Abstract
IRAK1 is a key regulatory protein in TLR/IL1R-mediated cell activation during the inflammatory response. Studies indicated that pending on the nature of the used inflammatory model, down-regulation of IRAK1 may be beneficial or detrimental. However the role of IRAK1 in affecting outcome in polymicrobial sepsis is unknown. We tested this question using an IRAK1 deficient mouse strain and the cecal ligation and puncture (CLP) procedure, which is a clinically relevant rodent septic model. Sepsis-induced mortality was markedly lower in IRAK1-deficient mice (35%) compared to WT (85%). Sepsis-induced increases in blood IL-6 and IL-10 levels were blunted at 6h post-CLP in IRAK1 deficiency compared to WT but cytokine levels were similar at 20h post-CLP. Sepsis induced blood granulocytosis and depletion of splenic B cells were also blunted in IRAK1 deficient mice as compared to WT. Analysis of TLR-mediated cytokine responses by IRAK1 deficient and WT macrophages ex vivo indicated a TLR4-dependent down-regulation of IL-6 and IL1β in IRAK1 deficiency, whereas TLR2 dependent responses were unaffected. TLR7/8-mediated IL-6, IL1β and IL-10 production was also blunted in IRAK1 macrophages as compared to WT. The study shows that IRAK1 deficiency impacts multiple TLR-dependent pathways and decreases early cytokine responses following polymicrobial sepsis. The delayed inflammatory response caused by the lack of IRAK1 expression is beneficial, as it manifests a markedly increased chance of survival after polymicrobial sepsis.
Keywords: Infection, TLR, signaling, macrophages, survival, Interleukin
Introduction
IRAK1 (Interleukin receptor associated kinase-1) is a regulatory protein with key functions in mediating immune cell activation (1–3). Upon certain physiological or pathological stimuli, ligands binding to TLR or IL-1R receptors initiate the reorganization of the intracellular domain of the receptor protein complex with subsequent recruitment of regulatory proteins including MyD88 and IRAK4. IRAK1 also associates with the complex and becomes phosphorylated by IRAK4 (1–3). Depending on the cell type, phosphorylated IRAK1 may become part of different signaling protein complexes including the TRAF6/TAK1/TAB1,2 complex or it may interact with interferon regulatory factors (IRFs) or Sumo proteins leading to the activation of nuclear factor kappa B, MAP kinases or IRFs with subsequent transcriptional activation of target genes (2,3). The regulatory role of IRAK1 in cell signaling is complex and depends not only on the initial TLR stimuli but also on the presence of redundant or interacting signaling pathways in a particular cell type. Nevertheless, because the effect of active IRAK1 promotes cell activation, it is expected that inhibition of IRAK1-dependent signaling would dampen cell activation and inflammatory responses.
After the development of IRAK1 knockout mice (4), the effects of IRAK1 deficiency have been tested in various in vitro and in vivo systems. Studies showed impaired NFκB activation and TNFα and IL-6 production following IL-1β stimulation in vitro and in vivo (4). Other studies indicated that IRAK1 regulates not only NFkB and MAPK-dependent cytokine productions (5), but also IL-10 (6) and type-I Interferon expression (7,8) through not yet fully elucidated cross talk among signaling pathways.
The impact of IRAK1 activation or the lack of, on clinical outcome is expected to be influenced by the unique pathology of the particular inflammatory condition. Consistent with this notion, it has been shown that IRAK1 deficiency improved myocardial contractile dysfunction following burn (9,10) and was beneficial in autoimmune conditions associated with hyperinflammation (11,12). Using acute endotoxicosis models, IRAK1-deficient mice presented decreased TNFα release, alleviated myocardial dysfunction and improved survival as compared to WT (10,13). Exhaustion of IRAK1 activity rendered by repeated endotoxin administration was shown to mediate endotoxin tolerance (14,15). In contrast, IRAK- deficient mice were more susceptible to iv administration of high dose live Staphylococcus aureus than WT controls (16). The direct clinical relevance of these observations however is not readily evident because high blood levels of bacterial endotoxins are seldom observed in human clinical conditions. Likewise, massive bacterial load through the blood stream, which is modeled by iv infusion of live bacteria, occurs rarely in clinical conditions especially in the absence of accompanying systemic or massive local inflammation. Therefore, it is important to further elucidate the effect of IRAK1 deficiency in clinically more relevant septic inflammatory models.
Septic peritonitis induced by the cecal ligation and puncture (CLP) procedure is accepted as a clinically relevant polymicrobial sepsis model in rodents (17–19). CLP initiates an acute peritonitis, which leads to an inflammatory response and septicemia that is reminiscent to that observed in septic patients. Therefore, the aim of the study was to test the effect of IRAK1 deficiency in CLP-initiated sepsis. We compared sepsis-induced mortality and level of bacteremia between WT and IRAK1 deficient subjects. Differences in the systemic inflammatory response were assessed by comparing blood and organ cytokine levels. Phagocyte and lymphocyte cell composition changes in selected organs were determined to assess cell trafficking and lymphocyte dysfunction. Finally, because multiple TLR-dependent pathways are activated during in vivo sepsis, we also tested TLR-induced cytokine responses by IRAK1 deficient and WT macrophages ex vivo.
