Abstract
Stroke has significant impact on dynamic balance during locomotion, with a 73% incidence rate for falls post-stroke. Current clinical assessments often rely on tasks and/or questionnaires that relate to the statistical probability of falls and provide little insight into the mechanisms that impair dynamic balance. Current quantitative measures that assess medial-lateral balance performance do not consider the angular motion of the body, which can be particularly impaired after stroke. Current control methods in bipedal robotics rely on the regulation of angular momentum (H) to maintain dynamic balance during locomotion. This study tests whether frontal-plane H is significantly correlated to clinical balance tests that could be used to provide a detailed assessment of medial-lateral balance impairments in hemiparetic gait. H was measured in post-stroke (n=48) and control (n=20) subjects. Post-stroke there were significant negative relationships between the change in frontal-plane H during paretic single-leg stance and two clinical tests: the Dynamic Gait Index (DGI) (r=-0.57, p<0.001) and the Berg Balance Scale (BBS) (r = -0.54, p<0.001). Control subjects showed timely regulation of frontal-plane H during the first half of single-leg stance, with the level of regulation depending on the initial magnitude. In contrast, the post-stroke subjects who made poorer adjustments to frontal-plane H during initial paretic leg single stance exhibited lower DGI and BBS scores (r = 0.45, p=0.003). We conclude that H is a promising balance indicator during steady-state hemiparetic walking and that paretic single-leg stance is a period with higher instability for stroke patients.
Keywords: Stability, Gait, Dynamic balance, Hemiparesis, Clinical measures
Introduction
There is a 73% incidence of falls among individuals post-stroke, with 37% that fall sustaining injuries requiring medical treatment [1] that ultimately leads to a significant decrease in activity due to the fear of falling [2, 3]. Additionally, balance needs are particular to the task being performed [4] and static balance measures may not reflect the complexities of dynamic balance. Studies of dynamic walking have suggested that active control is needed to regulate medial-lateral balance, but not sagittal-plane balance [5]. Quantitative measures of medial-lateral balance during walking have been developed based on the concept of the “extrapolated center-of-mass” [6, 7], but these have not been applied to the post-stroke population. Further, they assume that the angular acceleration of the trunk can be neglected, which may not be justified in the hemiparetic population. Thus, an important step toward understanding the increased falls risks in persons post-stroke may be developing a quantitative measure of medial-lateral dynamic balance performance during hemiparetic walking that incorporates the angular motion of the trunk.
A number of clinical balance measures have been proposed that attempt to assess balance [8-10]. However, Mancini et al. summarized the most used balance assessment tools and noted that while most successfully identify a balance problem, they typically fail to direct clinical rehabilitation towards solving the underlying balance disorders [11]. This highlights the need for a quantitative measure that can be linked to underlying mechanisms.
Two of the most common clinical measures to assess balance ability are the Berg Balance Scale (BBS) [12] and the Dynamic Gait Index (DGI) [13]. The BBS, which tests mostly static balance, has been shown to have a sensitivity of 91% and specificity of 82% to falls when coupling the test with a self-reported history of imbalance [14]. The DGI, which tests dynamic balance during walking tasks but allows assistive devices, has been described as a useful tool for evaluating balance with reported sensitivity of 77% and specificity of 90% to falls in persons with vestibular deficits [13]. Although these clinical measures provide good reliability, they are ordinal rating scores that are observational and not quantitative. Thus, they cannot provide a quantitative step-by-step measure by which to assess balance performance during walking and reveal little about the underlying mechanisms.
In contrast to ordinal assessment scales, continuous, quantitative measures can potentially provide insight into the mechanisms of dynamic balance regulation. Developments in bipedal robotics have used whole-body angular momentum (H) in trajectory planning to maintain dynamic balance in bipedal gait using the concept of Zero Moment Point (ZMP) [15-17]. The ZMP principle seeks to reduce the external moments about the center-of-mass (COM) to zero so that the whole-body angular orientation does not change from its initial condition. Studies in human gait suggest that H is highly regulated by the central nervous system and kept at a low value [18, 19]. However, since H is known to fluctuate in human walking, efforts associated with balance might be better represented by the change in the angular momentum, or more specifically the time derivative of H (Ḣ), which is equal to the sum of the external moments acting about the COM. Thus, frontal-plane Ḣ is directly related to medial-lateral and vertical ground reaction forces, and hence medial-lateral balance control.
