Abstract
Objective
To understand the regulation of adipocyte size and adipokine expression in relation to gender, anatomic location, adiposity, and metabolic risk factors in adolescents with morbid obesity.
Design and Methods
Adipocyte size and adipokine expression in paired abdominal subcutaneous (SAT) and omental (VAT) surgical adipose tissues were related to gender, anatomic location, adiposity, and metabolic risk factors in a group of morbidly obese adolescents.
Results
Significant depot- and/or gender-related differences in adipocyte size and adipokine expression were detected. Adjusted for BMI, adipocyte size in both depots was larger in males than in females and was a major predictor of mRNA levels of leptin, PAI-1, and adiponectin. Gender, but not adipocyte size, was significantly correlated with pro-inflammatory cytokine expression. BMI and waist circumference were correlated positively with VAT adipocyte size and negatively with SAT adipocyte size. VAT adiponectin and IL-6 expression levels were major predictors of HDL cholesterol concentrations, independent of gender, adiposity, and insulin sensitivity.
Conclusions
Adipose tissue morphology and function in obese adolescents are influenced by gender and anatomic location; the pattern of gender- and depot-related differences in adipocyte size and adipokine expression suggests that adolescent males, relative to the females, are at increased risk for obesity-related metabolic co-morbidities.
Introduction
Obesity is a leading cause of diabetes, hypertension, dyslipidemia, and coronary heart diseases. Abdominal obesity is associated with increased risks of developing obesity-related co-morbidities (1). Gender significantly influences body fat distribution and the risk of developing these co-morbidities (2,3). However, the molecular mechanisms that link male gender and abdominal obesity to increased metabolic risk are not fully understood (2–5).
As an active endocrine organ and the largest store of chemical energy, adipose tissue plays an important role in regulating energy homeostasis and metabolism. Obesity is associated with increased fat cell size and/or number (adipocyte hypertrophy and/or hyperplasia). Adipocyte hypertrophy is associated with insulin resistance and metabolic abnormalities, while adipocyte hyperplasia appears to be less harmful and may even provide protection against obesity-related co-morbidities (6). Adipose tissue secretes many bioactive molecules, known collectively as adipokines. Leptin and adiponectin, two hormones produced specifically in adipocytes, are crucial in the regulation of energy homeostasis and insulin sensitivity (7,8). Adipose tissues of obese individuals are characterized by increased macrophage infiltration and relative over-expression of pro-inflammatory cytokines, thromboembolic proteins, and proteins of the renin-angiotensin system (9), many of which have been linked to increased risk of metabolic and cardiovascular disorders (10). Although gender- and depot-related differences in adipocyte size, lipid metabolism, and expression levels of adipokines have been reported (4,5,11–17), there is limited understanding of the changes in adipocyte size in different fat depots in response to changes in general adiposity and the relationships among adipokine gene expression, gender, anatomic location, adipocyte size, and metabolic risk in obese individuals.
In this study, we systematically determined adipocyte size and expression levels of selected adipokine genes and the macrophage marker CD68 in paired abdominal subcutaneous and omental adipose tissue from morbidly obese adolescents. We asked the following specific questions: 1. Are gender- and depot-related differences in adipocyte size and adipokine gene expression in obese adolescents similar to those of obese adults? 2. How are expression levels of adipokines related to adipocyte size, anatomic site of origin, and gender? 3. Are there correlations of adipocyte size and expression levels of adipokines across depots? 4. How are BMI and waist circumference related to adipocyte size in these two depots? 5. How are adipokine expression levels in these fat depots related to metabolic risk factors after adjustment for adiposity? Because the subjects are young, adipose tissue function is less likely to be confounded by chronic metabolic phenotypes often present in similarly obese adults.
Methods and Procedures
Adipose tissue biopsy
Subjects were the participants of an FDA-approved study of the efficacy of laparoscopic adjustable gastric banding (LAGB) surgery for weight loss. Both the teens and their parents had given consents to this ancillary study of adipose tissue biology, including the biopsy of abdominal subcutaneous (SAT) and omental fat (VAT) and the release of biopsy specimen and the relevant medical information. Pre-surgical evaluation procedures, inclusion and exclusion criteria used to assign subjects to LAGB, and post-surgical follow up procedures have been described in the reports of the LAGB study (18,19). About two weeks prior to the LAGB surgery, the subjects underwent full pre-surgical evaluations that included assessment of developmental and pubertal status as well as height, weight, waist circumference (WC), blood lipids, oral glucose tolerance tests and plasma insulin levels. SAT samples were taken from the anterior abdominal wall, and VAT samples from omental tissue along the greater curvature of the stomach. The technique of sampling was identical for all patients. Samples placed in sterile containers were immediately transferred to the laboratory. After visible blood vessels and connective tissues were cleaned away, a portion of the sample was fixed in Z-fix for adipocyte sizing and the remainder was stored at −80°C for RNA analysis. The study protocol and the use of the relevant patient information were reviewed and approved by the Institutional Review Board of Columbia University Medical Center.
