Abstract
While driving is a complex task, it becomes relatively automatic over time although unfamiliar situations require increased cognitive effort. Much research has examined driving risk in cognitively impaired elders and found little effect. This study assessed whether mildly memory impaired elders made disproportionate errors in driving or story recall, under simultaneous simulated driving and story recall. Forty-six healthy (61% women; mean age = 76.4) and 15 memory impaired (66% women, mean age = 79.4) elders participated. Cognitive status was determined by neuropsychological performance. Results showed that during dual-task conditions, participants stayed in lane more, and recalled stories more poorly, than when they did the tasks separately. Follow-up analysis revealed that verbatim recall, in particular, was reduced while driving for healthy participants. While memory impaired participants performed more poorly than healthy controls on both tasks, cognitive status was not associated with greater dual-task costs when driving and story recall were combined.
Keywords: Older adults, Dual-task, Divided attention, Memory impairment, Driving
Age alone is a poor predictor of driving ability (Carr, Jackson, Madden, & Cohen, 1992). Driving is a complex task, involving sensory-perceptual, motor, social-personality and cognitive components. Despite the complexity, with practice, driving develops into an effortless task (Duchek, Hunt, Ball, Buckles, & Morris, 1997) allowing a skilled driver to focus on other information during driving (e.g., listening to the radio, having a conversation; Ward, 2004). While such automatic skills tend to be well preserved into old age and even into early dementia, the functions that allow driving to be goal-directed and purposeful may be adversely affected with dementia. A key loss with emerging cognitive impairment is the reduction of adaptive capacity to cope with novelty (Marson & Hebert, 2006). Therefore, when driving situations become unpredictable, or conditions become non-optimal (e.g., new neighborhood, thunderstorm, etc.), they require increased effort and controlled processing, placing drivers at heightened risk of driving failure, particularly if they are experiencing dementia-related losses in cognition (Dubinsky, Stein, & Lyons, 2000).
There is at least a two-fold increase in crash rate for those with Alzheimer’s disease (AD) compared to normal older adults (Carr & Ott, 2010) and approximately 30% of demented drivers will have a crash during the course of their disease (Morris, 2004). About half of drivers with dementia stop driving within 3 years of disease onset (Carr, Meuser, Berg-Weger, & Niewoehner, 2004). However, a diagnosis of dementia is not sufficient for driving cessation. Research has found that 62–76% of those with mild dementia pass an on-the-road driving evaluation (Brown et al., 2005; Hunt, Morris, Edwards, & Wilson, 1993). Nonetheless, given the progressive disease process, individuals with AD will most likely at some point become incapable of safe driving (Parasuraman & Nestor, 1991). Unfortunately, the timing is unpredictable at the individual level. When faced with such unpredictability, it is generally desirable to be conservative to ensure public safety (Brown & Ott, 2004; Molnar, Patel, Marshall, Man-Song-Hing, & Wilson, 2006).
Paradoxically, some research has failed to find effects of mild dementia on driving performance (Reger et al., 2004; Withaar, Brouwer, & van Zomeren, 2000). Two of the earliest studies on driving and dementia (see Friedland, Koss, & Kumar, 1988; Lucas-Blaustein, Filipp, & Dungan, 1988) found no significant relationship between severity of dementia, as measured by the Mini-Mental Status Examination (MMSE), and driving or crash history despite many of the drivers with dementia having crashes. Furthermore, MMSE scores could not discriminate who still drove or not, which is likely related to MMSE item content not being sufficiently sensitive to safe driving skills. Although, one would expect that individuals with very low MMSE scores would most likely not be safe for driving, while those with very high scores would most likely have no difficulty with driving.
To date, no single battery of neuropsychological tests has correlated adequately or consistently enough across studies to useful outcome measures of driving or driving risk (see Iverson et al., 2010 for recent neurology practice parameter on driving and Withaar et al., 2000 for a review on past cognitive studies of driving with cognitive impairment). In the recent practice parameter, the committee asserted that there is ‘insufficient evidence to support or refute the benefit of neuropsychological testing, after controlling for the presence and severity of dementia…’ (Iverson et al., 2010, p. 1320). In the second edition of the American Medical Association Physician’s Guide to Assessing and Counseling Older Drivers (Carr, Schwartzberg, Manning & Sempek, 2010), they suggest a battery of vision, physical, and cognitive measures including Trail Making Test Part B and the Clock Drawing Test to determine who should be referred for a formal driving evaluation due to possibly increased driving risk. However, the manual stresses that such tests ‘should not be the sole determinant as to whether an older adult can drive … it is unlikely that future fitness-to-drive evaluations will rely on one test but likely will employ a battery of tests’ (Carr et al., 2010, p. 35).
Such counter-intuitive findings may be due to weaknesses in the methods used for driving assessment in some studies. First, the use of crash history data relies on statistically improbable events (i.e., events with low base rates) to occur and is therefore unreliable. Second, the use of self-report or crash data is unreliable because crashes are not always reported and self-report from an individual with dementia could be inaccurate due to forgetting or social desirability. Studies have shown that self-report of driving skills in individuals with dementia is not useful in determining risk, as 94% of patients in one study rated themselves as safe while only 41% of them were able to pass an on-the-road evaluation (Iverson et al., 2010). Third, driving exposure in individuals with AD is less than older adults without cognitive impairment due to self-restriction, so the use of crash data for between group analyses is likely not well controlled (Withaar et al., 2000). Lastly, typical clinical and on-the-road driving research to date has included low-challenge, safe, optimal driving conditions, in which drivers can rely on their acquired automatic skills, likely contributing to why dementia-related driving impairments have been undetected.
While research has shown little effect of mild dementia on driving ability, many studies have found an effect of distracted driving or divided attention while driving during verbal activities (e.g., talking or listening; Engstrom, Johansson, & Ostlund, 2005). Concurrent tasks of verbal recall with simulated driving reaction time (Crook, West, & Larrabee, 1993) and verbal recall with visual search during simulated driving (McPhee, Scialfa, Dennis, Ho, & Caird, 2004) have both shown to be associated with reductions in performance on both the verbal and driving tasks, especially for older adults compared to younger adults. On the other hand, Kubose et al. (2006) found that both producing and comprehending language while driving had detrimental effects onmaintaining speed, but actually yielded better lane position control. One explanation for this is that participants may have prioritized driving due to the real-world dangers distracted driving entails, and sacrificed the verbal task.
Recent literature shows there is a well-documented impairment of performance on divided attention tasks involving verbal memory among older adults with mild AD (e.g., Della Sala & Logie, 2001; Belleville, Chertkow, & Gauthier, 2007). However, these impairments are not consistently observed among adults with mild cognitive impairment (MCI) relative to healthy agematched controls (for example, Foley, Kaschel, Logie, & Della Sala, 2011; Perry, Watson, & Hodges, 2000, found no group differences, and Belleville et al., 2007, found group differences). There is substantial heterogeneity of neuropsychological performance among adults with MCI, which may explain the inconsistency of these findings (Nordlund et al., 2005). For example, examination of different MCI subgroups suggests that those with pure amnestic MCI (i.e., impairment only in the domain of memory; aMCI) may not demonstrate greater dual-task performance costs despite attenuated prefrontal cortical activation during the task, relative to controls (Dannhauser et al., 2005), but those with multiple-domain MCI (i.e., impairment in multiple cognitive domains) do show greater dual-task costs (Lopez et al., 2006). In addition, a review of dual-task effects (Riby, Perfect, & Stollery, 2004) has suggested group differences in dual-task costs may be at least partly driven by the extent to which the tasks require effortful versus automatic processing. As alluded to earlier, MCI adults may not show differentially greater dual-task costs on tasks which are relatively automatic, but may show greater dual-task costs on cognitively-complex tasks requiring more controlled and effortful processing (Crossley Hiscock, & Foreman, 2004).
