Abstract
Endometrial cancer is the fourth most common malignancy among women and is a major cause of morbidity contributing to approximately 8,200 annual deaths in the USA. Despite advances to the understanding of endometrial cancer, novel interventions for the disease are necessary given that many tumors become refractory to therapy. As a strategy to identify novel therapies for endometrial carcinoma, in this study, we examined the contribution of the peroxisome proliferator-activated receptor β/δ (PPARβ/δ) to endometrial cancer cell proliferation and apoptosis. We found that when activated with the highly selective PPARβ/δ agonists, GW0742 and GW501516, PPARβ/δ inhibited the proliferation and markedly induced the apoptosis of three endometrial cancer cell lines. The specificity of the PPARβ/δ-induced effects on cell proliferation and apoptosis was demonstrated using PPARβ/δ-selective antagonists and PPARβ/δ small interfering RNA in combination with PPARβ/δ-selective agonists. Furthermore, we showed that PPARβ/δ activation increased phosphatase and tensin homolog expression, which led to protein kinase B (AKT) and glycogen synthase kinase-3β (GSK3β) dephosphorylation, and increased β-catenin phosphorylation associated with its degradation. Overall, our data suggest that the antitumorigenic effect of PPARβ/δ activation in endometrial cancer is mediated through the negative regulation of the AKT/GSK3β/β-catenin pathway. These findings warrant further investigation of PPARβ/δ as a therapeutic target in endometrial cancer.
Electronic supplementary material
The online version of this article (doi:10.1007/s12672-013-0157-7) contains supplementary material, which is available to authorized users.
Keywords: Endometrial Cancer, Endometrial Cancer Cell, Ishikawa Cell, Endometrial Cancer Cell Line, GW0742 Treatment
Introduction
Estimates predict that 49,500 women are affected with endometrial cancer every year and that 8,200 die as a result of the disease in the USA [1, 2]. Despite progress in endometrial cancer treatments, current treatments often fail to control tumor recurrence and metastasis. For example, although 75 % of all endometrial cancers are treated at an early stage, it is estimated that 15–20 % of all cases recur [3]. As no new targeted therapies are available in standard clinical care and response to conventional systemic therapy is limited, identification of novel therapeutic targets is urgently needed to improve endometrial cancer treatment efficacy [4].
An increasing body of evidence suggests that peroxisome proliferator-activated receptors (PPARs) contribute to the development of some solid tumors [5]. PPARs are members of the steroid hormone receptor superfamily; the three distinct isoforms—α, β/δ, and γ—act as ligand-activated transcription factors [6]. In the last 20 years, PPARs have gone from being virtually unknown to being recognized as major players in numerous physiological functions and pathological conditions, including the development of chronic diseases such as diabetes, obesity, atherosclerosis, and cancer [7, 8]. PPARβ/δ is found predominantly in the nucleus and can be co-immunoprecipitated with its heterodimerization partner, retinoid X receptor (RXR) [9]. Although there are limited reports describing the biological function of PPARβ/δ in endometrial cancer, in situ hybridization and immunohistochemistry demonstrate that PPARβ/δ levels are higher in endometrial cancer specimens compared with controls [10].
PPARα and γ subtypes are known to have fundamental roles in cell proliferation/apoptosis and carcinogenesis [11]. However, conflicting data regarding the role of PPARβ/δ in the development of cancer suggest that the exact contribution of PPARβ/δ to cancer cell proliferation or apoptosis has yet to be established. In particular, observations from previous studies raise the possibility of cell type- and organ-specific effects mediated by PPARβ/δ [12]. It is well-known that RARα and PPARβ/δ complement each other and contribute to the regulation of cell proliferation and apoptosis [13]. Schug et al. demonstrated this mechanism by elucidating that the proliferative effect of PPARβ/δ was mediated through a high fatty acid binding protein 5 (FABP5):cellular retinoic acid binding protein II (CRABP2) ratio, which diverted all-trans retinoic acid (atRA) away from RARα toward PPARβ/δ, which then led to anti-apoptotic events and increased proliferation [13]. However, studies in other cell types have found conflicting evidence, suggesting that the activity of PPARβ/δ is tissue specific [14].
Our lab previously demonstrated that the selective RARα agonist, AM580, directly regulates endometrial Ishikawa cell proliferation, and apoptosis; thus, RA signaling via RARα/RXR activation may play a critical role in mediating the carcinogenesis of human endometrial cancer [15]. However, the role of PPARβ/δ in endometrial carcinoma has not been established. Given the therapeutic potential of PPARβ/δ agonists, which have been examined in clinical trials [16], we wanted to understand the function and the underlying mechanisms of PPARβ/δ activation in endometrial cancer. We hypothesized that PPARβ/δ and its downstream pathways are effective targets that inhibit endometrial cancer cell proliferation and survival. To test our hypothesis, we evaluated the effects of ligand activation, antagonism, and silencing of PPARβ/δ on cell proliferation and apoptotic pathways in the Ishikawa, Sawano, and RL-95 human endometrial cancer cell lines.
Materials and Methods
Materials
Two highly selective PPARβ/δ-selective agonists, GW0742 and GW501516 [17], and a RARα-selective agonist, (E)-4-[2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthylenyl)-1-propenyl] benzoic acid (TTNPB), were purchased from Tocris Bioscience (Minneapolis, MN, USA). GW0742 and GW501516 were dissolved in dimethyl sulfoxide (DMSO). Two highly selective PPARβ/δ antagonists, GSK3787 and GSK0660, were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell Cultures
Endometrial cancer cell lines Ishikawa (grade 1 with phosphatase and tensin homolog (PTEN) and p53 mutations), RL95-2 (grade 2 GW501516 with PTEN and p53 mutations), and Sawano (naturally raised cisplatin-resistant cells) were purchased from ATCC (Manassas, VA, USA). Ishikawa cells were maintained in Dulbecco's modified eagle medium (DMEM)-F12 medium (GIBCO®, Life Technologies, New York, USA) supplemented with 5 % fetal bovine serum (FBS) and 500 units/ml penicillin/streptomycin. RL95-2 cells were maintained in DMEM-F12 medium (GIBCO, Life Technologies) supplemented with 10 % FBS, 500 units/ml penicillin/streptomycin, and 1 mM insulin. Sawano cells were maintained in MEM medium (GIBCO, Life Technologies, New York, USA) supplemented with 10 % FBS, 500 units/ml penicillin/streptomycin, and 1 mM glutamax. Human keratinocytes (HaCaT cells) were cultured in DMEM (GIBCO®, Life Technologies, New York, USA) supplemented with 2 mM l-glutamine, 10 % FBS, and with 500 units/ml penicillin/streptomycin. All cells were cultured at 37 °C and 5 % CO2.
Trypan Blue Staining Analysis
Endometrial cancer cells were plated on a 12-well plate at a density of 1 × 105 cells/well 24 h before cell counting at time 0. We used 0.4 % Trypan blue to stain cells and determined cell number using a Countess® Automated Cell Counter (Life Technologies, Carlsbad, CA, USA). Cells were serum-starved for 18 h prior to ligand treatment and then treated with control (DMSO), GW0742, or TTNPB for 24 or 48 h. The concentrations of GW0742 and TTNPB used for all experiments ranged from 0.1 to 10.0 μM; these were previously shown to specifically activate PPARβ/δ or RARα [18, 19]. Cells were counted every 24 h. Triplicate samples for each treatment were used for each time point and each replicate was counted three times. The average number of cells per well was calculated for each treatment group and recorded as mean ± standard deviation (SD).