Methods
Reagents
Endotoxin-free, cell culture grade buffers, media and reagents were used in the experiments. Flourochrome conjugated antibodies, assay diluents, lysing and permeabilizing flow cytometry solutions and kits were purchased from BD Biosiences and BD Pharmingen. All other reagents and chemicals of the highest grade available were purchased from Sigma-Aldrich Co. (St. Louis, MO).
Animals and Cecal Ligation and Puncture (CLP)
Male IRAK1 deficient (−/y) and WT (+/y, 57BL6J) mice were bred in parallel colonies at our animal facility. 10–16 week old animals were used in the experiments. The initial IRAK1-deficient breeders on BL6 background were obtained from James Thomas, University of Texas Southwestern Medical Center. Animals purchased from the Jackson Laboratory were used as breeders for the WT colony.
Polymicrobial septic peritonitis was induced using the cecal ligation and puncture (CLP) model as described earlier (20,21). Briefly, animals were anesthetized by an ip injection of Nembutal (5mg/100g bw). A midline abdominal incision was made. The cecum was exposed, ligated and punctured through opposing walls at two sites with a 20-gauge hypodermic needle. Animals were resuscitated by the subcutaneous injection of isotonic, pyrogen-free saline solution (0.04 ml/g bw) immediately postoperatively, and also at 20h post-CLP. When animals were followed for longer than 24 hours they received daily saline resuscitations at the same dose. In pilot experiment we compared naïve controls and sham operations (opening the abdomen, moving the intestine but no ligation or puncture) and found no remarkable increase in inflammatory markers (22). Thus, in these experiments we used non-treated naïve animals as controls.
The studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the New Jersey Medical School.
Genotyping
The IRAK1 KO mutant mouse was generated by replacing 7kb gene with neomycin selection cassette. The deleted region included 3.5kb of the upstream regulatory region and all of exons from 1–8 and a portion of exon9 (4). Total genomic DNA was isolated from tail clippings using the RED Extract-N-Amp Tissue PCR kit (Sigma-Aldrich). DNA was subjected to PCR amplification using forward primers complementary to the PGKneo insert or WT sequences, respectively, and a common downstream primer. Forward primers, WT: 5′-GCAAGCCAGAGCAGTACTGTG-3′; IRAK1 KO(NEO)-F: 5′-GCCTTCTATCGCCTTCTTGACG-3′; common reverse primer: 5′-GCCTCTGTAAGAGATCAGGTAG-3′.
PCR reaction was carried out in the presence of 2 mM MgCl2 with the following cycling: 94°C for 2 min; followed by 35 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 2 min. 30 s; with the final elongation of 72°C for 7 min. PCR amplicons were resolved on 0.8% agarose gels.
Blood, splenocyte and bone marrow (BM) cell isolation and incubations
Blood was collected into heparinized tubes via cardiac puncture from fully anesthetized animals. Following the exsanguination, femurs were collected from the same animals. Femurs were cut at the diaphyses and BM cells flushed out by repeated injections of phosphate buffered saline (PBS) containing 10 % FBS through the bone channel. BM cells were sedimented and washed by centrifugation and suspended in a final volume to obtain 10-million/ml cells in the same PBS/FBS buffer. Next, the spleen was removed and placed into DMEM containing 10% FBS and penicillin streptomycin solution. Hypodermic needles were used to pull apart the splenic capsule releasing spleen cells into suspension. The cell suspension together with the remaining splenic capsule was squeezed through a 70μm nylon mesh cell strainer. Isolated bone marrow cells or splenocytes were resuspended in DMEM containing 1 % FBS for subsequent analyses or in vitro incubations.
Macrophage isolation and treatments
Peritoneal macrophages were isolated as described previously (23). Briefly, mice were injected with 2.5 ml sterile Brewer’s thioglycolate broth ip. On day 4, peritoneal cells were harvested, washed repeatedly (300 × g for 15 min. at 4°C) and re-suspended in DMEM containing 20 mM HEPES, 4 mM glutamine, penicillin-streptomycin solution and 10% FBS. Cells were analyzed by flow cytometry for the dual expression of macrophage markers F4/80 and CD11b. More than 95% of cells were positive for these markers and yields were also similar in WT and IRAK1 deficient animals. Cells were seeded into Falcon 24-well tissue culture plates (0.6 × 105 cells/well) and incubated at 37°C (95% air/5%CO2) to allow cells to adhere for 60 min prior to administration of stimulants. Adherent cells were incubated with DMEM containing 1% FBS and penicillin streptomycin solution in the presence of vehicle, or different TLR agonists: TLR4 (ultrapure LPS 100 ng/ml), TLR2 (Pam3CSK4 200ng/ml), TLR7/8 agonist (Thiazoloquinoline, 2.5 μg/ml)) or opsonized heat-killed E. coli (HKB, 8×105/ml). All agents used in the experiments were dissolved in media as vehicle, unless otherwise stated. After various treatments, the media were collected and cytokine content was determined by enzyme linked immuno-absorbance assay (ELISA) according to the manufacturer’s protocol. Standards were run on each ELISA plate and analyzed in parallel with the test samples.