Determination of adipocyte size
Adipocyte size of fat samples was determined using the Image-Pro-Plus Program (IPP, Media Cybernetics, Inc. Bethesda, MD) as previously described (20). The mean adipocyte size of each sample was calculated based on the values of at least 500 adipocytes from 5 tissue sections, and was expressed as the mean cross-sectional area per cell, micrometer2/cell.
Quantification of adipose tissue gene expression
Quantitative RT-PCR (qRT-PCR) was used to determine mRNA levels as previously described (15). Briefly, total RNA was converted into single-stranded cDNA by reverse transcription. Quantitative amplification of cDNAs of interest by PCR was carried out using gene-specific primers (see Supplemental Table 1) and iQ SYBR Green Supermix (Bio-Rad Laboratories, CA). To prevent the amplification of any contaminating genomic DNA, the forward and reverse PCR primers were derived from two different exons that are separated by at least 1 kb intron. The size of the amplicon was confirmed by agarose gel electrophoresis. Cyclophilin A mRNA level was used to normalize total RNA input. The difference in PCR cycle numbers at the specified fluorescence thresholds (within the linear amplification range) for the gene of interest and cyclophilin A (delta Ct) was used to calculate the mRNA level of the gene of interest. All qRT-PCR were performed in triplicate and the arithmetic mean of the triplicate used in subsequent calculations.
Statistical analysis
Statistical analyses were performed using Statistica V6 (StatSoft, Tulsa OK). Adipocyte size and gene expression data were expressed as mean ± SEM. Two-way ANOVA was used to assess gender- and depot-related difference in adipocyte size and adipokine expression level. Factorial ANOVA was used to examine potential interactions between gender and depot origin. Simple correlation analysis was used to assess cross-depot correlations in adipocyte size and adipokine gene expression. Multiple regression analysis was used to determine the independent effects on adipokine gene expression of adipocyte size, and gender (coded as female=1 and male=2) and depot origin (coded as SAT=1 and VAT=2), which were included in the analysis as dichotomous variables. Forward stepwise multiple regression analysis was used to assess the size of the effect of independent variables (gender, depot origin, and adipocyte size) on the dependent variables (e.g., expression levels of adipokine genes). Independent variable(s) with F<1 were excluded from forward stepwise regression analysis. Multiple regression analysis and forward stepwise multiple linear regression analysis were also used to assess the relationships/effect sizes of gender, and SAT and VAT adipocyte size and adipokine expression levels with/on adiposity, plasma lipid concentrations, hemoglobin A1c (HbA1c), homeostatic model assessment-insulin resistance (HOMA-IR) and blood pressure.
Results
Anthropometric and metabolic characteristics of the study subjects
A total of 28 obese adolescents, 14 males and 14 females were included in this analysis. Ethnicities of the subjects were 39% Caucasian, 17% African American (AA); 28% Hispanic and 14% mixed (1 AA/Hispanic, 2 Caucasian/Hispanic, and 1 AA/Caucasian). The relevant anthopometric/clinical and metabolic data from the subjects, collected during the pre-surgical evaluation about two weeks prior to LAGB surgery and adipose tissue biopsy are shown in Table 1. All subjects were at Tanner stages IV–V. Males were taller and heavier than the females. BMI z-scores were higher in males than in females. HDL-cholesterol concentrations were lower in males than in females (Table 1).
Table 1.
Anthropometric and metabolic characteristics of ATS subjects
| Female | Male | |
|---|---|---|
| n | 14 | 14 |
| age (yr) | 16.6±0.26 | 17.1±0.29 |
| Tanner stage | 4.86±0.10 | 4.75±0.17 |
| height (cm) | 162.0 ±1.79 | 173.8 ±2.75** |
| weight (lb) | 268.5±14.2 | 327.4±17.5* |
| BMI (kg/m2) | 46.4±2.3 | 49.2±2.2 |
| BMI z-score | 2.53±0.04 | 3.02±0.09*** |
| waist circumference (cm) | 130.6±3.8 | 140.7±5.4 |
| SBP (mmHg) | 119.3±3.2 | 126.0±3.2 |
| DBP (mmHg) | 72.9±3.2 | 76.8±2.4 |
| HbA1c | 5.36±0.11 | 5.56±0.10 |
| HOMA-IR | 2.32±0.44 | 3.30±0.56 |
| TG (mg/dl) | 116.3±13.6 | 111.5±9.2 |
| Cholesterol (mg/dl) | 173.5±8.1 | 164.7±14.9 |
| HDL-C (mg/dl) | 47.2±2.7 | 36.3±2.2** |
| LDL-C (mg/dl) | 102.9±5.9 | 104.5±11.7 |
| CRP (mg/dl) | 10.8±2.9 | 9.0±2.8 |
indicate p<0.05, 0.01 and 0.001, respectively, female vs. male.