MTS Assays
Epithelial endometrial cancer cells (4 × 103 cells/well) were seeded in 96-well plates and grown to 70–80 % confluence in a mixture of DMEM and F12 (1:1) medium containing 10 % FBS. After overnight starvation, cells were continually cultured in FBS-free media for 24–48 h in the presence or absence of chemical reagents. Cell proliferation was determined using the CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay kit (Promega, Madison, WI, USA) according to the manufacturer's instructions. After treatment, 20 μL of methanethiosulfonate (MTS) assay solution was added to each well and the cells were incubated at 37 °C for 4 h. The amount of bioreduced formazan produced was estimated by measuring the absorbance at 490 nm. All experiments were performed in triplicate.
BrdU-Labeling Proliferation Assay
Cell proliferation in response to different treatments was confirmed using the Cell Proliferation ELISA 5-bromo-2′-deoxyuridine (BrdU; colorimetric) kit (Roche Diagnostics Canada, Quebec, Canada) and analysis was done according to the manufacturer's protocol. Briefly, cells were plated in 96-well plates and treated as described for the cell viability assays. A minimum of six replicates were assessed at each dose. BrdU (10 μM) was added 2 h prior to the end of each measurement period. The cells were fixed, lysed, and then treated for 3 min with peroxidase-conjugated and anti-BrdU antibody supplied by the manufacturer. The cells were washed three times followed by the addition of substrate solution. Absorbance was measured at 405 nm with a reference wavelength of 490 nm. Results were recorded as optical density measurements at 405/490 nm. In some wells, cells or BrdU were omitted to serve as negative controls and to estimate the extent of nonspecific binding of reagents.
Immunoblot Analysis
Endometrial cancer cells were cultured on 60-mm culture dishes. Cells were serum-starved for 18 h and then placed in DMEM without serum with DMSO (control), GW0742, or GW501516. After 24 h of treatment, the cells were washed and isolated using M-PER (Life Technologies, New York, USA) lysis buffer containing protease inhibitors.
A total of 20 μg of protein per sample was resolved using sodium dodecyl sulfate-polyacrylamide gels. Proteins were then electro-transferred onto PVDF membranes (90 V for 90 min). The membrane was blocked with 5 % non-fat milk powder in tris buffered saline-Tween 20 (TBS-T) buffer for 60 min. Membranes were then washed and incubated with primary antibodies overnight at 4 °C. Membranes were washed with TBS-T, incubated with a secondary peroxidase-conjugated antibody for 60 min, and washed. Bands were visualized by enhanced chemiluminescence and exposed to X-ray film. Beta-actin was detected to ensure equal protein loading. Independent triplicate samples were analyzed for each treatment group.
The following antibodies were used: poly(ADP-ribose) polymerase (PARP), caspase9, cleaved-caspase9, anti-protein kinase B (AKT), anti-phospho-AKT, anti-β-catenin, anti-phospho-β-catenin, anti-glycogen synthase kinase-3β (GSK3β) and anti-phospho-GSK3β (all from Cell Signaling Technology, Danvers, MA, USA), and anti-β-actin (Rockland, Gilbertsville, PA, USA). Antibodies against RARα and PPARβ/δ were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
RNA Extraction and Quantitative Real-Time RT-qPCR
Total RNA from cell samples was isolated using TRIzol reagent (Life Technologies, New York, USA) following the manufacturer's protocol. One microgram of RNA was then used to make cDNA using q-script cDNA SuperMix (QuantaBiosciences, Gaithersburg, MD, USA). Real-time quantitative polymerase chain reaction (PCR) was performed with the ABI 7900 Sequence Detection and the ABI Power Sybr Green gene expression systems (Applied Biosystems, Foster City, CA, USA). Messenger RNA levels for PPARβ/δ, RARα, CYP26A1, angiopoietin-like protein 4 (ANGPTL4), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were quantified. GAPDH was used for normalization. Relative quantification of mRNA species was done using the comparative threshold (CT) cycles method. For each sample, we normalized the gene CT value using the formula: ΔCT = CT gene − CT GAPDH. For relative expression levels, the following formula was used: ΔΔCT = ΔCT sample − ΔCT calibrator. This value was then used to plot the gene expression employing the formula 2−ΔΔCT. Commercially available primers were used for PPARβ/δ, RARα, CRABP2, FABP5, CYP26A1, ANGPTL4, and GAPDH (QIAGEN, Valencia, CA, USA).
Transient Transfection of siRNA
For in vitro knockdown experiments, three different double-stranded small interfering RNA (siRNAs) against PPAR β/δ as well as nontargeting siRNA, were designed and synthesized by IDT company (Integrated DNA Technologies, Inc. Iowa, USA). The detailed sequences are shown in Electronic Supplementary Material (ESM) 1. Cells were seeded in six-well plates at a concentration of 1 × 105 cells/well. When the cells reached 70 % confluence, 10 nM of siRNAs were transfected using Lipofectamine RNAiMAX (Invitrogen), according to the manufacturer's recommendations. We incubated the cells for 48–72 h to allow knockdown of PPAR β/δ. The transfection and silencing efficiency were monitored by quantitative PCR. The endometrial cancer cells were divided into three groups: a nontransfected control group, a negative control group that was transfected with negative control siRNA (siRNA-NC), and the experimental transfected group. Cells were used for further assays.
Phosphokinase Proteome Profiling
Endometrial cancer cells were plated in 100-mm tissue culture dishes and cultured in DMEM supplemented with 10 % FBS until they reached 70 % confluence. After serum starvation for 18 h, cells were then cultured in phenol red-free DMEM supplemented with or without 10 μM GW0742 for 24 h. Cells were then processed following the human phosphokinase array kit (Proteome Profiler™; R&D Systems, Minneapolis, USA) instructions. Phosphokinase array data were developed on X-ray films following exposure to chemiluminescent reagents.
Statistical Analysis
All data were reported as mean ± SE from at least three independent experiments. Statistical differences between samples were determined by one-way ANOVA followed by post hoc multiple comparison testing using the Newman–Keuls procedure. P < 0.05 was considered statistically significant.
Results
Human Endometrial Cancer Cell Lines Express PPAR β/δ and RARα
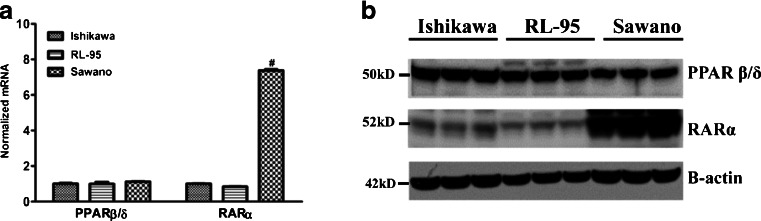
We investigated the expression of PPAR β/δ and RARα in the Ishikawa, RL-95, and Sawano human endometrial cancer cell lines using quantitative reverse-transcriptase-PCR (qRT-PCR). As shown in Fig. 1a, RARα mRNA expression varied among the cell lines, with the highest expression of RARα observed in the Sawano cells. Expression of PPAR β/δ mRNA was similar among the three endometrial cancer cell lines. Similar results were observed at the protein level; as shown in Fig. 1b, PPAR β/δ expression was comparable among all three cell lines and RARα protein levels were the highest in the Sawano cell line.