This study will test walking on an instrumented treadmill as opposed to overground, despite some previous concerns in the literature that treadmill walking has reduced asymmetry compared to overground walking in post stroke individuals [20, 21]. However, Kautz et al. [22] recently showed in a large study of 56 persons with hemiparesis that immediate improvements in symmetry (either temporal or spatial) do not occur when subjects walk on a treadmill without support (e.g., holding a hand rail or with body weight support from a harness). Instead, treadmill walking increased step length asymmetry. Treadmill walking appeared to provide a challenge and exacerbated hemiparetic participants’ existing motor control deficits because the differences observed between treadmill and overground walking did not influence key kinematic and EMG measures of motor control deficits [22]. Thus, we believe that treadmill walking is a valid method for studying control of angular momentum during walking.
This study aims to determine whether frontal-plane Ḣ differs between subjects with hemiparesis and speed-matched controls and whether frontal-plane Ḣ can serve as a quantitative measure of dynamic balance performance during walking. Specifically, we seek to determine the regions of the gait cycle where significant Ḣ differences occur between control and hemiparetic subjects. The relation between clinical balance assessment and Ḣ in these regions will also be tested. It is expected that, based on the robotics literature, larger values of Ḣ (i.e., large changes in H) will relate to poorer dynamic balance (and thus lower scores in clinical balance assessments). Since both BBS and DGI are reasonable predictors of falls [10, 12-14, 23-27], if this relationship proves true in hemiparetic walking, Ḣ may provide a valuable quantitative measure to assess balance disorders during walking. To help interpret any observed differences, we also quantified the variability in Ḣ and lateral foot placement.
Methods
Experimental procedure and demographics
Forty-eight subjects with post-stroke hemiparesis (29 males; age=58.3 ± 12.0 years; 5.1 ± 3.1 years post-stroke) walked at their comfortable self-selected treadmill walking speed on a split belt instrumented treadmill (Techmachine, Andrezieux Boutheon, France) for multiple trials of 30 seconds. The average number of steps per subject was 18.4 steps, with a range from 6 to 28 steps. They were also asked to walk over an instrumented mat (GaitRite, Havertown, PA) to determine their self-selected over-ground walking speed. BBS and DGI data were collected. The subjects were divided into three groups based on self-selected walking speeds on the treadmill to establish the effect of gait speed on the H. The slow group were subjects who walked slower than 0.4 m/s on the treadmill (n = 26). The medium speed group walked between 0.4 m/s and 0.8 m/s on the treadmill (n = 15) while the fast group walked between 0.8 m/s and 1.2 m/s (n = 7). Twenty control subjects (4 males; age = 65.1±10.4 years) also walked on the treadmill for 30 seconds at each of three different speeds (0.3 m/s, 0.6 m/s, and 0.9 m/s). The averages of the control group at 0.3 m/s, 0.6 m/s, and 0.9 m/s were used as speed matched controls for the slow, medium and fast post-stroke groups, respectively.
Data collection
Kinematic data were collected at 100 samples/sec by a 12 camera motion capture system (VICON, Los Angeles, USA). Reflective markers were placed in rigid clusters on 13 segments (pelvis, head, trunk and each foot, shank, thigh, upper arm, and lower arm). A custom model template was created in Visual3D (C-Motion, Germantown, MD, USA) and applied to the markers to determine body segment kinematics.
Data analysis
The gait cycle was divided into six regions. Region 1 begins with paretic foot strike and ends with non-paretic foot off (first double support). Regions 2 and 3 are defined as the first and second halves of paretic single-leg stance, respectively. Region 4 is the second double support and Regions 5 and 6 are the first and second halves of paretic swing, respectively.
