects may be sufficient to facilitate the development of metabolic disease. Chemical exposures may also synergize with the added insults of non-chemical disruptors of energy metabolism (e.g. caloric excess, physical inactivity). Furthermore, underlying genetics may augment an individual’s susceptibility to particular obesogenic or diabetogenic chemicals. Significantly, that underlying susceptibility may itself be influenced by developmental chemical exposures through modifications of the epigenome, an emerging area in the field of EDC research [reviewed in refs. [146,147]]. Understanding these complex interactions for an individual is therefore complicated; however, a more thorough understanding of environmental contaminants and their mechanisms of action will greatly advance our knowledge about this emerging risk factor, and may ultimately justify regulatory action to prevent the deleterious impact of environmental contaminants on human health.
Highlights.
Adipose tissue is a prime target for endocrine disrupting chemicals (EDCs).
EDCs promote changes in body weight in animals.
EDCs modulate adipocyte development and physiology through multiple mechanisms.
Disruption of adipocyte function can adversely affect global energy homeostasis.
Adipose tissue may regulate the systemic response to EDC exposure.
Acknowledgments
The project was supported by the National Institutes of Health [K08-ES019176 to R.M.S. and T32-HD007009 supporting S.M.R.].
Footnotes
The authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1:11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trust for America’s Health. F as in Fat: How Obesity Threatens America’s Future 2012. Robert Wood Johnson Foundation; Washington, DC: 2012. [Google Scholar]
- 5.International Diabetes Federation. Annual Report 2011. International Diabetes Federation; Brussels, Belgium: 2012. [Google Scholar]
- 6.International Diabetes Federation. IDF Diabetes Atlas 2012 Update. International Diabetes Federation; Brussels, Belgium: 2012. [Google Scholar]
- 7.Social Security Advisory Board. The unsustainable cost of health care. Government Printing Office; 2009. [Google Scholar]
- 8.Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- 9.Colborn T, Clement C. Chemically Induced Alterations in Sexual and Functional Development: The Wildlife-Human Connection. Princeton Scientific Pub, Princeton; New Jersey: 1992. [Google Scholar]
- 10.De Coster S, van Larebeke N. Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J Environ Public Health. 2012 doi: 10.1155/2012/713696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith BS. Male characteristics on female mud snails caused by antifouling bottom paints. J Appl Toxicol. 1981;1:22–25. doi: 10.1002/jat.2550010106. [DOI] [PubMed] [Google Scholar]
- 12.Hany J, Lilienthal H, Sarasin A, Roth-Härer A, Fastabend A, Dunemann L, Lichtensteiger W, Winneke G. Developmental exposure of rats to a reconstituted PCB mixture or aroclor 1254: effects on organ weights, aromatase activity, sex hormone levels, and sweet preference behavior. Toxicol Appl Pharmacol. 1999;158:231–243. doi: 10.1006/taap.1999.8710. [DOI] [PubMed] [Google Scholar]
- 13.Lilienthal H, Hack A, Roth-Härer A, Grande SW, Talsness CE. Effects of developmental exposure to 2,2,4,4,5-pentabromodiphenyl ether (PBDE-99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environ Health Perspect. 2006;114:194–201. doi: 10.1289/ehp.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svobodová K, Placková M, Novotná V, Cajthaml T. Estrogenic and androgenic activity of PCBs, their chlorinated metabolites and other endocrine disruptors estimated with two in vitro yeast assays. Sci Total Environ. 2009;407:5921–5925. doi: 10.1016/j.scitotenv.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Hu W, Liu H, Sun H, Shen O, Wang X, Lam MHW, Giesy JP, Zhang X, Yu H. Endocrine effects of methoxylated brominated diphenyl ethers in three in vitro models. Mar Pollut Bull. 2011;62:2356–2361. doi: 10.1016/j.marpolbul.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 16.Kunz PY, Fent K. Multiple hormonal activities of UV filters and comparison of in vivo and in vitro estrogenic activity of ethyl-4-aminobenzoate in fish. Aquat Toxicol. 2006;79:305–324. doi: 10.1016/j.aquatox.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Orton F, Rosivatz E, Scholze M, Kortenkamp A. Widely used pesticides with previously unknown endocrine activity revealed as in vitro antiandrogens. Environ Health Perspect. 2011;119:794–800. doi: 10.1289/ehp.1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann PJ, Schomburg L, Köhrle J. Interference of endocrine disrupters with thyroid hormone receptor-dependent transactivation. Toxicol Sci. 2009;110:125–137. doi: 10.1093/toxsci/kfp086. [DOI] [PubMed] [Google Scholar]
- 19.Heimeier RA, Shi YB. Amphibian metamorphosis as a model for studying endocrine disruption on vertebrate development: effect of bisphenol A on thyroid hormone action. Gen Comp Endocrinol. 2010;168:181–189. doi: 10.1016/j.ygcen.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Giera S, Bansal R, Ortiz-Toro TM, Taub DG, Zoeller RT. Individual polychlorinated biphenyl (PCB) congeners produce tissue- and gene-specific effects on thyroid hormone signaling during development. Endocrinology. 2011;152:2909–2919. doi: 10.1210/en.2010-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baillie-Hamilton PF. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med. 2002;8:185–192. doi: 10.1089/107555302317371479. [DOI] [PubMed] [Google Scholar]