Fig. 1.
PPAR β/δ and RARα expression in Ishikawa, RL-95, and Sawano endometrial cancer cells. a PPAR β/δ and RARα mRNA expressions by RT-qPCR. b Immunoblot of endometrial cancer cell lines to detect protein expression of PPAR β/δ and RARα
Endometrial Cancer Cell Proliferation is Suppressed in the Presence of PPAR β/δ and RARα-Selective Agonists
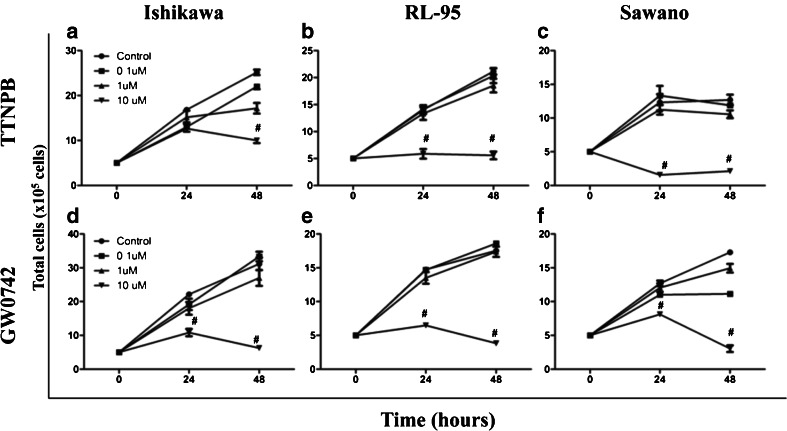
To determine the function of PPAR β/δ and RARα in endometrial cancer cells, we treated the cells for 24–48 h with either GW0742, a high affinity PPAR β/δ-selective agonist, or TTNPB, an RARα-selective agonist and quantified total cell number following treatment. As shown in (Fig. 2a–c), we found that after 48-h treatment, the total cell numbers significantly decreased in all three endometrial cancer cell lines treated with 10 μM TTNPB compared with controls. These results support previous data showing that activation of RARα inhibits cell proliferation [20]. Interestingly, a similar antiproliferative effect was observed in the three cell lines treated with GW0742, where total cell number decreased significantly 24 and 48 h following 10 μM GW0742 treatment (Fig. 2d–f), indicating that like RARα, PPAR β/δ may be a promising therapeutic target for endometrial cancer.
Fig. 2.
PPAR β/δ and RARα-selective agonists suppress proliferation of three different endometrial cancer cell lines (a–f). Cells were treated for up to 48 h with the indicated ligand concentrations and cell number was obtained using Trypan blue exclusion. a Ishikawa cells, b RL-95 cells, and c Sawano cells treated with increasing concentrations of TTNPB; d Ishikawa cells, e RL-95 cells, and f Sawano cells treated with increasing concentrations of GW0742. Values represent the mean ± standard error of the mean for experiments run in triplicate. # P < 0.05, significantly different than DMSO control
To further assess the effect on endometrial cancer cell viability, we used an MTS assay after TTNPB and GW0742 treatments. We observed that 10 μM TTNPB markedly decreased the viability of all three cell lines in a time-dependent manner (ESM 2a–c). A similar decrease in cell viability was observed with 10 μM GW0742 treatment (ESM 2d–f). These results demonstrate that, similar to RARα, ligand activation of PPAR β/δ inhibits the proliferation of human endometrial cancer cell lines. Given that the therapeutic potential of targeting RARα with selective agonists was previously established in endometrial carcinoma [15], we focused the rest of our studies on characterizing the therapeutic potential of PPAR β/δ in endometrial cancer.
PPAR β/δ-Selective Agonists Decrease Growth and Increase Apoptosis in Human Endometrial Cancer Cell Lines
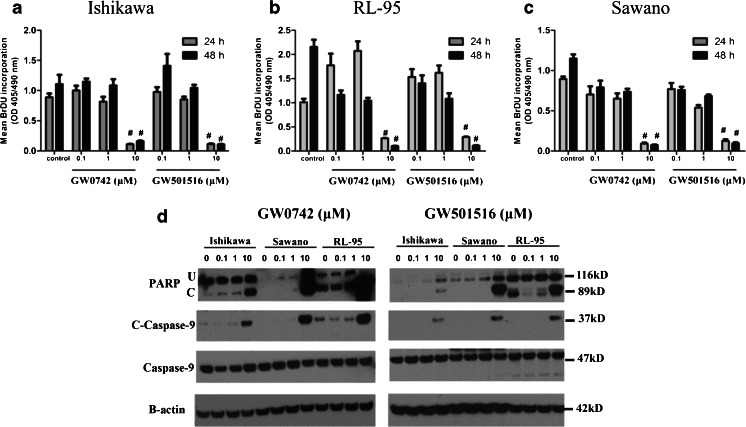
We used BrdU labeling to further confirm the growth-inhibiting effect of the PPAR β/δ-selective agonists GW0742 and GW501516 on the three endometrial cancer cell lines (Fig. 3). Although slight decreases in proliferation were observed after 24 and 48-h of PPAR β/δ-selective agonist treatment in the RL-95 and Sawano cell lines, only the 10 μM dose significantly decreased proliferation at these time points (Fig. 3a–c). Similar results were observed in the Ishikawa cell line, where 10 μM GW501516 and GW0742 significantly decreased cell proliferation after 24 and 48 h of treatments (Fig. 3a). Regarding apoptosis, we found that GW501516 and GW0742 also increased apoptosis in the three cell lines, as measured by cleaved PARP and cleaved caspase-9 levels (Fig. 3d). We observed that 10 μM GW501516 and GW0742 induced the cleavage of PARP and caspase-9 after 24 h of treatment. To verify the ability of the PPAR β/δ-selective agonists to induce apoptosis on an alternate human epithelial cell line, we treated the HaCaT with both GW0742 and GW501516 for 24 h (ESM 3). We observed that similar to the effects observed in the endometrial cancer cells, 10 μM GW501516 and GW0742 induced PARP cleavage in the HaCaT cells. Given that significant changes were observed within 24 h and using 10 μM GW501516 and 10 μM GW0742 treatment, we concluded that this dose was necessary to exert significant effects on cell proliferation and apoptosis.
Fig. 3.
PPARβ/δ-selective agonists GW0742 and GW501516 inhibit growth and activate apoptosis of endometrial cancer cells. Cells were treated for up to 48 h with the indicated ligand concentrations and cell proliferation was examined using BrdU incorporation assay in a Ishikawa, b RL-95, and c Sawano cells. d Immunoblot of apoptosis markers after treatment of cells with 0.1–10 μM GW0742 or GW501516 for 24 h. Cleaved PARP and cleaved caspase-9 were present in all three cell lines. Hybridization signals were normalized to β-actin. Values represent the mean ± standard error of the mean for experiments run in triplicate. # P < 0.05, significantly different from control. U Uncleaved PARP, C cleaved PARP. Blots are representative of n = 3 replicates
To confirm activation of PPARβ/δ by the two tested agonists, we quantified the gene expression levels of a well-characterized PPAR β/δ target gene, ANGPTL4, [21] in the three endometrial cancer cell lines after treatment with either GW0742 or GW501516 at all three doses. As expected, we observed that ANGPTL4 gene expression significantly increased in all cell lines in response to the 1 and 10 μM concentration of both agonists (ESM 4a, b).
