Summary
Background
During active (or REM) sleep, infant rats and other mammals exhibit myoclonic twitches of skeletal muscles throughout the body, resulting in jerky, discrete movements of the distal limbs. Hundreds of thousands of limb twitches are produced each day and sensory feedback from these movements is a substantial driver of infant brain activity, suggesting that these movements contribute to motor learning and sensorimotor integration. However, it is not known whether the production of twitches is random or spatiotemporally structured, or whether the patterning of twitching changes with age. Such information is critical if we are to understand how twitches contribute to development.
Results
We used high-speed videography and 3-D motion tracking to assess the spatiotemporal structure of twitching at forelimb joints in 2- and 8-day-old rats. At both ages, twitches exhibited highly structured spatiotemporal properties at multiple timescales, including synergistic and multi-joint movements within and across forelimbs. Hierarchical cluster analysis and latent class analysis revealed developmental changes in the quantity and patterning of twitching. Critically, we found evidence for a selectionist process whereby movement patterns at the early age compete for retention and expression over development.
Conclusions
These findings indicate that twitches are not produced randomly, but rather are highly structured at multiple timescales. This structure has important implications for our understanding of the brain and spinal mechanisms that produce twitching and the role that sensory feedback from twitching plays in the development of sensorimotor systems. We suggest that twitches represent a heretofore overlooked form of motor exploration that helps animals probe the biomechanics of their limbs, build motor synergies, and lay a foundation for complex, automatic, and goal-directed wake movements.
Keywords: REM sleep, active sleep, motor control, motor synergy, Bernstein's Problem, sensorimotor integration, kinematics, development, motor learning
Introduction
Sleep is conventionally characterized as an absence of behavior. But in fact, active (or REM) sleep comprises the paradoxical combination of profound inhibition of muscle tone punctuated by bursts of limb twitching. The causes and functions of these “storms of inhibition and brief whirlwinds of excitation” ([1] p. 560) constitute the central motor mystery of sleep. Until recently, limb twitches were generally considered mere fragments of motor output—generated by a dreaming cerebral cortex—that somehow penetrate the inhibitory medullary barrier that normally prevents us (and other animals) from acting out our dreams [2]. Accordingly, twitching has been considered “at best a caricature of a component of an organized behavioural act” ([3] p. 467) or perhaps “brief episodes of an otherwise integrated behavior that is suppressed by the presence of motor inhibition” ([1] p. 568).
Twitching is among the first behaviors expressed by fetuses [4-6]. In one classic study using fetal rats from embryonic day (E) 16 through the end of gestation at E20 [4], various categories of spontaneous motor behavior were identified, including localized “convulsive-type jerks and twitches” (p. 101) of the head, mouth, limbs, and tail. These fetal twitches appeared unintegrated, random, and unpredictable. In newborn rats, twitches occur exclusively against a background of muscle atonia, thereby helping to define the state of active sleep before the development of cortical delta activity [7]. Also, twitches are dependent for their expression on the functional integrity of neural circuits within the brainstem's mesopontine region [8, 9]. These and other observations suggest that postnatal twitches are not unintegrated, random, or unpredictable, but rather are generated by specific neural structures and are coordinated in time with other components of active sleep.
The common notion that twitches are by-products of a dreaming cerebral cortex is contradicted by studies showing that twitches appear unaffected by complete disconnection of the forebrain from the brainstem in infant rats [8] and adult cats [10]. Thus, twitches are produced directly and primarily by brainstem neural circuits [2]. And contrary to the perception of sleep as a period of relative isolation from peripheral sensory experience, twitches trigger sensory feedback that drives activity in primary somatosensory cortex, thalamus, and hippocampus [11-14]. Given that hundreds of thousands, if not millions, of twitches are produced each day in developing rats, it seems increasingly clear that twitching, like other forms of spontaneous activity in the developing nervous system (e.g., [15-17]), plays a critical role in the development, refinement, and maintenance of sensorimotor circuits in the spinal cord and brain across the lifespan [18-20].
If twitching is indeed a form of spontaneous motor activity that helps to shape the sensorimotor system (while also being shaped by it), then we need to better understand the structure of the limb movements that comprise it, as this structure could serve both as input to sensorimotor learning and a marker of motor organization (e.g., motor synergies). Therefore, the present study aimed to precisely characterize the structure of twitching at individual joints in infant rats, and determine if and how that structure changes over the first postnatal week. Our results provide clear evidence of within- and between-limb synergies at multiple timescales; these synergies exist at birth and are modified lawfully across the early postnatal period. These findings establish twitching as a distinct class of movement and motivate the goal of identifying the behavioral and neural processes underlying activity-dependent development of sensorimotor integration.
Results
Basic spatiotemporal properties of infant rat twitching
We studied twitching in ten P2 and six P8 rats using high-speed video analysis of forelimb twitching and 3-D motion tracking. From these rats, a total of 35 and 39 20-s videos were collected, respectively, yielding a total of 4966 and 5168 twitches (Table S1). The number of twitches at individual joints ranged from 228 (right wrist flexion at P2) to 551 (left shoulder adduction at P2; Table S2).
Twitches comprise rapid bursts of activity in multiple limbs, occurring in recognizable bouts with intervening, irregular periods of behavioral quiescence. Twitches at specific joints are often difficult to discern in real time. But, high-speed video of forelimb twitching readily reveals the discrete nature of twitching at the shoulder, elbow, and wrist joints (Figure 1A). Simultaneous twitches at multiple joints are relatively rare, but near-simultaneous twitches of varying complexity, both within and between limbs, are often observed (see Movie S1 for various examples of twitches corresponding to those described above).
Figure 1. Spatiotemporal organization of twitching.