In considering memory, story memory was an area of particular interest in this study. First, story memory is more functionally relevant for late life communication competence (Chapman, Anand, Sparks, & Cullum, 2006). Second, stories may be recalled not only verbatim (e.g., Wilson, Cockburn, & Baddeley, 1985), but also in more holistically- or globally-processed forms of recall (paraphrase or gist-based recall; i.e., Chapman et al., 2006) conveying memory for the information without reproducing it word-forword. Recounting ideas and events in narrative and abstracted (rather than veridical) form is generally more common than list recall (Gabrieli, 2004). Age-comparative research suggests that older adults tend to prefer gist-based story processing (e.g., Adams, 1991; Adams, Smith, Nyquist, & Perlmutter, 1997; Wingfield & Stine-Morrow, 2000), and story recall differences between young, young–old and old–old adults can be attenuated, if not eliminated, when gist-based scoring approaches are used (Chapman et al., 2006; Dixon et al., 2004; Johnson, 2003). With regard to cognitive status, as expected, memory-impaired older adults generally remember less on story memory tasks than healthy older adults (e.g., Gely-Nargeot, Ska, & Touchon, 2002; Robinson-Whelen & Storandt, 1992). Under gist-based scoring, differences between mild AD groups and healthy controls persist (Chapman et al., 2006; Johnson, Storandt, & Balota, 2003; Haut, Demarest, Keefover, & Rankin, 1994), but group differences between MCI and controls are inconsistent (Hudon et al., 2006). While gist, as an example of abstracted or non-verbatim recall, has received the lion’s share of attention in the story recall literature, paraphrasing is another form of assessing memory for ideas rather than words. Paraphrase recall has long been understood as providing evidence of the semantic encoding of to-be-remembered material (e.g., Anderson, 1971), and provides an index of inferential reading (e.g., Brewer, Sampaio, & Barlow, 2005), and is often taken as one index of how much information readers have accurately extracted from text (e.g., Lehman, Schraw, McCrudden, & Hartley, 2007).
The goal of the present study was to simulate challenging driving, under safe and controlled conditions, to assess whether increases in difficulty (as measured by manipulating driving speed on a curved roadway) and complexity (as measured by divided attention conditions), increased the risk of driving errors and/or reduced story recall in older adults, and whether these dual-task costs were disproportionately large in those with cognitive impairment. Four questions were examined. First, the within-person effect of task difficulty and between-persons effect of cognitive status on lane navigation were examined, hypothesizing that (a) as speed increased, lane navigation performance would decrease and (b) as speed increased, cognitively impaired participants’ lane navigation would be disproportionately reduced relative to healthy participants. Next, the within-person effect of task complexity and between-subjects effect of cognitive status on amount of driving ‘errors’ (operationalized as percent of time spent out of lane and percent of outlier trials [±3 SD from each participant’s mean]) were examined. The hypothesis was that (a) errors would be higher under dual compared to single task conditions and (b) that memory impaired participants would have disproportionate errors. Third, the within-person effect of task difficulty and between-persons effect of cognitive status on story recall was examined, hypothesizing that (a) as speed increased story recall would decrease and (b) as speed increased, cognitively impaired participants’ story recall would disproportionately decrease. Finally, we examined whether increases in task complexity, via divided attention conditions, differentially affected story memory as characterized by verbatim or paraphrased criteria. A comparison of single task (driving or remembering only) with dual-task (driving while remembering) permitted the investigation of dual-task costs on both the driving and verbal memory tasks. Potential differential effects of divided attention on verbatim versus paraphrase memory for healthy versus impaired older adults has, to our knowledge, not yet been investigated.
METHOD
Participants
Sixty-one community-dwelling older adults (age range 65–91) of varying cognitive levels participated in the study. Participants were recruited from the community through several research pools as well as local advertisements. Exclusionary criteria included: (a) history of neurological disease (other than Alzheimer’s disease), (b) heart attack within the last year, (c) stroke within the last year with residual motor signs (e.g., paralysis or weakness in any extremity), (d) current cancer treatment (except skin cancer), (e) history of cancer radiation treatment above the chest, (f) never had driver’s license or stopped driving more than 2 years ago, (g) visual or auditory deficits that precluded testing, (h) past drug or alcohol dependence, and (i) history of psychiatric hospitalization. The study was approved by the Institutional Review Board of the University of Florida and informed consent was obtained prior to participation.
Procedures
Participants were screened by telephone for exclusion criteria and included a brief cognitive assessment. After consent was obtained, participants completed the neuropsychological battery (to classify cognitive status; see later), questionnaires, and the experimental dual-task procedure, as described below. A Clinical Dementia Rating Scale (CDR; Morris, 1993) interview was completed by telephone with an identified informant of the participant for a select group of participants when members of the consensus panel decided that classification could not be made based solely on their neuropsychological data.
Following all data collection, a consensus conference panel (consisting of the authors as well as a neuropsychology faculty member) convened to assign participants to groups according to their cognitive performance and ability to carry out daily functions following established criteria for MCI and early-stage AD (following the Petersen criteria for diagnosing MCI, Petersen et al., 1999; and the CERAD criteria for diagnosing AD, Morris, Mohs, Rogers, Fillenbaum, & Heyman, 1988). All test scores were presented to the consensus panel in percentile format and attention was paid to test scores falling at or below the 7th percentile (which coincides with 1.5 SD definition of impairment as outlined by Petersen et al., 1999). CDR scores, where available, were also reviewed and considered in making the classification. (No participants subsequently classified as impaired had a CDR = 0 and only one participant classified as control had a CDR = 0.5.) Forty-six participants were classified as healthy controls, while 15 were classified as memory impaired (all had impairments in the memory domain and received a consensus classification of either Mild Cognitive Impairment [N = 12] or dementia [N = 3]).
Table 1 shows the demographic characteristics of the two groups. The only significant group differences were expected; the memory impaired group performed significantly worse on the MMSE, which is a broad indicator of cognitive functioning. However, despite the significant difference between groups on the MMSE, the mean of the memory impaired group indicates that the sub-sample was primarily non-demented based on the accepted cutoff of 24 (Holsinger, Deveau, Boustani, & Williams, 2007).
TABLE 1.
Mean (SD) or N (%) of demographic data
| Total sample (N=61) |
Healthy controls (N=46) |
Memory impaired (N=15) |
p-Value | |
|---|---|---|---|---|
| Age | 76.89 (6.78) | 76.07 (6.71) | 79.40 (6.59) | 0.10 |
| Education | 15.97 (2.51) | 15.93 (2.40) | 16.07 (2.91) | 0.86 |
| Sex | 0.80 | |||
| Male | 22 (36.0) | 17 (37.0) | 5 (33.3) | |
| Female | 39 (64.0) | 29 (63.0) | 10 (66.7) | |
| Race | 0.72 | |||
| Caucasian | 58 (95.08) | 44 (95.7) | 14 (93.3) | |
| Other | 3(4.92) | 2(4.3) | 1(6.7) | |
| MMSE Score | 28.26 (1.84) | 28.80 (1.28) | 26.60 (2.32) | <0.01 |
Measures
Participants were individually administered a neuropsychological battery. Detailed assessment of memory, including both verbal and visual memory, attention, working memory, speed of processing, and language were assessed in order to determine what, if any, domains were impaired. See Table 2 for measures included in the determination of cognitive status.
TABLE 2.