- 22.Grün F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147:S50–55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 23.Hatch EE, Nelson JW, Stahlhut RW, Webster TF. Association of endocrine disruptors and obesity: perspectives from epidemiological studies. Int J Androl. 2010;33:324–332. doi: 10.1111/j.1365-2605.2009.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Merrill M, Birnbaum LS. Childhood obesity and environmental chemicals. Mt Sinai J Med. 2011;78:22–48. doi: 10.1002/msj.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang-Péronard JL, Andersen HR, Jensen TK, Heitmann BL. Endocrine-disrupting chemicals and obesity development in humans: a review. Obes Rev. 2011;12:622–636. doi: 10.1111/j.1467-789X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- 26.Neel BA, Sargis RM. The paradox of progress: environmental disruption of metabolism and the diabetes epidemic. Diabetes. 2011;60:1838–1848. doi: 10.2337/db11-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klimentidis YC, Beasley TM, Lin HY, Murati G, Glass GE, Guyton M, Newton W, Jorgensen M, Heymsfield SB, Kemnitz J, Fairbanks L, Allison DB. Canaries in the coal mine: a cross-species analysis of the plurality of obesity epidemics. Proc Biol Sci. 2011;278:1626–1632. doi: 10.1098/rspb.2010.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White House Task Force on Childhood Obesity. Solving the Problem of Childhood Obesity within a Generation. White House Task Force on Childhood Obesity, Executive Office of the President of the United States; Washington, DC: 2010. [DOI] [PubMed] [Google Scholar]
- 29.NIH. Strategic Plan for NIH Obesity Research: A Report of the NIH Obesity Task Force. National Institutes of Health, U.S. Department of Health and Human Services; Bethesda, Maryland: 2011. [Google Scholar]
- 30.Holtcamp W. Obesogens: an environmental link to obesity. Environ Health Perspect. 2012;120:a62–68. doi: 10.1289/ehp.120-a62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müllerová D, Kopecký J. White adipose tissue: storage and effector site for environmental pollutants. Physiol Res. 2007;56:375–381. doi: 10.33549/physiolres.931022. [DOI] [PubMed] [Google Scholar]
- 32.Sargis RM, Johnson DN, Choudhury RA, Brady MJ. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring) 2010;18:1283–1288. doi: 10.1038/oby.2009.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janesick A, Blumberg B. Minireview: PPARγ as the target of obesogens. J Steroid Biochem Mol Biol. 2011;127:4–8. doi: 10.1016/j.jsbmb.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grün F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, Gardiner DM, Kanno J, Iguchi T, Blumberg B. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol. 2006;20:2141–2155. doi: 10.1210/me.2005-0367. [DOI] [PubMed] [Google Scholar]
- 35.Zuo Z, Chen S, Wu T, Zhang J, Su Y, Chen Y, Wang C. Tributyltin causes obesity and hepatic steatosis in male mice. Environ Toxicol. 2011;26:79–85. doi: 10.1002/tox.20531. [DOI] [PubMed] [Google Scholar]
- 36.le Maire A, Grimaldi M, Roecklin D, Dagnino S, Vivat-Hannah V, Balaguer P, Bourguet W. Activation of RXR–PPAR heterodimers by organotin environmental endocrine disruptors. EMBO Rep. 2009;10:367–373. doi: 10.1038/embor.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect. 2008;116:761–768. doi: 10.1289/ehp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hao CJ, Cheng XJ, Xia HF, Ma X. The endocrine disruptor diethylstilbestrol induces adipocyte differentiation and promotes obesity in mice. Toxicol Appl Pharmacol. 2012;263:102–110. doi: 10.1016/j.taap.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Hao CJ, Cheng XJ, Xia HF, Ma X. The endocrine disruptor 4-nonylphenol promotes adipocyte differentiation and induces obesity in mice. Cell Physiol Biochem. 2012;30:382–394. doi: 10.1159/000339032. [DOI] [PubMed] [Google Scholar]
- 40.Hao CJ, Cheng XJ, Xia HF, Ma X. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci Rep. 2012;32:619–629. doi: 10.1042/BSR20120042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt JS, Schaedlich K, Fiandanese N, Pocar P, Fischer B. Effects of di(2-ethylhexyl) phthalate (DEHP) on female fertility and adipogenesis in C3H/N mice. Environ Health Perspect. 2012;120:1123–1129. doi: 10.1289/ehp.1104016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah A, Mehta N, Reilly MP. Adipose inflammation, insulin resistance, and cardiovascular disease. JPEN J Parenter Enteral Nutr. 2008;32:638–644. doi: 10.1177/0148607108325251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leasure JL, Giddabasappa A, Chaney S, Johnson JE, Jr, Pothakos K, Lau YS, Fox DA. Low-level human equivalent gestational lead exposure produces sex-specific motor and coordination abnormalities and late-onset obesity in year-old mice. Environ Health Perspect. 2008;116:355–361. doi: 10.1289/ehp.10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001;109:675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S, Aubert ML, Hüppi PS. Perinatal exposure to bisphenol A alters early adipogenesis in the rat. Environ Health Perspect. 2009;117:1549–1555. doi: 10.1289/ehp.11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitalone A, Catalani A, Cinque C, Fattori V, Matteucci P, Zuena AR, Costa LG. Long-term effects of developmental exposure to low doses of PCB 126 and methylmercury. Toxicol Lett. 2010;197:38–45. doi: 10.1016/j.toxlet.