Whole body angular momentum (H) was calculated as the summation of the H of each segment about the COM. The time derivative of H (Ḣ) was then calculated and normalized by each subject’s weight and instantaneous COM vertical height to provide a non-dimensional measure. The mean Ḣ for each region (normalized by the time duration of the corresponding region) was considered the change in H for that region.
We also assessed two measures of variability for each subject: the Ḣ step-to-step variability (standard deviation of Ḣ over the paretic single stance phase for all steps of a walking trial) and the foot placement variability (standard deviation of the lateral distance between the paretic foot and the COM at the onset of region 2 for all steps of a walking trial).
Statistical analysis
Spearman’s correlation was used to correlate the H measures with the clinical measures. Pearson correlations were used to correlate H measures at two different points in the gait cycle on a step-to-step basis. Additionally, Spearman’s correlation was used to correlate the resulting correlation coefficients to DGI and BBS scores. Significant differences between groups were calculated using a two-tailed t-test. In all cases, significance was set at p < 0.05.
Results
Berg balance scale and dynamic gait index
Of the 48 subjects tested, 9 were considered at risk of falling according to the BBS (score < 45) [28] and 30 by the DGI (score < 19) [29]. All subjects classified as potential fallers by the BBS test were also classified as potential fallers by DGI. For simplicity, we will henceforth refer to potential fallers as “fallers” and those above the threshold score as “non-fallers”. Significant Spearman correlations existed between the two measures (r = 0.73 p<0.001). Both DGI (r = 0.72 p < 0.001) and BBS (r=0.71 p < 0.001) correlated strongly with the over ground self-selected walking speed in the persons with hemiparesis.
Walking speed and rate of change in angular momentum (ȦM)
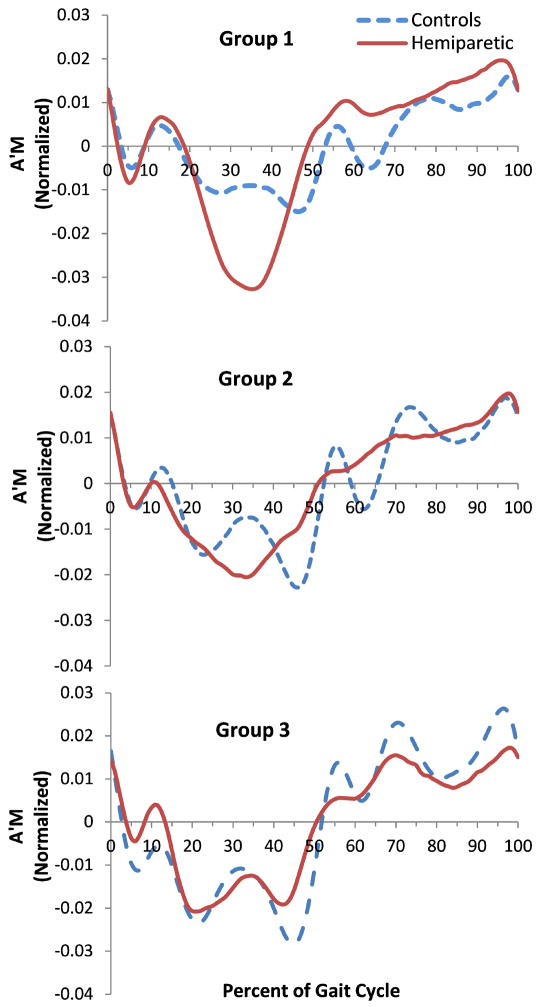
The frontal plane H for the different groups of hemiparetic subjects are shown for reference (Fig. 1). The control subjects showed characteristic Ḣ patterns in the frontal-plane when walking (Fig. 2), which changed directions during the double support periods with a double peak similar to typical medial/lateral and vertical ground reaction forces during single-leg stance. The peak Ḣ decreased with a decrease in speed. In hemiparetic the subjects, this was not the case for Regions 2 and 3 (paretic single-leg stance) of the gait cycle. The slow group showed increased Ḣ and a single peak compared to the controls’ double peak. The fast group showed more similarity with the control trajectories during matched speed walking. The largest difference in Ḣ occurred in Regions 2 and 3 (p = 0.023, p=0.003).