Measures used for consensus classification
| Cognitive Domain |
Test | Variables of interest | Published source |
|---|---|---|---|
| General cognitive screener | Mini-Mental Status Examination | Total score (using serial 7 subtraction) | Folstein, Folstein and McHugh (1975) |
| Memory | Hopkins Verbal Learning Test-Revised (HVLT-R) | Total Immediate, Delay, and Recognition | Brandt and Benedict (2001) |
| Wechsler Memory Scale -- Third Edition, Logical Memory | Total Immediate, Delay, and Recognition | Wechsler (1997) | |
| Boston Naming Test 15-item CERAD version | Total score (using serial 7 subtraction) | Morris, Heyman and Mohs (1989) | |
| Language | (BNT-15) | Benton and Hamsher (1989) | |
| Controlled Oral Word | |||
| Association (COWAT) | Total (F, A, S) | Goodglass and Kaplan (1972) | |
| Category Fluency | Total Animals | ||
| Psychomotor Speed | Trail Making Test A and | Time for A, Time for B, Errors for | |
| B (TrailsA, TrailsB) Wechsler Adult Intelligence Test -- Third | A, Errors for B | Reitan (1992) | |
| Edition, Digit Span | Wechsler (1997) | ||
| Attention | Subtest | Forward Span and Backward Span Automatic Detection Accuracy, Controlled Search Accuracy, Speed-Accuracy Difference) | |
| Ruff 2 & 7 Selective Attention Test | Ruff and Allen (1996) | ||
| Construction Ability | Rey-Osterreith Complex Figure | ||
| Copy Total | Rey (1941) | ||
| Geriatric Depression Scale (GDS) | Total score (using serial 7 subtraction) | Yesavage et al. (1983) | |
| Mood | |||
| Daily Functioning | Clinical Dementia Rating Scale (CDR) | Total score (using serial 7 subtraction) | Morris (1993) |
After completing the neuropsychological battery, participants completed an acclimation task (5 minutes) in which they practiced the driving simulator at slow speed without the secondary task to become familiar with the driving equipment. Following acclimation, the experimental task was administered in two 13.5-minute segments, with a 4.5-minute break period to prevent fatigue. In total, 6 trials were administered: 3 slow (30 mph) and 3 fast (60 mph), alternating slow and fast trials. Within each trial, a period of single-task driving was followed by dual-task driving and story recall. During the dual-task, participants heard, briefly mentally rehearsed, then verbally recalled a short story while driving.
A strong correlation (0.67) between simulated driving ability and onthe- road driving ability in those with dementia has been found, suggesting that simulators may be a valid way to assess driving ability in a secured environment (Freund, Gravenstein, Ferris, & Shaheen, 2002). Lane navigation was measured using scenarios created using STISIM Drive software (Systems Technology Inc, Hawthorn, CA). Scenarios were presented by desktop computer (Dell Optiplex GX270 CPU, 19-inch flat screen monitor, and Logitech MOMO Force Feedback Steering Wheel). Participants were seated at a desk with the steering wheel attached to the desk in front of them. The monitor was situated 18 inches from the front of participants’ heads. Participants did not control the speed; rather the software was configured to present the stimuli at the designated speeds for experimental control between participants. The amount of visual complexity (i.e., scenery; number of turns to be executed) was controlled such that the only difference between the slow and fast conditions was the speed of presentation and therefore the length of roadway driven. The dependent variable of interest was the standard deviation of how much they deviated from the center of the lane. This provided a measure, independent of the magnitude of lane deviation, about how consistently the participant stayed in the center of the lane for each trial (e.g., single/slow, single/fast, dual/slow, dual/fast). Higher levels of variability on this measure indicated more ‘weaving’. Driving data were collected at 10 Hz (i.e., 10 times per second) and trimmed for outliers by excluding datapoints ±3 SD from each individual participant’s own mean performance to account for any gross errors in driving.
Memory for stories was assessed using a paragraph recall task. A memory task was chosen for a few reasons. First, the impaired participants were likely to have deficits in this area. Thus, it was believed that a concurrent memory task would be particularly demanding of attentional and processing capability due to decreased resources secondary to their brain dysfunction. Memory is also an integral piece in driving (Parasuraman & Nestor, 1991), and therefore should be studied concurrently with simpler driving skills since this simultaneous processing is part of the regular task of driving. Four of the story recall paragraphs came from the Rivermead Behavioral Memory Test (Wilson et al., 1985). The other five had been used in a memory training study and were created using an algorithm created to make them to correspond to the Rivermead paragraphs in complexity, structure, and number of idea units (Rebok, 1998). ‘Idea unit’ refers to individual lexical story items; example, the following sample sentence from one of the stories is divided into ‘idea units’:
Ms. Virginia/Boone/a mother of two/won/the mother of the year award/on Sunday/during a community celebration/in Chicago.
For brevity, the Rivermead-type stories will be referred to as ‘Rivermead’ stories, acknowledging that not all of them came from the actual Rivermead Behavioral Memory Test. The order in which participants heard stories was randomized across participants. The stories were presented as sound files, recorded in a male voice, through the computer speakers. The stories were on average 28 seconds in length. After presentation, the participant was given 35 seconds to rehearse the story in their head and then the participant recalled the story as completely as possible. Responses were digitally recorded and then transcribed.
Scoring was based on the accuracy and completeness of recall. Each idea unit was scored as 0 (not recalled), 0.5 (paraphrased or incompletely recalled), or 1 point (verbatim or completely recalled). This scoring method was the same as that developed for the Rivermead paragraphs, and was adapted for the algorithm-based stories. Based on this rubric, stories were additionally scored for the proportion recalled verbatim (i.e., idea units scored as 1 point, or recalled with full accuracy, out of the 21 total idea units possible) and the proportion recalled in paraphrase (number of idea units scored as 0.5 points, or partially recalled, out of the 21 total idea units possible). In other words, if a participant recalled 7 idea units verbatim and 3 units paraphrased, their score would be (7/21×100) = 33.3% verbatim, and (3/21×100) = 14.3% paraphrased; therefore verbatim and paraphrase recall did not sum to 100% of total recall, and were not linearly dependent. To ensure reliability of scoring, the average of ratings by two independent raters was used in all analyses. The two sets of ratings were positively correlated for each story administered in each condition (ranging from r = .93 to r = .97, p < .001).
RESULTS
Preliminary analyses were conducted to examine the differences in performance between the healthy controls and memory impaired older adults on the neuropsychological tests. These measures were heavily used in making group assignment decisions during the consensus conference, and therefore Table 3 demonstrates how performance differed between groups; it provides a window into the classification rules used by the consensus team. As expected, the memory impaired group performed worse, and often within the clinically impaired range, than the healthy control group on most of the neuropsychological measures. The table also illustrates that effect sizes for between group differences varied from small to large.
TABLE 3.