Depot- and gender-related differences in adipocyte size and adipokine gene expression
Adipocyte size and mRNA levels of leptin, adiponectin, plasminogen activator inhibitor-1 (PAI-1), angiotensinogen, tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), monocyte attractant protein-1 (MCP-1), visfatin (pre-B cell colony-enhancing factor), resistin, and the macrophage marker CD68 were determined in the paired SAT and VAT samples from obese adolescents. The mean adipocyte size and adipokine mRNA levels in SAT and VAT of males and females (normalized to the respective values of female SAT for easy comparisons) are shown in Figure 1. The original data are shown in Supplemental Table 2. Two-way ANOVA showed that adipocyte size was significantly larger in males than in females in both fat depots (Table 2). Multiple regression analysis confirmed that male gender was associated with significantly larger adipocyte size in both SAT (β=0.52, p<0.01) and VAT (β=0.42, p<0.05) even when differences in adiposity (using BMI as a surrogate) were controlled for. Substituting BMI with BMI z-score or WC led to similar results (data not shown). Leptin, PAI-1, IL-6, and visfatin mRNA levels were higher in males than in females, whereas adiponectin and TNF-α mRNA levels were lower in males than in females. Adipocyte size was significantly larger in SAT than VAT, and leptin, PAI-1, CD68, and MCP-1 expression levels were higher in SAT than in VAT in both males and females (Table 2). No significant gender- or depot-related differences in the expression of resistin and angiotensinogen were observed (data not shown).
Fig 1.
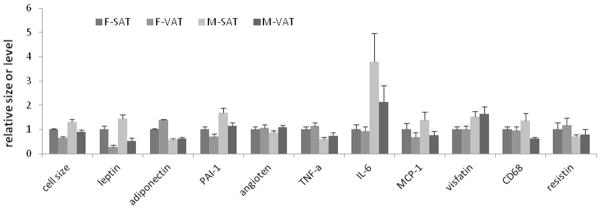
The average adipocyte size and mRNA levels of selected adipokines (mean±SE) in abdominal subcutaneous fat tissue (SAT) and omental fat tissue (VAT) from male (M) and female (F) obese adolescents. The values are expressed relative to the respective values in female SAT.
Table 2.
Depot- and gender-related differences in adipocyte size and adipokine gene expression by two-way ANOVA
| type of difference | variables | direction of difference | p |
|---|---|---|---|
| gender | adipocyte size | M > F | <0.001 |
| leptin | M > F | <0.05 | |
| PAI-1 | M > F | <0.001 | |
| IL-6 | M > F | <0.01 | |
| visfatin | M > F | <0.01 | |
| TNF-α | F > M | <0.01 | |
| adiponectin | F > M | <0.01 | |
| depot | adipocyte size | SAT > VAT | <0.001 |
| leptin | SAT > VAT | <0.001 | |
| PAI-1 | SAT > VAT | <0.01 | |
| CD68* | SAT > VAT | <0.05 | |
| MCP-1 | SAT > VAT | =0.057 |
indicates significant interaction between gender and depot (p=0.05)
Independent effects of adipocyte size, gender, and depot-of-origin on adipokine expression
We used multiple regression analysis to evaluate independent influences of adipocyte size, gender and depot origin on mRNA levels of the adipokines. Both adipocyte size and depot-of-origin were significant independent predictors of leptin mRNA levels in these obese adolescents (Table 3), accounting for 46% and 10% of the variation in leptin mRNA levels, respectively. Gender was not a significant independent predictor of leptin mRNA level once differences in adipocyte size and depot origin were accounted for. Like leptin, PAI-1 mRNA levels were positively correlated with adipocyte size. However, gender, but not depot-of-origin, was also a significant independent predictor of PAI-1 expression (Table 3). Adipocyte size and gender accounted, respectively, for 40% and 5% of variation in PAI-1 gene expression. In contrast to leptin and PAI-1, adiponectin mRNA level was negatively correlated with adipocyte size, which accounted for 26% of the variation in adiponectin expression (Table 3). Once variation in adipocyte size was controlled, neither gender nor depot was a significant predictor of adiponectin expression. Adipocyte size was not a significant predictor of mRNA levels of the other adipokines examined. Gender had modest effects on the expression levels of IL-6 and TNF-α, accounting for 15% and 19% of their variations, respectively (Table 3). Neither depot nor gender was a significant independent predictor of CD68, MCP-1, or visfatin expression levels once variation in adipocyte size was controlled for (Table 3).