PPAR β/δ Antagonists Abolish the Antiproliferative and Apoptotic Effects of PPAR β/δ Activation
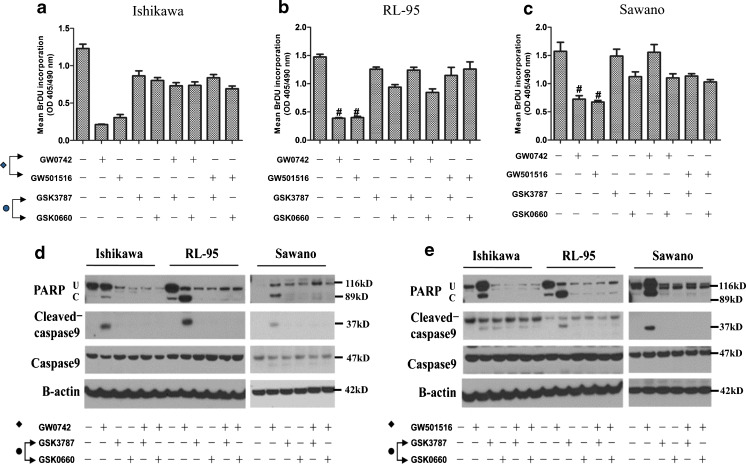
Next, we examined the ability of the PPAR β/δ-selective antagonists GSK3787 and GSK0660 to attenuate the observed apoptotic and antiproliferative effects of the PPAR β/δ selective agonists (GW0742 and GW501516) in the three human endometrial cancer cell lines (Fig. 4). BrdU labeling was performed after a 4-h pretreatment with either of the two PPAR β/δ-selective antagonists followed by a 24-h treatment with 10 μM concentrations of either PPAR β/δ-selective agonist. Pretreatment with either PPAR β/δ-selective antagonist completely abolished the antiproliferative effects elicited by the selective PPAR β/δ agonists in all three endometrial cancer cell lines (Fig. 4a–c). Apoptosis markers induced by the PPAR β/δ-agonists were also decreased in Ishikawa, RL-95, and Sawano cells following the same treatment conditions consisting of a 4-h antagonist pretreatment followed by 24-h treatment of 10 μM GW0742 or 10 μM GW501516 (Fig. 4d,e). These results demonstrate the specificity of PPAR β/δ effects on endometrial cancer cell proliferation and apoptosis.
Fig. 4.
Pretreatment with selective PPAR β/δ antagonists completely abolishes the antiproliferative and apoptotic effects of PPAR β/δ treatment. Cell proliferation measured by BrdU assay after a 4-h pretreatment of a Ishikawa, b RL-95, and c Sawano cell lines with either GSK3787 or GSK0660 PPAR β/δ antagonist followed by treatment with 10 μM of either GW0742 or GW501516 PPAR β/δ agonist for 24 h. Values represent the mean ± standard error of the mean for experiments run in triplicate. # P < 0.05, significantly different from control. d–e Immunoblot of apoptosis markers in cells pretreated for 4 h with either GSK3787 or GSK0660 PPAR β/δ selective antagonist followed by treatment with 10 μM concentration of d GW0742 or e GW501516 for 24 h. U uncleaved PARP and C cleaved PARP. Blots are representative of n = 3 replicates
PPAR β/δ Knockdown Eliminates the Effects of PPAR β/δ-Selective Agonists on Endometrial Cancer Cell Apoptosis and Gene Expression
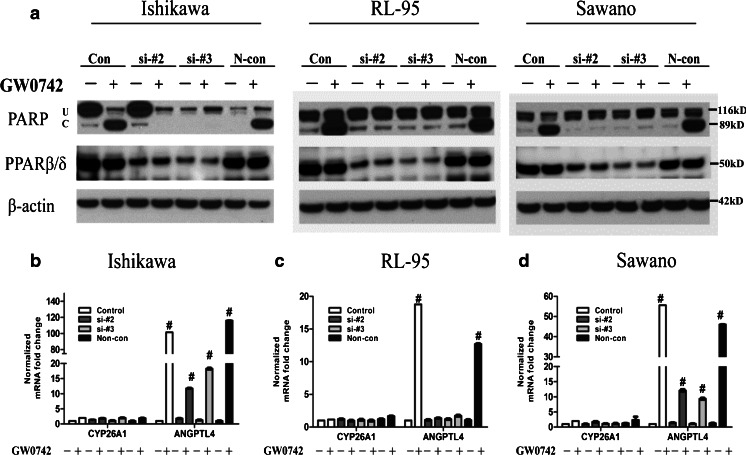
We knocked down PPAR β/δ expression with RNAi to confirm the functional importance of PPAR β/δ in mediating apoptosis in the three endometrial cancer cell lines. Successful PPAR β/δ gene silencing with two different siRNA oligos was confirmed by qRT-PCR (ESM 5a–c) and immunoblot (ESM 5d). Control and siRNA-transfected cells were treated with the PPAR β/δ-selective agonist GW0742 (10 μM, 24 h), and the protein levels of cleaved PARP were examined by immunoblot to determine apoptosis (Fig. 5a). As observed in our previous experiments, GW0742 induced PARP cleavage, whereas this effect was completely reversed with siRNA knockdown of PPAR β/δ. This indicates that the GW0742-induced apoptotic effects are mediated via PPAR β/δ activity.
Fig. 5.
PPARβ/δ knockdown attenuates the agonist-mediated apoptotic effects and target gene expression in endometrial cancer cells. a The effect of PPARβ/δ knockdown on PPARβ/δ-activated PARP cleavage was evaluated by immunoblot in the three endometrial cancer cell lines. Cells were treated with GW0742 (10 μM) for 24 h before assessing the effects on apoptosis. b–d The effect of PPARβ/δ knockdown on expression of the PPARβ/δ-dependent target gene ANGPTL4 and the RARα-target gene CYP26A1 was determined by qRT-PCR in b Ishikawa, c RL-95, and d Sawano cells following ligand activation of PPARβ/δ with 10 μM GW0742 for 24 h. Fold induction of mRNA was calculated from data normalized to GAPDH mRNA relative to control for each cell line. Values represent the mean ± standard error of the mean for experiments run in triplicate. # P < 0.05, significantly different from control. U Uncleaved PARP, C cleaved PARP. Blots are representative of n = 3 replicates
Others have suggested that the transactivation of RARα or PPAR β/δ is dependent not only on the ratio of FABP5 to CRABP-II but also on the RARα and PPAR β/δ expression levels [13]. This model suggests that in the absence of PPAR β/δ expression, the PPAR β/δ-selective agonist could transactivate RARα and induce target gene expression. Although we obtained significant PPAR β/δ knockdown, transactivation of the classic RARα target gene, CYP26A1, [15, 22] was not observed (Fig. 5b–d). These results suggest that the effects of GW0742 were primarily mediated by PPAR β/δ. However, we did observe a reduction in the classic PPAR β/δ target gene expression; ANGPTL4 mRNA levels significantly decreased after PPAR β/δ knockdown even in the presence of 10 μM GW0742 for 24 h (Fig. 5b–d). This indicates that siRNA-mediated knockdown of PPAR β/δ indeed decreases its transcriptional activity.
