Abstract
Context
Preterm birth is associated with increased mortality and morbidity. However, previous studies have been unable to rigorously examine whether confounding factors cause these associations rather than the harmful effects of being born preterm.
Objective
To estimate the extent to which the associations between early gestational age and offspring mortality and morbidity were due to confounds by using a quasi-experimental design, the sibling-comparison approach, and controlling for statistical covariates that varied within families.
Design, Setting, and Participants
A population-based cohort study, combining Swedish registries to identify all individuals born in Sweden from 1973–2008 (n=3,300,708 offspring of 1,736,735 mothers) and link them with multiple outcomes.
Main Outcome Measures
Offspring mortality (during infancy and throughout young adulthood) and psychiatric (psychotic or bipolar disorder, autism, ADHD, suicide attempts, substance use, and criminality), academic (failing grades and educational attainment), and social (partnering, parenthood, low income, social welfare benefits) outcomes through 2009.
Results
In the population, there was a dose-response relation between early gestation and the outcome measures. For instance, extreme preterm birth (23–27 weeks of gestation) was associated with infant mortality (OR=288.1, 95% CI=271.7–305.5), autism (HR=3.2, CI=2.6–4.0), low educational attainment (HR=1.7, CI=1.5–2.0), and social welfare benefits (HR=1.3, CI=1.2–1.5) compared to offspring born at term. The associations between early gestation and mortality and psychiatric morbidity generally were robust when comparing differentially exposed siblings and controlling for statistical covariates, whereas the associations with academic and some social problems were greatly or completely attenuated in the fixed effects models.
Conclusions
The mechanisms responsible for the associations between preterm birth and mortality and morbidity are outcome-specific. Associations between preterm birth and mortality and psychiatric morbidity were largely independent of shared familial confounds and measured covariates, consistent with a causal inference. Some associations, particularly predicting suicide attempt, educational attainment, and social welfare benefits were due to confounding factors, however.
Preterm birth is associated with increased risk of mortality during infancy1–2 and through young adulthood.3 Shortened gestational age (GA) also predicts offspring morbidity across the lifespan,4–5 including psychiatric disorders,2, 6–8 academic problems,2, 9–12 and social difficulties.2, 13–15
Precise estimates of the sequelae of shortened GA are critical for helping doctors and patients balance the benefits and risks of various interventions during pregnancy,16 and properly understanding the etiological mechanisms is crucial for designing effective prevention efforts.17 Most researchers have made strong causal inferences regarding the consequences of early GA. Research suggests that physical and immunological immaturity account for increased mortality,5 while brain abnormalities mediate the associations with cognitive and psychiatric problems.18–19 Yet, GA is associated with numerous environmental risks, such as poverty, that are themselves predictive of subsequent difficulties.20–21 Family- and twin-based studies also indicate that genetic factors, primarily passed down from the mother, influence GA.22–24 Environmental confounding and shared genetic liability, therefore, could account for part or all of the increased mortality and morbidity associated with GA.25
The existent human research has relied solely on controlling for statistical covariates to account for confounding factors, which only provides qualified support for causal inferences because of inability to account for unmeasured confounds.26–27 Randomized controlled studies in humans are impossible and animal studies of parturition have limited generalizability.16, 28 Researchers, therefore, must use other methods to rule out plausible confounding by genetic and environmental factors. Prestigious scientific working groups in Medicine26 and researchers across a number of other disciplines, including Psychiatry,29–30 Psychology,31–33 Epidemiology,34 Sociology,35 and Economics36, recently have stressed that quasi-experimental research, studies that use design features to account for confounding factors, play an essential role for drawing strong causal inferences. We know of only one study of GA, a sibling-comparison study that found an independent association with offspring ADHD medication in a single year,12 that has used such an approach, however.
The aim of the current study was to explore the associations between GA and numerous indices of mortality and morbidity in the largest population-based cohort study of GA to date. We also sought to rigorously rule out confounding factors by comparing differentially exposed siblings to account for all genetic and environmental factors that make siblings similar34, 37–39 and controlling for measured covariates that vary within families. Finally, we conducted several sensitivity analyses using various approaches39 to examine whether assumptions and limitations in the sibling-comparison design accounted for the results.
Methods
Study Design
After approval by the Institutional Review Boards at Karolinska Institutet and Indiana University to analyze the de-identified data, the data for this national cohort were obtained by linking information available in the following population-based registries: (1) the Medical Birth Registry includes data on more than 99% of pregnancies in Sweden since 1973; (2) the Multi-Generation Register contains information about biological relationships for all individuals living in Sweden since 1933; (3) the Migration Register supplies information on dates for migration in or out of Sweden; (4) the Cause of Death Register contains information on dates and causes of all deaths since 1958; (5) the Patient Registry provides diagnoses for all inpatient hospital admissions since 1973 and outpatient care since 2001; (6) the National Crime Register includes detailed information about all criminal convictions since 1973; (7) the National School Register includes grades in all subjects for all students at the end of grade nine since 1983; (8) the Education Register contains information on highest level of completed formal education through 2008; (9) the longitudinal integration database for health insurance and social studies (LISA) contains yearly assessments of income, marital status, social welfare status, and education for all individuals 15 years or older since 1990. More details on these and additional registries are available upon request (Author Material).
The current study consisted of singleton offspring born in Sweden between 1973 and 2008. Birth-related data for 3,619,712 offspring were obtained from the Swedish Medical Birth Registry. We sequentially removed multiple births (86,273), children with missing data on GA (8,290), those with a recorded GA less than 23 weeks (153) or more than 42 weeks and 6 days (41,440), missing maternal identification numbers (4,070), invalid or missing sex (2), invalid parity (23), and those who emigrated from Sweden (178,753) during this period. The resulting cohort of 3,300,708 offspring represents 91.2% of all recorded births to a total of 1,736,735 biological mothers. A large majority of offspring had siblings in the dataset (2,665,666, 80.1%), so that they were in families of mothers (1,101,693) with more than offspring. Sibling-comparisons were made among this subset of the population.