Table 3.
Independent influences of gender, depot origin, and adipocyte size on adipokine gene mRNA levels
| total R2 | depot | gender | adipocyte size | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| β | p | β | p | β | p | ||
| leptin | 0.57 | −0.4 | <0.01 | 0.09 | ns* | 0.43 | <0.01 |
| PAI-1 | 0.45 | 0.08 | ns | 0.26 | <0.05 | 0.49 | <0.01 |
| adiponectin | 0.31 | −0.12 | ns | −0.20 | ns | −0.5 | <0.01 |
| IL-6 | 0.18 | −0.21 | ns | 0.42 | <0.01 | −0.08 | ns |
| TNF-α | 0.22 | 0.07 | ns | −0.38 | <0.05 | −0.13 | ns |
| visfatin | 0.21 | 0.21 | ns | 0.26 | 0.08 | 0.32 | 0.07 |
| CD68 | 0.10 | −0.22 | ns | −0.04 | ns | 0.15 | ns |
| MCP-1 | 0.09 | −0.22 | ns | 0.10 | ns | 0.09 | ns |
| resistin | 0.05 | 0.08 | ns | −0.21 | ns | 0.01 | ns |
| AT-1 | 0.04 | 0.23 | ns | −0.10 | ns | 0.09 | ns |
ns, no significant correlation (p>0.1). p values between 0.05 and 0.1 are listed.
Cross-depot correlations in adipocyte size and adipokine gene expression
To further understand the influences of local versus systemic factors on adipocyte size and adipokine expression, we examined cross-depot correlations of these parameters. Significant cross-depot correlations in adipocyte size and expression levels of resistin, IL-6, TNF-α, PAI, and adiponectin were observed (Table 4). Among these, the expression levels of IL-6, TNF-α, PAI-1, and adiponectin also showed significant gender-related differences (Table 2), suggesting that gender and other systemic factors have significant influences on the expression of these adipokines. In contrast, no statistically significant cross-depot correlations in the mRNA levels of visfatin, MCP-1, angiotensinogen-1, leptin, and CD68 were observed (Table 4), suggesting that local or depot-specific factors, compared to systemic factors, may play more important roles in regulating the expression of these genes.
Table 4.
Cross-depot correlations in adipocyte size and adipokine gene expression
| Parameter | r | p |
|---|---|---|
| adipocyte size | 0.68 | <0.001 |
| resistin | 0.87 | <0.001 |
| IL-6 | 0.75 | <0.001 |
| PAI-1 | 0.73 | <0.001 |
| TNF-α | 0.65 | <0.001 |
| adiponectin | 0.60 | <0.01 |
| visfatin | 0.36 | ns |
| MCP-1 | 0.33 | ns |
| angiotensinogen-1 | 0.38 | ns |
| leptin | 0.25 | ns |
| CD68 | −0.07 | ns |
ns, not significant (p>0.05)
Distinct relationships of VAT adipocyte size and SAT adipocyte size with BMI and waist circumference
Adipocyte size in different fat depots may be independently or differentially regulated during the development of obesity. We examined the relationships of BMI and WC with adipocyte size in SAT and VAT. In males, both BMI (R2=0.47, p<0.05) and WC (R2=0.36, p=0.11) were positively correlated with VAT adipocyte size and negatively correlated with SAT adipocyte size (Table 5), suggesting that mechanisms underlying adipose tissue expansion may be different in these fat depots: for example, by adipocyte hypertrophy in VAT and by adipocyte hyperplasia in SAT. In females, WC (R2=0.30, p=0.14) displayed similar relationships with SAT and VAT adipocyte size as in males, but BMI (R2=0.09, p=0.59) showed no correlation with either SAT or VAT adipocyte size (Table 5), which may be explained by the predominantly lower body fat distribution pattern in females.
Table 5.