Activation of PPARβ/δ Inhibits the Proliferation of Endometrial Cancer Cells Through the AKT/GSK3β/β-Catenin Pathway
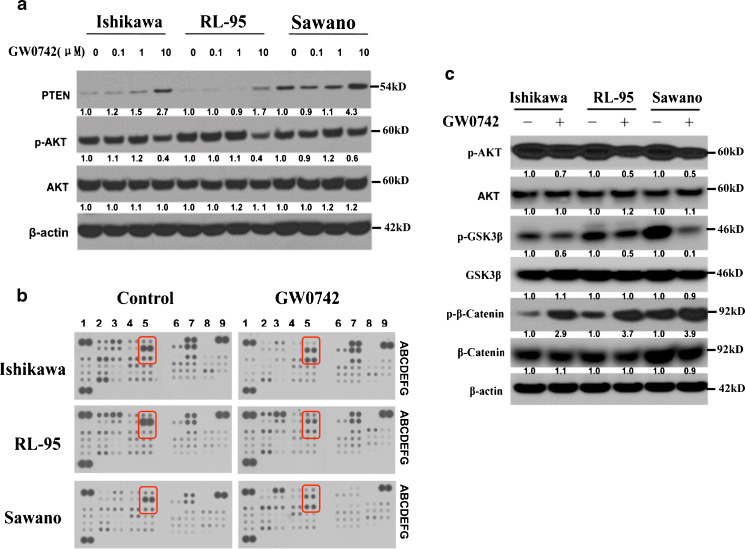
To characterize the mechanism by which PPAR β/δ activation exerts antiproliferative and apoptotic effects on endometrial cancer cells, we analyzed the expression of PTEN and AKT phosphorylation following GW0742 treatment. Others have shown that the AKT pathway is affected following the activation of PPAR β/δ by selective agonists [23]. Following treatment with increasing concentrations of the PPAR β/δ-selective agonist GW0742, all three cancer cell lines demonstrated increased expression of PTEN 10 (2.7-, 1.7-, 4.3-fold increase after 10 μM treatment in Ishikawa, RL-95, and Sawano cells, respectively, relative to vehicle) and lower phosphorylation of AKT (0.4-, 0.4-, and 0.6-fold decrease after 10 μM treatment in Ishikawa, RL-95, and Sawano cells, respectively, relative to vehicle; Fig. 6a). To fully characterize the signaling pathways affected by ligand activation of PPAR β/δ, we profiled 46 kinases and protein substrates using a phosphokinase array (Fig. 6b). All three cell lines were treated with 10 μM GW0742 for 24 h and cell lysates were hybridized to membranes to identify signaling pathways that were affected by PPAR β/δ activation. We found changes in the phosphorylation of GSK3β, AKT, and β-catenin after GW0742 treatment that was consistent among all three endometrial cancer cell lines (see coordinates A5, B5, and C5 on Fig. 6b and highlighted rows in Table 1). PPAR β/δ activation also resulted in decreased phosphorylation of AKT at Ser473 and decreased GSK3β phosphorylation, whereas β-catenin phosphorylation was markedly increased upon GW0742 treatment (Fig. 6b). The changes observed in the phosphokinase array were further validated in the endometrial cancer cell lines by immunoblot (Fig. 6c). The immunoblot results in fact validated the GW0742-induced changes on the phosphorylation states of AKT (0.7-, 0.5-, and 0.5-fold decrease after 10 μM GW0742 treatment in Ishikawa, RL-95, and Sawano cells, respectively), GSK3β (0.6-, 0.5-, and 0.1-fold decrease in Ishikawa, RL-95, and Sawano cells, respectively) and β-catenin (2.9-, 3.7-, and 3.9-fold increase in Ishikawa, RL-95, and Sawano cells, respectively), indicating that ligand activation of PPAR β/δ by GW0742 affects the AKT/GSK3β/β-catenin signaling pathway.
Fig. 6.
PPAR β/δ activation regulates components and protein targets of the AKT signaling pathway. a Immunoblots showing increased PTEN and decreased phosphorylation of AKT in all three cell lines following 24 h of increasing GW0742 concentrations. b Representative images from the phosphokinase arrays of Ishikawa, RL-95, and Sawano cells treated with GW0742 (10 μM) for 24 h. The red boxes outline the proteins that changed consistently after GW0742 treatment in all three cell lines. The red boxes correspond to the following coordinates, A5 (GSK3β), B5 (pAKT), and C5 (β-catenin). Each kinase is spotted in duplicate and the pairs of dots in each corner are positive controls. c Immunoblots demonstrating the effect of GW0742 treatment (10 μM, 24 h) on the phosphorylation of AKT and the downstream effectors GSK3β, β-catenin, and other protein targets of the AKT pathway. β-actin served as the loading control. Hybridization signals were normalized to β-actin and are presented as the fold change as compared to the control. Blots are representative of n = 3 replicates
Table 1.