Figure 1.
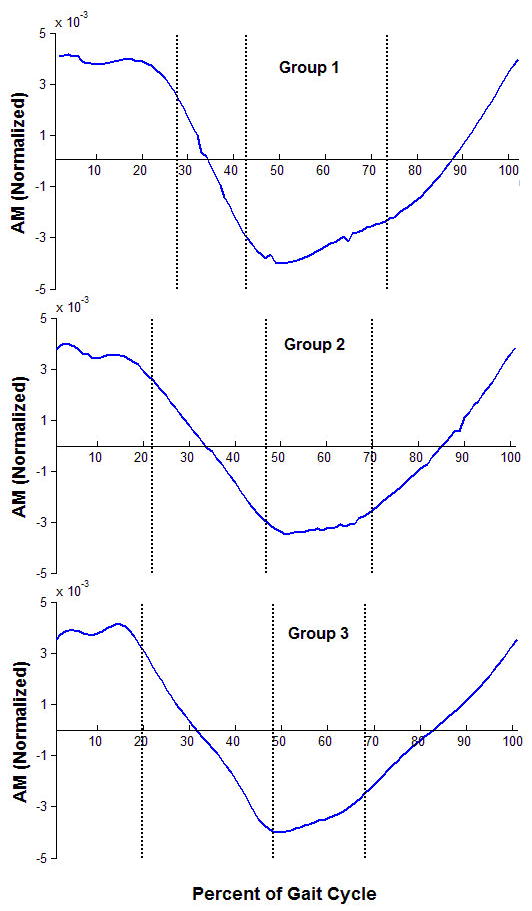
Group average data for H for three groups of persons with hemiparesis: Slow walkers (0-0.4m/s, top), medium walkers (0.4-0.8m/s, middle), and fast walkers (0.8-1.2m/s, bottom).The three vertical lines represent the average transitions between Regions 1-2, 3-4, and 4-5 (i.e., the transitions between double support phases – Regions 1 and 4). The transitions between Regions 2-3 and 5-6 are not shown but are at the midpoint of the single support phases.
Figure 2.
Group average data for Ḣ for persons with hemiparesis and controls. Slow walkers (top) were compared to controls walking at 0.3 m/s, the medium walkers (middle) were compared to controls walking at 0.6 m/s, and the fast walkers (bottom) were compared to controls walking at 0.9 m/s.
Frontal-plane angular momentum during single-leg support
The average initial value of H at the start of Region 2 (first half of single support) for controls was negatively correlated (r = -0.78, p < 0.001) with the average change in H over the region (Fig. 3). Thus, when control subjects started Region 2 with high H, there was a greater reduction in H over the remainder of that region. The initial Region 2 value was not correlated with the final Region 2 value (p=0.94) (Fig. 3). In contrast, subjects with hemiparesis showed varied relationships between initial H at the start of Region 2 and the change in H during Region 2. The correlation (for all steps in the trial) between each subjects’ change in H during Region 2 and their initial H in Region 2 correlated to BBS (r = 0.46, p = 0.002) and DGI scores (r = 0.44, p = 0.004). Therefore, subjects with a higher Pearson correlation between initial H and change in H over Region 2 also had better BBS and DGI scores (Fig. 4). As a group, DGI fallers also had a significantly less negative correlation between initial H and the change in H over Region 2 compared to the DGI non-fallers (p = 0.030).
Figure 3.