Relationships of SAT and VAT adipocyte size with BMI and waist circumference (WC) in males and females separately
| Male | Female | ||||
|---|---|---|---|---|---|
|
| |||||
| Dependent variable: BMI (R2=0.47, p<0.05) | Dependent variable: BMI (R2=0.09, p=0.59) | ||||
| β | p | β | p | ||
| SAT adipocyte size | −0.69 | 0.057 | SAT adipocyte size | −0.28 | ns |
| VAT adipocyte size | 0.95 | <0.05 | VAT adipocyte size | 0.17 | ns |
|
| |||||
| Dependent variable: WC (R2=0.36, p=0.11) | Dependent variable: WC (R2=0.30, p=0.14) | ||||
| β | p | β | p | ||
| SAT adipocyte size | −0.46 | ns | SAT adipocyte size | −0.48 | ns |
| VAT adipocyte size | 0.81 | <0.05 | VAT adipocyte size | 0.35 | ns |
Influences of VAT IL-6 and adiponectin expression levels on cholesterol metabolism
One of the goals of the study was to assess possible relationships between SAT and VAT adipokine expression and metabolic risk factors. Simple correlation analyses revealed that IL-6 levels, particularly VAT IL-6 levels, were negatively correlated with total, HDL- and LDL-cholesterol levels (r=−0.51, −0.49, and −0.47, respectively, all p<0.05). The negative correlation between the cholesterol parameters and VAT IL-6 expression levels persisted even when adiposity (using WC or BMI as a surrogate) was controlled for. HDL concentrations were also strongly correlated with VAT adiponectin levels (r=0.68, p<0.001), and to a lesser degree with SAT adiponectin levels (r=0.37, p<0.06). Multiple regression analysis indicated that both VAT adiponectin levels and IL-6 levels were significant independent predictors of HDL concentration in this cohort, accounting for 46% and 11% of variation in HDL, respectively (Table 6). Inclusion of BMI, WC, HOMA-IR, VAT and SAT adipocyte size or SAT adiponectin and IL-6 mRNA levels in the regression analyses had no significant effect on the results. Moreover, gender was no longer a significant predictor of circulating HDL cholesterol when VAT adiponectin and IL-6 expression levels were included as independent variables (Table 6). No significant correlation of blood pressures, HbA1c, HOMA-IR, TG, or CRP levels, to SAT and/or VAT adipokine expression was observed when variation in adiposity (using WC as surrogate) was controlled for, although there was a trend of correlation between plasma CRP levels and VAT CD68 levels (β=0.33, p=0.08).
Table 6.
VAT adiponectin and IL-6 expression levels are major independent predictors of plasma HDL-cholesterol concentration
| variables | delta R2 | β | p |
|---|---|---|---|
| VAT adiponectin | 0.46 | 0.52 | <0.01 |
| VAT IL-6 | 0.11 | −0.30 | <0.05 |
| gender | 0.03 | −0.20 | NS |
Discussion
The four main findings of this study of obese adolescents are: 1. Depot- and gender- related differences in adipocyte size, leptin, adiponectin and PAI-1mRNA levels in the obese adolescents are similar to those observed in obese adults (12,13,16,17). However, in contrast to the obese adults, pro-inflammatory cytokine expression levels are not elevated in VAT relative to SAT in the obese adolescents (4,5,21,22). 2. Adipocyte size is a major predictor of expression levels of leptin, PAI-1 and adiponectin, but not of pro-inflammatory cytokines such as IL-6 and TNF-α. Variation in adipocyte size appears to mediate some of the gender- and/or depot-related differences in leptin, PAI-1 and adiponectin expression levels. 3. BMI and WC in males, and WC but not BMI in females, are correlated positively with VAT adipocyte size and negatively with SAT adipocyte size, suggesting that mechanisms regulating adipose tissue expansion may differ significantly by depots and gender. 4. VAT adiponectin and IL-6 expression levels are strong predictors HDL-cholesterol concentrations, independent of gender, adiposity and insulin sensitivity, consistent with the “portal theory” which suggests that substrates and adipokines released directly into the portal circulation have important effects on hepatic glucose and lipid metabolism (5,23). It is important to note that only mRNA levels of the adipokines were measured in this study, which could potentially be distinct from protein secretion although results in the literature suggest that mRNA levels and protein secretion rates of leptin, adiponectin, PAI-1, IL-6, and TNF-alpha are well correlated (14,16,24–28).
Gender-related differences in adipocyte size and adipokine gene expression and their relationships with metabolic risk factors
Males are at a higher risk for obesity-related metabolic disorders, compared to pre-menopausal women; differences in body fat distribution and adipose tissue function may contribute to these gender-related differences (2,3). Obese adult males have larger omental fat cells than women whereas women have larger femoral and gluteal adipocytes than men (11,29). Differences in adipocyte size and function among three main subcutaneous fat depots - abdominal, gluteal and femoral - are also well recognized (11,29). In Tanner stage IV–V obese adolescents, we find that males, independent of the degree of adiposity, have larger adipocytes in both SAT and VAT than females. Similar to the findings in obese adults (16,17), PAI-1 expression is higher in male than female adolescents, even when adipocyte size is controlled for. Adiponectin expression is lower in males than in females whereas leptin is higher in males than in females, both of which appears to be mainly due to the difference in adipocyte size in these obese adolescents because once adipocyte size is controlled for, gender no longer has a significant effect on either adiponectin or leptin mRNA levels. Direct effects of gonadal steroids on leptin gene expression and plasma leptin level remain controversial (30,31). Plasma leptin concentrations are higher in female than male Tanner stage IV–V adolescents (32), which may be due in part to the greater proportion of subcutaneous fat in females than males. Gender also exerts significant though small effects on IL-6 and TNF-α expression in the obese adolescents. It has been shown in mice that HF diet exposure elicits a much greater inflammatory response, i.e., up-regulation of genes involved in cytokine-cytokine receptor interactions and acute-phase protein synthesis, in males than in females, and that this sexual dimorphism is partially reversed after ovariectomy (33).