Means, variance, and group differences for performance on neuropsychological measures by cognitive status
| Mean | SD | Levene’s Test |
Independent sample |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measure | HC | MI | HC | MI | F | p-value | t | p-value | Cohen’ s d |
Effect size r |
| TICS-M Score | 36.50 | 32.07 | 6.27 | 3.47 | 0.49 | ns | 2.52 | 0.02 | 0.77 | 0.36 |
| MMSE Score | 28.80 | 26.60 | 1.28 | 2.32 | 10.81 | <0.01 | 3.51 | <0.01 | 1.38 | 0.57 |
| HVLT-R Total | 27.20 | 17.80 | 4.42 | 4.93 | 0.14 | ns | 6.95 | <0.01 | 2.07 | 0.72 |
| HVLT-R Delay | 9.83 | 3.00 | 1.77 | 2.24 | 1.13 | ns | 12.15 | <0.01 | 3.61 | 0.87 |
| WMS LMI | 45.76 | 28.60 | 8.34 | 7.95 | 0.07 | ns | 7.00 | <0.01 | 2.08 | 0.72 |
| WMS LMII | 28.67 | 13.47 | 6.33 | 8.30 | 2.02 | ns | 7.47 | <0.01 | 2.22 | 0.74 |
| COWA Total | 16.39 | 13.00 | 4.39 | 3.98 | 0.47 | ns | 2.21 | 0.03 | 0.28 | 0.14 |
| Category Fluency | 20.76 | 16.13 | 5.74 | 5.10 | 0.01 | ns | 2.78 | <0.01 | 0.83 | 0.38 |
| BNT Total Score | 14.67 | 13.80 | 0.56 | 1.15 | 12.7 | <0.01 | 2.84 | 0.01 | 1.18 | 0.51 |
| Rey–Osterrieth | 29.53 | 25.03 | 4.89 | 5.21 | 0.71 | ns | 3.05 | <0.01 | 0.91 | 0.41 |
| Score Trails A | ||||||||||
| Time Trails B | 33.57 | 45.07 | 11.93 | 10.97 | 0.18 | ns | −3.31 | <0.01 | 0.98 | 0.44 |
| Time WAIS | 85.95 | 128.45 | 43.45 | 62.69 | 2.37 | ns | −2.93 | <0.01 | 0.87 | 0.40 |
| Digit Span Ruff | 18.74 | 16.33 | 4.66 | 3.81 | 1.01 | ns | 1.81 | ns | 0.54 | 0.26 |
| Accuracy | 94.26 | 73.87 | 16.64 | 27.98 | 7.61 | <0.01 | 2.67 | 0.02 | 1.02 | 0.46 |
| GDS | 4.43 | 6.47 | 5.02 | 6.76 | 1.60 | ns | −1.25 | ns | 0.37 | 0.18 |
Note: HC, Healthy Control Group (N = 46); MI, Memory Impaired Group (N = 15); TICSM, Modified Telephone Interview for Cognitive Status; MMSE, Mini-Mental Status Examination; HVLT-R, Revised Hopkins Verbal Learning Test; WMS LM= Wechsler Memory Scale Logical Memory Subscale; COWA, Controlled Oral Word Association Test; WAIS, Wechsler Adult Intelligence Test; GDS, Geriatric Depression Scale; ns, Not significant at .05 level; Levene’s test examined the homogeneity of variance between the Normal and Impaired groups; significant tests suggest unequal variance between groups. Independent samples t-tests compared group differences in mean on each variable. Where Levene’s test was significant, a t-test with adjusted degrees of freedom was used; Cohen’s D and Effect size r were adjusted for different sample sizes and the absolute value is presented.
Lane Navigation – Task Difficulty and Impairment Group
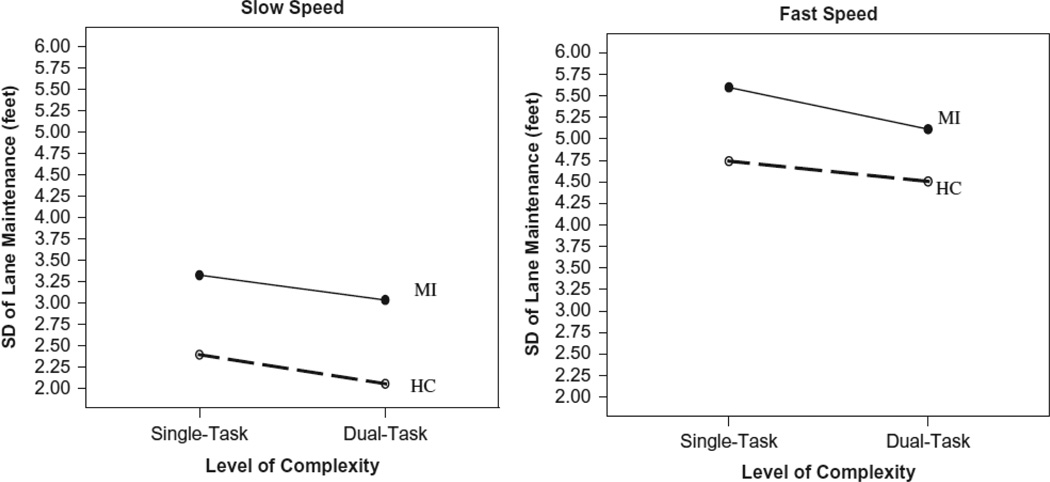
A repeated measures analysis of variance (RM-ANOVA) was conducted to examine the effect of task difficulty and complexity and the potential added effect of impairment level on the dependent variable of interest (standard deviation of lane deviation – a measure, independent of the magnitude of lane deviation, that captured how consistently the participant stayed in the center of the lane, with higher levels of variability on this measure indicating more weaving, measured in feet). The 2 × 2 × 2 RM-ANOVA had two levels of within-person task difficulty (Slow and Fast) crossed with two levels of task complexity (Single and Dual) and two levels of the between-person factor of impairment group (Control vs. Memory Impaired) to examine their effect on the standard deviation of lane deviation. Means of task difficulty (speed) show that there was more deviation in lane maintenance at the faster speed (M ± SE = 4.99 ± 0.26) than at the slower speed (M ± SE = 2.70 ± 0.31). This observation was supported with a main effect of task difficulty, F(1, 53) = 73.20, p < .01, η2 = .58. Counter-intuitively, means of task complexity showed that there was more deviation in lane maintenance in the single task (M ± SE = 4.02 ± 0.24) than in the dual-task (M ± SE = 3.68 ± 0.29). This was supported with a main effect of task complexity, F(1, 53) = 7.12, p = .01, η2 = .12. While there was only a trend toward a main effect of impairment, F(1, 53) = 2.78, p = .10, η2 = .05, the means of the memory impairment group revealed that they tended to have slightly more deviation of lane maintenance (M ± SE = 4.27 ± 0.44) than the control group (M ± SE = 3.43 ± 0.25).
There were no significant interactions found (task difficulty by impairment group, F(1, 53) = 0.18, p = .68, η2 = .01; task complexity by impairment group, F(1, 53) = 0.16, p = .70, η2 = .01; and task difficulty by task complexity, F(1, 53) = 0.02, p = .88, η2 = .01). The three-way interaction between task difficulty, task complexity, and impairment group was also nonsignificant, F(1, 53) = 0.24, p = .62, η2 = .01. Table 4 displays the means for this three-way interaction and Figure 1 graphically shows these findings.
TABLE 4.
Means ± standard errors of three-way interaction for deviation of lane maintenance
| Task difficulty: slow | Task difficulty: fast | |||
|---|---|---|---|---|
| Impairment group |
Single task | Dual task | Single task | Dual task |
| Healthy control | 2.40±0.31 | 2.06 ±0.31 | 4.74 ±0.24 | 4.51 ±0.31 |
| Memory impaired | 3.33 ±0.56 | 3.04 ±0.55 | 5.60 ±0.43 | 5.11 ±0.56 |
FIGURE 1.
Line graphs of level of difficulty and level of complexity by impairment status for standard deviation of lane maintenance (feet). Memory Impaired participants (MI) show more variability in lane maintenance than controls (HC), and participants showed more variability at fast speeds than at slow speeds. However, under dual-task conditions, participants showed marginally less variability when a secondary memory task was added.
The amount of driving ‘errors’, operationalized as percent of time spent out of lane and percent of trials that were outliers (±3 SD from each participants mean), were also examined. Participants spent more time out of lane and in outlier trials under the single task condition (18% of time out of lane, 2.7% outlier trials) than under the dual-task condition (16% of time out of lane, 1.8% outlier trials). Consistent with earlier findings, there was no effect of impairment status and impairment did not interact with single or dual conditions, possibly related to low power to detect differences.