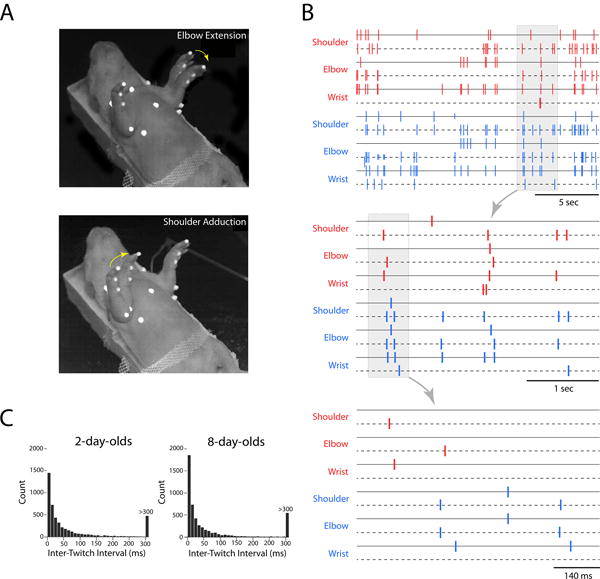
(A) Time-lapse photographs, compiled from two sequential high-speed video frames, of a supine 8-day-old rat exhibiting discrete twitches of the left elbow (top) and right shoulder (bottom). Yellow arrows indicate direction of movement. The white dots are used for motion tracking of joint movements. (B) Spatiotemporal organization of twitching in an 8-day-old rat at three timescales. Each tick mark indicates the occurrence of a twitch in the right (red) or left (blue) forelimb at the shoulder, elbow, or wrist, as determined using high-speed video and motion tracking. For each joint, two movements are depicted: adduction and abduction for the shoulder and flexion and extension for the elbow and wrist (denoted by solid and dashed lines for each joint). Non-random distribution of twitching is evident at each timescale, especially at the two smaller timescales in which the “bouts-within-bouts” structure of twitching is most apparent. (C) Frequency distribution of inter-twitch intervals for P2 and P8 rats across shoulder, elbow, and wrist joints in the two forelimbs (pooled over >5000 intervals).
A full rendering of a single 20-s video of twitch events across all six joints and joint directions for both forelimbs is shown in the top panel of Figure 1B. At the broadest temporal scale (i.e., 20 s), periods of twitching and interposed periods of quiescence were apparent. At a finer timescale of several seconds (Figure 1B, middle panel), distinct bouts of twitching spanning joints in the two forelimbs were observed. Finally, at an even finer timescale of less than 1 s (Figure 1B, bottom panel), additional bouts of twitching were revealed. This “bouts-within-bouts” temporal structure was typical.
As shown in Figure 1C for P2 and P8 subjects, the majority of inter-twitch intervals were shorter than 100 ms, thus roughly defining the temporal boundaries of a twitch bout at these ages. However, the “bouts-within-bouts” structure of twitching cautions against the expectation of a single boundary that distinguishes twitching bouts at all scales [21]. Indeed, twitching might be better characterized as a hierarchically organized structure comprising sets of partially overlapping events.
The analyses described below focus only on shoulder and elbow movements. We excluded wrist movements because they had smaller amplitudes than shoulder and elbow movements, making it harder to detect them independently, especially when other joints were moving.
Pairwise temporal relations of twitching at individual joints
Figures 2 and 3 show perievent histograms that capture the temporal relations between pairs of joint movements for P2 and P8 subjects, respectively. Each histogram indicates the total number of target events that co-occurred with the trigger event (at time 0) with in each 50-ms time bin around the trigger. At both P2 and P8, there were many instances of significant co-expression of joint movements. For example, consider the four types of homologous twitches of the right and left forelimbs (e.g., right and left shoulder adduction; highlighted in green; see Movie S1). In all four instances, a twitch in one forelimb was likely to be preceded or succeeded within 50 ms by a homologous twitch in the other forelimb. Similarly, for pairwise movements within a forelimb (e.g., left shoulder adduction and left elbow flexion; highlighted in red), movements most often occurred within 50 ms of each other (the exception being the relatively weak relations between elbow flexions and shoulder abductions). Finally, although antagonist movements (e.g., elbow flexion and extension; highlighted in blue) could not physically occur at the same time, they did co-occur within a 100-ms window and were more strongly expressed at P8 than at P2.
Figure 2. Perievent histograms showing the temporal pairwise relations between twitch movements at individual joints for infant subjects at 2 days of age.
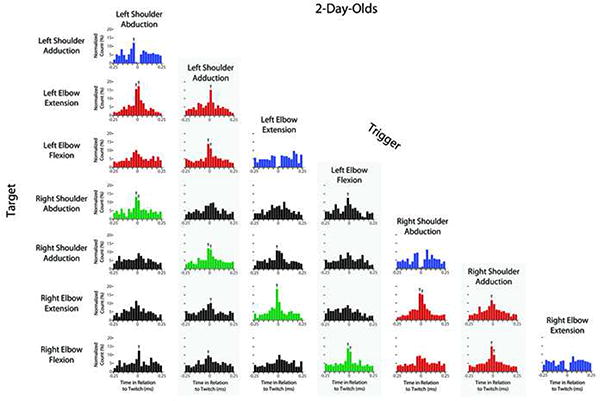
For each histogram, the joint movement identified along the left-hand column (i.e., the target) is plotted in relation to the joint movement identified in each column (i.e., the trigger). Because the data were pooled across all 2-day-old subjects, each y-axis indicates the total number of target twitches within each 50-ms bin before and after each trigger twitch; these counts are normalized and presented as percentages in relation to the total number of target twitches within the 500-ms histogram window. An arrow above a bin denotes statistical significance at p < .01. Color shading of plots highlights several categories of movements: across-limb twitches within homologous pairs of movements (green), within-limb synergies (red), and antagonist movements at the same joints (blue). Data for wrist movements are not shown.
Figure 3. Perievent histograms showing the temporal pairwise relations between twitch movements at individual joints for infant subjects at 8 days of age.
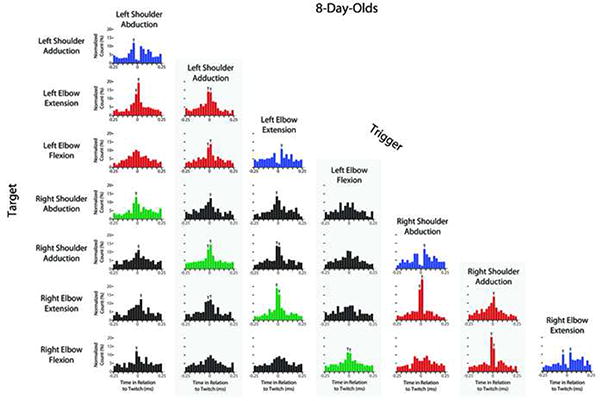
The details are identical to those described for Figure 2.