Flow cytometry
BM, blood and spleen flow cytometry analyses and gating strategy has been described in detail previously (24). Briefly, the number of PMNs and lymphocyte subsets in blood, spleen and thymus was determined by the number of total cell counts and the percent distribution of CD3+CD4+, CD3+CD8+ T-cells, CD19+ B-cells and CD11b+ myeloid cells using antibodies against CD markers conjugated with FITC, APC, PERCP or PE (BD Biosciences) in three or four-color incubations. BM cell composition was determined by the cell distribution of CD45+CD19+CD11b− (B-cells), CD11b+CD45+CD19− (myeloid cells). Aliquots of 0.1 ml whole blood, splenocyte or BM cell suspension were incubated with the respective markers for 15 min followed by incubation with BD FACS lysing solution (BD Biosciences) for 7 min at 37°C. Cells were washed twice with BD FACS wash buffer and then fixed with 1% methanol free formaldehyde. FACS acquisitions were performed in a centralized flow cytometry facility. At least 30,000 events were collected for each analysis.
Blood differentials and cell counts in BM, spleen and thymus were determined using a computerized cell counter (Hematrue Veterinary Hematology Analyzer, Heska Corporation, Loveland, CO).
ELISAs, enzyme assays and determination of blood bacterial counts
ELISA kits for IL-10, IL-6, IL1β were purchased from BD biosciences (San Jose, CA), Mip-2 (Dy452E) from R&D Systems (Minneapolis, MN). Plasma from freshly drawn heparinized blood was stored at −85°C till analysis. ELISAS were performed according to the manufacturer’s protocol. All the compared samples from different genotypes were run simultaneously in duplicates on one plate. Values were determined from a calibration curve run parallel with the samples.
Bacterial counts were determined from a 0.1-ml blood sample collected under sterile conditions and diluted serially in sterile physiological saline. 0.05ml of each dilution was aseptically plated on trypticase blood agar plates (BD Biosciences, San Jose, CA) and after 24h incubation at 37 °C, the number of bacterial colonies was counted.
Statistical analysis
Statistical calculations were performed using JMP software (SAS Institute Inc., Cary, NC). Results were analyzed using ANOVA followed by t-test for pair-wise comparisons or Tukey-Kramer’s test for multiple comparisons. We used the Long Rank Test to assess survival differences among groups. Different study components were performed on 6–8 different animals from each of the in vivo treatment groups unless indicated otherwise. Significant difference was concluded at p<0.05 unless otherwise noted.
Results
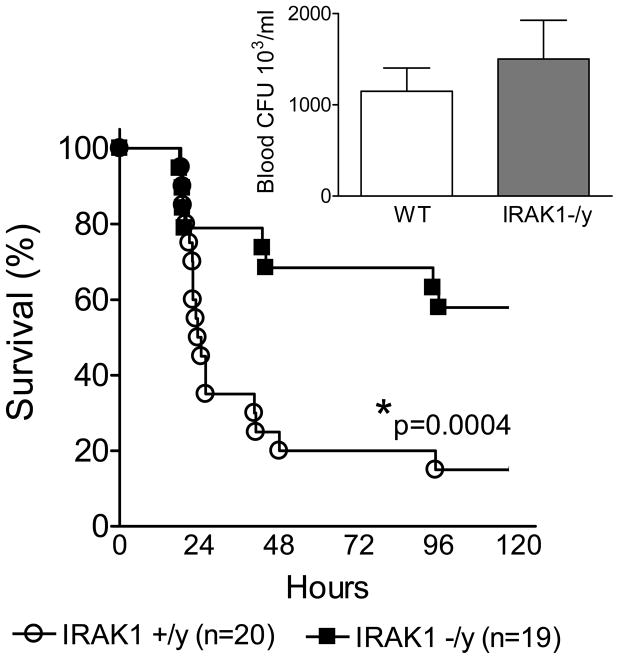
Cecal ligation and puncture-induced sepsis (CLP) manifests many similarities to that observed in human peritonitis-induced clinical sepsis (18,19,25,26). First, we compared CLP-induced mortality between IRAK1 deficient and WT animals. Fig 1 indicates that CLP resulted in about 85 % mortality in WT mice whereas mortality was only about 35% in IRAK1 deficient animals. The difference in sepsis induced mortality was statistically significant between WT and IRAK1 deficient group (p<0.001).
Fig 1. IRAK1 deficiency improves sepsis survival.