Phosphokinase array coordinates used to identify PPARβ/δ-regulated signaling pathways in endometrial cancer cells
| Membrane/coordinate | Target | Cell type | ||
|---|---|---|---|---|
| Ishikawa | RL-95 | Sawano | ||
| A1 | Reference | |||
| A2 | p38α | ↓ | ↓ | |
| A3 | ERK1/2 | ↓ | ↑ | |
| A4 | JNK pan | ↓ | ||
| A5 | GSK-3α/β, S21/S9 | ↓ | ↓ | ↓ |
| A7 | p53 | ↓ | ||
| A9 | Reference | |||
| B2 | MEK1/2 | ↓ | ||
| B3 | MSK1/2 | ↓ | ||
| B4 | AMPKα1 | ↓ | ||
| B5 | AKT, S473 | ↓ | ↓ | ↓ |
| B7 | p53(s15) | ↓ | ||
| C1 | TOR | ↓ | ↑ | |
| C2 | CREB | ↓ | ↑ | |
| C3 | HSP27 | ↓ | ||
| C5 | β-catenin | ↑ | ↑ | ↑ |
| C6 | p70 S6 kinase | |||
| C8 | P27 | ↑ | ||
| D1 | Src | ↓ | ||
| D2 | Lyn | ↓ | ||
| E1 | Fyn | ↓ | ||
| E2 | Yes | ↓ | ||
| F1 | Hck | ↓ | ||
| F2 | Chk-2 | ↓ | ||
| F3 | FAK | ↓ | ||
| F4 | STAT6 | ↓ | ||
| F9 | Negative control | |||
| G1 | Reference spot | |||
| G3 | Negative control | |||
Effect of Ligand Activation of PPAR β/δ on the AKT/GSK3β/β-Catenin Pathway can be Attenuated by PPAR β/δ Knockdown and Lithium
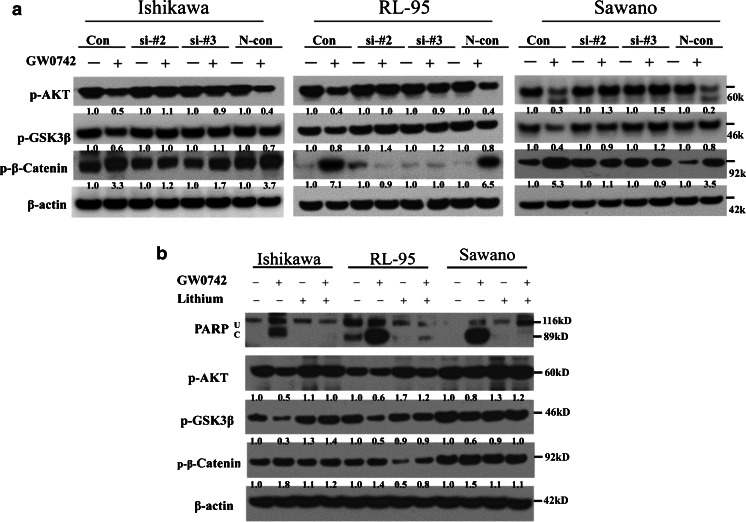
To assess the specificity of PPAR β/δ-induced changes on the regulation of the AKT/GSK3β/β-catenin signaling pathway, we performed siRNA knockdown of the receptor in combination with 10 μM GW0742 for 24 h. siRNA knockdown of PPAR β/δ in the three endometrial cancer cell lines reversed the GW0742-induced effects on AKT, GSK3β and β-catenin (Fig. 7a). Specifically, we observed that compared to the controls, PPAR β/δ knockdown reversed the GW0742-induced decrease in AKT phosphorylation (from 0.5 to 1.1, 0.4 to 1.0, and 0.3 to 1.3-fold increase in Ishikawa, RL-95, and Sawano cells treated with siPPAR β/δ #2, respectively), and abolished the observed increase in phospho-β-catenin (from 3.3 to 1.2, 7.1 to 0.9, and 5.3 to 1.1-fold decrease in Ishikawa, RL-95, and Sawano cells treated with siPPAR β/δ #2, respectively; Fig. 7a). Similarly, the GW0742-induced decrease in GSK3β phosphorylation was reversed by PPAR β/δ knockdown (from 0.6 to 1.0, 0.8 to 1.4, and 0.4 to 0.9-fold increase in Ishikawa, RL-95, and Sawano cells treated with siPPAR β/δ #2, respectively) in Fig. 7a. These results demonstrate the specificity of the receptor in regulating the AKT/GSK3β/β-catenin signaling pathway.
Fig. 7.
PPAR β/δ knockdown or lithium treatment attenuate the PPAR β/δ-effects on the AKT/GSK3β/β-catenin pathway and apoptosis. a Immunoblot of phosphorylated AKT, GSKβ, and β-catenin in control cells or cells transfected with PPAR β/δ siRNA or a nontargeting siRNA (N-con) and treated with GW0742 (10 μM, 24 h). b Immunoblot of PARP and phosphorylated AKT, GSK3β, and β-catenin after pretreatment of cancer cells with lithium and subsequent 24 h GW0742 (10 μM, 24 h) treatment. U Uncleaved PARP, C cleaved PARP. Hybridization signals were normalized to β-actin and are presented as the fold change as compared to the control. Blots are representative of n = 3 replicates
We used lithium as a signaling pathway inhibitor to test the specificity of PPAR β/δ on the AKT/GSK3β/β-catenin signaling pathway. Lithium is known to induce the phosphorylation and activation of AKT and to have inhibitory effects on GSK3β [24, 25]. We pretreated the cancer cell lines with lithium prior to GW0742 treatment to test the ability of lithium to reduce the GW0742-mediated effects. In the samples treated with the combination of lithium and GW0742, lithium attenuated the apoptotic effects of PPAR β/δ activation as indicated by decreased levels of cleaved PARP (Fig. 7b). We also observed that compared to the GW0742-treated samples, the combination of lithium and GW0742 restored the phosphorylation of both AKT (from 0.5 to 1.0, 0.6 to 1.2, and 0.8 to 1.2-fold increase in Ishikawa, RL-95, and Sawano cells lines, respectively) and GSK3β (from 0.3 to 1.4, 0.5 to 0.9, 0.6 to 1.0-fold increase, in Ishikawa, RL-95, and Sawano cells, respectively; Fig. 7b). Similarly, the combination of GW0742 and lithium restored the levels of β-catenin phosphorylation (from 1.8 to 1.2, 1.4 to 0.8, and 1.5 to 1.1-fold change in Ishikawa, RL-95, and Sawano cells, respectively), leading to a modest decrease in β-catenin phosphorylation in all three endometrial cancer cell lines. Overall, these results indicate that the ligand-mediated effects of PPAR β/δ on endometrial cancer cell apoptosis can be abrogated through the lithium-based pharmacologic activation of the AKT/GSK3β/β-catenin pathway.
Discussion
In this study, we demonstrated that ligand-activated PPARβ/δ regulates the proliferation and apoptosis of three different human endometrial cancer cell lines, and that the AKT/GSK3β/β-catenin pathway is involved in mediating these effects. PPARβ/δ activated with highly selective synthetic ligands led to a decrease in cell proliferation and an increase in markers of apoptosis (cleaved PARP and caspases). PPARβ/δ knockdown as well as pretreating the cells with PPARβ/δ antagonists or lithium could attenuate these effects. Our work reveals a unique function of PPARβ/δ in endometrial cancer cells and strongly supports the hypothesis that modulating PPARβ/δ activity may be a key therapeutic target for endometrial cancer interventions.
As a nuclear receptor involved in multiple physiological regulatory pathways, PPARβ/δ is an ideal drug target for the treatment of disease. However, the role of PPARβ/δ in human cancer has remained controversial because of disparities in the literature. Previous work by some laboratories suggested that activation of PPARβ/δ increased cell growth, while others showed that this effect was seen in only some cell types. Given the potential of targeting PPARβ/δ for the prevention and/or treatment of cancer, it is important to clarify the functional role of PPARβ/δ in cell proliferation and survival.
PPARβ/δ can be activated by several commercially available high-affinity synthetic PPARβ/δ ligands, including GW0742, GW501516, and L165041. These ligands can activate PPARβ/δ at concentrations in the low nanomolar o micromolar range [17, 26]. In this study, we exposed three different endometrial cancer cell lines to the ligands GW0742 and GW501516. Our results provide convincing evidence that in endometrial cancer cells, PPARβ/δ agonists decrease endometrial cancer cell proliferation and survival. We find that while PPARβ/δ agonists exert dose-dependent effects on target gene expression, the high 10 μM dose was necessary to significantly affect the AKT/GSK3β/β-catenin pathway and induce changes in proliferation and apoptosis. It is plausible that lower concentrations of these agonists can induce expression of individual genes, whereas high doses are necessary to alter more complex cellular events such as apoptosis or signal pathway activation, which may require coordinated changes in expression of a large number of genes. Overall, the observation of PPARβ/δ regulating cell proliferation and survival is consistent with previous work showing the PPARβ/δ-dependent inhibition of cell proliferation in keratinocytes and various human cancers [7, 27–30]
Because of the conflicting evidence, no unified, universally accepted mechanism of action has been put forward to describe the role of PPARβ/δ in cancer. Several mechanisms have been proposed to explain the observed procarcinogenic effects of PPARβ/δ, partly based on data from normal mouse primary keratinocytes [31]. Analyses of these cells suggested that the high ratio of intracellular FABP5 to CRABPII diverts atRA or PPARβ/δ ligands to PPARβ/δ rather than to the RARα. This is thought to increase PDPK1 to protect against apoptosis and increase cell survival. However, follow-up studies, including ours, have not validated this model [13, 14].