Age-related changes in twitching
To statistically confirm the observations above and determine how twitching changes over development, we created a “windowed dataset.” We constructed this dataset by stepping through the raw data in 100-ms increments and identifying the twitches that occurred within each of these windows (or events; see Supplemental Methods). Only events with at least two twitches were included.
Figure 4A shows the proportion of events containing twitches at homologous joints in the left and right forelimbs between P2 and P8. An age (2) × joint (4) mixed ANOVA revealed no main effect of age (F[1,11] < 1, NS), but a significant main effect of joint (F[3,33] = 12.0, p < 0.001) and a significant joint × age interaction (F[3,33] = 4.3, p < 0.05). Thus, there are age-related changes in the co-expression of homologous twitches across the two limbs, but the effect of age is not unidirectional.
Figure 4. Quantitative differences in twitching.
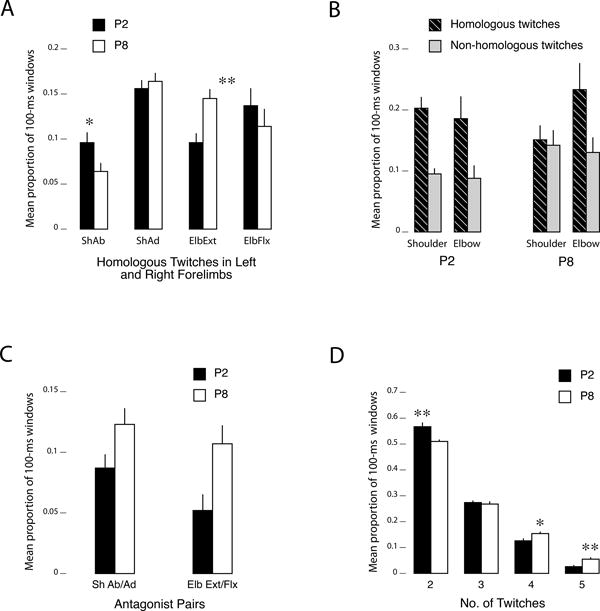
(A) Mean proportion of 100-ms windows (per pup/litter) containing antagonist twitch movements at the shoulder (adduction and abduction) and elbow (flexion and extension) at P2 (black bars) and P8 (white bars). (B) Mean proportion of 100-ms windows containing homologous (striped bars) or non-homologous (gray bars) twitch movements at the left and right shoulder or elbow at P2 and P8. For this analysis only, only those windows containing two movements, one on each side of the body, were included. (C) Mean proportion of 100-ms windows containing twitches at homologous joints in the left and right forelimbs at P2 (black bars) and P8 (white bars). (D) Mean proportion of 100-ms windows containing 2, 3, 4, or 5 twitches at P2 (black bars) and P8 (white bars). See Figure S1 for a corresponding analysis in relation to chance. Events containing 0-1 twitches were excluded from this analysis. All means + SE. Abbreviations as in Figure 2. N = 7 (P2) and 6 (P8). * p < .05, ** p < .01.
We next assessed the relative occurrence of homologous and non-homologous twitches. We limited this analysis (and this analysis only) to the subset of events in which there were only two twitches, one on each side of the body (P2: 46.1 events/pup; P8: 35.2 events/pup). Within these events, we classified (for each joint) whether the events were homologous (e.g., left and right shoulder adduction) or non-homologous (e.g., left shoulder adduction and right shoulder abduction). The results (Figure 4B) show that homologous movements at the shoulder and elbow were more likely than non-homologous movements. A joint (shoulder/elbow) × twitch-type (homologous/non-homologous) × age ANOVA indicated that there was no main effect of joint (F[1,11] < 1) or age (F[1,11] = 1.9, NS). However, the main effect of twitch-type was significant (F[1,11]=14.2, p = .003) and this did not interact with joint or age. Overall, homologous twitches (mean = .38 + .03) were about 1.7 times more prevalent than non-homologous twitches (mean = .22 + .03).
We next examined antagonist movements within a joint (Figure 4C). A joint × age ANOVA revealed significant main effects of joint movement (F[1,11] = 8.5, p < 0.05) and age (F[1,11] = 6.1, p < 0.05), but no joint movement × age interaction (F[1,11] = 2.8, NS). This age-related increase in antagonist twitches at both joints is consistent with the perievent histograms presented in Figures 2 and 3 (highlighted in blue).
Finally, as a prelude to the next analyses of twitching across more than two joints, we examined the proportion of events containing two or more twitches (Figure 4D). A twitch-count × age ANOVA revealed significant main effects of number of twitches per event (F[3,33] = 369.8, p < 0.001) and age (F[1,11] = 11.3, p < 0.01), and a significant twitch-count × age interaction (F[3,33] = 9.2, p < 0.001). There were more twitch movements within the same 100-ms windows at P8 than at P2 (i.e., larger proportions of 4- and 5-twitch events, and fewer 2-twitch events), suggesting that twitching becomes more complex with age. A follow-up analysis using Monte Carlo randomizations indicated that at both ages 3-, 4-, and 5-twitch events were more likely than expected by chance, whereas 2-twitch events were not (Figure S1).
Hierarchical cluster analysis reveals complex spatiotemporal structure of twitching
To determine if twitching exhibits complex structure among more than two joints, hierarchical cluster analysis (HCA) with seriation was performed separately on the windowed dataset at P2 and P8 [22]. Unlike traditional HCA, this analysis simultaneously extracts structure on two dimensions: clusters among the limbs (the dendrograms at the top of Figure 5), and clusters among the events (the rotated dendrograms on the sides). By extracting clusters on two dimensions simultaneously, seriation provides a more powerful way to visualize structure in complex datasets. For comparison, we performed identical analyses using randomized datasets (Figure S2).
Figure 5. Hierarchical cluster analyses, with seriation, of multi-joint twitches at the shoulder and elbow of both forelimbs in (A) 2- and (B) 8-day-old rats.
