Abstract
During mitosis, kinetochores coordinate the attachment of centromeric DNA to the dynamic plus ends of microtubules, which is hypothesized to pull sister chromatids toward opposing poles of the mitotic spindle. The outer kinetochore Ndc80 complex acts synergistically with the Ska (spindle and kinetochore-associated) complex to harness the energy of depolymerizing microtubules and power chromosome movement. The Ska complex is a hexamer consisting of two copies of the proteins Ska1, Ska2 and Ska3, respectively. The C-terminal domain of the spindle and kinetochore-associated protein 1 (Ska1) is the microtubule-binding domain of the Ska complex. We solved the solution structure of the C. elegans microtubule-binding domain (MTBD) of the protein Ska1 using NMR spectroscopy. Here, we report the resonance assignments of the MTBD of C. elegans Ska1.
Keywords: Ska complex, kinetochore, mitosis, microtubule, NMR resonance assignments
Biological Context
During mitosis, kinetochores assemble on a specialized region of each chromosome termed the centromere to coordinate the attachment to the dynamic plus ends of microtubules (McEwen et al. 2007). The kinetochore is a proteinaceous structure, composed of the inner kinetochore, which is responsible for the interaction with the centromere, and the outer kinetochore, which establishes the interactions to the spindle microtubules. Proteins associated with the outer kinetochore interact with plus ends of microtubules and harness the energy of depolymerizing microtubule ends to power the movement of the chromosomes (Cheeseman and Desai 2008; Schmidt et al. 2012). The control of chromosome segregation is critical, as the loss or addition of chromosomes (aneuploidy) has severe consequences for daughter cells (Sheltzer et al. 2011). The spindle-assembly checkpoint (SAC) monitors interactions between the kinetochore and spindle microtubules. The SAC prevents the onset of anaphase as long as unattached kinetochores are present (Pereira and Maiato 2012). At the onset of anaphase, cohesion between sister chromatids is cleaved and microtubule depolymerization is thought to pull sister chromatids toward opposing poles of the mitotic spindle (Diaz-Martinez and Clarke 2009; Welburn et al. 2009). The Ska (spindle and kinetochore-associated) complex was recently identified as a component of the outer kinetochore, where it plays an important role in the dynamic interaction between the kinetochore and mitotic-spindle microtubules during chromosome segregation. The complex is a hexamer containing two copies of its subunits Ska1, Ska2 and Ska3, respectively (Hanisch et al. 2006; Jeyaprakash et al. 2012; Welburn et al. 2009). The Ska complex mediates kinetochore connections to dynamic microtubules by interacting with both the microtubule lattice and the bent protofilaments present at the depolymerizing ends of microtubules. Importantly, the Ska complex acts synergistically with the Ndc80 complex. Together, the outer kinetochore Ndc80 complex and the Ska complex are responsible for the direct kinetochore-microtubule attachments that occur in vertebrate cells (DeLuca and Musacchio 2012; Schmidt et al. 2012).
We have solved the three-dimensional structure of the C-terminal domain of the C. elegans orthologue of the Ska1 protein by NMR spectroscopy. The C-terminal domain of Ska1 is the microtubule-binding domain (MTBD) of the Ska complex. A cluster of positively charged arginine residues facilitates microtubule-binding, while Aurora B kinase phosphorylation reduces the microtubule-binding activity of the Ska complex in vitro (Schmidt et al. 2012). The sequences of the MTBDs of human Ska1 (residues 132-255) and C. elegans Ska1 (residues 118-243) share 29.8% identity and 62.1% similarity, respectively (Figure 1).
Figure 1.

Sequence alignment of the microtubule-binding domains of hSka1 and ceSka1. Aurora B phosphorylation sites identified in human Ska1 are highlighted in green; conserved arginine residues important for microtubule-binding are highlighted in magenta (Schmidt et al. 2012). The sequence alignment was obtained with ClustalW2.
We recombinantly produced the C. elegans Ska1 MTBD (ceSka1 MTBD-7xHis) in E. coli and solved the structure of the purified protein with NMR spectroscopy (Schmidt et al. 2012). Here, we report the NMR resonance assignments.