Low HDL-cholesterol is a risk factor for cardiovascular disease. HDL-cholesterol was 23% lower in males than females in this group of obese adolescents, similar to the effect size observed in lean and obese adults (34). We found that VAT adiponectin and IL-6 mRNA levels were strong predictors of HDL-cholesterol concentrations. Visceral adipose tissues are major determinants of plasma IL-6 concentration in the portal vein, which is ~50% higher than that of the radial artery in morbidly obese subjects (35). Adiponectin has been shown to regulate HDL assembly (36), and affects plasma HDL-cholesterol levels independent of body fat mass (37). Taken together, our finding suggests that VAT adiponectin and IL-6 may influence hepatic cholesterol metabolism directly via the portal circulation. Gender differences in HDL-cholesterol are no longer significant once adiponectin and IL-6 gene expression levels in VAT are controlled for, suggesting that the differences in the expression levels of adiponectin and IL-6 may contribute to some of the gender-related differences in HDL-cholesterol concentration.
Depot specific regulation of adipocyte size and adipokine gene expression
Anatomically distinct fat depots may have distinct developmental origins and are subjected to independent regulation in adipogenesis and function (38). We found that adipocyte size was significantly greater in abdominal subcutaneous than in omental fat in both male and female obese adolescents, similar to the differences observed in obese adults (11,12). Interestingly, VAT and SAT adipocyte sizes also display distinct relationships with the degree of adiposity (BMI and WC as surrogates). In the linear regression model, BMI and WC in males, and WC in females, all display a positive correlation with VAT adipocyte size and a negative correlation with SAT adipocyte size. Although the mass of these two fat depots was not measured here, an inference from these divergent correlations of adipocyte size with adiposity is that adipogenesis/adipose tissue expansion in these fat depots may be differentially regulated during the development of obesity – VAT being more likely to expand by increases in adipocyte size while SAT preferentially increases in adipocyte number. Spalding et al report that in adults adiposity is mainly correlated with the total number of adipocytes, which appears to be determined in early puberty and remains relatively consistent throughout of life despite ongoing turnover of adipocytes (39). The fractional rates of adipocyte turnover are not significantly different between lean and obese subjects (39). In the context of this model, our observations in obese adolescents suggest that the expansion of adipocyte number in the development of obesity during early adolescence occurs preferentially in subcutaneous fat depots.
As in obese adults, both leptin and PAI-1 mRNA levels are higher in SAT than in VAT in obese adolescents (12,16). However, we found that depot-related differences in leptin and PAI-1 expression in the obese adolescents are correlated mainly with adipocyte size, although depot per se remains a significant predictor of leptin mRNA level (SAT >VAT), consistent with the findings of our earlier studies in lean and obese mice (14,15). The lack of significant cross-depot correlation in leptin gene expression further suggests that local factors, e.g., adipocyte size, levels of glucocorticoid receptor and adrenergic receptors (14,40), play important roles in determining leptin mRNA levels in specific fat depots. We did not find a consistent pattern of depot-related differences in the expression levels of CD68 and pro-inflammatory cytokines. Previous studies in obese adults found that visceral adipose tissues exhibit elevated levels of IL-6 and MCP-1 expression compared to subcutaneous adipose tissues (21,22). Differences in obese adolescents may relate to age, duration of obesity, metabolic status of the study cohorts and molecules assayed. Additionally, macrophage marker CD68 levels showed little correlation between depots or with adipocyte size in our subjects, suggesting that the degree of macrophage infiltration in different fat depots may be regulated locally but is not necessarily related to the degree of adipocyte hypertrophy.
In summary, despite the evidence that many adipose tissue functions are co-regulated to various degrees by gender and other systemic factors, depot-related differences in adipocyte size and leptin gene expression observed in this group of obese adolescents support the contention that fat depots are in some ways independent mini organs and that their functions are substantially influenced by developmental origins and local environmental factors. The finding that morbidly obese adolescent males are more likely to develop adipocyte hypertrophy, express lower adiponectin and higher PAI-1 levels, and have lower circulating HDL-cholesterol than females suggests that obese adolescent males are at higher risk to develop obesity-related metabolic co-morbidities. Early nutritional and medical interventions, such as weight loss, exercise, and cholesterol control, may prevent or delay the onset of metabolic complications and improve their long term health.
Supplementary Material
What is already known about this subject?