Story Recall – Task Difficulty and Impairment Group
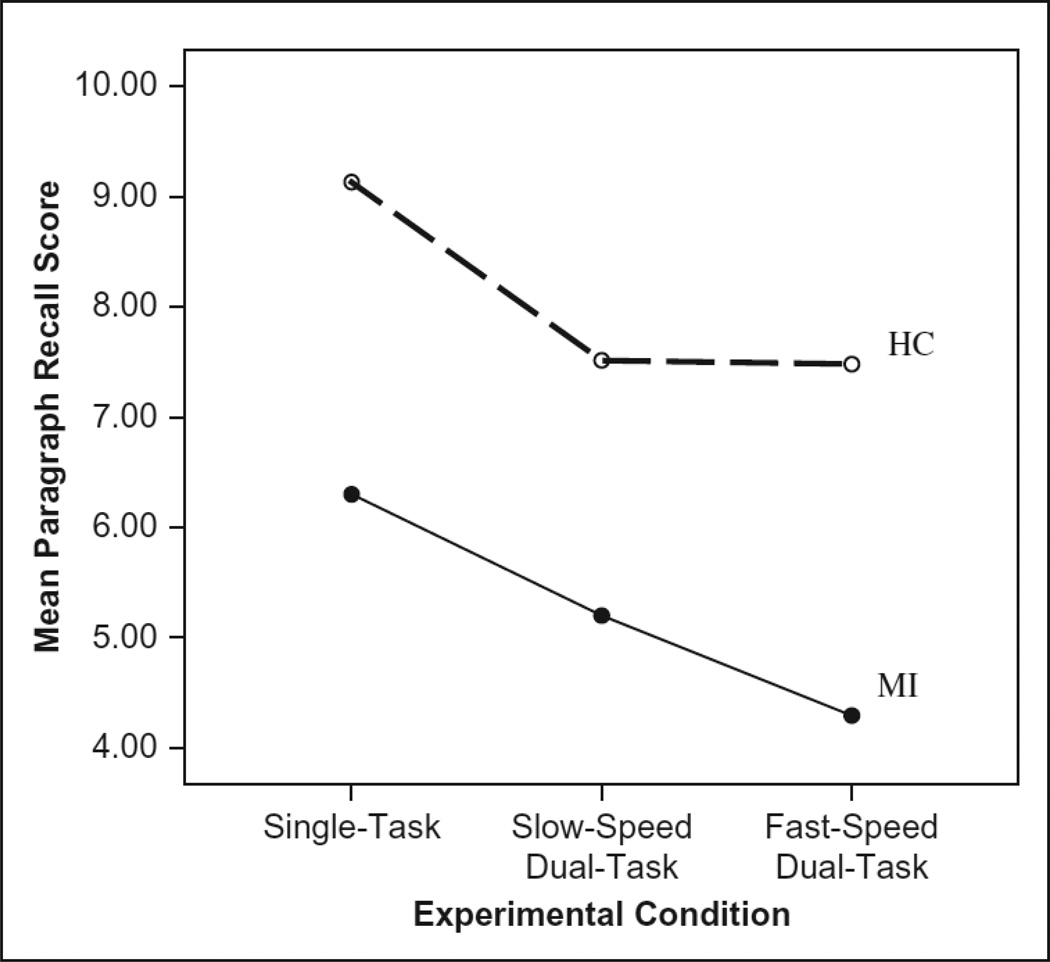
Participants may have prioritized the driving task at the cost of the story recall task, which might help explain the unexpected pattern of lane navigation performance. This hypothesis was investigated with a 3 × 2 RM-ANOVA with three levels of within-person story recall (total score during single task, slow conditions, fast conditions) and the between person factor of impairment group. There was a significant main effect of experimental condition, F(2, 102) = 13.77, p < .01, η2 = .21, such that the participants remembered more story information under single task (M ± SE = 7.71 ± 0.39) than slow dual-task conditions (M ± SE = 6.36 ± 0.39) and fast dual-task conditions (M ± SE = 5.89 ± 0.43). A Bonferroni corrected comparison of the experimental conditions showed that single-task story recall was significantly better than in both the slow condition (p < .01) and the fast condition (p < .01), but recall did not differ between the slow and fast conditions (p = .21). There was also a main effect of impairment group, F(1, 51) = 16.38, p < .01, η2 = 0.24, such that the control group performed better (M ± SE = 8.04 ± 0.33) than the memory impaired group (M ± SE = 5.26 ± 0.60) on average. However, the interaction between experimental condition and impairment was not significant, F(2, 102) = 0.74, p = .47, η2 = .01 (see Figure 2).
FIGURE 2.
Line graph for each experimental condition by impairment status for paragraph recall score.
Story Recall – Verbatim versus Paraphrase Recall
A 3 × 2 × 3 RM-ANOVA with three levels of within-person story recall (total score during single task, slow conditions, fast conditions) and type of story recall (verbatim, paraphrase) and the between-person factor of impairment group (healthy, memory impaired) was conducted. Significant effects were decomposed using Bonferroni-corrected post hoc comparisons. Task difficulty and impairment status did not significantly interact. Recall type, however, interacted with impairment, F(1, 51) = 17.52, p < .001, η2 = .09, such that healthy participants recalled significantly (p < .001) more verbatim (M ± SE = 0.32 ± 0.02) than they did paraphrase (M ± SE = 0.14 ± 0.01), but the impaired group recalled approximately equal proportions of verbatim and paraphrased information (and less information than healthy participants overall, as reported earlier).
Recall type also interacted with within-person task difficulty, F(2, 51) = 6.43, p = .002, η2 = .02, such that switching from single to dual task difficulty significantly reduced verbatim recall (p = .001; Single task: M ± SE = 0.3 ± 0.02; Dual task: M ± SE = 0.25 ± 0.02), but did not affect paraphrase recall. The fast driving condition did not additionally reduce verbatim recall compared to the slow driving condition. Importantly the three-way interaction of recall type, task difficulty, and impairment was not significant (p = .75), suggesting that the dual-task effect on verbatim but not paraphrased recall did not differ between the healthy and impaired participant groups. Table 5 illustrates the mean proportion of verbatim and paraphrase recall for healthy and impaired participant groups across occasions.
TABLE 5.
Means ± standard errors of three-way interaction for recall type; values represent proportion of idea units recalled verbatim or paraphrase out of the total possible
| Task difficulty | ||||
|---|---|---|---|---|
| Group | Recall type | Single task | Slow dual-task | Fast dual-task |
| Healthy | Verbatim | 0.37 ±0.018 | 0.31 ±0.018 | 0.28 ±0.016 |
| Paraphrase | 0.15±0.008 | 0.14 ±0.007 | 0.14 ±0.010 | |
| Impaired | Verbatim | 0.23 ±0.033 | 0.19 ±0.034 | 0.14 ±0.030 |
| Paraphrase | 0.16±0.015 | 0.14 ±0.012 | 0.13 ±0.019 | |
DISCUSSION
This study manipulated difficulty (slow versus fast speed) and complexity (single versus dual-task) in a simulated lane navigation driving situation and investigated whether performance on lane navigation and story recall decreased disproportionately in those with memory impairment, and examined whether added task difficulty and complexity affected verbatim or paraphrased text recall. The results confirmed the hypothesis that participants’ story recall performance would decrease as task complexity (dual-task) and difficulty (speed) increased. However, the hypothesis that increasing task complexity (dual-task) would decrease lane navigation performance was not supported. Rather, participants’ lane navigation was significantly better and percent of driving ‘errors’ were lower under dual-task conditions while the story recall performance declined. Thus, participants became more vigilant to the driving task, while compromising their performance on the story memory task. Interestingly, the hypothesis that participants with memory impairment would perform disproportionately worse in the dual-task conditions was not supported, as there was no interaction in either the driving task or the story recall task. In both cases, while the impaired participants generally performed more poorly, they were not disproportionately affected by the addition of a secondary task.
Since the addition of a second simultaneous task negatively affected story recall but not lane navigation, we sought to understand in greater depth what aspects of recall were sacrificed in the dual-task scenario, and if there were group differences in dual-task costs, what aspects of recall were differentially affected for impaired participants. The results reflected that both groups recalled fewer verbatim idea units with increasing task difficulty, and there was no difference in the extent to which verbatim or paraphrased recall were affected by simultaneous lane navigation between cognitive status groups.