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 47.Meggs WJ, Brewer KL. Weight gain associated with chronic exposure to chlorpyrifos in rats. J Med Toxicol. 2007;3:89–93. doi: 10.1007/BF03160916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slotkin TA. Does early-life exposure to organophosphate insecticides lead to prediabetes and obesity? Reprod Toxicol. 2011;31:297–301. doi: 10.1016/j.reprotox.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim S, Ahn SY, Song IC, Chung MH, Jang HC, Park KS, Lee KU, Pak YK, Lee HK. Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. PLoS ONE. 2009;4:e5186. doi: 10.1371/journal.pone.0005186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, Pham HT, Janesick AS, Blumberg B. Triflumizole is an obesogen in mice that acts through peroxisome proliferator activated receptor gamma (PPARγ) Environ Health Perspect. 2012;120:1720–1726. doi: 10.1289/ehp.1205383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie Y, Yang Q, Nelson BD, DePierre JW. Characterization of the adipose tissue atrophy induced by peroxisome proliferators in mice. Lipids. 2002;37:139–146. doi: 10.1007/s11745-002-0873-7. [DOI] [PubMed] [Google Scholar]
- 52.Ohhira S, Matsui H, Nitta K. Subchronic study of the metabolism of triphenyltin in hamsters. Vet Hum Toxicol. 1996;38:206–209. [PubMed] [Google Scholar]
- 53.Feige JN, Gerber A, Casals-Casas C, Yang Q, Winkler C, Bedu E, Bueno M, Gelman L, Auwerx J, Gonzalez FJ, Desvergne B. The pollutant diethylhexyl phthalate regulates hepatic energy metabolism via species-specific PPARα-dependent mechanisms. Environ Health Perspect. 2010;118:234–241. doi: 10.1289/ehp.0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miura Y, Naito M, Ablake M, Terayama H, Yi SQ, Qu N, Cheng LX, Suna S, Jitsunari F, Itoh M. Short-term effects of di-(2-ethylhexyl) phthalate on testes, liver, kidneys and pancreas in mice. Asian J Androl. 2007;9:199–205. doi: 10.1111/j.1745-7262.2007.00220.x. [DOI] [PubMed] [Google Scholar]
- 55.Sakurai T, Miyazawa S, Hashimoto T. Effects of di-(2-ethylhexyl)phthalate administration on carbohydrate and fatty acid metabolism in rat liver. J Biochem. 1978;83:313–320. doi: 10.1093/oxfordjournals.jbchem.a131906. [DOI] [PubMed] [Google Scholar]
- 56.Martinelli MI, Mocchiutti NO, Bernal CA. Effect of di(2-ethylhexyl) phthalate (DEHP) on lipolysis and lipoprotein lipase activities in adipose tissue of rats. Hum Exp Toxicol. 2010;29:739–745. doi: 10.1177/0960327110361750. [DOI] [PubMed] [Google Scholar]
- 57.Shepherd PR, Gnudi L, Tozzo E, Yang H, Leach F, Kahn BB. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem. 1993;268:22243–22246. [PubMed] [Google Scholar]
- 58.Halvorsen YD, Bond A, Sen A, Franklin DM, Lea-Currie YR, Sujkowski D, Ellis PN, Wilkison WO, Gimble JM. Thiazolidinediones and glucocorticoids synergistically induce differentiation of human adipose tissue stromal cells: biochemical, cellular, and molecular analysis. Metab Clin Exp. 2001;50:407–413. doi: 10.1053/meta.2001.21690. [DOI] [PubMed] [Google Scholar]
- 59.Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 2012;81:715–736. doi: 10.1146/annurev-biochem-052110-115718. [DOI] [PubMed] [Google Scholar]
- 60.Kirchner S, Kieu T, Chow C, Casey S, Blumberg B. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol Endocrinol. 2010;24:526–539. doi: 10.1210/me.2009-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biemann R, Navarrete Santos A, Navarrete Santos A, Riemann D, Knelangen J, Blüher M, Koch H, Fischer B. Endocrine disrupting chemicals affect the adipogenic differentiation of mesenchymal stem cells in distinct ontogenetic windows. Biochem Biophys Res Commun. 2012;417:747–752. doi: 10.1016/j.bbrc.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 62.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture II Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 63.Student AK, Hsu RY, Lane MD. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980;255:4745–4750. [PubMed] [Google Scholar]
- 64.Gregoire FM. Adipocyte differentiation: from fibroblast to endocrine cell. Exp Biol Med (Maywood) 2001;226:997–1002. doi: 10.1177/153537020122601106. [DOI] [PubMed] [Google Scholar]
- 65.Richard AJ, Stephens JM. Emerging roles of JAK–STAT signaling pathways in adipocytes. Trends Endocrinol Metab. 2011;22:325–332. doi: 10.1016/j.tem.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sul HS. Minireview: Pref-1: role in adipogenesis and mesenchymal cell fate. Mol Endocrinol. 2009;23:1717–1725. doi: 10.1210/me.2009-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 68.Feige JN, Gelman L, Rossi D, Zoete V, Métivier R, Tudor C, Anghel SI, Grosdidier A, Lathion C, Engelborghs Y, Michielin O, Wahli W, Desvergne B. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J Biol Chem. 2007;282:19152–19166. doi: 10.1074/jbc.M702724200. [DOI] [PubMed] [Google Scholar]
- 69.Riu A, Grimaldi M, le Maire A, Bey G, Phillips K, Boulahtouf A, Perdu E, Zalko D, Bourguet W, Balaguer P. Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ Health Perspect. 2011;119:1227–1232. doi: 10.1289/ehp.1003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saito T, Abe D, Sekiya K. Flavanone exhibits PPARgamma ligand activity and enhances differentiation of 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2009;380:281–285. doi: 10.1016/j.bbrc.2009.01.058. [DOI] [PubMed] [Google Scholar]