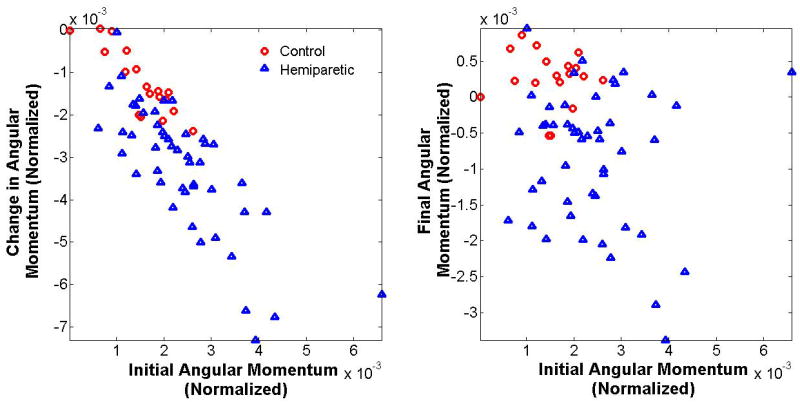
The final (left) and change (right) in angular momentum versus the initial angular momentum in Region 2 of the gait cycle. The initial angular momentum correlates strongly with the change in angular momentum. Subjects that exhibited a deviation from this trend also exhibited lower BBS and DGI scores.
Figure 4.
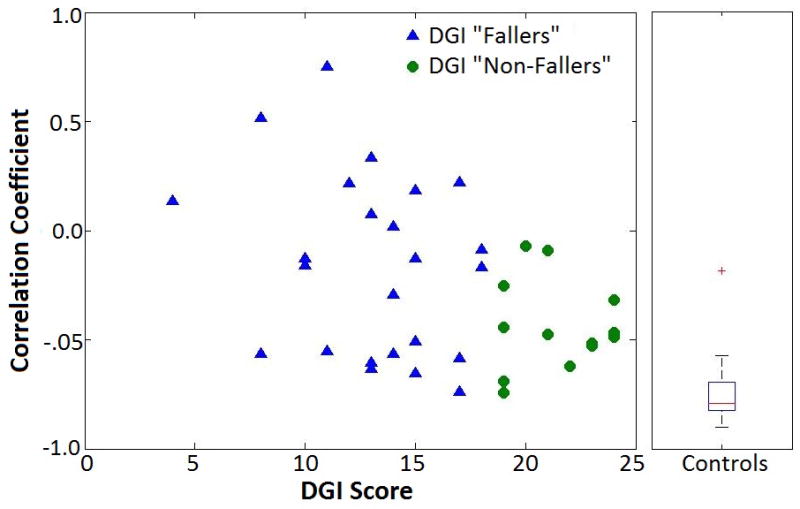
The correlation coefficients between initial angular momentum and change in angular momentum over Region 2 for DGI “Fallers” and “Non-Fallers” (left) and controls (right) as calculated from all steps during their self-selected walking trial.
Mean Ḣ during single-leg support
Frontal-plane Ḣ was negatively correlated with the BBS (r = -0.51, p < 0.001) and DGI (r = -0.50, p = 0.001) scores during Regions 2 and 3. Significant differences in Ḣ were noted between BBS fallers and non-fallers (p = 0.014) in paretic single-leg stance only (Regions 2 and 3) (Fig. 5). These differences were also evident for the DGI fallers and non-fallers (p=0.002) (Fig. 5). During each double support region there were no significant differences between the BBS fallers and non-fallers (p=0.091, p=0.625) and DGI fallers (p=0.279, p=0.100). There were also no significant differences between fallers and non-fallers during non-paretic single-leg stance (Regions 5 and 6) according to BBS (p=0.403) and DGI (p=0.336).
Figure 5.
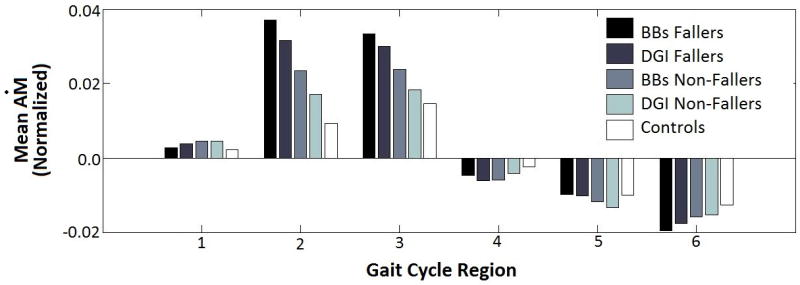
Mean Ḣ is equal to the change in angular momentum over a region of the gait cycle divided by the time spent in that region. There is a significant difference in mean Ḣ between the fallers and non-fallers during paretic single-leg stance only.