WT and IRAK1 deficient animals were made septic by cecal ligation and puncture (CLP). Animals received fluid resuscitation postoperatively and then repeatedly at every 24h and were observed for mortality. Deficient animals showed improved survival as compared to WT. * Statistically significant difference compared to WT (n=19–20 in each group). Insert indicates bacterial counts in blood 20h post-CLP from a separate set of experiments (n=19 mice in each group).
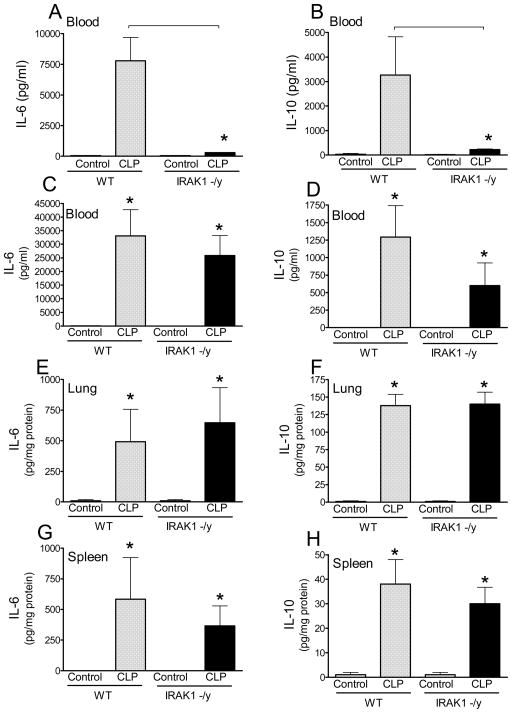
We also tested bacterial colony forming units in blood 20h after CLP, which is a time point when animal loss is negligible and thus observations are not affected by survival bias. We found that bacteremia was similar in WT and IRAK1 deficient mice (Fig 1 insert). This indicated that the survival advantage in IRAK1 deficiency is likely to be associated with changes in the severity of inflammation rather than differences in bacterial load. Therefore, next we compared blood and tissue concentrations of IL-6 and IL-10 which are important cytokine mediators of the inflammatory response during sepsis (27–30). Fig 2 indicates that blood IL-6 and IL-10 was elevated in WT mice at 6h after CLP whereas IRAK1 deficient mice showed negligible levels of these cytokines (Fig 2AB). In contrast, blood IL-6 and IL-10 levels were similar in WT and IRAK1 deficient mice 20h after CLP (Fig 2CD). IL-6 and IL-10 content in lung and spleen was below detection limit at 6h post-CLP (data not shown) and similar at 20h post-CLP in IRAK1 and WT mice (Fig 2E–H).
Fig 2. Role of IRAK1 in early cytokine release following sepsis.
Blood and tissues were collected at 6h and 20h after CLP and plasma cytokine content was determined using ELISA as described in the materials and methods section. Mean ± S.E.M., n=5–8 animals in each group. *Statistically significant difference as compared to control or as indicated by connecting line (p<0.05, Mean ± S.E.M.).
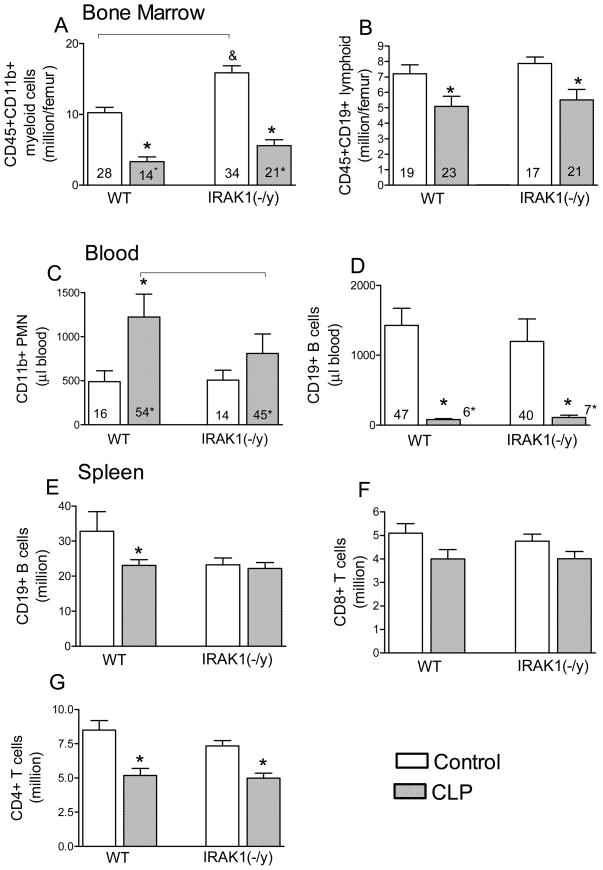
Sepsis results in changes in immune cell composition in blood as well as immune competent organs due to inter-organ cell trafficking, cell proliferation or apoptosis. Thus, we determined CLP-induced cell composition changes in blood and selected organs from WT and IRAK1 deficient mice at the 20h post-CLP when animal death is still negligible (Fig 3). Myeloid cell content in BM was elevated in IRAK1 deficient mice as compared to WT under control non-challenged conditions. Sepsis depleted BM myeloid as well as lymphoid cell content in WT and deficient mice similarly (Fig 3A,B). Sepsis-induced increase in the number of circulating neutrophils was blunted in IRAK1 animals as compared to WT but the marked depletion of circulating B cells was similar in WT and IRAK1 deficient mice (Fig 3C,D). In contrast, splenic B-cell content was depleted in WT but not in IRAK1 deficient septic mice (Fg 3 E), whereas sepsis-induced changes in CD8 and CD 4 T cell content in spleen (Fig 3F,G) or thymus (not shown) were similar in WT and IRAK1 deficient animals.