Consistent with prior literature, memory impaired participants remembered less story information than healthy older adults. The group difference seemed primarily restricted to verbatim recall: impaired participants remembered less information verbatim, but the proportion of information recalled in paraphrase was roughly equivalent between the two cognitive status groups. Regarding the addition of a simultaneous task, impaired participants’ memory for story information was no more greatly affected by increases in task difficulty than that of healthy participants’, suggesting that at increases in attentional load due to the visuospatial task of simulated driving did not disproportionately affect memory impaired persons’ recall. Taken together, these results suggest that while memory-impaired older adults remember less verbatim overall compared to healthy older adults, under conditions of divided attention, the total amount of information recalled is not differentially reduced for impaired participants.
While our finding of increased lane maintenance under dual-task conditions seems counterintuitive, past research in other populations has previously suggested similar findings. For example, Kubose et al. (2006) found that maintenance of lane position was better during concurrent speech production than driving alone (although this was not true for concurrent speech comprehension). Engstrom et al. (2005) also reported about a meta-analysis on the effect of cognitive load on lane-keeping ability, indicating that one study (Brookhuis, de Vries, & deWard, 1991) also found increased lane navigation performance during a mobile-phone conversation. Correspondingly, lower memory performance under dual-task conditions was observed in the present study, although there was little evidence that memory was progressively reduced with increasing driving challenge (e.g., as participants moved from slow to fast driving). However, a key challenge in most dual-task research, including the present study, is that the attentional allocation ratios (i.e., the amount of priority each participant gave each task) of participants was not systematically varied; thus, there may have been individual differences in the proportion of attention allocated to the driving and memory tasks. This therefore emerged as a possible source of heterogeneity between participants. A second possibility is that the level of arousal in the participants under dual-task conditions may also have lead to the finding of increased performance. Many studies have confirmed that the level of physical arousal increases with cognitive workload during driving (e.g., De Waard, 1996). In fact, more recent research has investigated the use of different difficulty levels and types of cognitive tasks in dual-task scenarios to increase alertness while driving in monotonous conditions (e.g., highways; for example, see Oron-Gilad, Ronen, & Shinar, 2008; Takayama & Nass, 2008).
Throughout the analyses in this study, cognitive status essentially never interacted with any of the other study variables. Despite consistent hypotheses that persons with memory impairment would be disproportionately affected by distraction and increased task difficulty, the study did not support this expectation. While there was generally a main effect of impairment (such that amnestic participants performed more poorly, meaning they were less adept at lane navigation), this did not appear to interact with any other factors. The power to detect significant group differences was, of course, low due to the small sample sizes, especially for detecting interactions.
Another potential explanation for the lack of interaction effect is that the two tasks in the dual-task condition required different (i.e., non-competing) cognitive resources. While the secondary task was chosen to be heavily memory-based, in order to maximize cognitive load in the domain on which amnestic individuals are most impaired (and the results corroborated that the memory impaired group performed worse on the story recall task than the control group), the lack of interaction could be due to low interference the secondary task placed on the primary driving task. Specifically, the attention needed to support driving (visuospatial) may have represented a different set of cognitive resources from the attention needed to support memory (verbal/acoustic). Additionally, the level of challenge in either or both of the driving and memory tasks may not have been adequate to truly challenge the attention allocation system of either healthy control or mild memory impaired elders. Some studies have found that the dual-task costs are reduced significantly when using two relatively simple tasks that are processed via different perceptual modalities (Verhaeghen, Steitz, Sliwinski, & Cerella, 2003). However, other studies utilizing cross-modality dual-task designs have found significant interference effects (for example, Lindenberger, Marsiske, & Baltes, 2000; Maylor & Wing, 1996; Weeks, Forget, & Mouchnino, 2003; Woollacott & Shumway-Cook, 2002).
The counterintuitive effect obtained (lane navigation was better with divided attention) suggests that the specific tasks chosen for this study, and their specific levels of difficulty, may need to have been better calibrated for this population with a pilot study. On the more positive side, the absence of strong negative dual-task effects and disproportionately negative effects for the cognitively impaired lends further credence to the literature that suggests that memory impairment, at least in its early stages, may not be a strong risk factor for driving (Reger et al., 2004; Withaar et al., 2000). Additionally, this study is consistent with prior work suggesting that changes in performance under dual-task driving conditions may be seen more on the secondary task (which is ‘sacrificed’) than on the primary driving task (Becic et al., 2010). Another explanation may relate to the focal nature of early cognitive impairment in the memory impaired group included in this study. Since the majority of the memory impaired group had amnestic memory losses without other affected cognitive domains, they could still be expected to have relatively spared attentional abilities (Duchek & Balota, 2005; Parasuraman & Haxby, 1993), and the secondary task may not have been optimal to reveal any subtle changes in attentional capacity during the driving lanemaintenance task. Research has shown that individuals with MCI may still have compensatory mechanisms for dealing with their mild cognitive losses, and it is not until further in the disease process that multiple domains are often detected with testing. Although, a recent study suggested that a problem-solving task utilizing multiple domains was sensitive enough to detect impairment in MCI (Beversdorf et al., 2007). It may be that despite the poorer memory performance of the impaired participants, the ‘limiting variable’ in the dual-task condition was attention, and this was still quite intact, or well-compensated for, in most of the impaired participants.
Study Limitations and Future Directions
Several limitations of this study need to be considered. First, the participants were a highly select group consisting mainly of white, healthy, highly educated elders, which is not representative of the general elder population in the United States. Second, the study did not include a medical or neurological examination to rule out other potential causes of memory problems, such as a vitamin B deficiency or untreated hypothyroidism. Third, with regard to the driving task, while there was between-person experimental control, the ecological validity of the task used in the study is questionable. Participants were not able to control speed on their own. Additionally, while participants given breaks throughout the study, it is unclear how fatigue might have affected results of the driving task. Lastly, the instructions for the driving task did not include which task, the driving task or the secondary memory task, should have been prioritized, permitting participants to vary in their resource allocation during the dual-task conditions.
Driving is central to maintaining independence in old age. The memory impaired group was not disproportionately affected by the dual-task scenario in the present study, which is consistent with other studies that suggest no clear cognitive impairment effect early in a neurodegenerative disease process. Future work is needed to clarify the best assessment strategies for determining at which point driving safely is problematic. In addition, examining the extent to which driving safety in old age may be modifiable, with training or cognitive enhancing medications for example, remains to be empirically determined.
ACKNOWLEDGEMENTS
Supported in part by the AARP Scholars Program (Sarah E. Cook), National Institutes of Health (T32- AG- 020499: Sarah E. Cook and Shannon M. Sisco and F31 AG034002: Shannon M. Sisco). STISIM Software support was provided by Systems Technology Incorporated, Hawthorne, CA. Support was also received from the University of Florida’s National Older Drivers Research and Training Center, now known as The Institute for Mobility, Activity and Participation (I-MAP, http://mobility.phhp.ufl.edu/). This manuscript represents findings from both a dissertation (Sarah E. Cook) and master’s thesis (Shannon M. Sisco). Both authors contributed equally to the writing of this manuscript. A portion of this work has been presented in poster format at the 36th Annual meeting of the International Neuropsychological Society, Waikoloa, HI (Sarah E. Cook), the 2007 Annual meeting of the Gerontological Society of America (Shannon M. Sisco), and the 2008 International Conference on Aging, Disability, and Independence, St. Petersburg, FL (Shannon M. Sisco).