- 71.Takahashi N, Goto T, Taimatsu A, Egawa K, Katoh S, Kusudo T, Sakamoto T, Ohyane C, Lee JY, Kim YI, Uemura T, Hirai S, Kawada T. Bixin regulates mRNA expression involved in adipogenesis and enhances insulin sensitivity in 3T3-L1 adipocytes through PPARgamma activation. Biochem Biophys Res Commun. 2009;390:1372–1376. doi: 10.1016/j.bbrc.2009.10.162. [DOI] [PubMed] [Google Scholar]
- 72.Xu HE, Li Y. Ligand-dependent and -independent regulation of PPAR gamma and orphan nuclear receptors. Sci Signal. 2008;1:pe52. doi: 10.1126/scisignal.148pe52. [DOI] [PubMed] [Google Scholar]
- 73.Prasanth GK, Divya LM, Sadasivan C. Bisphenol-A can bind to human glucocorticoid receptor as an agonist: an in silico study. J Appl Toxicol. 2010;30:769–774. doi: 10.1002/jat.1570. [DOI] [PubMed] [Google Scholar]
- 74.Johansson M, Johansson N, Lund BO. Xenobiotics and the glucocorticoid receptor: additive antagonistic effects on tyrosine aminotransferase activity in rat hepatoma cells. Basic Clin Pharmacol Toxicol. 2005;96:309–315. doi: 10.1111/j.1742-7843.2005.pto960406.x. [DOI] [PubMed] [Google Scholar]
- 75.Neel BA, Brady MJ, Sargis RM. The endocrine disrupting chemical tolylfluanid alters adipocyte metabolism via glucocorticoid receptor activation. Mol Endocrinol. 2013;27:394–406. doi: 10.1210/me.2012-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Atanasov AG, Nashev LG, Tam S, Baker ME, Odermatt A. Organotins disrupt the 11beta-hydroxysteroid dehydrogenase type 2-dependent local inactivation of glucocorticoids. Environ Health Perspect. 2005;113:1600–1606. doi: 10.1289/ehp.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Atanasov AG, Tam S, Röcken JM, Baker ME, Odermatt A. Inhibition of 11 beta-hydroxysteroid dehydrogenase type 2 by dithiocarbamates. Biochem Biophys Res Commun. 2003;308:257–262. doi: 10.1016/s0006-291x(03)01359-7. [DOI] [PubMed] [Google Scholar]
- 78.Zhao B, Chu Y, Huang Y, Hardy DO, Lin S, Ge RS. Structure-dependent inhibition of human and rat 11beta-hydroxysteroid dehydrogenase 2 activities by phthalates. Chem Biol Interact. 2010;183:79–84. doi: 10.1016/j.cbi.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 79.Zhao B, Lian Q, Chu Y, Hardy DO, Li XK, Ge RS. The inhibition of human and rat 11β-hydroxysteroid dehydrogenase 2 by perfluoroalkylated substances. J Steroid Biochem Mol Biol. 2011;125:143–147. doi: 10.1016/j.jsbmb.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 80.Nashev LG, Vuorinen A, Praxmarer L, Chantong B, Cereghetti D, Winiger R, Schuster D, Odermatt A. Virtual screening as a strategy for the identification of xenobiotics disrupting corticosteroid action. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pereira CD, Azevedo I, Monteiro R, Martins MJ. 11β-Hydroxysteroid dehydrogenase type 1: relevance of its modulation in the pathophysiology of obesity, the metabolic syndrome and type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14:869–881. doi: 10.1111/j.1463-1326.2012.01582.x. [DOI] [PubMed] [Google Scholar]
- 82.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 83.Vantyghem MC, Balavoine AS, Douillard C, Defrance F, Dieudonne L, Mouton F, Lemaire C, Bertrand-Escouflaire N, Bourdelle-Hego MF, Devemy F, Evrard A, Gheerbrand D, Girardot C, Gumuche S, Hober C, Topolinski H, Lamblin B, Mycinski B, Ryndak A, Karrouz W, et al. How to diagnose a lipodystrophy syndrome. Ann Endocrinol (Paris) 2012;73:170–189. doi: 10.1016/j.ando.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 84.Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr. 1956;4:20–34. doi: 10.1093/ajcn/4.1.20. [DOI] [PubMed] [Google Scholar]
- 85.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 86.Lassiter TL, Brimijoin S. Rats gain excess weight after developmental exposure to the organophosphorothionate pesticide, chlorpyrifos. Neurotoxicol Teratol. 2008;30:125–130. doi: 10.1016/j.ntt.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 87.Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, Nadal A. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect. 2010;118:1243–1250. doi: 10.1289/ehp.1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kortenkamp A. Low dose mixture effects of endocrine disrupters: implications for risk assessment and epidemiology. Int J Androl. 2008;31:233–240. doi: 10.1111/j.1365-2605.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- 89.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1α-dependent myokine that drives browning of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, Accili D. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of PPARγ. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arner P. The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones. Trends Endocrinol Metab. 2003;14:137–145. doi: 10.1016/s1043-2760(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 94.Kolditz CI, Langin D. Adipose tissue lipolysis. Curr Opin Clin Nutr Metab Care. 2010;13:377–381. doi: 10.1097/MCO.0b013e32833bed6a. [DOI] [PubMed] [Google Scholar]
- 95.Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;7:346–353. doi: 10.1038/nrendo.2011.56. [DOI] [PubMed] [Google Scholar]
- 96.Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock EJ, Lillefosse H, Ma T, Pesenti S, Sonne SB, Marstrand TT, Malde MK, Du ZY, Chavey C, Fajas L, Lundebye AK, Brand CL, Vidal H, Kristiansen K, Frøyland L. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect. 2010;118:465–471. doi: 10.1289/ehp.0901321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ibrahim MM, Fjære E, Lock EJ, Naville D, Amlund H, Meugnier E, Le Magueresse Battistoni B, Frøyland L, Madsen L, Jessen N, Lund S, Vidal H, Ruzzin J. Chronic consumption of farmed salmon containing persistent organic pollutants causes insulin resistance and obesity in mice. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin Y, Wei J, Li Y, Chen J, Zhou Z, Song L, Wei Z, Lv Z, Chen X, Xia W, Xu S. Developmental exposure to di(2-ethylhexyl) phthalate impairs endocrine pancreas and leads to long-term adverse effects on glucose homeostasis in the rat. Am J Physiol Endocrinol Metab. 2011;301:E527–538. doi: 10.1152/ajpendo.00233.2011. [DOI] [PubMed] [Google Scholar]