Another mechanism suggests that PPARβ/δ promotes cell survival via regulation of the ILK-PDPK1-PTEN-AKT pathway. The tumor suppressor gene PTEN, a negative regulator of the PI3K/AKT signaling pathway, can modulate cell growth, proliferation, and survival. PPARβ/δ was originally shown to inhibit the expression of PTEN in keratinocytes during wound healing [23]. Other studies found that this change in PTEN is not consistent [29, 32–34] and that the upregulation of PTEN expression by activated PPARβ/δ is context specific. Our study showed increased PTEN expression in the endometrial cancer cells treated with the PPARβ/δ agonist GW0742. Although we did not directly determine the effects of activated PPARβ/δ on PI3K activity, we did detect a loss of phosphorylated AKT, a known downstream target of PI3K. These results suggest that activated PPARβ/δ increases PTEN expression and downregulates the AKT signaling pathway to suppress proliferation and stimulate apoptosis in endometrial cancer cells.
GSK3 proteins are serine/threonine kinases that were originally identified as key regulatory enzymes in glucose metabolism [35]. There are two isoforms, GSK3α and GSK3β, that are encoded by separate genes [36]. GSK3β is an important effector of AKT activity and is also an important element of the PI3K/AKT cell survival pathway [37]. The activity of GSK3β is regulated by phosphorylation on the Ser9 residue; abnormal regulation of GSK3β is associated with various pathological conditions. Many upstream protein kinases, including AKT/PKB [38], are known to phosphorylate GSK3β at Ser9, depending on the cellular context and on various upstream regulators. In recent years, the role of GSK3β in cancer has become firmly established [39]. For example, it is known that GSK3β plays a major role in epithelial cell homeostasis and is a key factor in human cancers, regulating transcription, contributing to accelerated cell cycle progression, and to cancer cell metastasis and anti-apoptosis [40]. Despite its well-established role in other cancers, a detailed analysis of GSK3β in endometrial cancer and its therapeutic potential is still lacking.
In our study, GW0742 inactivated AKT and effectively decreased the phosphorylation of AKT on Ser473 in the endometrial cancer cell lines we tested. AKT phosphorylation negatively regulates GSK3β activity by phosphorylating it on Ser9 [41]. In our study, we observed that ligand activation of PPAR β/δ decreased the phosphorylation levels of AKT and GSK3β. GSK3β is known to phosphorylate β-catenin, an integral component of the canonical Wnt signaling pathway [42]. Unphosphorylated β-catenin is stable and accumulates in the cytoplasm; its nuclear translocation allows it to act as a transcriptional cofactor to promote cell survival and proliferation [43]. In our study, ligand activation of PPAR β/δ in endometrial cancer cell lines led to the increased phosphorylation of β-catenin, which has been previously shown to target it for proteosomal degradation [42]. Furthermore, lithium, which is known to indirectly regulate GSK3β through the PI3K/AKT pathway [24, 44], can enhance GSK3β activation and rescue the endometrial cancer cell lines from PPARβ/δ-induced apoptosis. Together, these results may partly explain the mechanism by which the PPAR β/δ-selective agonists cause apoptosis in endometrial cancer cells. These data also suggest that GSK3β may be a promising therapeutic target in endometrial cancer and pursuit of this therapeutic strategy is warranted.
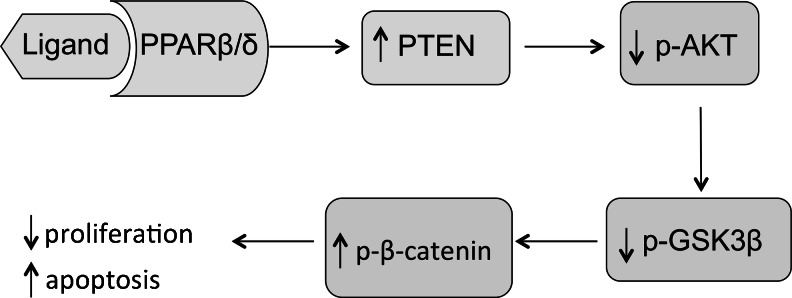
Based on the findings described above, we propose a model (Fig. 8) for the role of PPARβ/δ in modulating cell proliferation and apoptosis in endometrial cancer cells. After activation of PPARβ/δ, increased PTEN generation leads to decreased phosphorylation of AKT and GSK3β. These changes increase the phosphorylation of β-catenin, a post-translational modification that targets it for ubiquitin-dependent proteosomal degradation. Taken together, our findings indicate that in endometrial cancer cells, PPARβ/δ activation prevents cell proliferation and causes apoptosis, and that the regulation of the AKT/GSK3β/β-catenin pathway is involved.
Fig. 8.
Diagram demonstrating the ligand activated-PPAR β/δ regulation of proliferation and apoptosis of endometrial cancer cells. Ligand activation of PPARβ/δ increases PTEN expression and leads to decreased AKT phosphorylation on S473. Consequently, this reduction in AKT phosphorylation results in the reduced phosphorylation of GSK-3β that, in turn, decreases the stability of the β-catenin complex. Finally, destabilization and degradation of β-catenin is observed as its phosphorylation increases, resulting in decreased proliferation and apoptotic activation of the endometrial cancer cells
In conclusion, we demonstrated that ligand-activated PPARβ/δ regulates cell proliferation and apoptosis in endometrial cancer cells and that upregulation of PTEN and modulation of the AKT/GSK3β/β-catenin pathway may be involved. A full characterization of this pathway will be addressed in future studies. These results have important implications not only for understanding the molecular mechanisms of PPARβ/δ in endometrial cancer, but also by providing novel insights into the treatment of endometrial cancer.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
DOCX 47,422 kb
Footnotes
J.J.M. and D.M. contributed equally to this work.