Measures
Gestational Age
The analyses used two different representations for GA. For the ordinal representation, children were divided into five subgroups: (a) 23 weeks to 27 weeks 6 days, (b) 28 weeks to 30 weeks 6 days, (c) 31 weeks to 33 weeks 6 days, (d) 34 weeks to 36 weeks 6 days, and (e) 37 weeks to 42 weeks and 6 days, consistent with previous studies.2
For a continuous assessment, we converted GA to a linear scale that was referenced at 40 weeks and ranged from −17.0 weeks (23 weeks) to +2.9 weeks (42 weeks, 6 days).
Offspring Outcomes
Two mortality outcomes were created from the Cause of Death Registry. Infant mortality indexed children who were born alive but died before their first birthday. A separate right-censored variable was used to index mortality after one year (up to 36 years old).
Six indices of psychiatric morbidity were modeled. Psychotic or bipolar disorder (up to 37 years old) was measured as age of first inpatient hospitalization for schizophrenia, bipolar disorder, or other non-organic psychotic disorders according to ICD-8, -9, and -10 criteria, which are valid indices of these disorders.40 Autism and attention-deficit/hyperactivity disorder (ADHD) were identified using inpatient and outpatient diagnoses according to ICD -9 and ICD-10 for individuals born 1980–2001 (up to 19 years old). It is important to note that both the diagnosis of Autism41 and ADHD42 have been validated. Age of first suicide attempt (up to 37 years old) was identified using the ICD codes for any primary or secondary diagnosis for individuals 12 years of age or older in the Patient Register.43 Substance use problem (up to 37 years old) was defined as first inpatient hospitalization involving a primary or secondary diagnosis of alcohol- or any other, non-nicotine, substance use disorder for individuals 12 years of age or older.44 Criminality was indexed by the age of the first occurrence of any criminal conviction (from 15 years, the age of legal responsibility in Sweden, up to 37 years old).45–46 More details about the measurement of psychiatric morbidity are available upon request (Author Material).
Three indices of academic problems were included. Failing grades indexed poor school performance in grade 9 (when the offspring were approximately 15 years old), commensurate with a mean failing grade across 16 academic subjects.47–48 Highest level of educational attainment was available in the Register of Education.49 Education under 10 years was an index of low educational attainment. The higher education group completed 3 or more years of postsecondary education; only individuals born 1973–1983 whose age made it possible to achieve that level were included in the analysis of high education.
Three indices of social adversity were incorporated, which included assessments of individuals up to 38 years old. First, parenthood was indexed as age when they first became biological parents. Second, ever partnered was based on age of first civil or marital partnership using information recorded in the LISA database. Third, social welfare benefits was based on age of first receiving government social welfare subsidies during the previous year in the LISA.
Covariates
Offspring gender, birth order, and year of birth were obtained from the medical birth records. The measured maternal and paternal covariates included were (1) age at the child’s birth, (2) highest level of completed education in 2008, and (3) lifetime history of any criminal conviction. Because of the coverage of the Swedish registers there was little missing data (<1.2% of each covariate). To account for the missing values in the covariates we created dummy codes to compare individuals with missing values to the observations with low risk.
Statistical Analyses
We used Cox survival analyses for right-censored outcomes and logistic regression analyses for dichotomous outcomes. We fitted a series of models for each outcome. All models controlled for offspring sex and birth order, while the logistic models also controlled for offspring year of birth. First, we used the ordinal assessment of GA to provide estimates of increased risks consistent with previous research. Second, we compared a linear and quadratic model using the continuous representation of GA as a baseline model; model selection was based on the AIC fit statistic. We refer to the analysis as the baseline model, which estimated the associations between GA and each outcome in the population. Third, we included both offspring-specific (offspring gender, birth order, and year of birth, as well as maternal and paternal age at childbearing) and parental covariates (maternal and paternal highest level of education and history of criminal conviction) to account statistically for the measures; we refer to the analysis as the adjusted model. Fourth, we fit a fixed-effects model50 at the maternal level that accounted for all factors that siblings share, including all genetic and environmental factors that make siblings similar,37–38 while controlling for offspring-specific covariates (we refer to the analysis as the fixed-effects model). The final model, therefore, compared siblings born at different gestational ages and statistically controlled for measured covariates that varied among siblings.
We also ran several sensitivity analyses to test assumptions in sibling-comparison studies, and we examined whether historical changes throughout the study period altered our conclusions concerning infant mortality.2
Results
Table E1 in the Supplemental Material presents the sample by different GA categories and the number of cases for each outcome. The table illustrates how the covariates and outcome variables varied across the ordinal subgroups of GA.
Mortality
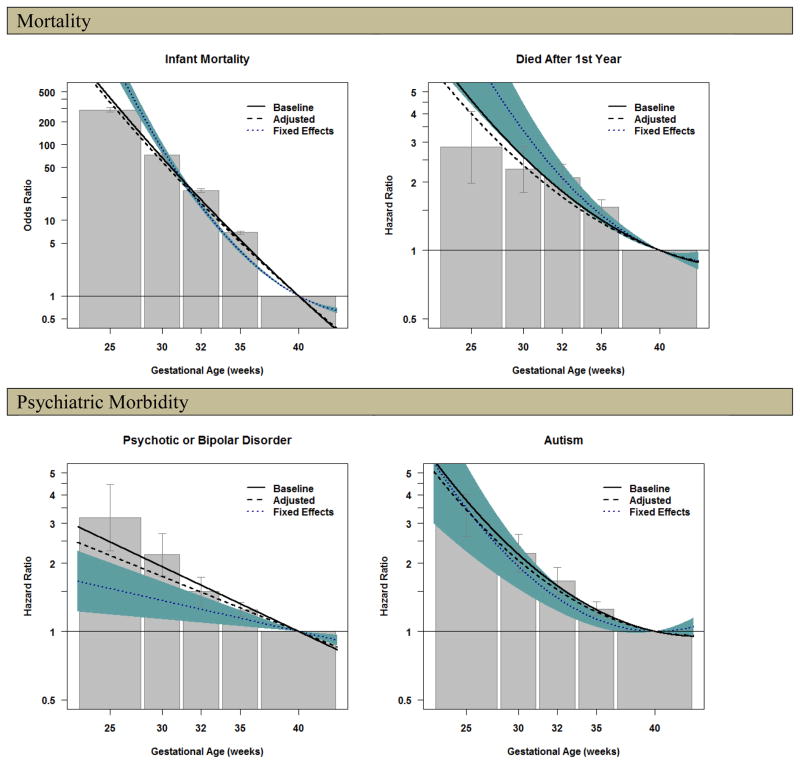
The initial analyses, which used the ordinal assessment of GA to provide estimates consistent with previous research, are presented in Figure 1, where the bars represent risk estimates with 95% confidence intervals (all ordinal parameter estimates are available in the Author Material). There was a strong association between GA and risk of infant mortality in the population. For example, offspring born 23–27 weeks of gestation had much higher odds of infant mortality (288.1, CI=271.7–305.5) compared to offspring born at term. Offspring born 28–30 (72.8, CI=68.6–77.3), 31–33 (24.6, CI=23.3–26.1) and 34–36 weeks (6.9, CI=6.6–7.2) also had higher odds.