REFERENCES
- Adams C. Qualitative age differences in memory for text: A life-span developmental perspective. Psychology and Aging. 1991;6:323–336. doi: 10.1037//0882-7974.6.3.323. PMid: 1930750. [DOI] [PubMed] [Google Scholar]
- Adams C, Smith M, Nyquist L, Perlmutter M. Adult age-group differences in recall for the literal and interpretive meanings of narrative text. Journal of Gerontology: Psychological Sciences. 1997;4:187–195. doi: 10.1093/geronb/52b.4.p187. [DOI] [PubMed] [Google Scholar]
- Anderson RC. Encoding processes in the storage and retrieval of sentences. Journal of Experimental Psychology. 1971;91:338–340. [Google Scholar]
- Becic E, Dell GS, Bock K, Garnsey SM, Kubose T, Kramer AF. Driving impairs talking. Psychosomatic Bulletin & Review. 2010;17:15–21. doi: 10.3758/PBR.17.1.15. [DOI] [PubMed] [Google Scholar]
- Benton A, Hamsher K. Multilingual aphasia examination. Iowa City: AJA Associates; 1989. [Google Scholar]
- Beversdorf DQ, Ferguson JLW, Hillier A, Sharma UK, Nagaraja HN, Bornstein RA, Scharre DW. Problem solving ability in patients with mild cognitive impairment. Cognitive and Behavioral Neurology. 2007;20:44–47. doi: 10.1097/WNN.0b013e31802e5101. [DOI] [PubMed] [Google Scholar]
- Brandt J, Benedict RHB. Hopkins Verbal Learning Test – Revised: Professional Manual. Odessa, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- Brewer WF, Sampaio C, Barlow MR. Confidence and accuracy in the recall of deceptive and nondeceptive sentences. Journal of Memory and Language. 2005;52:618–627. [Google Scholar]
- Brookhuis KA, de Vries G, de Ward D. The effects of mobile telephoning on driving performance. Accident Analysis and Prevention. 1991;23:309–316. doi: 10.1016/0001-4575(91)90008-s. [DOI] [PubMed] [Google Scholar]
- Brown LB, Ott BR. Driving and dementia: A review of the literature. Journal of Geriatric Psychiatry and Neurology. 2004;17:232–240. doi: 10.1177/0891988704269825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LB, Ott BR, Papandonatos GD, Sui Y, Ready RE, Morris JC. Prediction of on-road driving performance in patients with early Alzheimer’s disease. Journal of the American Geriatrics Society. 2005;53:94–98. doi: 10.1111/j.1532-5415.2005.53017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr D, Jackson TW, Madden DJ, Cohen HJ. The effect of age on driving skills. Journal of the American Geriatric Society. 1992;40:567–573. doi: 10.1111/j.1532-5415.1992.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Carr DB, Meuser TM, Berg-Weger M, Niewoehner P. Driving and dementia in older adults: Evidence-based assessment and cessation strategies. Washington, DC: Paper presented at the 57th Annual Meeting of the Gerontological Society of America; Nov, 2004. PMid: 1587972. [Google Scholar]
- Carr DB, Ott BR. The older adult driver with cognitive impairment. Journal of the American Medical Association. 2010;303:1632–1641. doi: 10.1001/jama.2010.481. PMid: 20424254; PMCid: 2915446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Schwartzberg JG, Manning L, Sempek J. Physician’s guide to assessing and counseling older drivers. 2nd ed. Washington, DC: National Highway Traffic and Safety Administration; 2010. [Google Scholar]
- Chapman SB, Anand R, Sparks G, Cullum CM. Gist distinctions in healthy cognitive aging versus mild Alzheimer’s disease. Brain Impairment. 2006;7:223–233. [Google Scholar]
- Crook TH, West RL, Larrabee GJ. The driving-reaction time test assessing age declines in dual-task performance. Developmental Neuropsychology. 1993;9:31–39. [Google Scholar]
- Crossley M, Hiscock M, Foreman JB. Dual-task performance in early stage dementia: Differential effects for automatized and effortful processing. Journal of Clinical and Experimental Neuropsychology. 2004;26:332–346. doi: 10.1080/13803390490510068. PMid:15512924. [DOI] [PubMed] [Google Scholar]
- Dannhauser TM, Walker Z, Stevens T, Lee L, Seal M, Shergill SS. The functional anatomy of divided attention in amnestic mild cognitive impairment. Brain. 2005;128:1418–1427. doi: 10.1093/brain/awh413. [DOI] [PubMed] [Google Scholar]
- De Waard D. PhD thesis, University of Groningen. Haren, The Netherlands: University of Groningen, Traffic Research Centre; 1996. The measurement of drivers’ mental workload. [Google Scholar]
- Della Sala S, Logie RH. Theoretical and practical implications of dual-task performance in Alzheimer’s disease. Brain. 2001;124:1479–1481. doi: 10.1093/brain/124.8.1479. PMid:11459740. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Wahlin A, Maitland SB, Hultsch DF, Hertzog C, Backman L. Episodic memory change in late adulthood: Generalizability across samples and performance indices. Memory and Cognition. 2004;32:768–778. doi: 10.3758/bf03195867. [DOI] [PubMed] [Google Scholar]
- Dubinsky RM, Stein AC, Lyons K. Practice parameter: Risk of driving and Alzheimer’s disease (An evidence-based review) Neurology. 2000;54:2205–2211. doi: 10.1212/wnl.54.12.2205. PMid:10881240. [DOI] [PubMed] [Google Scholar]
- Duchek JM, Balota DA. Failure to control prepotent pathways in early stage dementia of the Alzheimer’s type: Evidence from dichotic listening. Neuropsychology. 2005;19:687–695. doi: 10.1037/0894-4105.19.5.687. PMid: 16187887. [DOI] [PubMed] [Google Scholar]
- Duchek JM, Hunt L, Ball K, Buckles V, Morris JC. The role of selective attention in driving and dementia of the Alzheimer type. Alzheimer Disease and Associated Disorders. 1997;11:48–56. doi: 10.1097/00002093-199706001-00011. [DOI] [PubMed] [Google Scholar]
- Engstrom J, Johansson E, Ostlund J. Effects of visual and cognitive load in real and simulated motorway driving. Transportation Research Part F. 2005;8:97–120. [Google Scholar]
- Foley JA, Kaschel R, Logie RH, Della Sala S. Dual-task performance in Alzheimer’s disease, mild cognitive impairment, and normal ageing. Archives of Clinical Neuropsychology. 2011;26:340–348. doi: 10.1093/arclin/acr032. PMid: 21576091. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-Mental State’: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Freund B, Gravenstein S, Ferris R, Shaheen E. Evaluating driving performance of cognitive impaired and healthy older adults: A pilot study comparing on-road testing and driving simulation. Journal of the American Geriatrics Society. 2002;50:1309–1310. doi: 10.1046/j.1532-5415.2002.50325.x. PMid: 12133033. [DOI] [PubMed] [Google Scholar]
- Friedland RP, Koss E, Kumar A. Motor vehicle crashes in dementia of the Alzheimer type. Annals of Neurology. 1988;24:782–786. doi: 10.1002/ana.410240613. PMid: 3207361. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE. Memory: Pandora’s hippocampus? Cerebrum. 2004;6:39–48. [PubMed] [Google Scholar]
- Gely-Nargeot MC, Ska B, Touchon J. Text structure and content modulate the recall of patients with dementia of the Alzheimer’s type. Brain and Cognition. 2002;48:371–375. PMid: 12030470. [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Assessment of aphasia and related disorders. Philadelphia, PA: Lea & Febiger; 1972. [Google Scholar]
- Haut MW, Demarest D, Keefover RW, Rankin ED. Semantic sensitivity for prose in patients with probable Alzheimer’s disease. Aging Neuropsychology, and Cognition. 1994;1:238–246. [Google Scholar]
- Holsinger T, Deveau J, Boustani M, Williams JW. Does this patient have dementia? Journal of the American Medical Association. 2007;297:2391–2404. doi: 10.1001/jama.297.21.2391. PMid: 17551132. [DOI] [PubMed] [Google Scholar]
- Hudon C, Belleville S, Souchay C, Gely-Nargeot MC, Chertkow H, Gauthier S. Memory for gist and detail information in Alzheimer’s disease and mild cognitive impairment. Neuropsychology. 2006;20:566–577. doi: 10.1037/0894-4105.20.5.566. PMid: 16938019. [DOI] [PubMed] [Google Scholar]