- 99.Rajesh P, Sathish S, Srinivasan C, Selvaraj J, Balasubramanian K. Exposure to diethyl hexyl phthalate (DEHP) to adult male rat is associated with insulin resistance in adipose tisssue: Protective role of antioxidant vitamins (C & E) J Cell Biochem. 2012 doi: 10.1002/jcb.24399. [DOI] [PubMed] [Google Scholar]
- 100.Hoppe AA, Carey GB. Polybrominated diphenyl ethers as endocrine disruptors of adipocyte metabolism. Obesity (Silver Spring) 2007;15:2942–2950. doi: 10.1038/oby.2007.351. [DOI] [PubMed] [Google Scholar]
- 101.Hsu HF, Tsou TC, Chao HR, Kuo YT, Tsai FY, Yeh SC. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on adipogenic differentiation and insulin-induced glucose uptake in 3T3-L1 cells. J Hazard Mater. 2010;182:649–655. doi: 10.1016/j.jhazmat.2010.06.081. [DOI] [PubMed] [Google Scholar]
- 102.Sargis RM, Neel BA, Brock CO, Lin Y, Hickey AT, Carlton DA, Brady MJ. The novel endocrine disruptor tolylfluanid impairs insulin signaling in primary rodent and human adipocytes through a reduction in insulin receptor substrate-1 levels. Biochim Biophys Acta. 2012;1822:952–960. doi: 10.1016/j.bbadis.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Odermatt A, Gumy C, Atanasov AG, Dzyakanchuk AA. Disruption of glucocorticoid action by environmental chemicals: potential mechanisms and relevance. J Steroid Biochem Mol Biol. 2006;102:222–231. doi: 10.1016/j.jsbmb.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 104.White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283:E413–422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- 105.Sun XJ, Liu F. Phosphorylation of IRS proteins yin-yang regulation of insulin signaling. Vitam Horm. 2009;80:351–387. doi: 10.1016/S0083-6729(08)00613-4. [DOI] [PubMed] [Google Scholar]
- 106.Sakurai K, Kawazuma M, Adachi T, Harigaya T, Saito Y, Hashimoto N, Mori C. Bisphenol A affects glucose transport in mouse 3T3-F442A adipocytes. Br J Pharmacol. 2004;141:209–214. doi: 10.1038/sj.bjp.0705520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ropero AB, Alonso-Magdalena P, García-García E, Ripoll C, Fuentes E, Nadal A. Bisphenol-A disruption of the endocrine pancreas and blood glucose homeostasis. Int J Androl. 2008;31:194–200. doi: 10.1111/j.1365-2605.2007.00832.x. [DOI] [PubMed] [Google Scholar]
- 108.Irigaray P, Ogier V, Jacquenet S, Notet V, Sibille P, Méjean L, Bihain BE, Yen FT. Benzo[a]pyrene impairs beta-adrenergic stimulation of adipose tissue lipolysis and causes weight gain in mice: a novel molecular mechanism of toxicity for a common food pollutant. FEBS J. 2006;273:1362–1372. doi: 10.1111/j.1742-4658.2006.05159.x. [DOI] [PubMed] [Google Scholar]
- 109.Zhang W, Sargis RM, Volden PA, Carmean CM, Sun XJ, Brady MJ. PCB 126 and other dioxin-like PCBs specifically suppress hepatic PEPCK expression via the aryl hydrocarbon receptor. PLoS ONE. 2012;7:e37103. doi: 10.1371/journal.pone.0037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11:351–356. doi: 10.1016/s1043-2760(00)00323-4. [DOI] [PubMed] [Google Scholar]
- 112.Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS. Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab. 1996;81:4406–4413. doi: 10.1210/jcem.81.12.8954050. [DOI] [PubMed] [Google Scholar]
- 113.Keim NL, Stern JS, Havel PJ. Relation between circulating leptin concentrations and appetite during a prolonged, moderate energy deficit in women. Am J Clin Nutr. 1998;68:794–801. doi: 10.1093/ajcn/68.4.794. [DOI] [PubMed] [Google Scholar]
- 114.Leibel RL. The role of leptin in the control of body weight. Nutr Rev. 2002;60:S15–19. doi: 10.1301/002966402320634788. discussion S68–84, 85–87. [DOI] [PubMed] [Google Scholar]
- 115.Kennedy AJ, Ellacott KLJ, King VL, Hasty AH. Mouse models of the metabolic syndrome. Dis Model Mech. 2010;3:156–166. doi: 10.1242/dmm.003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Myers MG, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21:643–651. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miyawaki J, Sakayama K, Kato H, Yamamoto H, Masuno H. Perinatal and postnatal exposure to bisphenol A increases adipose tissue mass and serum cholesterol level in mice. J Atheroscler Thromb. 2007;14:245–252. doi: 10.5551/jat.e486. [DOI] [PubMed] [Google Scholar]
- 118.Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol. 2009;304:97–105. doi: 10.1016/j.mce.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 119.Phrakonkham P, Viengchareun S, Belloir C, Lombès M, Artur Y, Canivenc-Lavier MC. Dietary xenoestrogens differentially impair 3T3-L1 preadipocyte differentiation and persistently affect leptin synthesis. J Steroid Biochem Mol Biol. 2008;110:95–103. doi: 10.1016/j.jsbmb.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 120.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 121.Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008;116:1642–1647. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kusminski CM, McTernan PG, Kumar S. Role of resistin in obesity, insulin resistance and Type II diabetes. Clin Sci. 2005;109:243–256. doi: 10.1042/CS20050078. [DOI] [PubMed] [Google Scholar]