Figure 1.
Model Fitting Results for the Association between Gestational Age and Offspring Mortality, Psychiatric Morbidity, Academic Problems, and Social Adversity.
Note. The shaded bars present the results of the ordinal analyses for the baseline association between gestational age and the indices of offspring mortality and morbidity (the analyses did not control for confounding factors). The bars represent the magnitude of increased risk from being born earlier compared to offspring born at term, with the 95% confidence intervals represented by the error bars. The solid black line presents the association of the best fitting model (either the linear or quadratic model) for the baseline model, considering gestational age as a continuous measure (referenced at 40 weeks of gestation). The dashed line presents the results of the analyses that included measured covariates to account for confounds. The dotted line presents the results of the analyses that used fixed effects model that compared differentially exposed siblings and controlled for statistical covariates. The line, therefore, presents the increased risk associated with early gestational age when accounting for all genetic and environmental factors that make siblings similar and the statistical covariates that varied within families. The 95% confidence region of the association between gestational age and each offspring outcome in the fixed effects model is presented in shaded blue.
Figure 1 also summarizes the results from the continuous analyses of GA in the baseline model, where we present the results from either the linear or quadratic model of GA, depending on which model fitted the best based on fit indices (Author Material). A quadratic model fitted significantly better than the linear model when predicting infant mortality. As can be seen with the parameter estimates in Table 1, the estimated association from the baseline model was based on a quadratic model (blinear=−0.363, p<0.001; bquadratic=0.004, p<0.001). The solid line closely follows the point estimates from the ordinal analyses when plotted in Figure 1, providing a similar interpretation to the results from the ordinal analysis. The association remained robust when controlling for covariates in the adjusted model (blinear=−0.346, p<0.001; bquadratic=0.004, p<0.001). Figure 1 illustrates how the adjusted model (the dashed line) was comparable to the baseline model, suggesting the statistical covariates did not account for the association between GA and infant mortality.
Table 1.
Comparison of the unstandardized linear and quadratic regression coefficients for the Baseline, Adjusted, and Fixed Effects Models.
| Outcome | Baseline Model | Adjusted Model | Fixed Effects Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Linear Term | Quadratic Term | Linear Term | Quadratic Term | Linear Term | Quadratic Term | |||||||
|
| ||||||||||||
| b | SE | b | SE | b | SE | b | SE | b | SE | b | SE | |
|
Mortality
| ||||||||||||
| Infant Mortalitya | −0.3633 | 0.0051 | 0.0036 | 0.0004 | −0.3464 | 0.0053 | 0.0041 | 0.0004 | −0.2108 | 0.0112 | 0.0207 | 0.0015 |
| Died After 1st Yearb | −0.0525 | 0.0058 | 0.0036 | 0.0008 | −0.0474 | 0.0058 | 0.0033 | 0.0008 | −0.0532 | 0.0116 | 0.0060 | 0.0017 |
|
| ||||||||||||
|
Psychiatric Morbidity
| ||||||||||||
| Psychotic or Bipolar Disorderb | −0.0626 | 0.0038 | - | - | −0.0531 | 0.0038 | - | - | −0.0299 | 0.0093 | - | - |
| Autismb | −0.0298 | 0.0055 | 0.0043 | 0.0006 | −0.0256 | 0.0055 | 0.0041 | 0.0006 | −(0.0017) | 0.0123 | 0.0058 | 0.0015 |
| ADHD Diagnosisb | −0.0507 | 0.0041 | 0.0015 | 0.0005 | −0.0394 | 0.0041 | 0.0016 | 0.0005 | −(0.0101) | 0.0100 | 0.0031 | 0.0012 |
| Suicide Attemptb | −0.0380 | 0.0031 | - | - | −0.0263 | 0.0031 | - | - | (0.0072) | 0.0068 | - | - |
| Substance Use Problemb | −0.0358 | 0.0034 | −0.0040 | 0.0006 | −0.0249 | 0.0034 | −0.0046 | 0.0006 | −(0.0048) | 0.0067 | −0.0040 | 0.0011 |
| Criminalityb | 0.0026 | 0.0011 | - | - | 0.0122 | 0.0011 | - | - | 0.0216 | 0.0024 | - | - |
|
| ||||||||||||
|
Academic Problems
| ||||||||||||
| Failing Gradesa | −0.0149 | 0.0015 | 0.0038 | 0.0003 | −(0.0008) | 0.0016 | 0.0035 | 0.0003 | 0.0129 | 0.0035 | 0.0027 | 0.0006 |
| Education under 10 yrsa | 0.0201 | 0.0013 | 0.0055 | 0.0002 | 0.0321 | 0.0014 | 0.0055 | 0.0002 | 0.0603 | 0.0029 | 0.0052 | 0.0005 |
| Higher Educationa | −0.0044 | 0.0017 | −0.0061 | 0.0004 | −0.0095 | 0.0018 | −0.0043 | 0.0004 | −0.0065 | 0.0047 | −0.0005 | 0.0009 |
|
| ||||||||||||
|
Social Adversity
| ||||||||||||
| Parenthoodb | −0.0272 | 0.0010 | −0.0043 | 0.0002 | −0.0284 | 0.0010 | −0.0049 | 0.0002 | −0.1558 | 0.0325 | −0.0187 | 0.0058 |
| Ever Partneredb | −0.1694 | 0.0017 | −0.0301 | 0.0004 | −0.1736 | 0.0017 | −0.0304 | 0.0004 | −0.1617 | 0.0045 | −0.0275 | 0.0009 |
| Social Welfare Benefitsb | −(0.0001) | 0.0012 | 0.0039 | 0.0002 | 0.0054 | 0.0012 | 0.0026 | 0.0002 | 0.0115 | 0.0027 | 0.0015 | 0.0005 |
Note:
Based on logistic regression models.