Gender and body fat distribution affect the risk for obesity-related co-morbidities
Adipose tissue secretes peptides and metabolites influencing energy homeostasis, inflammation, coagulation, and other phenotypes.
The production of these molecules is influenced by gender and anatomic location of adipose tissue.
What does this study add?
Demonstration of independent effects of adipocyte size, gender, and anatomic location on expression of multiple adipokines in obese adolescents.
Adiposity in morbidly obese adolescents is positively correlated with visceral fat cell size, and negatively correlated with subcutaneous fat cell size.
Observation of strong association between expression levels of adiponectin and IL-6 in omental fat with circulating concentrations of HDL.
Acknowledgments
We thank the participants and their parents, nurses, and staff members for their support to this work. The work was funded in part by grants from National Institutes of Health, R01DK063034 (YZ), R01DK52431 (RLL), P30 DK26687, and P30 DK63608.
Abbreviations used
- SAT
abdominal subcutaneous adipose tissue
- VAT
omental adipose tissue
- LAGB
laparoscopic adjustable gastric banding
- WC
waist circumference
- BMI
body mass index
- IL-6
interleukin-6
- TNF-α
tumor necrosis factor-alpha
- PAI-1
plasminogen activator inhibitor-1
- MCP-1
monocyte chemoattractant protein-1
- HDL-C
high density lipoprotein cholesterol
- LDL-C
low density lipoprotein cholesterol
- TG
triglycerides
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- CRP
c-reactive protein
- HbA1c
hemoglobin A1c
- HOMA-IR
homeostatic model of assessment-insulin resistance
Footnotes
Competing interests: the authors have no competing interests.
References
- 1.Bjorntorp P. Abdominal obesity and the metabolic syndrome. Ann Med. 1992;24:465–468. doi: 10.3109/07853899209166997. [DOI] [PubMed] [Google Scholar]
- 2.Nedungadi TP, Clegg DJ. Sexual dimorphism in body fat distribution and risk for cardiovascular diseases. J Cardiovasc Transl Res. 2009;2:321–327. doi: 10.1007/s12265-009-9101-1. [DOI] [PubMed] [Google Scholar]
- 3.Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol. 2006;95:136–147. doi: 10.1007/s00392-006-0351-5. [DOI] [PubMed] [Google Scholar]
- 4.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: mplication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34:1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Item F, Konrad D. Visceral fat and metabolic inflammation: the portal theory revisited. Obes Rev. 2012;13 (Suppl 2):30–39. doi: 10.1111/j.1467-789X.2012.01035.x. [DOI] [PubMed] [Google Scholar]
- 6.Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59:105–109. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 8.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 9.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8:709–716. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- 11.Fried SK, Kral JG. Sex differences in regional distribution of fat cell size and lipoprotein lipase activity in morbidly obese patients. Int J Obes. 1987;11:129–140. [PubMed] [Google Scholar]
- 12.Van Harmelen V, Reynisdottir S, Eriksson P, Thorne A, Hoffstedt J, Lonnqvist F, et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. 1998;47:913–917. doi: 10.2337/diabetes.47.6.913. [DOI] [PubMed] [Google Scholar]
- 13.Montague CT, Prins JB, Sanders L, Digby JE, SOR Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes. 1997;46:342–347. doi: 10.2337/diab.46.3.342. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Guo KY, Diaz PA, Heo M, Leibel RL. Determinants of leptin gene expression in fat depots of lean mice. Am J Physiol Regul Integr Comp Physiol. 2002;282:R226–R234. doi: 10.1152/ajpregu.00392.2001. [DOI] [PubMed] [Google Scholar]
- 15.Guo KY, Halo P, Leibel RL, Zhang Y. Effects of obesity on the relationship of leptin mRNA expression and adipocyte size in anatomically distinct fat depots in mice. Am J Physiol Regul Integr Comp Physiol. 2004;287:112–119. doi: 10.1152/ajpregu.00028.2004. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson P, Van Harmelen V, Hoffstedt J, Lundquist P, Vidal H, Stemme V, et al. Regional variation in plasminogen activator inhibitor-1 expression in adipose tissue from obese individuals. Thromb Haemost. 2000;83:545–548. [PubMed] [Google Scholar]