Methods and experiments
A pET3aTr plasmid containing the ceSka1 MTBD-7xHis sequence was transformed into Escherichia coli BL21 (DE3) cells (Stratagene). Uniformly labeled ceSka1 MTBD-7xHis was grown at 25 °C in M9 media containing 1 g 15N-NH4Cl and 2 g 13C-glucose per liter to an OD600 of 0.6 and induced with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG). FILV labeled proteins for 3D and 4D methyl NOESY experiments were grown in perdeuterated M9 with 1 g 15N-NH4Cl and 2 g 2H-12C-glucose in 2H2O. We added 0.1 g 15N-phenylalanine, 75 mg ketobutyrate sodium (13C/1H methyl labeled and deuterated (Ile methyl precursor)), 125 mg isoketovalerate sodium (13C/1H methyl labeled and deuterated (Leu and Val methyl precursor)) one hour before induction with IPTG. The leucine and valine methyl precursors were stereospecificly 13C labeled and protonated at the λ-2 and γ-2 positions, respectively (Gans et al. 2010; Gardner 1997).
After 12–15 h of induction, the cells were harvested by centrifugation, resuspended in buffer-1 (50 mM Tris-HCl pH 8.0, 350 mM NaCl, 10 mM imidazole), 3.5 mM β-mercaptoethanol, 750 U Benzonase* Nuclease HC (purity >90%, Novagen), lysozyme (at a final concentration of 1 mg ml-1), a tablet of protease inhibitor cocktail (Complete, EDTA-free Tabs-Roche) and lysed by sonication at 4 °C. Cell debris and other insoluble particles were pelleted by centrifugation at 30000 g for 40 min, the cleared solution with the ceSKA1 MTBD-7xHis was then applied to Ni-NTA resin (Qiagen) and incubated for 2 hours at 4 °C. The Ni-NTA beads were subsequently washed with 10-column volumes (CV) buffer-2 (50 mM Tris-HCl pH 8.0, 1 M NaCl, 10 mM imidazole) and with 10 CV buffer-3 (50 mM Tris-HCl pH 8.0, 350 mM NaCl, 40 mM imidazole). We eluted ceSka1 MTBD-7xHis with 35 ml buffer-4 (50 mM Tris-HCl pH 8.0, 1 M NaCl, 250 mM imidazole) and dialyzed it in two steps against 4 l of buffer-5 (20 mM potassium phosphate pH 6.5, 150 mM NaCl, 1.5 mM DTT). Amicon Ultra concentrators with 10-kDa molecular weight cutoff (Millipore) were used to concentrate the dialyzed sample. Finally, 0.01% NaN3 and 5% D2O were added to prepare the sample for NMR spectroscopy.
NMR spectroscopy
Spectra were recorded on a Varian/Agilent Inova 500 MHz with a room temperature probe, on a Varian/Agilent Inova 600 MHz with a cryogenically cooled probe and Bruker 700 MHz and 900 MHz spectrometers with cryogenically cooled probes at 298 K. Backbone and side chain resonances were assigned with standard triple resonance experiments (HNCA/HN(CO)CA, HNCO/HN(CA)CO, HNCACB, (H)C(C-CO)NH-TOCSY, H(CC-CO)NH-TOCSY and HCCH-TOCSY) (Ferentz and Wagner 2000). Aromatic side chains were assigned using 2D HBCBCGCDHD and HBCBCGCDCEHE experiments (Yamazaki 1993). Stereospecific assignment of the respective λ-2 and γ-2 methyl groups for Leu and Val residues was achieved with selective methyl labeling (Gans et al. 2010). All spectra were processed with NMRpipe (Delaglio et al. 1995) and analyzed with CcpNmr (Vranken et al. 2005) and CARA (Keller 2004).