Based on Cox survival models. All models controlled for offspring gender and birth order, while the logistic models also controlled for offspring year of birth. The Adjusted Model included additional offspring-specific (maternal and paternal age at childbearing) and parental covariates (maternal and paternal highest level of education and history of criminal conviction). The Fixed Effects Model included a fixed variance term at the maternal level (shared by all siblings within a family) and statistically controlled for the offspring-specific covariates. b = maximum likelihood estimate of the unstandardized regression coefficient; SE = estimated standard error. A dash (-) indicates that no quadratic term was included in the model because the fit indices indicated that a linear model was a better fit to the data. All bracketed coefficients have an estimated p-value > 0.05.
Finally, the fixed-effect analyses also are summarized in Figure 1 with the dotted line and 95% confidence interval presented in the shaded blue area. Consistent with a causal effect, GA significantly predicted infant mortality within differentially exposed siblings across the entire range of GA while also controlling for offspring-specific covariates (blinear=−0.211, p<0.001; bquadratic=0.021, p<0.001).
Similar to the results for infant mortality, there was a non-linear association between GA and mortality after age one that was substantial in the population (e.g., HRGA: 23–27 weeks=2.9, CI=2.0–4.1), albeit of smaller magnitude than the association between GA and infant mortality. As can be seen in Table 1 and Figure 1 the association was not attenuated in subsequent adjusted or fixed effects models, indicating the association between GA and mortality after one year was also robust to all confounding factors shared by siblings and the measured covariates. It is important to note that offspring born moderate-to-late preterm and very-preterm were also at increased risk for early mortality.
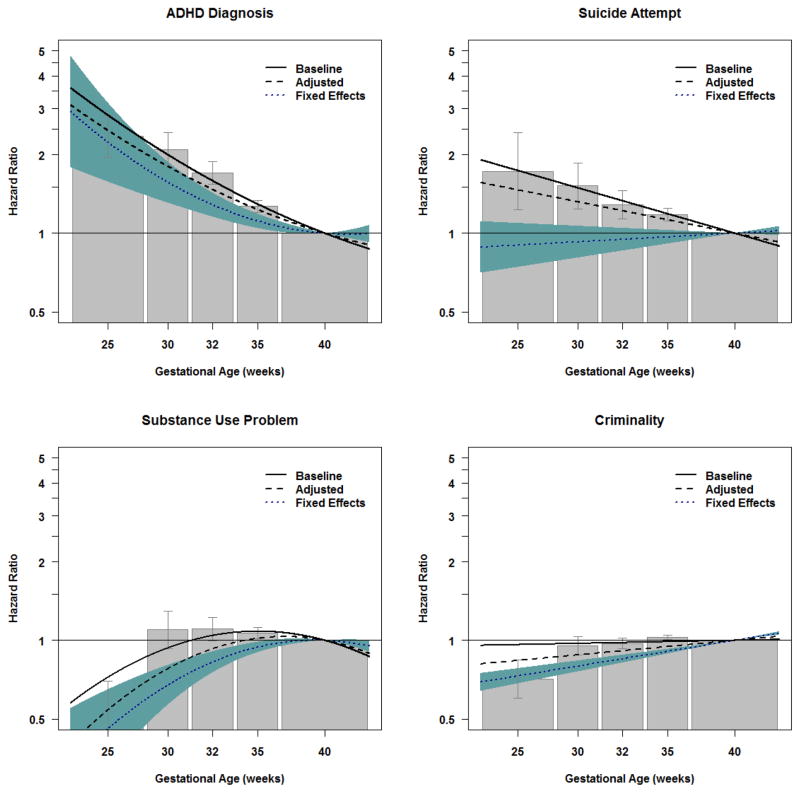
Psychiatric Morbidity
The pattern of findings for psychiatric morbidity was domain-specific. In the baseline models earlier GA was highly associated with each increased risk of each psychiatric outcome. For example, extreme preterm birth was associated with psychotic or bipolar disorders (HRGA: 23–27 weeks=3.2, CI=2.3–4.4), autism (HRGA: 23–27 weeks=3.2, CI=2.6–4.0), and ADHD (HRGA: 23–27 weeks=2.3, CI=2.0–2.8 for ADHD diagnosis; the Author Material presents commensurate results using prescriptions as an index of ADHD, consistent a previous study12). When predicting psychotic or bipolar disorder, the magnitude of the association with earlier GA was slightly attenuated in the adjusted model, and the association was further attenuated in the fixed effects model, suggesting confounds account for some, but not all, of the increased risk with earlier GA. The adjusted and fixed effects models for autism and ADHD found that the associations with GA were principally independent of the measured covariates and familial factors shared by siblings. A different pattern occurred when predicting suicide attempts. Earlier GA was associated with increased risk of suicide attempts in the population (HRGA: 23–27 weeks=1.7, CI=1.2–2.4). The association was slightly attenuated but still robust in the adjusted model. In contrast, the association between GA and suicide attempts was completely attenuated when comparing differentially exposed siblings, suggesting shared familial confounds account for the statistical association in the population.
It is important to note that early GA was associated with decreased risk for problematic substance use (HRGA: 23–27 weeks=0.5, CI=0.4–0.7) and criminality (HRGA: 23–27 weeks=0.7, CI=0.6–0.8) in the population. These decreased associations remained robust to the statistical controls and the comparison of siblings, suggesting GA had a specific relation with lower odds of substance use problems and criminality.
Academic Problems
The figures for Academic Problems are presented in the Supplemental Material E2. Early GA was also associated with multiple indicators of academic problems in the population, including greater risk of failing grades (HRGA: 23–27 weeks=2.0, CI=1.7–2.3), odds of completing less than 10 years of education (HRGA: 23–27 weeks=1.7, CI=1.5–2.0), and lower likelihood of completing 3 or more years of post-secondary education (HRGA: 23–27 weeks=0.5, CI=0.4–0.6). When controlling for statistical covariates in the adjusted model the associations between GA and each outcome were somewhat attenuated. In the fixed effects models, however, the magnitude of the associations with GA were further reduced. The association between early GA and failing school grades was attenuated (compared to the adjusted model) but remained independent of the confounding factors, especially in the lowest GA range (the Author Matrial provides additional results with IQ measured in males). In contrast, the association with completed education (both risk of low education and likelihood of completing advanced schooling), was greatly attenuated across the range of GA, with associations remaining only at the very lowest gestational ages, if at all. The results for highest level of completed education, therefore, suggest confounding factors shared by siblings account for the association with GA.