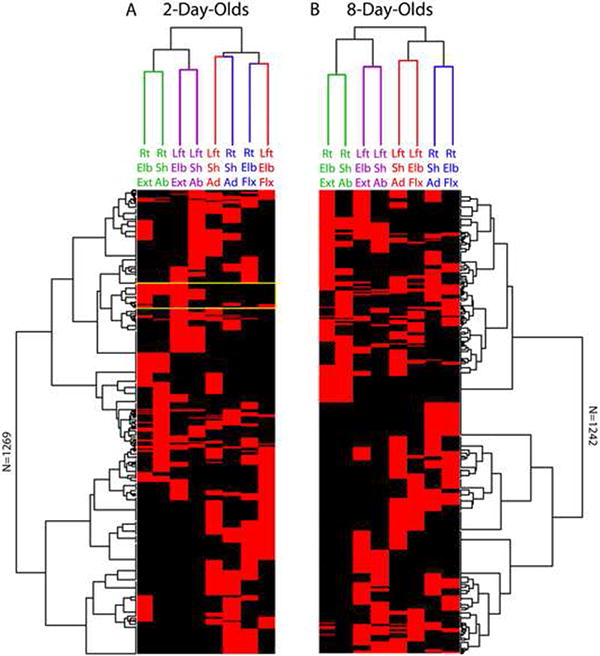
These analyses were performed on the 100-ms windowed dataset. Each row of data flows vertically down each figure, with red corresponding to the presence of a twitch and black to its absence. There are a total of 1269 rows (or events) at 2 days of age and 1242 rows at 8 days of age. In addition to the dendrograms depicted at the top of each figure depicting relationships among the joints, seriation is used to produce the dendrograms along the rows to reveal structure among the events in the data. The color-coding for the dendrograms at the top highlights similar and dissimilar clustering at the two ages. The yellow box is discussed in the text. For comparison with randomized data, see Figure S2. Abbreviations: Rt, right; Lft, left; Sh, shoulder; Elb, elbow; Ad, adduction; Ab, abduction; Flx, flexion; Ext, extension.
The dendrograms describing clustering among limbs (Figure 5, top clusters) exhibit clear functional structure. At both ages, shoulder abductions are tightly clustered with elbow extensions within each of the left and right forelimbs (green and purple branches). In contrast, we observed a developmental change in shoulder adductions and elbow flexions: at P2 the primary clustering occurs for homologous twitches on different sides (i.e., right/left shoulder adduction, right/left elbow flexion), whereas at P8 this shifts to complementary twitches within a side (i.e., shoulder adduction and elbow flexion). It is important to note, however, that at the second level of clustering these four joints movements are grouped similarly at both ages, suggesting that the observed age-related change does not represent a complete reorganization, but rather a shift in the prominence of within- vs. between-limb structure. In short, all low-level clusters at P8 exhibit within-limb linkages, with higher-level clusters linking homologous twitches across the two limbs. In contrast, the linkages at P2 are less systematic.
Although HCA provided a clear picture of structure among twitches, it offered a more complex picture of structure among events (Figure 5, rotated dendrograms). This is crucial: in addition to wanting to know, for example, that right elbow extensions are closely linked with right shoulder abductions, we also want to know if there were specific types of twitch movements that co-occurred. The rotated clusters suggest a wide variety of multi-twitch patterns with a complex overlapping structure. For example, in Figure 5A, the yellow box highlights one region (a group of events) in which right elbow extensions are often linked with right shoulder abductions (the top half of this region); however, just below it is a cluster of events illustrating a linkage between the same right elbow extension and a left elbow extension (homologous twitches). These linkages contribute to the first two cluster levels observed in this region. However, within this region there are also clusters illustrating weaker linkages between the right elbow extension and left shoulder abduction (also contributing to the 2nd-level clustering) as well as a smattering of other joint movements. This complexity suggests that twitching at any given time reflects the overlapping influence of multiple movement patterns. Whereas HCA can only link each event to a single cluster, if events were probabilistically assignable to more than one cluster, a more coherent structure might emerge. To move beyond this limitation we turned to latent class analysis.
Latent class analysis reveals the development of complex multi-joint patterns of twitches
Latent class analysis (LCA) was performed separately on the windowed dataset at P2 and P8, yielding 28 clusters at P2 and 21 clusters at P8. Of these, 19 clusters at P2 and 16 clusters at P8 showed contributions from three or more joints, further supporting the multi-joint structure of twitching (see Figure S1). LCA provides a set of profile plots for each cluster, with each plot showing how strongly particular twitch movements associated are with that cluster. Each profile plot was initially examined visually to assess the degree to which similar twitch patterns occurred at P2 and P8. Noting many instances of similar profile plots, we devised an objective method to match specific clusters across ages (see Supplemental Methods). In total, 18 matched clusters were identified and nearly all of these were also identified during our initial visual inspection.
Eight representative pairs of matched clusters are shown in Figure 6. Many of the matched clusters comprised twitches at two joints within the same limb (e.g., shoulder adduction and elbow flexion; Figure 6A). However, other matched clusters were transformed from two-joint between-limb movements at P2 into more complex multi-joint limb movements at P8 with additional joints added on a partial basis (Figure 6B, top two rows). But no single pattern describes all changes in clusters between P2 and P8 (Figure 6B, bottom two rows).
Figure 6. Profile plots of multi-joint patterns of twitching identified using latent class analysis (LCA).
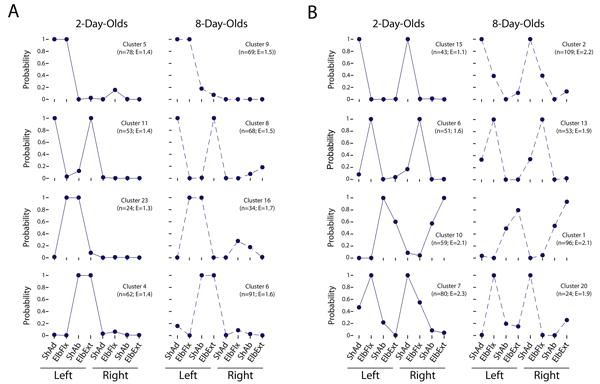
As in the hierarchical cluster analysis presented in Figure 5, the LCA analysis was performed using the 100-ms windowed dataset. In total, 28 clusters were identified at 2 days of age and 21 clusters were identified at 8 days of age. Subsequently, we used two methods to match similar clusters at the two ages (see Materials and Methods); only the profile plots for matched clusters are presented in the figure (out of a total of 18 match clusters). Each plot can be interpreted as the likelihood that, given the existence of a cluster, a particular joint movement would be included within it. The figure presents a sampling of profile plots for clusters comprised primarily of movements at (A) two joints and (B) more than two joints. For each cluster, its frequency (n) and entropy (E) are shown. Abbreviations: ShAd, shoulder adduction; ElbFlx, elbow flexion; ShAb, shoulder abduction; ElbExt, elbow extension.