Mean Ḣ step-to-step variability during Regions 2 and 3 showed a negative correlation with the BBS (r = -0.54, p = 0.002) and DGI (r = -0.45, p =0.001) scores. Both BBS fallers (p < 0.001) and DGI fallers (p < 0.001) had significantly increased variability compared to controls during paretic single-leg stance, while BBS non-fallers (p = 0.17) and DGI non-fallers (p = 0.80) did not have a difference compared to controls. The variability of the lateral distance between the paretic foot and the COM at the onset of Region 2 correlated with the Ḣ variability (r=0.58, p < 0.001). Lateral distance variability was also inversely correlated with the BBS (r = -0.41 p = 0.006) and DGI (r = -0.40, p = 0.007) scores (Fig. 6).
Figure 6.
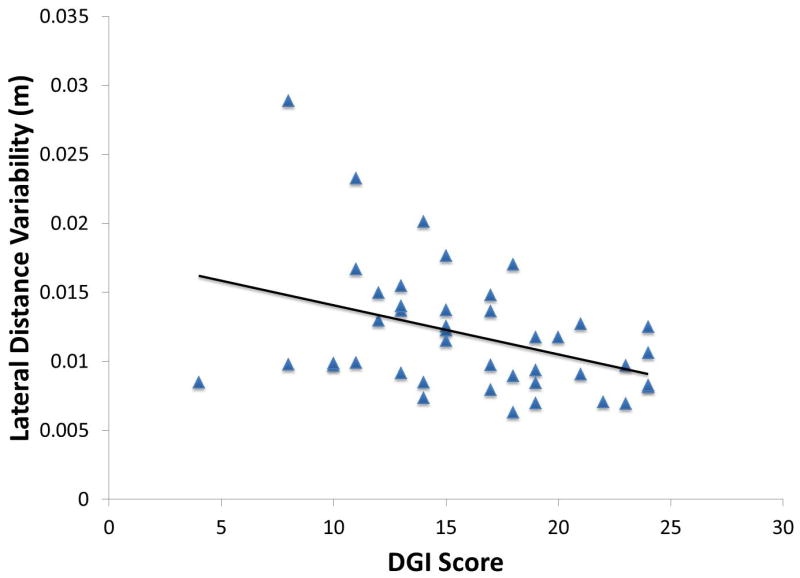
The correlation between lateral foot variability and DGI. Those subjects with poorer dynamic balance (i.e., lower DGI) had greater variability in lateral foot placement.
Discussion
Our primary finding was that there is a significant inverse correlation between two widely used clinical measures (BBS and DGI) and Ḣ during paretic single-leg stance (r = -0.57, p=<0.001; r = -0.54, p<0.001). Subjects that had lower clinical scores showed greater changes in H in paretic single-leg stance. Furthermore, H of control subjects during the first half of single-leg stance is such that they start rotating toward the swing leg by mid-swing. Subjects with lower clinical balance scores did not have this control and tended to have changes in H that were less dependent on the initial value in that region. Further, the variability of the lateral foot placement at the onset of the first half of single-leg stance was correlated with the Ḣ variability and inversely correlated with the BBS and DGI scores. Thus, these results provide evidence that increased variability in lateral foot placement and Ḣ are both related to decreased clinical balance measures.
Inverse correlation between the change in H and the clinical balance measures suggest that angular motion of the trunk, which is neglected in current quantitative assessments of balance performance (e.g., the margin of stability based on the position of the extrapolated center of mass [6, 7]), may be an important element to consider in balance assessment. Since margin of stability has not been reported in the stroke population, previous comparisons have not been made with angular momentum-based measures. However, research in walking after spinal cord injury suggests that person with poor balance likely had increased margin of stability relative to control subjects walking at a matched speed [30]. Thus, it appears that relationships do exist between margin of stability and changes in H, BBS and DGI (i.e., higher margin of stability would be associated with higher change in H and lower clinical balance measures). Future work is needed to further investigate these relationships.