Fig 3. The effect of IRAK1 deficiency on BM, blood and spleen cell composition following sepsis.
From control or CLP-subjected mice (20h), the total numbers of major WBC subtypes (bars) as well as percent distribution of cells (numbers within bars) were determined. *Statistically significant difference as compared to control or as indicated by connecting line (p<0.05, Mean ± S.E.M.; n=6–8 animals in each group).
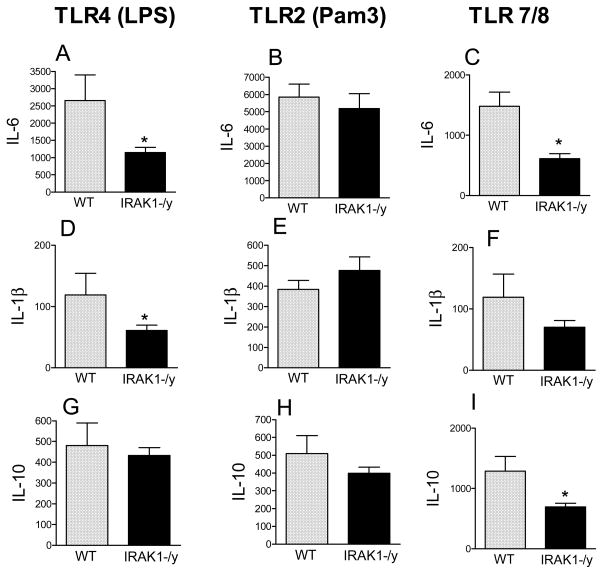
Because macrophages are major producers of cytokines during sepsis we compared cytokine responses by peritoneal macrophages from WT or IRAK1 deficient mice following stimulation by various TLR agonists in vitro (Fig 4). Ultrapure LPS and Pam3CSK4 were used as selective TLR4 and TLR2 activators, whereas thiazoloquinoline was employed to stimulate TLR7/TLR8-mediated responses.
Fig 4. The impact of IRAK1 deficiency depends on the TLR stimulus.
Peritoneal macrophages from WT and IRAK1 deficient mice were incubated in the presence of different TLR agonists; TLR4 (ultrapure LPS 100 ng/ml), TLR2 (Pam3CSK4 200ng/ml) or TLR7/8 (Thiazoloquinoline, 2.5 μg/ml). Following 20h incubation, cytokines were measured in media. *Statistically significant difference as compared to WT (p<0.05, Mean ± S.E.M.; n=5–7 animals in each group).
Fig 4 shows that after TLR4 activation, IL-6 and IL-1β responses were blunted in IRAK1 deficient macrophages as compared to WT (Fig 4A,D). IL-6 and IL-1β responses were also partially decreased following TLR7/8 activation in IRAK1 deficiency (Fig 4C,F). However, IL-6 and IL-1β production was similar after TLR2 stimulus in WT and IRAK1-deficient macrophages (FIG 4B,E). IL-10 production was unaffected by IRAK1 deficiency after TLR4 or TLR2 stimulus, however, IL-10 production was lower in IRAK1 deficient than WT macrophages following TLR7/8 activation (Fig 3G,H,I). In the presence of vehicle, cytokine production was negligible in WT or IRAK1 deficient macrophages (not shown).
Discussion
This study demonstrates for the first time that the lack of IRAK1-mediated signaling down-regulates the early phase of cytokine cascade during peritonitis-initiated sepsis and improves the survival of septic subjects. The observations from this clinically relevant septic rodent model are in agreement with human investigations which demonstrated that a variant IRAK1 haplotype with an increased functional IRAK1 activity worsens outcome from sepsis and septic shock (31,32). Thus, our current findings together with other independent studies (2,9,10,13,31–33) support the notion that IRAK1-mediated signaling is a key component of the regulatory pathways, which have direct and determining roles in clinical outcome during inflammatory conditions.