- Hunt L, Morris JC, Edwards D, Wilson BS. Driving performance in persons with mild senile dementia of the Alzheimer type. Journal of the American Geriatric Society. 1993;41:747–753. doi: 10.1111/j.1532-5415.1993.tb07465.x. PMid: 8315186. [DOI] [PubMed] [Google Scholar]
- Johnson R. Aging and the remembering of text. Developmental Review. 2003;23:261–346. [Google Scholar]
- Johnson DK, Storandt M, Balota DA. Discourse analysis of logical memory recall in normal aging and in dementia of the Alzheimer type. Neuropsychology. 2003;17:82–92. PMid: 12597076. [PubMed] [Google Scholar]
- Kubose TT, Bock K, Dell GS, Garnsey SM, Kramer AF, &Mayhugh J. The effects of speech production and speech comprehension on simulated driving performance. Applied Cognitive Psychology. 2006;20:43–63. [Google Scholar]
- Lehman S, Schraw G, McCrudden MT, Hartley K. Processing and recall of seductive details in scientific text. Contemporary Educational Psychology. 2007;32:569–587. [Google Scholar]
- Lindenberger U, Marsiske M, Baltes PB. Memorizing while walking: Increase in dual-task costs from young adulthood to old age. Psychology and Aging. 2000;15:417–436. doi: 10.1037//0882-7974.15.3.417. PMid: 11014706. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Jagust WJ, Fitzpatrick A, Carlson MC, DeKosky ST, Breitner J, Lyketsos CG, Jones B, Kawas C, Kuller LH. Neuropsychological characteristics of mild cognitive impairment subgroups. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;77:159–165. doi: 10.1136/jnnp.2004.045567. PMid: 50411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Blaustein MJ, Filipp L, Dungan C. Driving in patients with dementia. Journal of the American Geriatric Society. 1988;36:1087–1091. doi: 10.1111/j.1532-5415.1988.tb04394.x. PMid: 3192886. [DOI] [PubMed] [Google Scholar]
- Marson D, Herbert KR. Functional assessment. In: Attix DK, Welsh-Bohmer KA, editors. Geriatric neuropsychology: Assessment and intervention. New York, NY: The Guilford Press; 2006. [Google Scholar]
- Maylor EA, Wing AM. Age differences in postural stability are increased by additional cognitive demands. Journal of Gerontology Psychological Science. 1996;51B:P143–P154. doi: 10.1093/geronb/51b.3.p143. [DOI] [PubMed] [Google Scholar]
- McPhee LC, Scialfa CT, Dennis WM, Ho G, Caird JK. Age differences in visual search for traffic signs during a simulated conversation. Human Factors. 2004;46:674–685. doi: 10.1518/hfes.46.4.674.56817. [DOI] [PubMed] [Google Scholar]
- Molnar FJ, Patel A, Marshall SC, Man-Song-Hing M, Wilson KG. Clinical utility of office-based cognitive predictors of fitness to drive in persons with dementia: A systematic review. Journal of the American Geriatrics Society. 2006;54:1809–1824. doi: 10.1111/j.1532-5415.2006.00967.x. PMid: 17198485. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC. Driving and dementia in older adults: Evidence-based assessment and cessation strategies. St. Louis, MO: Washington University School of Medicine; 2004. [Google Scholar]
- Morris JC, Heyman A, Mohs RC. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. PMid: 2771064. [DOI] [PubMed] [Google Scholar]
- Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A. Consortium to Establish a Registry for Alzheimer’s disease (CERAD) Clinical and neuropsychological assessment of Alzheimer’s disease. Psychopharmacology Bulletin. 1988;24:641–652. PMid: 3249766. [PubMed] [Google Scholar]
- Nordlund A, Rolstad S, Hellström P, Sjögren M, Hansen S, Wallin A. The Goteberg MCI study: Mild cognitive impairment is a heterogeneous condition. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:1485–1490. doi: 10.1136/jnnp.2004.050385. PMid: 50411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oron-Gilad T, Ronen A, Shinar D. Alertness maintaining tasks while driving. Accident Analysis and Prevention. 2008;40:851–860. doi: 10.1016/j.aap.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Haxby JV. Attention and brain function in Alzheimer’s disease: A review. Neuropsychology. 1993;7:242–272. [Google Scholar]
- Parasuraman R, Nestor PG. Attention and driving skills in aging and Alzheimer’s disease. Human Factors. 1991;33:539–557. doi: 10.1177/001872089103300506. PMid: 1769674. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Watson P, Hodges JR. The nature and staging of attention dysfunction in early (minimal and mild) Alzheimer’s disease: Relationship to episodic and semantic memory impairment. Neuropsychologia. 2000;38:252–271. doi: 10.1016/s0028-3932(99)00079-2. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. PMid: 10190820. [DOI] [PubMed] [Google Scholar]
- Rebok G. ACTIVE Memory Training Manual. Unpublished document, Johns Hopkins University; 1998. [Google Scholar]
- Reger MA, Welsh RK, Watson GS, Cholerton B, Baker LD, Craft S. The relationship between neuropsychological functioning and driving ability in dementia: A meta-analysis. Neuropsychology. 2004;18:85–93. doi: 10.1037/0894-4105.18.1.85. PMid: 14744191. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Trail Making Test: Manual for administration and scoring. Tucson, AZ: Reitan Neuropsychology Lab; 1992. [Google Scholar]
- Rey A. L’examen clinique en psychologie. Paris: Presses Universitaires de France; 1941. [Google Scholar]
- Riby LM, Perfect TJ, Stollery BT. The effects of age and task domain on dual task performance: A meta-analysis. European Journal of Cognitive Psychology. 2004;16:863–891. [Google Scholar]
- Robinson-Whelen S, Storandt M. Immediate and delayed prose recall among normal and demented adults. Archives of Neurology. 1992;49:32–34. doi: 10.1001/archneur.1992.00530250036012. PMid: 1728260. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Allen CC. Ruff 2 & 7 Selective Attention Test. Odessa, FL: Psychological Assessment Resources, Inc; 1996. [Google Scholar]
- Takayama L, Nass C. Assessing the effectiveness of interactive media in improving drowsy driver safety. Human Factors. 2008;50:772–781. doi: 10.1518/001872008X312341. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Steitz DW, Sliwinski MJ, Cerella J. Aging and dual-task performance: A meta-analysis. Psychology and Aging. 2003;18:443–460. doi: 10.1037/0882-7974.18.3.443. PMid: 14518807. [DOI] [PubMed] [Google Scholar]
- Ward A. Attention: A neuropsychological approach. New York, NY: Psychology Press; 2004. [Google Scholar]
- Wechsler D. Wechsler Memory Scale –Third Edition: Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Weeks DL, Forget R, Mouchnino L. Interaction between attention demanding motor and cognitive tasks and static postural stability. Gerontology. 2003;49:225–232. doi: 10.1159/000070402. PMid: 12792157. [DOI] [PubMed] [Google Scholar]
- Wilson B, Cockburn J, Baddeley A. The Rivermead Behavioural Memory Test. Bury St. Edmunds, England: Thames Valley Test Company; 1985. [Google Scholar]
- Wingfield A, Stine-Morrow EAL. Language and speech. In: Craik IM, Salthouse TA, editors. Handbook of aging and cognition. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. pp. 359–416. [Google Scholar]
- Withaar FK, Brouwer WH, van Zomeren AH. Fitness to drive in older drivers with cognitive impairment. Journal of the International Neuropsychological Society. 2000;6:480–490. doi: 10.1017/s1355617700644065. PMid: 10902417. [DOI] [PubMed] [Google Scholar]
- Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: A review of an emerging area of research. Gait & Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a Geriatric Depression Screening Scale: A preliminary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]