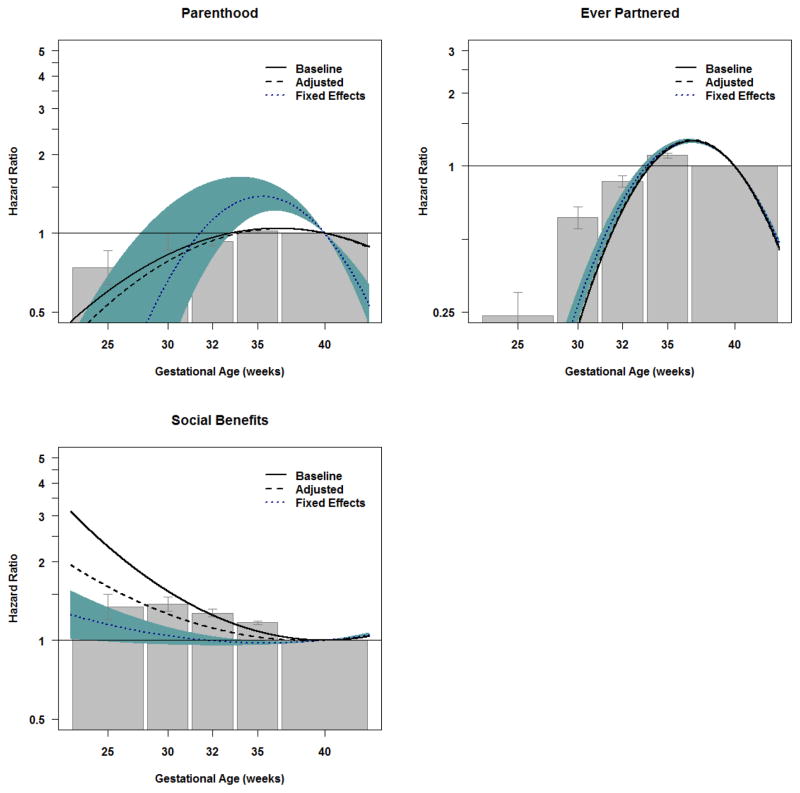
Social Adversity
Early GA was strongly associated with social adversity, such as decreased likelihood of parenthood (HRGA: 23–27 weeks=0.7, CI=0.6–0.9) and ever being married/in a registered partnership (HRGA: 23–27 weeks=0.2, CI=0.2–0.3). Controlling for measured covariates in the adjusted models and shared familial confounds in the fixed effects models did not reduce these associations. These findings are in contrast to those with receiving social welfare benefits. Earlier GA predicted social welfare benefits in the population (HRGA: 23–27 weeks=1.3, CI=1.2–1.5). The magnitude of the association was reduced in the adjusted model, and the association was largely attenuated when controlling for statistical covariates and shared familial confounds in the fixed effects model (the Author Matrial presents comparable results when predicting income).
Sensitivity Analyses
The sibling-comparison design includes a number of limitations and assumptions that could influence the interpretation of the results.34, 37–39, 51 To address concerns about the generalizability of findings from offspring with siblings to offspring without siblings26 we ran three sets of sensitivity analyses. First, we compared the population estimates in families with multiple children to families with only one child (Author Material). The population estimates were not lower in offspring with siblings (except for when predicting criminality), which indicates that the lower fixed-effects estimates (when they occurred) were not due to lower population estimates in the subset of the data that included offspring with siblings. Second, we also ran the fixed-effects analyses with the ordinal distribution of GA to test whether misspecification of the shape of the models (e.g., linear or quadratic) could account for the findings (Author Material). The sensitivity analysis relaxed the assumption that the shape of the models in families with multiple children, as these families can only provide information for the sibling-comparison models. The results of the ordinal analyses gave commensurate interpretations to the models using continuous GA. Third, we conducted cousin-comparisons (Supplemental Material E3) to address concerns about the generalizability of the findings from differentially exposed siblings to other populations.51 The analyses provided a commensurate pattern of results as in the main analyses, which strongly suggest that the sibling-comparison conclusions do not rely on idiosyncratic comparisons that do not generalize to other populations.
We examined the possibility of exposure of one sibling influencing the outcome of another (i.e., carry-over effects)26 by conducting two sets of analyses. First, we fit bidirectional, case-crossover models52 (Author Material), which explored whether different patterns of early GA within families (i.e., either the first- or second-born offspring had lower GA) moderated the sibling-comparison results. The analyses compared the sibling-comparison estimates in families where the first child had an earlier GA to families where the second child had an earlier GA. The results were only consistent with a carry-over effect for one outcome variable, low education. The bidirectional case-crossover model fitting, however, also suggested the opposite pattern for two outcomes, infant mortality and psychotic and bipolar disorder, although the effect sizes were large in both types of sibling pairs. The results imply that the analyses suggesting carry-over effects of early GA for the first-born sibling on the low educational attainment of the second-born sibling may be a chance finding. To further test for the possibility of carry-over effects, we also relied on the cousin-comparison models (Supplemental Material E3), in which carry-over effects are less of a concern. The cousin comparisons, again, gave a commensurate pattern of results as in the main analyses. The sensitivity analyses, therefore, do not support the hypothesis that carry-over effects account for the attenuation of the associations in the sibling-comparison models.
Sibling-comparisons do not test for moderating factors.37 As such, we ran supplemental analyses (Author Material) to examine whether year of birth decreased the association between GA and infant mortality, which has been reported elsewhere.2 The analyses support the overall conclusions regarding infant mortality. Finally, siblings-comparisons are sensitive to measurement error.39, 51 To begin to address this concern we removed observations with extreme values for birth weight relative to their GA53 because these may be misclassifications.12 The results of the baseline and fixed effects models based on the subset of the data are commensurate with those presented in the main analyses (Author Material).