Extent of the assignments and data deposition
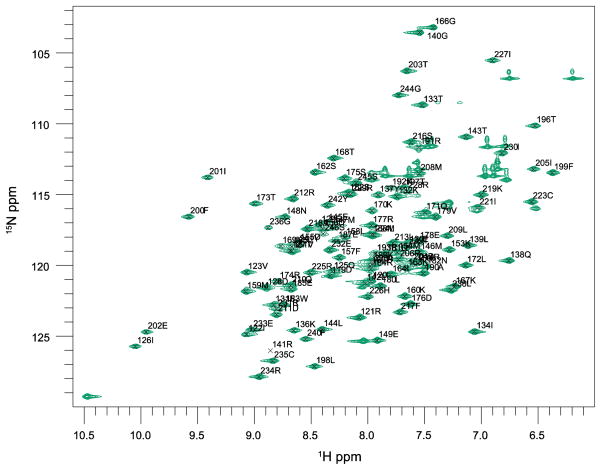
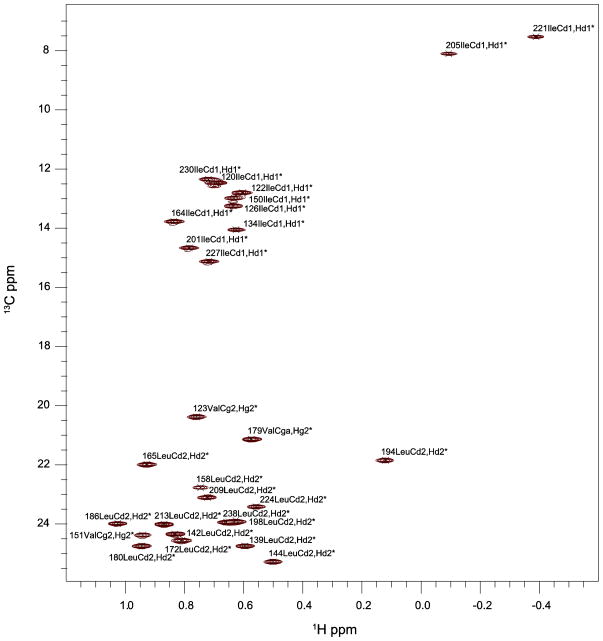
We assigned the backbone resonances of ceSka1-MTBD-7xHis almost completely (96% - excluding the highly flexible C-terminal His7-tag and the N-terminal Met residue - Figures 2 and 3) and 83% of aliphatic carbon and proton side chain resonances. We used selective methyl labeling to assign Leu-Hλ and Val-Hγ resonances stereospecifically (Figure 4) (Gans et al. 2010). The aromatic side chain resonances Hλ and Hε - with the exception of F41-Hε, H43-Hε, Hλ and W67-Hε - were assigned with 2D experiments as described by Yamazaki and colleagues (Yamazaki 1993).
Figure 2.

Primary sequence of ceSka1-MTBD (residues 118-243). Backbone resonances for all residues with the exception of the prolines, Asn118, Lys161 and Leu194 (no borders) are assigned (grey borders). The purified protein also a carried an N-terminal Met for translation initiation and a C-terminal His-7 tag connected by a short GSS linker region.
Figure 3.
2D 1H-15N HSQC of ceSka1-MTBD, recorded at 600 MHz and 298 K. Backbone peaks are annotated with their single-letter code and their sequence positions.
Figure 4.
2D 1H-13C methyl-HSQC of ceSka1-MTBD, recorded at 900 MHz and 298 K. Methyl peaks are annotated with their three-letter code, their sequence position and their corresponding atom names.
The chemical shifts derived from the assignments were used to calculate dihedral angles with the TALOS+ program (Cornilescu et al. 1999), the dihedral angles were used together with distance restraints for structure calculation of C.elegans Ska1 MTBD (Schmidt et al. 2012). The 1H, 15N and 13C chemical shifts were deposited in the Biological Magnetic Resonance Data Bank (http://www.bmrb.wisc.edu) with the BMRB accession number 18717.
Acknowledgments
Support for this project is provided by project P22170, and the doctoral school “DK Molecular Enzymology” (W901-B05), which are both funded by the Austrian Science Fund (FWF). This work was supported by NIH grants NIDDK-K01-DK085198 (HA), GM047467 (GW) and by a grant from the NIH/National Institute of General Medical Sciences to IMC (GM088313).