The observation that IRAK1 deficiency inhibited systemic cytokine responses only at the early phase of the septic response indicates that cellular activation is delayed initially however, at later stages of sepsis redundant signaling or other mechanisms may compensate for lack of IRAK1. Additionally, sepsis survival was markedly improved in IRAK1 deficiency despite the fact that the degree of inflammation, as assessed by blood, BM, spleen and lung cytokine levels, was similar at the pre-mortal (20h) stage of sepsis. These observations suggest that the early phase of the septic response may be critical in determining outcome. This conclusion is in agreement with previous studies, which indicated that although IL-6 was not causative in mediating outcome, elevated blood IL-6 concentration measured at 6h post-CLP was a good predictor of mortality (27,28).
The findings indicating delayed inflammation in IRAK1 deficient mice by reduced mobilization of circulating neutrophils and blunted splenic B-cell depletion following sepsis are important because both neutrophils and B-cells play important roles in sepsis pathology. Mobilization and infiltration of activated neutrophils contribute to sepsis-associated organ dysfunction and damage (34,35) whereas B cell depletion is an important component of septic immune-paralysis (36–38). Thus, the blunted mobilization of IRAK1-deficient neutrophils together with lessened depletion of tissue-resident B cells is a possible contributing mechanism resulting in improved sepsis survival of IRAK1 deficient subjects.
Our in vitro observations on isolated macrophages following the activation of different TLRs suggests an IRAK1-dependent interplay among various signaling pathways (39,40). The observed decrease in TLR4-mediated IL1β and IL-6 production in IRAK1 deficiency is in agreement with previous studies (4). The lack of impact of IRAK1 deficiency on cytokine production following TLR2 stimulus indicates the presence of compensatory mechanisms independent of IRAK1 for TLR2-mediated IL-6 and IL1β production in macrophages (39,41). Polymicrobial sepsis is expected to activate multiple TLR signaling pathways thus, the observation that IRAK1 deficiency delayed the onset of inflammation suggests that IRAK1 is important for early post-sepsis signaling via TLR4 and TLR7/8, whereas the inflammatory response at the later stages of sepsis is driven by other TLR-dependent pathways or redundant signaling mechanisms. It remains to be elucidated whether the presence of alternative signaling mechanisms in IRAK1 deficiency is the result of modulated differentiation during development or alternatively, the signaling pathways mediating the inflammatory response during polymicrobial sepsis are inherently redundant.
Efficient elimination of invading bacteria without harming the septic host requires a balanced inflammatory response. Thus, complete and broad inhibition of either pro or anti-inflammatory components during the septic response is unlikely to benefit the host. In contrast, partial inhibition or delay in the onset of cell activation and associated inflammatory responses is likely to be advantageous in septic conditions. The fact that our observations indicated improvement in survival in IRAK1 deficiency even in the evident presence of redundant signaling mechanisms supports the notion that a partial inhibition or delay in the onset of cell activation is likely to be advantageous during septic conditions.
In summary, our study indicates a positive effect of IRAK1 inhibition during a polymicrobial septic response, which is manifested in a delayed onset of inflammation and improved survival. Lack of IRAK1 signaling only partially alleviates inflammation due to the presence of redundant pathways following mixed TLR stimuli. These observations together with other animal (9,10,14,15) and human studies (31,32) support the notion that down regulation of cell activation through targeting IRAK1 may be beneficial in clinical conditions, which predisposes the host to subsequent septic complications.
Acknowledgments
This study was supported by NIH-NIGMS grant GM084932
Abbreviations
- PMN
polymorphonuclear neutrophils
- BM
Bone marrow
Reference List
- 1.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 2.Gottipati S, Rao NL, Fung-Leung WP. IRAK1: a critical signaling mediator of innate immunity. Cell Signal. 2008;20:269–276. doi: 10.1016/j.cellsig.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Janssens S, Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell. 2003;11:293–302. doi: 10.1016/s1097-2765(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 4.Thomas JA, Allen JL, Tsen M, Dubnicoff T, Danao J, Liao XC, Cao Z, Wasserman SA. Impaired cytokine signaling in mice lacking the IL-1 receptor-associated kinase. J Immunol. 1999;163:978–984. [PubMed] [Google Scholar]