Comment
This large population-based cohort study replicates previous reports—early GA is associated with increased risk of early mortality and psychiatric, academic, and social problems.2, 6–15 Early GA also was associated with decreased likelihood of criminality and substance use problems, consistent with some but not all previous research.54 The current study used a sibling-comparison design and controlled for measured covariates to examine the degree to which confounding factors account for the associations. Several of the statistical associations (e.g., with mortality during infancy and through young adulthood, autism, ADHD, substance use problems, criminality, parenthood, and ever partnered) were largely independent of shared familial confounds and the statistical covariates, consistent with a causal inference. The findings support theories associated with mediating role of physical and immunological immaturity,5 as well as problems with brain development,15,16 on subsequent mortality and morbidity. In contrast, the associations between GA and other outcomes were either greatly (e.g., with psychotic or bipolar disorder, grades, and educational attainment) or completely attenuated (e.g., with suicide and receiving social welfare benefits). The latter results, therefore, suggest that confounding factors, such as environmental factors correlated with early GA,20–21 and not early GA in itself, account for these statistical associations. The findings for grades, educational attainment, suicide, and social welfare benefits contradict the results of previous studies and meta-analyses of the associations between GA and these outcomes,2, 8, 11, 13–15 as well as the general conclusions in reviews of the field,4–5 although no previous studies of these outcomes used a quasi-experimental approach.
The current study provides critical insight into the consequences associated with early GA because of six key advances. First and foremost, the study combined design features to rule out all confounds shared by siblings31,35–37 with statistical controls to rule out plausible alternative hypotheses for the observed associations. This is one of the first studies of GA to use a quasi-experimental design, which is essential for drawing stronger causal inferences.23,24 It is important to note that the statistical associations between GA and many outcomes (e.g., receiving social welfare benefits) were only attenuated in the fixed-effects models, which highlights the limitations of relying solely on statistical covariates to control for confounding factors. Second, the study explicitly tested several assumptions about sibling-comparison studies, including the generalizability from offspring with siblings to offspring without siblings, the generalizability of findings from differentially exposed siblings to other populations, and the possibility of carryover effects from one sibling to another.26 The sensitivity analyses suggest these alternative explanations do not account for the general conclusions, which further strengthen the inferences we were able to draw. But, additional quasi-experimental research, relying on methods with different assumptions and limitations, and research in other populations is necessary to strengthen causal inferences.23,24
Third, this is the largest epidemiological study to date of GA, providing a comprehensive view from an entire country. The sample size and measurement allowed us to more precisely estimate the risks for rare outcomes that were difficult to predict in previous research (e.g., autism7). Fourth, the analyses explored associations with the continuum of GA. The study, therefore, sheds light on extremely and very preterm births, in addition to moderate/late preterm births.
Fifth, the inclusion of multiple valid indices of morbidity in the current study allowed us the opportunity to find converging evidence—commensurate results were found when using different indices of key constructs. For example, we obtain the same results when predicting ADHD diagnosis and when predicting prescriptions for ADHD; we likewise found comparable results when predicting school grades and IQ. And, we obtained the same pattern when predicting both low and high income as when predicting social welfare benefits. As such, the results do not appear to be dependent on single observations or indices of important constructs. Sixth, predicting multiple domains of functioning with valid indices of morbidity also allowed us to explore the specificity of the predictions and underlying etiological mechanisms associated with early GA. As such, the current study provides novel insight because the mechanisms responsible for the associations with early GA are outcome-specific. In particular, researchers will need to explore risk factors shared by siblings that account for the statistical association between early GA and suicide attempts, educational outcomes, and need for social welfare benefits.
The current study also has a number of limitations. The findings will need to be replicated to examine whether the results from a country with universal health care coverage and the quality of prenatal care in Sweden generalize to other countries. Quasi-experimental studies are not randomized controlled studies and, therefore, cannot rule out all confounds. The sibling-comparison design does not account for offspring-specific genetic factors that could influence GA.37 Twin and family quantitative genetic studies,22–23, 55–56 including in this cohort,24 have indicated that fetal-specific genetic factors do not account for much variability in GA, although recent research suggests such genetic factors may play a larger role than previous estimates.57 We controlled for offspring-specific covariates, but as is true of all human studies of GA,2 the current study also cannot rule out the possibility that medical problems could cause preterm birth and the offspring outcomes.37 Nevertheless, the results suggest that risks specifically associated with early GA influence subsequent mortality and morbidity. The current study also may have misestimated the magnitude of some associations because the measurement of GA can misclassify some offspring.1 Sibling- and cousin-comparisons are sensitive to random measurement error and bias from confounders shared by siblings that are unrelated to the outcomes.39, 51 Fixed-effects models also have lower statistical power than population-based estimates,50 but our use of a continuous index of GA helped us more precisely estimate the associations.
The current study, one of the first quasi-experimental studies of GA, stresses the importance of prevention efforts aimed at reducing preterm birth, as well as wrap-around services that target familial risks that co-occur with preterm birth. The findings should inform etiological theory, risk assessment, and follow-up practices to prevent adverse outcomes associated with preterm birth.
Supplementary Material
Acknowledgments
The manuscript was supported by grants from the National Institute of Child Health and Human Development (HD061817), National Institute of Mental Health (MH094011), the Swedish Research Council (Medicine), and the Swedish Prison and Probation Services.
Footnotes
The following authors were responsible for the design and conduct of the study (all); collection (Larsson, Långström, Lichtenstein), management (Lichtenstein), analysis (D’Onofrio, Rickert), and interpretation of the data (all); and preparation (D’Onofrio, Rickert, Class), review, or approval of the manuscript (all). Dr. Martin Rickert had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
There were no financial or other conflicts of interest for any of the authors. Neither this manuscript nor one with substantially similar content under our authorship has been published or is being considered for publication elsewhere.