We conducted a regression analysis to determine if there were subtler shifts over development. We focused on two key measures for each cluster (at each age): frequency of occurrence and coherence. To measure the coherence of a cluster, we computed each cluster's Shannon entropy, which measures the degree of structure in the twitches. Here, random clusters (e.g., with all limbs involved to some degree) will have higher entropies, and clusters with a smaller number of frequently occurring twitches will have lower entropies (see Supplemental Methods).
To determine how cluster frequency and entropy change over time, regression analyses were performed on cluster frequency (log transformed) and entropy at P2 and P8. Figures 7A and 7B show that within each age there were no significant relationships between cluster frequency and entropy (P2: r2 = 0.13, β = -.32, F[1,16] = 2.3, NS; P8: r2 = .04, β = -.17, F[1,16] < 1, NS; vertical arrows in 7E and 7F). That is, higher frequency clusters were not more or less coherent at either age. Similarly, there was moderate stability in a cluster's entropy between P2 and P8 (P2: r2 = .34, β = .59, F[1,16] = 8.3, β < .05; lower horizontal arrows in Figure 7E and 7F). This was expected since clusters were matched across ages using the same probabilities over which their entropies were computed. There was also stability in a cluster's frequency between P2 and P8 (r2 = .25, β = .50, F[1,16] = 5.3, p < .05; upper horizontal arrows in Figure 7E and 7F).
Figure 7. Regression analyses of the relations between cluster frequency and entropy.
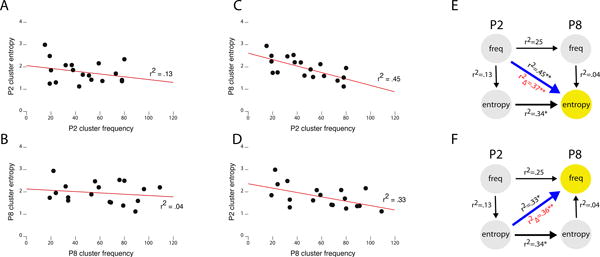
Using the frequency and entropy values for only the matched clusters identified using LCA (see Figure 5), linear regression analyses were performed. At P2 (A) and P8 (B), cluster frequency is unrelated to cluster entropy. However, as shown in (C), clusters that were more frequent at P2 became clusters with lower entropy at P8. Conversely, as shown in (D), clusters that were more frequent at P8 were the clusters with lower entropy at P2. (E) Hierarchical regression analysis with entropy at P8 as the dependent variable (yellow circle) reveals that cluster frequency at P2 significantly accounts for the variance in entropy at P8 (blue arrow), over and above the effects of cluster entropy at P2 and cluster frequency at P8 (r2∆, in red). (F) Hierarchical regression analysis with frequency at P8 as the dependent variable (yellow circle) reveals that cluster entropy at P2 significantly accounts for the variance in cluster entropy at P8 (blue arrow), over and above the effects of cluster frequency at P2 and cluster entropy at P8 (r2∆, in red). * p < .05, ** p < .005.
Quite strikingly, however, P2 cluster frequency was significantly related to cluster entropy at P8 (r2 = .45, β = -.58, F[1,16] = 12.8, p < .005 Figure 7C, diagonal in Figure 7E). This was true even after partialling out P8 cluster entropy (the same-age correlation) and P2 cluster entropy (the auto-correlation) in a hierarchical regression (Figure 7E, diagonal path; r2∆ = .37, F[1,14] = 20.1, p < 0.001).Thus, higher-frequency clusters at P2 became more highly organized (lower entropy) at P8. This suggests that with “practice” the animal prunes secondary movements from the cluster.
Conversely, P2 cluster entropy predicted cluster frequency at P8 (r2 = .33, β=-.57, F[1,16] = 7.8, p < .05). Here, more organized (lower entropy) clusters at P2 became more frequent clusters at P8 (Figure 7D, diagonal in Figure 7F). Again, this effect was confirmed over and above the effect of cluster entropy at P8 (the same-age correlation) and cluster frequency at P2 (the auto-correlation) in a hierarchical regression (Figure 7F; r2∆ = 0.36, F[1,14] = 14.1, p < .005).
Discussion
Twitches have long been considered mere jetsam of a dreaming brain—unstructured and largely unnoticed fragments of behavior [2]. In contrast, the present results indicate that twitches are highly structured behaviors and suggest that they provide functionally meaningful content for the developing nervous system. These results are surprising in light of prior research. For example, a seminal study of behavior in rat fetuses [4], discussed above, failed to find evidence of interlimb coordination. Specifically, at E16 (i.e., five days before birth) the right and left forelimbs were “not mirror-imaged or otherwise coordinated” (p. 106) and at E19 still “no coordination of left and right [fore]limbs was detected” (p. 108). In a subsequent investigation, Robinson et al. [6] provided evidence of bout structure from E17 through P9; however, they did not find evidence of complex patterns across joints or limbs, such as multi-joint movements within a limb. As they noted, however, this failure could have resulted from the limitations of the conventional video methods used in their study.
By using high-speed video and 3-D reconstruction of movements at individual forelimb joints, we more accurately assessed the content of twitching and how it changes across the first postnatal week. Our results reveal—within and across limbs— heretofore undetected and unexpected complex spatiotemporal structure that is expressed over multiple timescales and modified lawfully across age. The motor synergies inherent in twitching provide clues to the underlying neural circuitry generating these movements and point toward possible mechanisms of sensorimotor development.
Brainstem and spinal circuits may contribute to twitching at different timescales
What neural mechanisms underlie the patterns of twitching observed here, including the “bouts-within-bouts” structure? One possibility is that the spatiotemporal structure of twitching arises from spinal circuits alone, as may be the case at E20 [6]. However, disrupting midbrain circuits during the first postnatal week significantly affects the expression of twitching [8, 23]. Moreover, given that twitching at P2 is tightly coupled with muscle atonia, brainstem mechanisms must already be coordinating sleep components at this age (see [7]). Thus, the neural control of twitching appears to migrate from autonomous spinal control in fetuses to substantial brainstem control early in postnatal development.