- 5.Tiwari RL, Singh V, Singh A, Barthwal MK. IL-1R-associated kinase-1 mediates protein kinase Cdelta-induced IL-1beta production in monocytes. J Immunol. 2011;187:2632–2645. doi: 10.4049/jimmunol.1002526. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y, Li T, Sane DC, Li L. IRAK1 serves as a novel regulator essential for lipopolysaccharide-induced interleukin-10 gene expression. J Biol Chem. 2004;279:51697–51703. doi: 10.1074/jbc.M410369200. [DOI] [PubMed] [Google Scholar]
- 7.Uematsu S, Sato S, Yamamoto M, Hirotani T, Kato H, Takeshita F, Matsuda M, Coban C, Ishii KJ, Kawai T, Takeuchi O, Akira S. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction. J Exp Med. 2005;201:915–923. doi: 10.1084/jem.20042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu YC, Simmons DP, Li X, Abbott DW, Boom WH, Harding CV. TLR2 signaling depletes IRAK1 and inhibits induction of type I IFN by TLR7/9. J Immunol. 2012;188:1019–1026. doi: 10.4049/jimmunol.1102181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas JA, Tsen MF, White DJ, Horton JW. IRAK contributes to burn-triggered myocardial contractile dysfunction. Am J Physiol Heart Circ Physiol. 2002;283:H829–H836. doi: 10.1152/ajpheart.00416.2001. [DOI] [PubMed] [Google Scholar]
- 10.Thomas JA, Haudek SB, Koroglu T, Tsen MF, Bryant DD, White DJ, Kusewitt DF, Horton JW, Giroir BP. IRAK1 deletion disrupts cardiac Toll/IL-1 signaling and protects against contractile dysfunction. Am J Physiol Heart Circ Physiol. 2003;285:H597–H606. doi: 10.1152/ajpheart.0655.2001. [DOI] [PubMed] [Google Scholar]
- 11.Deng C, Radu C, Diab A, Tsen MF, Hussain R, Cowdery JS, Racke MK, Thomas JA. IL-1 receptor-associated kinase 1 regulates susceptibility to organ-specific autoimmunity. J Immunol. 2003;170:2833–2842. doi: 10.4049/jimmunol.170.6.2833. [DOI] [PubMed] [Google Scholar]
- 12.Jacob CO, Zhu J, Armstrong DL, Yan M, Han J, Zhou XJ, Thomas JA, Reiff A, Myones BL, Ojwang JO, Kaufman KM, Klein-Gitelman M, McCurdy D, Wagner-Weiner L, Silverman E, Ziegler J, Kelly JA, Merrill JT, Harley JB, Ramsey-Goldman R, Vila LM, Bae SC, Vyse TJ, Gilkeson GS, Gaffney PM, Moser KL, Langefeld CD, Zidovetzki R, Mohan C. Identification of IRAK1 as a risk gene with critical role in the pathogenesis of systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2009;106:6256–6261. doi: 10.1073/pnas.0901181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swantek JL, Tsen MF, Cobb MH, Thomas JA. IL-1 receptor-associated kinase modulates host responsiveness to endotoxin. J Immunol. 2000;164:4301–4306. doi: 10.4049/jimmunol.164.8.4301. [DOI] [PubMed] [Google Scholar]
- 14.Albrecht V, Hofer TP, Foxwell B, Frankenberger M, Ziegler-Heitbrock L. Tolerance induced via TLR2 and TLR4 in human dendritic cells: role of IRAK-1. BMC Immunol. 2008;9:69. doi: 10.1186/1471-2172-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong Y, Qiu F, Piao W, Song C, Wahl LM, Medvedev AE. Endotoxin tolerance impairs IL-1 receptor-associated kinase (IRAK) 4 and TGF-beta-activated kinase 1 activation, K63-linked polyubiquitination and assembly of IRAK1, TNF receptor-associated factor 6, and IkappaB kinase gamma and increases A20 expression. J Biol Chem. 2011;286:7905–7916. doi: 10.1074/jbc.M110.182873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verdrengh M, Thomas JA, Hultgren OH. IL-1 receptor-associated kinase 1 mediates protection against Staphylococcus aureus infection. Microbes Infect. 2004;6:1268–1272. doi: 10.1016/j.micinf.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Ebong S, Call D, Nemzek J, Bolgos G, Newcomb D, Remick D. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect Immun. 1999;67:6603–6610. doi: 10.1128/iai.67.12.6603-6610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13:110–116. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
- 19.Remick DG, Ward PA. Evaluation of endotoxin models for the study of sepsis. Shock. 2005;24(Suppl 1):7–11. doi: 10.1097/01.shk.0000191384.34066.85. [DOI] [PubMed] [Google Scholar]
- 20.Baker CC, I, Chaudry H, Gaines HO, Baue AE. Evaluation of Factors Affecting Mortality-Rate After Sepsis in A Murine Cecal Ligation and Puncture Model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 21.Chandra R, Federici S, Nemeth ZH, Horvath B, Pacher P, Hasko G, Deitch EA, Spolarics Z. Female X-chromosome mosaicism for NOX2 deficiency presents unique inflammatory phenotype and improves outcome in polymicrobial sepsis. J Immunol. 2011;186:6465–6473. doi: 10.4049/jimmunol.1100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemeth ZH, Csoka B, Wilmanski J, Xu D, Lu Q, Ledent C, Deitch EA, Pacher P, Spolarics Z, Hasko G. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol. 2006;176:5616–5626. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilmanski J, Siddiqi M, Deitch EA, Spolarics Z. Augmented IL-10 production and redox-dependent signaling pathways in glucose-6-phosphate dehydrogenase-deficient mouse peritoneal macrophages. J Leukoc Biol. 2005;78:85–94. doi: 10.1189/jlb.0105010. [DOI] [PubMed] [Google Scholar]