References
- 1.EXPRESS Group members. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA. 2009;301:2225–2233. doi: 10.1001/jama.2009.771. [DOI] [PubMed] [Google Scholar]
- 2.Moster D, Terje L, Markestad T. Long-term medical and social consequences of preterm birth. The New England Journal of Medicine. 2008;359:262–273. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 3.Crump C, Sundquist K, Sundquist J, Winkleby MA. Gestational age at birth and mortality in young adulthood. JAMA. 2011;306:1233–1240. doi: 10.1001/jama.2011.1331. [DOI] [PubMed] [Google Scholar]
- 4.Doyle LW, Anderson PJ. Adult outcome of extremely preterm infants. Pediatrics. 2010;126:342–351. doi: 10.1542/peds.2010-0710. [DOI] [PubMed] [Google Scholar]
- 5.McCormick MC, Litt JS, Smith VC, Zupancic JAF. Prematurity: An overview and public health implications. Annual Review Public Health. 2011;32:367–379. doi: 10.1146/annurev-publhealth-090810-182459. [DOI] [PubMed] [Google Scholar]
- 6.Crump C, Winkleby MA, Sundquist K, Sundquist J. Preterm birth and psychiatric medication prescription in young adulthood: a Swedish national cohort study. International Journal of Epidemiology. 2010;39:1522–1530. doi: 10.1093/ije/dyq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: A comprehensive meta-analysis. Pediatrics. doi: 10.1542/peds.2010-1036. Published online July 11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mittendorfer-Rutz E, Rasmussen F, Wasserman D. Restricted fetal growth and adverse maternal psychosocial and socioeconomic conditions as risk factors for suicidal behaviour of offspring: a cohort study. The Lancet. 364(9440):1135–1140. doi: 10.1016/S0140-6736(04)17099-2. [DOI] [PubMed] [Google Scholar]
- 9.McGowan JE, Alderdice FA, Holmes VA, Johnston L. Early childhood development of late-preterm infants: A systematic review. Pediatrics. 2011;127:1111–1124. doi: 10.1542/peds.2010-2257. [DOI] [PubMed] [Google Scholar]
- 10.Lindstrom K, Lindblad F, Hjern A. Preterm birth and attention-deficit/hyperactivity disorder in schoolchildren. Pediatrics. 2011;127:858–865. doi: 10.1542/peds.2010-1279. [DOI] [PubMed] [Google Scholar]
- 11.Bhutta At CMACPHCMMAKS. Cognitive and behavioral outcomes of school-aged children who were born preterm: A meta-analysis. JAMA. 2002;288(6):728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 12.Lindström K, Lindblad F, Hjern A. Preterm Birth and Attention-Deficit/Hyperactivity Disorder in Schoolchildren. Pediatrics. 2011 Apr 18;2011 doi: 10.1542/peds.2010-1279. [DOI] [PubMed] [Google Scholar]
- 13.Mathiasen R, Hansen BM, Anderson AN, Greisen G. Socio-economic achievements of individuals born very preterm at the age of 27 to 29 years: a nationwide cohort study. Developmental Medicine and Child Neurology. 2009;51:901–908. doi: 10.1111/j.1469-8749.2009.03331.x. [DOI] [PubMed] [Google Scholar]
- 14.Saigal S, Streiner D. Socio-economic achievements of individuals born very preterm at the age 0f 27 to 29. Developmental Medicine and Child Neurology. 2009;51:845–850. doi: 10.1111/j.1469-8749.2009.03374.x. [DOI] [PubMed] [Google Scholar]
- 15.Lindström K, Winbladh B, Haglund B, Hjern A. Preterm infants as young adults: A Swedish national cohort study. Pediatrics. 2007;120(1):70–77. doi: 10.1542/peds.2006-3260. [DOI] [PubMed] [Google Scholar]
- 16.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. The New England Journal of Medicine. 2010;362:529–535. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 17.Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371:164–175. doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]
- 18.Whitaker AH, Feldman JF, Lorenz JM, et al. Neonatal head ultrasond abnormalities in preterm infants and adolescent psychiatric disorders. Archivies of General Psychiatry. 2011;68:742–752. doi: 10.1001/archgenpsychiatry.2011.62. [DOI] [PubMed] [Google Scholar]
- 19.Woodward LJ, Anderson PJ, Austin N, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. The New England Journal of Medicine. 2006;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 20.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm births. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hack M, Taylor HG, Schluchter M, Andreias L, Drotar D, Klein N. Behavioral outcomes of extremely low birth weight children at age 8 years. Journal of Developmental and Behavioral Pediatrics. 2009;30:122–130. doi: 10.1097/DBP.0b013e31819e6a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilcox AJ, Skjaerven R, Lie RT. Familial patterns of preterm delivery: Maternal and fetal contributions. American Journal of Epidemiology. 2007;167:474–479. doi: 10.1093/aje/kwm319. [DOI] [PubMed] [Google Scholar]
- 23.Clausson B, Lichtenstein P, Cnattingius S. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. British Journal of Obstetrics and Gynaecology. 2000;107:375–381. doi: 10.1111/j.1471-0528.2000.tb13234.x. [DOI] [PubMed] [Google Scholar]
- 24.Svensson AC, Sandin S, Cnattingius S, et al. Maternal effects for preterm birth: A genetic epidemiologic study of 630,000 families. American Journal of Epidemiology. 2009;170:1365–1372. doi: 10.1093/aje/kwp328. [DOI] [PubMed] [Google Scholar]
- 25.Thapar A, Rutter M. Do prenatal risk factors cause psychiatric disorder? Be wary of causal claims. British Journal of Psychiatry. 2009;195:100–101. doi: 10.1192/bjp.bp.109.062828. [DOI] [PubMed] [Google Scholar]
- 26.Academy of Medical Sciences Working Group. Identifying the environmental causes of disease: How should we decide what to believe and when to take action? London: Academy of Medical Sciences; 2007. [Google Scholar]
- 27.Rutter M. Proceeding from observed correlation to causal inference: The use of natural experiments. Perspectives on Psychological Science. 2007;2:377–395. doi: 10.1111/j.1745-6916.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2009;297:R525–R545. doi: 10.1152/ajpregu.00153.2009. [DOI] [PubMed] [Google Scholar]
- 29.Kendler KS. Psychiatric genetics: A methodological critique. American Journal of Psychiatry. 2005;162:3–11. doi: 10.1176/appi.ajp.162.1.3. [DOI] [PubMed] [Google Scholar]