- 123.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 124.Kim MJ, Pelloux V, Guyot E, Tordjman J, Bui LC, Chevallier A, Forest C, Benelli C, Clément K, Barouki R. Inflammatory pathway genes belong to major targets of persistent organic pollutants in adipose cells. Environ Health Perspect. 2012;120:508–514. doi: 10.1289/ehp.1104282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee MJ, Lin H, Liu CW, Wu MH, Liao WJ, Chang HH, Ku HC, Chien YS, Ding WH, Kao YH. Octylphenol stimulates resistin gene expression in 3T3-L1 adipocytes via the estrogen receptor and extracellular signal-regulated kinase pathways. Am J Physiol, Cell Physiol. 2008;294:C1542–1551. doi: 10.1152/ajpcell.00403.2007. [DOI] [PubMed] [Google Scholar]
- 126.Ben-Jonathan N, Hugo ER, Brandebourg TD. Effects of bisphenol A on adipokine release from human adipose tissue: implications for the metabolic syndrome. Mol Cell Endocrinol. 2009;304:49–54. doi: 10.1016/j.mce.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bergman RN, Van Citters GW, Mittelman SD, Dea MK, Hamilton-Wessler M, Kim SP, Ellmerer M. Central role of the adipocyte in the metabolic syndrome. J Investig Med. 2001;49:119–126. doi: 10.2310/6650.2001.34108. [DOI] [PubMed] [Google Scholar]
- 128.Chamorro-García R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B. Transgenerational Inheritance of Increased Fat Depot Size, Stem Cell Reprogramming, and Hepatic Steatosis Elicited by Prenatal Obesogen Tributyltin in Mice. Environ Health Perspect. 2013 doi: 10.1289/ehp.1205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marmugi A, Ducheix S, Lasserre F, Polizzi A, Paris A, Priymenko N, Bertrand-Michel J, Pineau T, Guillou H, Martin PGP, Mselli-Lakhal L. Low doses of bisphenol A induce gene expression related to lipid synthesis and trigger triglyceride accumulation in adult mouse liver. Hepatology. 2012;55:395–407. doi: 10.1002/hep.24685. [DOI] [PubMed] [Google Scholar]
- 130.Chang H, Wang YJ, Chang LW, Lin P. A histochemical and pathological study on the interrelationship between TCDD-induced AhR expression, AhR activation, and hepatotoxicity in mice. J Toxicol Environ Health Part A. 2005;68:1567–1579. doi: 10.1080/15287390590967513. [DOI] [PubMed] [Google Scholar]
- 131.Polyzos SA, Kountouras J, Deretzi G, Zavos C, Mantzoros CS. The emerging role of endocrine disruptors in pathogenesis of insulin resistance: a concept implicating nonalcoholic fatty liver disease. Curr Mol Med. 2012;12:68–82. doi: 10.2174/156652412798376161. [DOI] [PubMed] [Google Scholar]
- 132.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MRS, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Civitarese AE, Ukropcova B, Carling S, Hulver M, DeFronzo RA, Mandarino L, Ravussin E, Smith SR. Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab. 2006;4:75–87. doi: 10.1016/j.cmet.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 135.Brown JEP, Conner AC, Digby JE, Ward KL, Ramanjaneya M, Randeva HS, Dunmore SJ. Regulation of beta-cell viability and gene expression by distinct agonist fragments of adiponectin. Peptides. 2010;31:944–949. doi: 10.1016/j.peptides.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 136.Wijesekara N, Krishnamurthy M, Bhattacharjee A, Suhail A, Sweeney G, Wheeler MB. Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J Biol Chem. 2010;285:33623–33631. doi: 10.1074/jbc.M109.085084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Karam JH, Lewitt PA, Young CW, Nowlain RE, Frankel BJ, Fujiya H, Freedman ZR, Grodsky GM. Insulinopenic diabetes after rodenticide (Vacor) ingestion: a unique model of acquired diabetes in man. Diabetes. 1980;29:971–978. doi: 10.2337/diab.29.12.971. [DOI] [PubMed] [Google Scholar]
- 138.Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114:106–112. doi: 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fu J, Woods CG, Yehuda-Shnaidman E, Zhang Q, Wong V, Collins S, Sun G, Andersen ME, Pi J. Low-level arsenic impairs glucose-stimulated insulin secretion in pancreatic beta cells: involvement of cellular adaptive response to oxidative stress. Environ Health Perspect. 2010;118:864–870. doi: 10.1289/ehp.0901608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kurata Y, Katsuta O, Doi T, Kawasuso T, Hiratsuka H, Tsuchitani M, Umemura T. Chronic cadmium treatment induces islet B cell injury in ovariectomized cynomolgus monkeys. Jpn J Vet Res. 2003;50:175–183. [PubMed] [Google Scholar]
- 141.Chen YW, Yang CY, Huang CF, Hung DZ, Leung YM, Liu SH. Heavy metals, islet function and diabetes development. Islets. 2009;1:169–176. doi: 10.4161/isl.1.3.9262. [DOI] [PubMed] [Google Scholar]
- 142.Ryan KK, Haller AM, Sorrell JE, Woods SC, Jandacek RJ, Seeley RJ. Perinatal exposure to bisphenol-a and the development of metabolic syndrome in CD-1 mice. Endocrinology. 2010;151:2603–2612. doi: 10.1210/en.2009-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21:199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 144.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 145.Han J, Lee JE, Jin J, Lim JS, Oh N, Kim K, Chang SI, Shibuya M, Kim H, Koh GY. The spatiotemporal development of adipose tissue. Development. 2011;138:5027–5037. doi: 10.1242/dev.067686. [DOI] [PubMed] [Google Scholar]