- 24.Chandra R, Villanueva E, Feketova E, Machiedo GW, Hasko G, Deitch EA, Spolarics Z. Endotoxemia down-regulates bone marrow lymphopoiesis but stimulates myelopoiesis: the effect of G6PD deficiency. J Leukoc Biol. 2008;83:1541–1550. doi: 10.1189/jlb.1207838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deitch EA. Rodent models of intra-abdominal infection. Shock. 2005;24(Suppl 1):19–23. doi: 10.1097/01.shk.0000191386.18818.0a. [DOI] [PubMed] [Google Scholar]
- 26.Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19:198–208. doi: 10.1016/j.tim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Remick DG, Bolgos G, Copeland S, Siddiqui J. Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect Immun. 2005;73:2751–2757. doi: 10.1128/IAI.73.5.2751-2757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotake Y, Moore DR, Vasquez-Walden A, Tabatabaie T, Sang H. Antioxidant amplifies antibiotic protection in the cecal ligation and puncture model of microbial sepsis through interleukin-10 production. Shock. 2003;19:252–256. doi: 10.1097/00024382-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Hiraki S, Ono S, Tsujimoto H, Kinoshita M, Takahata R, Miyazaki H, Saitoh D, Hase K. Neutralization of interleukin-10 or transforming growth factor-beta decreases the percentages of CD4+ CD25+ Foxp3+ regulatory T cells in septic mice, thereby leading to an improved survival. Surgery. 2012;151:313–322. doi: 10.1016/j.surg.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Toubiana J, Courtine E, Pene F, Viallon V, Asfar P, Daubin C, Rousseau C, Chenot C, Ouaaz F, Grimaldi D, Cariou A, Chiche JD, Mira JP. IRAK1 functional genetic variant affects severity of septic shock. Crit Care Med. 2010;38:2287–2294. doi: 10.1097/CCM.0b013e3181f9f9c7. [DOI] [PubMed] [Google Scholar]
- 32.Arcaroli J, Silva E, Maloney JP, He Q, Svetkauskaite D, Murphy JR, Abraham E. Variant IRAK-1 haplotype is associated with increased nuclear factor-kappaB activation and worse outcomes in sepsis. Am J Respir Crit Care Med. 2006;173:1335–1341. doi: 10.1164/rccm.200603-341OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lye E, Mirtsos C, Suzuki N, Suzuki S, Yeh WC. The role of interleukin 1 receptor-associated kinase-4 (IRAK-4) kinase activity in IRAK-4-mediated signaling. J Biol Chem. 2004;279:40653–40658. doi: 10.1074/jbc.M402666200. [DOI] [PubMed] [Google Scholar]
- 34.Souto FO, Alves-Filho JC, Turato WM, Auxiliadora-Martins M, Basile-Filho A, Cunha FQ. Essential role of CCR2 in neutrophil tissue infiltration and multiple organ dysfunction in sepsis. Am J Respir Crit Care Med. 2011;183:234–242. doi: 10.1164/rccm.201003-0416OC. [DOI] [PubMed] [Google Scholar]
- 35.Andrades M, Ritter C, de Oliveira MR, Streck EL, Fonseca Moreira JC, Dal-Pizzol F. Antioxidant treatment reverses organ failure in rat model of sepsis: role of antioxidant enzymes imbalance, neutrophil infiltration, and oxidative stress. J Surg Res. 2011;167:e307–e313. doi: 10.1016/j.jss.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Iwata A, de Claro RA, Morgan-Stevenson VL, Tupper JC, Schwartz BR, Liu L, Zhu X, Jordan KC, Winn RK, Harlan JM. Extracellular administration of BCL2 protein reduces apoptosis and improves survival in a murine model of sepsis. PLoS One. 2011;6:e14729. doi: 10.1371/journal.pone.0014729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nature Immunology. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 38.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4(+) T lymphocytes in humans. Journal of Immunology. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 39.Liu YC, Simmons DP, Li X, Abbott DW, Boom WH, Harding CV. TLR2 signaling depletes IRAK1 and inhibits induction of type I IFN by TLR7/9. J Immunol. 2012;188:1019–1026. doi: 10.4049/jimmunol.1102181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berglund M, Thomas JA, Hornquist EH, Hultgren OH. Toll-like receptor cross-hyporesponsiveness is functional in interleukin-1-receptor-associated kinase-1 (IRAK-1)-deficient macrophages: differential role played by IRAK-1 in regulation of tumour necrosis factor and interleukin-10 production. Scand J Immunol. 2008;67:473–479. doi: 10.1111/j.1365-3083.2008.02096.x. [DOI] [PubMed] [Google Scholar]
- 41.Kawagoe T, Sato S, Matsushita K, Kato H, Matsui K, Kumagai Y, Saitoh T, Kawai T, Takeuchi O, Akira S. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol. 2008;9:684–691. doi: 10.1038/ni.1606. [DOI] [PubMed] [Google Scholar]