- 30.Lahey BB, D’Onofrio BM, Waldman ID. Using epidemiologic methods to test hypotheses regarding causal influences on child and adolescent mental disorders. Journal of Child Psychology and Psychiatry. 2009;50:53–62. doi: 10.1111/j.1469-7610.2008.01980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. New York: Houghton Mifflin; 2002. [Google Scholar]
- 32.Rutter M, Pickles A, Murray R, Eaves LJ. Testing hypotheses on specific environmental causal effects on behavior. Psychological Bulletin. 2001;127:291–324. doi: 10.1037/0033-2909.127.3.291. [DOI] [PubMed] [Google Scholar]
- 33.D’Onofrio BM, Lahey BB. Biosocial influences on the family: A decade review. Journal of Marriage and Family. 2010;72:762–782. doi: 10.1111/j.1741-3737.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Susser E, Eide MG, Begg M. Invited Commentary: The Use of Sibship Studies to Detect Familial Confounding. Am J Epidemiol. 2010 Sep 1;172(5):537–539. doi: 10.1093/aje/kwq196. [DOI] [PubMed] [Google Scholar]
- 35.Freese J. Genetics and the social science explanation of individual outcomes. American Journal of Sociology. 2008;114:S1–S35. doi: 10.1086/592208. [DOI] [PubMed] [Google Scholar]
- 36.Duncan GJ. Give us this day our daily breadth. Child Development. 2012;83(1):6–15. doi: 10.1111/j.1467-8624.2011.01679.x. [DOI] [PubMed] [Google Scholar]
- 37.Lahey BB, D’Onofrio BM. All in the family: Comparing siblings to test causal hypotheses regarding environmental influences on behavior. Current Directions in Psychological Science. 2010;19:319–323. doi: 10.1177/0963721410383977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donovan SJ, Susser E. Commentary: Advent of sibling designs. International Journal of Epidemiology. 2011;40:345–349. doi: 10.1093/ije/dyr057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Onofrio BM, Lahey BB, Turkheimer E, Lichtenstein P. The critical need for family-based, quasi-experimental research in integrating genetic and social science research. American Journal of Public Health. doi: 10.2105/AJPH.2013.301252. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lichtenstein P, Yip BH, Björk C, et al. Common genetic influences for schizophrenia and bipolar disorder: A population-based study of 2 million nuclear families. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Idring S, Rai D, Dal H, et al. Autism Spectrum Disorders in the Stockholm Youth Cohort: Design, Prevalence and Validity. PLOS One. 2012;7:e41280. doi: 10.1371/journal.pone.0041280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larsson H, Rydén E, Boman M, Långström N, Lichtenstein P, Landén M. Does attention deficit hyperactivity disorder share etiologic factors with bipolar disorder and schizophrenia? British Journal of Psychiatry. doi: 10.1192/bjp.bp.112.120808. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tidemalm D, Långström N, Lichtenstein P, Runeson B. Risk of suicide after suicide attempt according to coexisting psychiatric disorder: Swedish cohort study with long term follow-up. BMJ. 2008:337. doi: 10.1136/bmj.a2205. 2008-11-18 00:00:00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Onofrio BM, Rickert ME, Långström N, et al. Familial confounding of the associations between maternal smoking during pregnancy and offspring substance use problems: Converging evidence across samples and measures. Archives of General Psychiatry. 2012;69:1140–1150. doi: 10.1001/archgenpsychiatry.2011.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fazel S, Grann M, Carlström E, Lichtenstein P, Långström N. Risk factors for violent crime in schizophrenia: a national cohort study of 13,806 patients. J Clin Psychiatry. 2009;70:362–369. doi: 10.4088/jcp.08m04274. [DOI] [PubMed] [Google Scholar]
- 46.D’Onofrio BM, Singh AL, Iliadou A, et al. Familial confounding of the association between maternal smoking during pregnancy and offspring criminality: A population-based study in Sweden. Archives of General Psychiatry. 2010;67:529–538. doi: 10.1001/archgenpsychiatry.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lambe M, Hultman C, Torrang A, MacCabe J, Cnattingius S. Maternal smoking during pregnancy and school performance at age 15. Epidemiology. 2006;17:524–530. doi: 10.1097/01.ede.0000231561.49208.be. [DOI] [PubMed] [Google Scholar]
- 48.D’Onofrio BM, Singh AL, Iliadou A, et al. A quasi-experimental study of maternal smoking during pregnancy and offspring academic achievement. Child Development. 2010;81:80–100. doi: 10.1111/j.1467-8624.2009.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Statistics Sweden. Educational attainment of the population. http://www.scb.se/templates/Product____9577.asp.
- 50.Allison PD. Fixed effects regression models. Washington DC: Sage; 2009. [Google Scholar]
- 51.Frisell T, Oberg S, Kuja-Halkola R, Sjolander A. Sibling comparison designs: Bias from non-shared confounders and measurement error. Epidemiology. 2012;23:713–720. doi: 10.1097/EDE.0b013e31825fa230. [DOI] [PubMed] [Google Scholar]
- 52.Meyer KA, Williams P, Hernandez-Diaz S, Cnattingius S. Smoking and risk of oral clefts: Exploring the impact of study designs. Epidemiology. 2004;15:671–678. doi: 10.1097/01.ede.0000142148.51230.60. [DOI] [PubMed] [Google Scholar]
- 53.Haglund B. Birthweight distributions by gestational age: Comparison of LMP-based and ultrasound-based estimates of gestational age using data from the Swedish Birth Registry. Paediatr Perinat Epidemiol. 2007;21:72–78. doi: 10.1111/j.1365-3016.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- 54.Hack M. Adult outcomes of preterm children. Journal of Developmental and Behavioral Pediatrics. 2009;30:460–470. doi: 10.1097/DBP.0b013e3181ba0fba. [DOI] [PubMed] [Google Scholar]
- 55.Boyd HA, Poulsen G, Wohlfahrt J, Murray JC, Bjarke F, Melbye M. Maternal contribution to preterm delivery. Am J Epidemiol. 2009;170:1358–1364. doi: 10.1093/aje/kwp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ward K, Argyle V, Meade M, Nelson L. The heritability of preterm delivery. Obstetrics & Gynecology. 2005;106:1235–1239. doi: 10.1097/01.AOG.0000189091.35982.85. [DOI] [PubMed] [Google Scholar]
- 57.York T, Eaves L, Lichtenstein P, et al. Fetal and Maternal Genes Influence Gestational Age in a Quantitative Genetic Analysis of 244,000 Swedish Births. The American Journal of Epidemiology. doi: 10.1093/aje/kwt005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.