Since both BBS and DGI are reasonable predictors of falls [10, 12-14, 23-27], we believe that they are the appropriate clinical tests of balance with which to compare new quantitative measures of dynamic balance performance. Additionally, BBS and DGI provide consistent assessment of balance as there was a significant positive correlation between the two (r = 0.72, p < 0.001). Since, DGI identified a significantly larger portion of subjects as fallers (46%) and relates to dynamic balance, we believe it may be a more appropriate measure for dynamic balance assessment in post-stroke subjects. Note that others have found that BBS under-predicts falls when used as a dichotomous scale [31].
An interesting finding was that frontal-plane H appears highly regulated during the first half of paretic single-leg stance (Region 2), and persons with hemiparesis who had a higher correlation between initial H and change in H over Region 2 also had better BBS and DGI scores. In controls, H appears to be regulated according to the value at the beginning of contralateral swing (Region 2) because there is a strong negative correlation between the initial H value and the change in H over Region 2. The change in H in Region 2 is almost equal and opposite to the initial value such that H is close to zero by mid-single-leg stance (this allows the person to start falling toward the swinging leg at mid stance) (Fig. 4). Subjects with hemiparesis who do not exhibit this pattern have lower clinical scores. Those that did not regulate their H across Region 2 in response to the initial value at the beginning of the region had lower DGI (r = 0.48, p = 0.002) and BBS (r=0.042, p = 0.004) scores.
Paretic single-leg stance appears to be a vulnerable period in post-stroke hemiparetic subjects when dynamic balance is compromised, as Regions 2 and 3 were the only regions in which Ḣ measures differed significantly between fallers and non-fallers. This vulnerability may be related to inconsistent lateral foot placement. Variability in lateral paretic foot placement relative to the COM at the beginning of Region 2 correlated significantly with the variability of the mean ȦM for the region (r = 0.58, p < 0.001) and negatively to the clinical scores (r = -0.41, p= 0.007).
Lateral foot placement alters H as it dictates the lever arm length of the external moment about the COM from the ground reaction forces, and therefore has a significant role in frontal-plane stability during gait. Note that a wider step width will lead to a greater external moment (e.g., moment arm between the center-of-pressure of the ground reaction forces of the paretic (stance) leg and the COM of the body), which might be expected to increase the H fluctuations. Nevertheless, the placement of the foot is not the entire story as the fallers tend to not control H (i.e., they do not have changes in H that are proportional to the initial H) when they are in paretic single-leg stance and their COM is moving toward the paretic side. They are also more variable in where the foot is placed laterally relative to the COM of the body. Thus, wide and variable paretic foot placement relative to the COM may lead to large H fluctuations and reflect poor balance during paretic single-leg support. While our results suggest that subjects who have these characteristics during walking also score poorly on clinical balance assessments, further work is needed to determine the overall influence of foot placement and H fluctuations on walking stability.
Conclusion
Similar to Silverman et al., who showed that below knee amputee H was different from non-amputees at various speeds [32], this study shows that H in post-stroke subjects differs from controls. Subjects who are unable to regulate their H trajectory during steady-state walking have been shown to have reduced clinical balance scores. H measures appear to confirm the intuitive assumption that persons with hemiparesis are more unstable when the paretic leg is the stance leg by showing large fluctuations during this period in the gait cycle, which correlates with clinical tests. The data also shows that hemiparetic subjects do not regulate their H once they are in single-leg support. Furthermore, paretic leg foot placement variability was also associated the variability in H (as revealed by Ḣ) and was inversely correlated with the BBS and DGI scores. Thus, difficulty placing the paretic foot in the correct position (e.g., if it is too wide) is likely associated with poorer H control during the paretic stance phase that leads to poorer dynamic balance. Thus, H can be used as an effective balance assessment tool during steady-state hemiparetic walking and paretic single-leg stance is a period with greater fluctuations and less H control for persons post-stroke.
Research Highlights.
We measured dynamic balance based on angular momentum.
Measures were made in persons post stroke and control subjects.