It may be that twitches are produced by a combination of spinal mechanisms interacting with descending brainstem motor systems, including the rubrospinal, vestibulospinal, and reticulospinal pathways [24]. Each of these pathways contributes differentially to the control of skeletal muscles and could, therefore, contribute to twitching. Some evidence for this comes from neurophysiological recordings in the red nucleus—the source of the rubrospinal tract—in adult cats [25]; red nucleus activity increased phasically during active sleep, especially just before rapid eye movements and myoclonic twitches. However, lesions to the red nucleus did not disrupt the quantity or patterning of twitching. Unfortunately, from these and other studies (e.g., [3]), we still lack definitive information about the relative contributions of descending motor systems to twitching in adults; even less is known about these systems early in development.
Leaving aside the specific brainstem pathways, the “bouts-within-bouts” structure could arise from different neural components contributing at different timescales. For example, a brainstem signal could initiate a bout of twitching and, in doing so, trigger a cascade of subsequent twitches that are structured and/or mediated by spinal mechanisms. What kinds of spinal mechanisms might be involved in this process? One possibility is that a twitching limb, via proprioceptive or tactile feedback, triggers additional twitches via reflexes. However, in adult cats, monosynaptic and polysynaptic spinal reflexes are powerfully inhibited during periods of twitching [26-28]. Indeed, without these inhibitory mechanisms, one wonders what would stop a twitch from reverberating (e.g., between agonist and antagonist movements at a single joint). Regardless, it is not known whether these inhibitory mechanisms are functional in early infancy.
A second and more likely possibility is that a descending signal to a spinal motoneuron triggers a twitch and also activates, in parallel, additional components of spinal circuitry. For example, consider the strong propensity for homologous twitches to occur in the left and right forelimbs (e.g., Figure 4B). Although such patterns could be produced by bilaterally descending twitch-production nuclei in the brainstem, they could also reflect the action of commissural interneurons (CINs) [29]. CINs, which are functional in newborn mice [30], coordinate synchronous and alternating limb movements through excitatory or inhibitory actions on spinal motoneurons controlling functionally similar muscles on the two sides of the body. Accordingly, the homologous pattern of twitching could arise from a combination of descending brainstem activation of spinal motoneurons and their associated CINs, followed in succession by the activation of motoneurons in the contralateral spinal cord. Similar intrinsic spinal circuits, including those controlling flexor-extensor movements at the same joint [29], could contribute to other twitch patterns that we observed. Finally, the combined actions of spinal circuit activation and sensory feedback from twitching could contribute to the development, refinement, and maintenance of these functional spinal circuits.
Solving Bernstein's problem in our sleep: Twitching, motor synergies, and exploratory behavior
The discovery of motor synergies expressed within the context of sleep suggests that twitching is a form of exploratory movement that contributes to the development of goal-directed behaviors like reaching. With regard to such movements, Nikolai Bernstein classically described the challenge of selecting a single movement trajectory from a wide array of possible trajectories [31]. One of his solutions to this so-called degrees-of-freedom problem was to propose motor synergies as the functional units of motor planning. As Sporns and Edelman summarize Bernstein's perspective, “synergies are used by the developing nervous system to reduce the number both of controlled parameters and of afferent signals needed to generate and guide ongoing movement” ([32] p. 963). Motor control theorists continue to elaborate upon Bernstein's concepts and propose new solutions to the degrees-of-freedom problem [33].
In attempting to understand how human infants solve Bernstein's problem, developmental psychologists have focused on exploratory behavior as a key contributor to the emergence of goal-directed behaviors [32, 34-37]. As but one example, Sporns and Edelman proposed a solution in which “somatic selection of neuronal groups” leads to the “progressive transformation of a primary movement repertoire into a set of motor synergies and adaptive action patterns” ([32] p. 960, italics added). Our results also suggest a selectionist process whereby certain motor synergies, based on their prevalence and structure, are retained and elaborated across the first postnatal week during sleep (Figure 7). These nascent synergies could form at least part of the primary movement repertoire of the developing infant from which more complex movements are built. Therefore, in contrast to most conceptions of motor development, our results introduce a non-obvious factor in building movement repertoires. Accordingly, motor practice and exploration need not be restricted to waking movements. Instead, the enormous quantity of twitches produced by the sleeping infant may provide critical early experiences that help shape and refine motor synergies and perhaps even contribute to the development of so-called “motor primitives” [38].
Twitching may also help the animal develop more precise expectations of the sensorimotor consequences of an action. By generating a movement and observing its proprioceptive consequences—including sensory consequences arising from passively moving joints—the animal can learn kinematic and biomechanical relationships among muscles, joints, and effectors and their perceptual correlates [18, 35, 39, 40], much as “motor babbling” may help an animal learn the visual consequences of an action [41]. In that sense, the particular co-occurrence patterns embedded in the twitch events could prepare the organism to perceive the consequences of highly likely multi-joint actions.
Additional clues to the functions of twitching are starting to emerge from developmental robotics, an interdisciplinary field that is shifting our understanding of how development contributes to the emergence of flexible and adaptable behavior [42]. Specifically, in a robotic or simulated limb equipped with joints, muscles, and sensors, a training regimen comprising unstructured and intermittent “twitches” of the synthetic muscles resulted in discrete movements of the joints and distinct sensory responses conveying force and stretch information to the “nervous system” [18, 40]. Incredibly, even just a brief regimen of twitching resulted in the self-organization of spinal reflexes, including stretch and withdrawal reflexes.
Developmental changes in the patterns of twitching suggest experience-based pruning of organization: Although twitching might initially be unstructured (e.g., in fetal rats), over time the more prevalent patterns are refined and the less refined patterns are eliminated. This selectionist process is broadly consistent with what is known about development in many other domains including speech perception [43], mathematical reasoning [44], face perception [45], and word learning [46]. In many of these cases, Hebbian and anti-Hebbian mechanisms have been posited as a mechanism of self-organization [44, 46]; similar processes could be at play with respect to the developmental consequences of twitching [18].