- 146.Newbold RR. Developmental exposure to endocrine-disrupting chemicals programs for reproductive tract alterations and obesity later in life. Am J Clin Nutr. 2011;94:1939S–1942S. doi: 10.3945/ajcn.110.001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Janesick A, Blumberg B. Obesogens, stem cells and the developmental programming of obesity. Int J Androl. 2012;35:437–448. doi: 10.1111/j.1365-2605.2012.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Patisaul HB, Bateman HL. Neonatal exposure to endocrine active compounds or an ERbeta agonist increases adult anxiety and aggression in gonadally intact male rats. Horm Behav. 2008;53:580–588. doi: 10.1016/j.yhbeh.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 149.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of endocrine disruptors. Reprod Toxicol. 2011;31:337–343. doi: 10.1016/j.reprotox.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Davies MN, Volta M, Pidsley R, Lunnon K, Dixit A, Lovestone S, Coarfa C, Harris RA, Milosavljevic A, Troakes C, Al-Sarraj S, Dobson R, Schalkwyk LC, Mill J. Functional annotation of the human brain methylome identifies tissue-specific epigenetic variation across brain and blood. Genome Biol. 2012;13:R43. doi: 10.1186/gb-2012-13-6-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Noer A, Boquest AC, Collas P. Dynamics of adipogenic promoter DNA methylation during clonal culture of human adipose stem cells to senescence. BMC Cell Biol. 2007;8:18. doi: 10.1186/1471-2121-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures I Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. 2003;111:994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Palanza P, Parmigiani S, vom Saal FS. Effects of prenatal exposure to low doses of diethylstilbestrol, o,p′DDT, and methoxychlor on postnatal growth and neurobehavioral development in male and female mice. Horm Behav. 2001;40:252–265. doi: 10.1006/hbeh.2001.1697. [DOI] [PubMed] [Google Scholar]
- 154.Tuomisto JT, Pohjanvirta R, Unkila M, Tuomisto J. TCDD-induced anorexia and wasting syndrome in rats: effects of diet-induced obesity and nutrition. Pharmacol Biochem Behav. 1999;62:735–742. doi: 10.1016/s0091-3057(98)00224-x. [DOI] [PubMed] [Google Scholar]
- 155.Zhu BT, Gallo MA, Burger CW, Jr, Meeker RJ, Cai MX, Xu S, Conney AH. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin administration and high-fat diet on the body weight and hepatic estrogen metabolism in female C3H/HeN mice. Toxicol Appl Pharmacol. 2008;226:107–118. doi: 10.1016/j.taap.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Makita Y, Omura M, Tanaka A, Kiyohara C. Effects of concurrent exposure to tributyltin and 1,1-dichloro-2,2 bis (p-chlorophenyl) ethylene (p,p′-DDE) on immature male Wistar rats. Basic Clin Pharmacol Toxicol. 2005;97:364–368. doi: 10.1111/j.1742-7843.2005.pto_199.x. [DOI] [PubMed] [Google Scholar]
- 157.Hennig B, Ormsbee L, McClain CJ, Watkins BA, Blumberg B, Bachas LG, Sanderson W, Thompson C, Suk WA. Nutrition can modulate the toxicity of environmental pollutants: implications in risk assessment and human health. Environ Health Perspect. 2012;120:771–774. doi: 10.1289/ehp.1104712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stein MS, Caasi PI, Nair PP. Influence of dietary fat and di-2-ethylhexyl phthalate on tissue lipids in rats. J Nutr. 1974;104:187–191. doi: 10.1093/jn/104.2.187. [DOI] [PubMed] [Google Scholar]
- 159.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Baker NA, Karounos M, English V, Fang J, Cassis LA. Coplanar polychlorinated biphenyls impair glucose homeostasis in lean C57BL/6 mice and mitigate beneficial effects of weight loss on glucose homeostasis in obese mice. Environ Health Perspect. 2013;121:105–110. doi: 10.1289/ehp.1205421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chevrier J, Dewailly E, Ayotte P, Mauriège P, Després JP, Tremblay A. Body weight loss increases plasma and adipose tissue concentrations of potentially toxic pollutants in obese individuals. Int J Obes Relat Metab Disord. 2000;24:1272–1278. doi: 10.1038/sj.ijo.0801380. [DOI] [PubMed] [Google Scholar]
- 162.Pelletier C, Doucet E, Imbeault P, Tremblay A. Associations between weight loss-induced changes in plasma organochlorine concentrations, serum T(3) concentration, and resting metabolic rate. Toxicol Sci. 2002;67:46–51. doi: 10.1093/toxsci/67.1.46. [DOI] [PubMed] [Google Scholar]
- 163.Kim MJ, Marchand P, Henegar C, Antignac JP, Alili R, Poitou C, Bouillot JL, Basdevant A, Le Bizec B, Barouki R, Clément K. Fate and Complex Pathogenic Effects of Dioxins and Polychlorinated Biphenyls in Obese Subjects before and after Drastic Weight Loss. Environ Health Perspect. 2011;119:377–383. doi: 10.1289/ehp.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lim JS, Son HK, Park SK, Jacobs DR, Jr, Lee DH. Inverse associations between long-term weight change and serum concentrations of persistent organic pollutants. Int J Obes (Lond) 2011;35:744–747. doi: 10.1038/ijo.2010.188. [DOI] [PubMed] [Google Scholar]
- 165.Hurst CH, Waxman DJ. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci. 2003;74:297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- 166.Li X, Ycaza J, Blumberg B. The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3-L1 preadipocytes. J Steroid Biochem Mol Biol. 2011;127:9–15. doi: 10.1016/j.jsbmb.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Carfi’ M, Croera C, Ferrario D, Campi V, Bowe G, Pieters R, Gribaldo L. TBTC induces adipocyte differentiation in human bone marrow long term culture. Toxicology. 2008;249:11–18. doi: 10.1016/j.tox.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 168.Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K. Bisphenol A accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci. 2005;84:319–327. doi: 10.1093/toxsci/kfi088. [DOI] [PubMed] [Google Scholar]