- 17.Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 18.Conroy R, Lee EJ, Jean A, Oberfield SE, Sopher A, Kiefer K, et al. Effect of laparoscopic adjustable gastric banding on metabolic syndrome and its risk factors in morbidly obese adolescents. J Obes. 2011;2011:906384. doi: 10.1155/2011/906384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zitsman JL, Fennoy I, Witt MA, Schauben J, Devlin M, Bessler M. Laparoscopic adjustable gastric banding in adolescents: short-term results. J Pediatr Surg. 2011;46:157–162. doi: 10.1016/j.jpedsurg.2010.09.082. [DOI] [PubMed] [Google Scholar]
- 20.Guo K, Mogen J, Struzzi S, Zhang Y. Preadipocyte transplantation: an in vivo study of direct leptin signaling on adipocyte morphogenesis and cell size. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1339–1347. doi: 10.1152/ajpregu.90691.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 22.Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90:2282–2289. doi: 10.1210/jc.2004-1696. Epub 2005 Jan 2225. [DOI] [PubMed] [Google Scholar]
- 23.Rytka JM, Wueest S, Schoenle EJ, Konrad D. The portal theory supported by venous drainage-selective fat transplantation. Diabetes. 2011;60:56–63. doi: 10.2337/db10-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffstedt J, Eriksson P, Mottagui-Tabar S, Arner P. A polymorphism in the leptin promoter region (−2548 G/A) influences gene expression and adipose tissue secretion of leptin. Horm Metab Res. 2002;34:355–359. doi: 10.1055/s-2002-33466. [DOI] [PubMed] [Google Scholar]
- 25.Fisher FM, McTernan PG, Valsamakis G, Chetty R, Harte AL, Anwar AJ, et al. Differences in adiponectin protein expression: effect of fat depots and type 2 diabetic status. Horm Metab Res. 2002;34:650–654. doi: 10.1055/s-2002-38246. [DOI] [PubMed] [Google Scholar]
- 26.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fain JN, Bahouth SW, Madan AK. Involvement of multiple signaling pathways in the post-bariatric induction of IL-6 and IL-8 mRNA and release in human visceral adipose tissue. Biochem Pharmacol. 2005;69:1315–1324. doi: 10.1016/j.bcp.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Arvidsson E, Viguerie N, Andersson I, Verdich C, Langin D, Arner P. Effects of different hypocaloric diets on protein secretion from adipose tissue of obese women. Diabetes. 2004;53:1966–1971. doi: 10.2337/diabetes.53.8.1966. [DOI] [PubMed] [Google Scholar]
- 29.Tchoukalova YD, Koutsari C, Karpyak MV, Votruba SB, Wendland E, Jensen MD. Subcutaneous adipocyte size and body fat distribution. Am J Clin Nutr. 2008;87:56–63. doi: 10.1093/ajcn/87.1.56. [DOI] [PubMed] [Google Scholar]
- 30.Wu-Peng S, Rosenbaum M, Nicolson M, Chua SC, Leibel RL. Effects of exogenous gonadal steroids on leptin homeostasis in rats. Obes Res. 1999;7:586–592. doi: 10.1002/j.1550-8528.1999.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 31.Machinal F, Dieudonne MN, Leneveu MC, Pecquery R, Giudicelli Y. In vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones. Endocrinology. 1999;140:1567–1574. doi: 10.1210/endo.140.4.6617. [DOI] [PubMed] [Google Scholar]
- 32.Horlick MB, Rosenbaum M, Nicolson M, Levine LS, Fedun B, Wang J, et al. Effect of puberty on the relationship between circulating leptin and body composition. J Clin Endocrinol Metab. 2000;85:2509–2518. doi: 10.1210/jcem.85.7.6689. [DOI] [PubMed] [Google Scholar]
- 33.Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes (Lond) 2010;34:989–1000. doi: 10.1038/ijo.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner A, Simon C, Oujaa M, Platat C, Schweitzer B, Arveiler D. Adiponectin is associated with lipid profile and insulin sensitivity in French adolescents. Diabetes Metab. 2008;34:465–471. doi: 10.1016/j.diabet.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 36.Oku H, Matsuura F, Koseki M, Sandoval JC, Yuasa-Kawase M, Tsubakio-Yamamoto K, et al. Adiponectin deficiency suppresses ABCA1 expression and ApoA-I synthesis in the liver. FEBS Lett. 2007;581:5029–5033. doi: 10.1016/j.febslet.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 37.Baratta R, Amato S, Degano C, Farina MG, Patane G, Vigneri R, et al. Adiponectin relationship with lipid metabolism is independent of body fat mass: evidence from both cross-sectional and intervention studies. J Clin Endocrinol Metab. 2004;89:2665–2671. doi: 10.1210/jc.2003-031777. [DOI] [PubMed] [Google Scholar]
- 38.Billon N, Dani C. Developmental origins of the adipocyte lineage: new insights from genetics and genomics studies. Stem Cell Rev. 2012;8:55–66. doi: 10.1007/s12015-011-9242-x. [DOI] [PubMed] [Google Scholar]
- 39.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 40.Wahrenberg H, Lonnqvist F, Arner P. Mechanisms underlying regional differences in lipolysis in human adipose tissue. J Clin Invest. 1989;84:458–467. doi: 10.1172/JCI114187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