Conclusions
Sensory feedback from twitches exerts a powerful influence over infant nervous system activity, from spinal cord [20] to forebrain [11-14]. The present results close the loop by showing that the structure of twitching evolves over time, suggesting that developmental experience—including sensory feedback from twitches—modifies the neural structures that produce subsequent twitches. Delineating this process of feedback modulation and sensorimotor integration remains a future challenge, as does resolving the contributions of twitching to other aspects of activity-dependent development—from synapse formation and elimination to topographic organization [18].
The multi-joint patterning of twitching at P2 and its modification across the first postnatal week suggests that twitching is part of a learning system whereby basic motor synergies are explored and refined, and retained or eliminated. In time, these motor synergies might be automatized and flexibly linked with other synergies to produce the more complex patterns of behavior that characterize waking life. There is as yet little information concerning the structural and functional relations among twitching and waking movements (e.g., locomotion [47]) in healthy subjects of any age or species. However, a recent investigation of motor behavior in human adults with REM sleep behavior disorder should encourage more work in this area [48]. Regardless, the present findings highlight twitching—arguably the most prevalent behavior of early infancy in rats and other mammals—as an unexpected form of coordinated behavior that could provide useful insights to scientists and clinicians about the functional status of the healthy and damaged nervous system across the lifespan.
Experimental Procedures
Subjects
Subjects were male and female Sprague-Dawley Norway rats (Rattus norvegicus). A total of 10 P2 rats from seven litters (body weight: 6.1-8.2 g) and six P8 rats from six litters (body weight: 17.2-20.1 g) were used.
High-speed videorecording and data acquisition
A pup was secured in a supine position inside a humidified incubator maintained at thermoneutrality. When the pup was cycling between sleep and wakefulness, two highspeed digital video cameras were used to record twitching behavior. During each 20-s recording period, the experimenter closely monitored the pup and confirmed that only twitches were expressed. Multiple 20-s recordings were acquired from each pup.
Data reduction and analysis
Automatic motion tracking was supplemented by frame-by-frame confirmation and, when necessary, manual correction. We identified six joint angles or line distances that reliably identified shoulder abduction and adduction, elbow extension and flexion, and wrist extension and flexion. These angles and distances were converted to discrete twitch-events indicating movement onset times for the six joints across the two forelimbs. Because of the short duration of each individual 20-s recording, most analyses were performed on pooled data at each age. These pooled datasets were used for the analyses performed here.
Complete experimental procedures are presented in the Supplemental Information.
Supplementary Material
Highlights.
High-speed video was used to investigate limb twitching in sleeping newborn rats
Twitches exhibit structured spatiotemporal properties at multiple timescales
Twitch patterns are retained and refined by a selectionist process over development
Twitching may be a form of motor exploration that builds motor synergies
Acknowledgments
Preparation of this article was made possible by National Institutes of Health research grants to MSB (HD63071) and BM (DC008089) and an Independent Scientist Award to MSB (MH66424). We thank Roger Bakeman, Magnus Magnusson, and Kristian Markon for analytical assistance, Josie Delgado for technical assistance, and Hugo Gravato Marques for helpful comments on an earlier draft of the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chase M, Morales F. The atonia and myoclonia of active (REM) sleep. Annu Rev Psych. 1990;41:557–584. doi: 10.1146/annurev.ps.41.020190.003013. [DOI] [PubMed] [Google Scholar]
- 2.Blumberg MS. Beyond dreams: Do sleep-related movements contribute to brain development? Front Neurol. 2010;1:140. doi: 10.3389/fneur.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gassel MM, Marchiafava PL, Pompeiano O. Phasic changes in muscular activity during desynchronized sleep in unrestrained cats. An analysis of the pattern and organization of myoclonic twitches. Arch Ital Biol. 1964;102:449. [PubMed] [Google Scholar]
- 4.Narayanan CH, Fox MW, Hamburger V. Prenatal development of spontaneous and evoked activity in the rat (Rattus norvegicus albinus) Behaviour. 1971:100–134. doi: 10.1163/156853971x00357. [DOI] [PubMed] [Google Scholar]
- 5.Corner M. Sleep and the beginnings of behavior in the animal kingdom --Studies of ultradian motility cycles in early life. Prog Neurobiol. 1977;8:279–295. doi: 10.1016/0301-0082(77)90008-9. [DOI] [PubMed] [Google Scholar]
- 6.Robinson SR, Blumberg MS, Lane MS, Kreber LS. Spontaneous motor activity in fetal and infant rats is organized into discrete multilimb bouts. Behav Neurosci. 2000;114:328–336. doi: 10.1037//0735-7044.114.2.328. [DOI] [PubMed] [Google Scholar]
- 7.Blumberg MS, Seelke AMH. The form and function of infant sleep: From muscle to neocortex. In: Blumberg MS, Freeman JH, Robinson SR, editors. The Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press; 2010. pp. 391–423. [Google Scholar]
- 8.Kreider J, Blumberg MS. Mesopontine contribution to the expression of active “twitch” sleep in decerebrate week-old rats. Brain Res. 2000;872:149–159. doi: 10.1016/s0006-8993(00)02518-x. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson KÆ, Gall A, Mohns EJ, Seelke AMH, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biol. 2005;3:e143. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villablanca J. Behavioral and polygraphic study of “sleep” and “wakefulness” in chronic decerebrate cats. Electroencephalogr Clin Neurophysiol. 1966;21:562–577. doi: 10.1016/0013-4694(66)90175-1. [DOI] [PubMed] [Google Scholar]