- 169.Chamorro-García R, Kirchner S, Li X, Janesick A, Casey SC, Chow C, Blumberg B. Bisphenol A diglycidyl ether induces adipogenic differentiation of multipotent stromal stem cells through a peroxisome proliferator-activated receptor gamma-independent mechanism. Environ Health Perspect. 2012;120:984–989. doi: 10.1289/ehp.1205063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Barnes DM, Hanlon PR, Kircher EA. Effects of inorganic HgCl2 on adipogenesis. Toxicol Sci. 2003;75:368–377. doi: 10.1093/toxsci/kfg195. [DOI] [PubMed] [Google Scholar]
- 171.Park KW, Waki H, Choi SP, Park KM, Tontonoz P. The small molecule phenamil is a modulator of adipocyte differentiation and PPARgamma expression. J Lipid Res. 2010;51:2775–2784. doi: 10.1194/jlr.M008490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Styner M, Sen B, Xie Z, Case N, Rubin J. Indomethacin promotes adipogenesis of mesenchymal stem cells through a cyclooxygenase independent mechanism. J Cell Biochem. 2010;111:1042–1050. doi: 10.1002/jcb.22793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yang Y, Shang W, Zhou L, Jiang B, Jin H, Chen M. Emodin with PPARgamma ligand-binding activity promotes adipocyte differentiation and increases glucose uptake in 3T3-Ll cells. Biochem Biophys Res Commun. 2007;353:225–230. doi: 10.1016/j.bbrc.2006.11.134. [DOI] [PubMed] [Google Scholar]
- 174.Zhang X, Ji J, Yan G, Wu J, Sun X, Shen J, Jiang H, Wang H. Sildenafil promotes adipogenesis through a PKG pathway. Biochem Biophys Res Commun. 2010;396:1054–1059. doi: 10.1016/j.bbrc.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 175.Wei J, Lin Y, Li Y, Ying C, Chen J, Song L, Zhou Z, Lv Z, Xia W, Chen X, Xu S. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology. 2011;152:3049–3061. doi: 10.1210/en.2011-0045. [DOI] [PubMed] [Google Scholar]
- 176.Ravanan P, Harry GJ, Awada R, Hoareau L, Tallet F, Roche R, Lefebvre d’Hellencour C. Exposure to an organometal compound stimulates adipokine and cytokine expression in white adipose tissue. Cytokine. 2011;53:355–362. doi: 10.1016/j.cyto.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Newbold RR, Padilla-Banks E, Snyder RJ, Phillips TM, Jefferson WN. Developmental exposure to endocrine disruptors and the obesity epidemic. Reprod Toxicol. 2007;23:290–296. doi: 10.1016/j.reprotox.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kurita H, Yoshioka W, Nishimura N, Kubota N, Kadowaki T, Tohyama C. Aryl hydrocarbon receptor-mediated effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on glucose-stimulated insulin secretion in mice. J Appl Toxicol. 2009;29:689–694. doi: 10.1002/jat.1459. [DOI] [PubMed] [Google Scholar]
- 179.Enan E, Liu PC, Matsumura F. 2,3,7,8-Tetrachlorodibenzo-p-dioxin causes reduction of glucose transporting activities in the plasma membranes of adipose tissue and pancreas from the guinea pig. J Biol Chem. 1992;267:19785–19791. [PubMed] [Google Scholar]
- 180.Somm E, Schwitzgebel VM, Vauthay DM, Camm EJ, Chen CY, Giacobino JP, Sizonenko SV, Aubert ML, Hüppi PS. Prenatal nicotine exposure alters early pancreatic islet and adipose tissue development with consequences on the control of body weight and glucose metabolism later in life. Endocrinology. 2008;149:6289–6299. doi: 10.1210/en.2008-0361. [DOI] [PubMed] [Google Scholar]
- 181.Kamath V, Rajini PS. Altered glucose homeostasis and oxidative impairment in pancreas of rats subjected to dimethoate intoxication. Toxicology. 2007;231:137–146. doi: 10.1016/j.tox.2006.11.072. [DOI] [PubMed] [Google Scholar]
- 182.Ueyama J, Kamijima M, Asai K, Mochizuki A, Wang D, Kondo T, Suzuki T, Takagi K, Takagi K, Kanazawa H, Miyamoto K, Wakusawa S, Hasegawa T. Effect of the organophosphorus pesticide diazinon on glucose tolerance in type 2 diabetic rats. Toxicol Lett. 2008;182:42–47. doi: 10.1016/j.toxlet.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 183.Panahi P, Vosough-Ghanbari S, Pournourmohammadi S, Ostad SN, Nikfar S, Minaie B, Abdollahi M. Stimulatory effects of malathion on the key enzymes activities of insulin secretion in langerhans islets, glutamate dehydrogenase and glucokinase. Toxicol Mech Methods. 2006;16:161–167. doi: 10.1080/15376520500191623. [DOI] [PubMed] [Google Scholar]
- 184.Pournourmohammadi S, Farzami B, Ostad SN, Azizi E, Abdollahi M. Effects of malathion subchronic exposure on rat skeletal muscle glucose metabolism. Environ Toxicol Pharmacol. 2005;19:191–196. doi: 10.1016/j.etap.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 185.Lassiter TL, Ryde IT, Levin ED, Seidler FJ, Slotkin TA. Neonatal exposure to parathion alters lipid metabolism in adulthood: interactions with dietary fat intake and implications for neurodevelopmental deficits. Brain Res Bull. 2010;81:85–91. doi: 10.1016/j.brainresbull.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Boberg J, Metzdorff S, Wortziger R, Axelstad M, Brokken L, Vinggaard AM, Dalgaard M, Nellemann C. Impact of diisobutyl phthalate and other PPAR agonists on steroidogenesis and plasma insulin and leptin levels in fetal rats. Toxicology. 2008;250:75–81. doi: 10.1016/j.tox.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 187.Martinelli MI, Mocchiutti NO, Bernal CA. Dietary di(2-ethylhexyl)phthalate-impaired glucose metabolism in experimental animals. Hum Exp Toxicol. 2006;25:531–538. doi: 10.1191/0960327106het651oa. [DOI] [PubMed] [Google Scholar]