References
- 1.Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet. 2012;379:1352–1360. doi: 10.1016/S0140-6736(12)60442-5. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Nout RA, Smit VT, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010;375:816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 4.Slomovitz BM, Coleman RL. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin Cancer Res. 2012;18:5856–5864. doi: 10.1158/1078-0432.CCR-12-0662. [DOI] [PubMed] [Google Scholar]
- 5.Panigrahy D, Kaipainen A, Kieran MW, Huang S. PPARs: a double-edged sword in cancer therapy? PPAR Res. 2008;2008:350351. doi: 10.1155/2008/350351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CH, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144:2201–2207. doi: 10.1210/en.2003-0288. [DOI] [PubMed] [Google Scholar]
- 7.Burdick AD, Kim DJ, Peraza MA, Gonzalez FJ, Peters JM. The role of peroxisome proliferator-activated receptor-beta/delta in epithelial cell growth and differentiation. Cell Signal. 2006;18:9–20. doi: 10.1016/j.cellsig.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer. 2012;12:181–195. doi: 10.1038/nrc3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 10.Tong BJ, Tan J, Tajeda L, Das SK, Chapman JA, et al. Heightened expression of cyclooxygenase-2 and peroxisome proliferator-activated receptor-delta in human endometrial adenocarcinoma. Neoplasia. 2000;2:483–490. doi: 10.1038/sj.neo.7900119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer. 2004;4:61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 12.Youssef J, Badr M. Peroxisome proliferator-activated receptors and cancer: challenges and opportunities. Br J Pharmacol. 2011;164:68–82. doi: 10.1111/j.1476-5381.2011.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rieck M, Meissner W, Ries S, Muller-Brusselbach S, Muller R. Ligand-mediated regulation of peroxisome proliferator-activated receptor (PPAR) beta/delta: a comparative analysis of PPAR-selective agonists and all-trans retinoic acid. Mol Pharmacol. 2008;74:1269–1277. doi: 10.1124/mol.108.050625. [DOI] [PubMed] [Google Scholar]
- 15.Cheng YH, Utsunomiya H, Pavone ME, Yin P, Bulun SE. Retinoic acid inhibits endometrial cancer cell growth via multiple genomic mechanisms. J Mol Endocrinol. 2011;46:139–153. doi: 10.1530/JME-10-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelton P. GW-501516 GlaxoSmithKline/ligand. Curr Opin Investig Drugs. 2006;7:360–370. [PubMed] [Google Scholar]
- 17.Sznaidman ML, Haffner CD, Maloney PR, Fivush A, Chao E, et al. Novel selective small molecule agonists for peroxisome proliferator-activated receptor delta (PPARdelta)—synthesis and biological activity. Bioorg Med Chem Lett. 2003;13:1517–1521. doi: 10.1016/S0960-894X(03)00207-5. [DOI] [PubMed] [Google Scholar]
- 18.Palkar PS, Borland MG, Naruhn S, Ferry CH, Lee C, et al. Cellular and pharmacological selectivity of the peroxisome proliferator-activated receptor-beta/delta antagonist GSK3787. Mol Pharmacol. 2010;78:419–430. doi: 10.1124/mol.110.065508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bissonnette RP, Brunner T, Lazarchik SB, Yoo NJ, Boehm MF, et al. 9-cis retinoic acid inhibition of activation-induced apoptosis is mediated via regulation of fas ligand and requires retinoic acid receptor and retinoid X receptor activation. Mol Cell Biol. 1995;15:5576–5585. doi: 10.1128/mcb.15.10.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spinella MJ, Kitareewan S, Mellado B, Sekula D, Khoo KS, et al. Specific retinoid receptors cooperate to signal growth suppression and maturation of human embryonal carcinoma cells. Oncogene. 1998;16:3471–3480. doi: 10.1038/sj.onc.1201876. [DOI] [PubMed] [Google Scholar]
- 21.Heinaniemi M, Uski JO, Degenhardt T, Carlberg C. Meta-analysis of primary target genes of peroxisome proliferator-activated receptors. Genome Biol. 2007;8:R147. doi: 10.1186/gb-2007-8-7-r147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahony S, Mazzoni EO, McCuine S, Young RA, Wichterle H, et al. Ligand-dependent dynamics of retinoic acid receptor binding during early neurogenesis. Genome Biol. 2011;12:R2. doi: 10.1186/gb-2011-12-1-r2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di-Poi N, Tan NS, Michalik L, Wahli W, Desvergne B. Antiapoptotic role of PPARbeta in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol Cell. 2002;10:721–733. doi: 10.1016/S1097-2765(02)00646-9. [DOI] [PubMed] [Google Scholar]
- 24.Chalecka-Franaszek E, Chuang DM. Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc Natl Acad Sci U S A. 1999;96:8745–8750. doi: 10.1073/pnas.96.15.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noble W, Planel E, Zehr C, Olm V, Meyerson J, et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berger J, Leibowitz MD, Doebber TW, Elbrecht A, Zhang B, et al. Novel peroxisome proliferator-activated receptor (PPAR) gamma and PPARdelta ligands produce distinct biological effects. J Biol Chem. 1999;274:6718–6725. doi: 10.1074/jbc.274.10.6718. [DOI] [PubMed] [Google Scholar]
- 27.Michalik L, Desvergne B, Tan NS, Basu-Modak S, Escher P, et al. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)alpha and PPARbeta mutant mice. J Cell Biol. 2001;154:799–814. doi: 10.1083/jcb.200011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters JM, Lee SS, Li W, Ward JM, Gavrilova O, et al. Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor beta(delta) Mol Cell Biol. 2000;20:5119–5128. doi: 10.1128/MCB.20.14.5119-5128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollingshead HE, Killins RL, Borland MG, Girroir EE, Billin AN, et al. Peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) ligands do not potentiate growth of human cancer cell lines. Carcinogenesis. 2007;28:2641–2649. doi: 10.1093/carcin/bgm183. [DOI] [PubMed] [Google Scholar]
- 30.Fukumoto K, Yano Y, Virgona N, Hagiwara H, Sato H, et al. Peroxisome proliferator-activated receptor delta as a molecular target to regulate lung cancer cell growth. FEBS Lett. 2005;579:3829–3836. doi: 10.1016/j.febslet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Peters JM, Gonzalez FJ. Sorting out the functional role(s) of peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) in cell proliferation and cancer. Biochim Biophys Acta. 2009;1796:230–241. doi: 10.1016/j.bbcan.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim DJ, Bility MT, Billin AN, Willson TM, Gonzalez FJ, et al. PPARbeta/delta selectively induces differentiation and inhibits cell proliferation. Cell Death Differ. 2006;13:53–60. doi: 10.1038/sj.cdd.4401713. [DOI] [PubMed] [Google Scholar]
- 33.Marin HE, Peraza MA, Billin AN, Willson TM, Ward JM, et al. Ligand activation of peroxisome proliferator-activated receptor beta inhibits colon carcinogenesis. Cancer Res. 2006;66:4394–4401. doi: 10.1158/0008-5472.CAN-05-4277. [DOI] [PubMed] [Google Scholar]
- 34.Burdick AD, Bility MT, Girroir EE, Billin AN, Willson TM, et al. Ligand activation of peroxisome proliferator-activated receptor-beta/delta(PPARbeta/delta) inhibits cell growth of human N/TERT-1 keratinocytes. Cell Signal. 2007;19:1163–1171. doi: 10.1016/j.cellsig.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fung TK, Gandillet A, So CW. Selective treatment of mixed-lineage leukemia leukemic stem cells through targeting glycogen synthase kinase 3 and the canonical Wnt/beta-catenin pathway. Curr Opin Hematol. 2012;19:280–286. doi: 10.1097/MOH.0b013e3283545615. [DOI] [PubMed] [Google Scholar]
- 38.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 39.Mishra R. Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol Cancer. 2010;9:144. doi: 10.1186/1476-4598-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo J. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;273:194–200. doi: 10.1016/j.canlet.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muyllaert D, Kremer A, Jaworski T, Borghgraef P, Devijver H, et al. Glycogen synthase kinase-3beta, or a link between amyloid and tau pathology? Genes Brain Behav. 2008;7(Suppl 1):57–66. doi: 10.1111/j.1601-183X.2007.00376.x. [DOI] [PubMed] [Google Scholar]
- 42.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/S0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 43.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowe MK, Chuang DM. Lithium neuroprotection: molecular mechanisms and clinical implications. Expert Rev Mol Med. 2004;6:1–18. doi: 10.1017/S1462399404008385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DOCX 47,422 kb