- 11.Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- 12.Mohns EJ, Blumberg MS. Neocortical activation of the hippocampus during sleep in newborn rats. J Neurosci. 2010;30:3438–3449. doi: 10.1523/JNEUROSCI.4832-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiriac A, Uitermarkt BD, Fanning AS, Sokoloff G, Blumberg MS. Rapid whisker movements in sleeping newborn rats. Curr Biol. 2012;22:2075–2080. doi: 10.1016/j.cub.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McVea DA, Mohajerani MH, Murphy TH. Voltage-sensitive dye imaging reveals dynamic spatiotemporal properties of cortical activity after spontaneous muscle twitches in the newborn rat. J Neurosci. 2012;32:10982–10994. doi: 10.1523/JNEUROSCI.1322-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong R. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- 17.Tritsch N, Yi E, Gale J, Glowatzki E, Bergles D. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- 18.Blumberg MS, Marques HG, Iida F. Twitching in sensorimotor development from sleeping rats to robots. Curr Biol. 2013;23:R532–R537. doi: 10.1016/j.cub.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006;29:414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Petersson P, Waldenström A, Fåhraeus C, Schouenborg J. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature. 2003;424:72–75. doi: 10.1038/nature01719. [DOI] [PubMed] [Google Scholar]
- 21.Slater P, Lester N. Minimising errors in splitting behaviour into bouts. Behaviour. 1982;79:153–161. [Google Scholar]
- 22.Caraux G, Pinloche S. PermutMatrix: a graphical environment to arrange gene expression profiles in optimal linear order. Bioinformatics. 2005;21:1280–1281. doi: 10.1093/bioinformatics/bti141. [DOI] [PubMed] [Google Scholar]
- 23.Blumberg MS, Lucas D. Dual mechanisms of twitching during sleep in neonatal rats. Behav Neurosci. 1994;108:1196–1202. doi: 10.1037//0735-7044.108.6.1196. [DOI] [PubMed] [Google Scholar]
- 24.Lemon R. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 25.Gassel M, Marchiafava P, Pompeiano O. Activity of the red nucleus during deep desynchronized sleep in the unrestrained cat. Arch Ital Biol. 1965;103:369–396. [PubMed] [Google Scholar]
- 26.Baldissera F, Broggi G, Mancia M. Monosynaptic and polysynaptic spinal reflexes during physiological sleep and wakefulness. Arch Ital Biol. 1966;104:112. [PubMed] [Google Scholar]
- 27.Kubota K, Tanaka R. Fusimotor unit activities and natural sleep in cat. Jpn J Physiol. 1968;18:43–58. doi: 10.2170/jjphysiol.18.43. [DOI] [PubMed] [Google Scholar]
- 28.Gassel MM, Marchiafava PL, Pompeiano O. Tonic and phasic inhibition of spinal reflexes during deep, desynchronized sleep in unrestrained cats. Arch Ital Biol. 1964;102:471. [PubMed] [Google Scholar]
- 29.Kiehn O. Development and functional organization of spinal locomotor circuits. Curr Opin Neurobiol. 2011;21:100–109. doi: 10.1016/j.conb.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Quinlan KA, Kiehn O. Segmental, synaptic actions of commissural interneurons in the mouse spinal cord. J Neurosci. 2007;27:6521–6530. doi: 10.1523/JNEUROSCI.1618-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernstein NA. The co-ordination and regulation of movements. Oxford Pergamon Press; 1967. [Google Scholar]
- 32.Sporns O, Edelman G. Solving Bernstein's problem: A proposal for the development of coordinated movement by selection. Child Dev. 1993;64:960–981. [PubMed] [Google Scholar]
- 33.Latash ML, Scholz JP, Schöner G. Toward a new theory of motor synergies. Motor Control. 2007;11:276–308. doi: 10.1123/mcj.11.3.276. [DOI] [PubMed] [Google Scholar]
- 34.Gibson EJ. Exploratory behavior in the development of perceiving, acting, and the acquiring of knowledge. Annu Rev Psych. 1988;39:1–41. [Google Scholar]
- 35.Berthier NE, Rosenstein MT, Barto AG. Approximate optimal control as a model for motor learning. Psychol Rev. 2005;112:329–346. doi: 10.1037/0033-295X.112.2.329. [DOI] [PubMed] [Google Scholar]
- 36.Schlesinger M. Evolving agents as a metaphor for the developing child. Dev Sci. 2004;7:1–7. doi: 10.1111/j.1467-7687.2004.00333.x. [DOI] [PubMed] [Google Scholar]
- 37.Adolph K, Berger S. Motor development. Damon W, editor. Handbook of child psychology: Vol 2: Cognition, perception, and language. 2006:161–213. [Google Scholar]
- 38.Dominici N, Ivanenko YP, Cappellini G, d'Avella A, Mondi V, Cicchese M, Fabiano A, Silei T, Di Paolo A, Giannini C, et al. Locomotor primitives in newborn babies and their development. Science. 2011;334:997–999. doi: 10.1126/science.1210617. [DOI] [PubMed] [Google Scholar]
- 39.MacNeilage PF, Davis BL. On the origin of internal structure of word forms. Science. 2000;288:527–531. doi: 10.1126/science.288.5465.527. [DOI] [PubMed] [Google Scholar]
- 40.Marques HG, Imtiaz F, Iida F, Pfeifer R. Self-organization of reflexive behavior from spontaneous motor activity. Biol Cybern. 2013;107:25–37. doi: 10.1007/s00422-012-0521-7. [DOI] [PubMed] [Google Scholar]
- 41.Kuperstein M. Neural model of adaptive hand-eye coordination for single postures. Science. 1988;239:1308–1311. doi: 10.1126/science.3344437. [DOI] [PubMed] [Google Scholar]
- 42.Lungarella M, Metta G, Pfeifer R, Sandini G. Developmental robotics: a survey. Connect Sci. 2003;15:151–190. [Google Scholar]
- 43.Werker JF, Tees RC. Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behav Dev. 1984;7:49–63. [Google Scholar]
- 44.Siegler RS. Mechanisms of cognitive development. Annu Rev Psych. 1989;40:353–379. doi: 10.1146/annurev.ps.40.020189.002033. [DOI] [PubMed] [Google Scholar]
- 45.Lewkowicz DJ, Ghazanfar AA. The emergence of multisensory systems through perceptual narrowing. Trends Cogn Sci. 2009;13:470–478. doi: 10.1016/j.tics.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 46.McMurray B, Horst JS, Samuelson LK. Word learning emerges from the interaction of online referent selection and slow associative learning. Psychol Rev. 2012;119:831–877. doi: 10.1037/a0029872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shriner AM, Drever FR, Metz GA. The development of skilled walking in the rat. Behav Brain Res. 2009;205:426–435. doi: 10.1016/j.bbr.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oudiette D, Leu-Semenescu S, Roze E, Vidailhet M, De Cock VC, Golmard JL, Arnulf I. A motor signature of REM sleep behavior disorder. Mov Disord. 2011;27:428–431. doi: 10.1002/mds.24044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