References
- Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nature reviews Molecular cell biology. 2008;9(1):33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- Cornilescu G, Delaglio F, Bax A. Protein backbone angle restraints from searching a database for chemical shift and sequence homology. Journal of biomolecular NMR. 1999;13 (3):289–302. doi: 10.1023/a:1008392405740. [DOI] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. Journal of biomolecular NMR. 1995;6 (3):277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- DeLuca JG, Musacchio A. Structural organization of the kinetochore-microtubule interface. Current opinion in cell biology. 2012;24(1):48–56. doi: 10.1016/j.ceb.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Martinez LA, Clarke DJ. Chromosome cohesion and the spindle checkpoint. Cell cycle. 2009;8 (17):2733–2740. doi: 10.4161/cc.8.17.9403. [DOI] [PubMed] [Google Scholar]
- Ferentz AE, Wagner G. NMR spectroscopy: a multifaceted approach to macromolecular structure. Quarterly reviews of biophysics. 2000;33 (1):29–65. doi: 10.1017/s0033583500003589. [DOI] [PubMed] [Google Scholar]
- Gans P, Hamelin O, Sounier R, Ayala I, Dura MA, Amero CD, Noirclerc-Savoye M, Franzetti B, Plevin MJ, Boisbouvier J. Stereospecific isotopic labeling of methyl groups for NMR spectroscopic studies of high-molecular-weight proteins. Angewandte Chemie. 2010;49(11):1958–1962. doi: 10.1002/anie.200905660. [DOI] [PubMed] [Google Scholar]
- Gardner KHK, LE Production and Incorporation of 15N, 13C, 2H (1H-d1 Methyl) Isoleucine into Proteins for Multidimensinoal NMR Studies. Journal of the American Chemical Society. 1997;119 (32):7599–7600. [Google Scholar]
- Hanisch A, Sillje HH, Nigg EA. Timely anaphase onset requires a novel spindle and kinetochore complex comprising Ska1 and Ska2. The EMBO journal. 2006;25(23):5504–5515. doi: 10.1038/sj.emboj.7601426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaprakash AA, Santamaria A, Jayachandran U, Chan YW, Benda C, Nigg EA, Conti E. Structural and functional organization of the Ska complex, a key component of the kinetochore-microtubule interface. Molecular cell. 2012;46(3):274–286. doi: 10.1016/j.molcel.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Keller R. The Computer Aided Resonance Assignment Tutorial. Goldau: CANTINA Verlag; 2004. [Google Scholar]
- McEwen BF, Dong Y, VandenBeldt KJ. Using electron microscopy to understand functional mechanisms of chromosome alignment on the mitotic spindle. Methods in cell biology. 2007;79:259–293. doi: 10.1016/S0091-679X(06)79011-2. [DOI] [PubMed] [Google Scholar]
- Pereira AJ, Maiato H. Maturation of the kinetochore-microtubule interface and the meaning of metaphase. Chromosome research : an international journal on the molecular, supramolecular and evolutionary aspects of chromosome biology. 2012;20(5):563–577. doi: 10.1007/s10577-012-9298-8. [DOI] [PubMed] [Google Scholar]
- Schmidt JC, Arthanari H, Boeszoermenyi A, Dashkevich NM, Wilson-Kubalek EM, Monnier N, Markus M, Oberer M, Milligan RA, Bathe M, Wagner G, Grishchuk EL, Cheeseman IM. The kinetochore-bound Ska1 complex tracks depolymerizing microtubules and binds to curved protofilaments. Developmental cell. 2012;23(5):968–980. doi: 10.1016/j.devcel.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheltzer JM, Blank HM, Pfau SJ, Tange Y, George BM, Humpton TJ, Brito IL, Hiraoka Y, Niwa O, Amon A. Aneuploidy drives genomic instability in yeast. Science. 2011;333(6045):1026–1030. doi: 10.1126/science.1206412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, Llinas M, Ulrich EL, Markley JL, Ionides J, Laue ED. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins. 2005;59(4):687–696. doi: 10.1002/prot.20449. [DOI] [PubMed] [Google Scholar]
- Welburn JP, Grishchuk EL, Backer CB, Wilson-Kubalek EM, Yates JR, 3rd, Cheeseman IM. The human kinetochore Ska1 complex facilitates microtubule depolymerization-coupled motility. Developmental cell. 2009;16(3):374–385. doi: 10.1016/j.devcel.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Forman-Kay JD, Kay LE. Two-dimensional NMR experiments for correlating carbon-13-b and proton-d/e chemical shifts of aromatic residues in 13C-labeled proteins via scalar couplings. Journal of the American Chemical Society. 1993;115:11054–11055. [Google Scholar]