The measures were larger in persons after stroke.
The measure differentiated those with poor scores on clinical measures.
Acknowledgments
Supported by NIH Grant R01 HD46820.
This material is the result of work supported with resources and the use of facilities at the Ralph H. Johnson VA Medical Center in Charleston, SC and the NF/SG Veterans Health System in Gainesville, FL. The contents do not represent the views of the NIH, NICHD, Department of Veterans Affairs or the United States Government.
The authors would like to thank Mark Bowden, PhD, PT, Erin Carr, BS and Chitra Balasubramanian, PhD, PT for their assistance with data collection and Ryan Knight, MS for his assistance in data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Forster A, Young J. Incidence and consequences of falls due to stroke: a systematic inquiry. BMJ. 1995;311(6997):83–6. doi: 10.1136/bmj.311.6997.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tinetti ME, et al. Fear of falling and fall-related efficacy in relationship to functioning among community-living elders. J Gerontol. 1994;49(3):M140–7. doi: 10.1093/geronj/49.3.m140. [DOI] [PubMed] [Google Scholar]
- 3.Legters K, et al. Fear of falling, balance confidence and health-related quality of life in individuals with postpolio syndrome. Physiother Theory Pract. 2006;22(3):127–35. doi: 10.1080/09593980600724196. [DOI] [PubMed] [Google Scholar]
- 4.Huxham FE, Goldie PA, Patla AE. Theoretical considerations in balance assessment. Aust J Physiother. 2001;47(2):89–100. doi: 10.1016/s0004-9514(14)60300-7. [DOI] [PubMed] [Google Scholar]
- 5.Bauby CE, Kuo AD. Active control of lateral balance in human walking. Journal of Biomechanics. 2000;33(11):1433–40. doi: 10.1016/s0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 6.Hof AL. The ‘extrapolated center of mass’ concept suggests a simple control of balance in walking. Human Movement Science. 2008;27(1):112–125. doi: 10.1016/j.humov.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Hof AL, Gazendam MGJ, Sinke WE. The condition for dynamic stability. Journal of Biomechanics. 2005;38(1):1–8. doi: 10.1016/j.jbiomech.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Lord SR, Clark RD. Simple Physiological and Clinical Tests for the Accurate Prediction of Falling in Older People. Gerontology. 1996;42(4):199–203. doi: 10.1159/000213793. [DOI] [PubMed] [Google Scholar]
- 9.Duncan PW, et al. Functional Reach: A New Clinical Measure of Balance. Journal of Gerontology. 1990;45(6):M192–M197. doi: 10.1093/geronj/45.6.m192. [DOI] [PubMed] [Google Scholar]
- 10.Perell KL, et al. Fall Risk Assessment Measures. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2001;56(12):M761–M766. doi: 10.1093/gerona/56.12.m761. [DOI] [PubMed] [Google Scholar]
- 11.Mancini M, Horak FB. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur J Phys Rehabil Med. 2010;46(2):239–48. [PMC free article] [PubMed] [Google Scholar]
- 12.Blum, et al. Usefulness of the Berg Balance Scale in Stroke Rehabilitation : A Systematic Review. Vol. 88. Alexandria, VA, ETATS-UNIS: American Physical Therapy Association; 2008. p. 8. [DOI] [PubMed] [Google Scholar]
- 13.Jonsdottir J, Cattaneo D. Reliability and Validity of the Dynamic Gait Index in Persons With Chronic Stroke. Archives of Physical Medicine and Rehabilitation. 2007;88(11):1410–1415. doi: 10.1016/j.apmr.2007.08.109. [DOI] [PubMed] [Google Scholar]
- 14.Shumway-Cook A, et al. Predicting the Probability for Falls in Community-Dwelling Older Adults. Physical therapy. 1997;77(8):812–819. doi: 10.1093/ptj/77.8.812. [DOI] [PubMed] [Google Scholar]
- 15.Mousavi PN, Bagheri A. Mathematical simulation of a seven link biped robot on various surfaces and ZMP considerations. Applied Mathematical Modelling. 2007;31(1):18–37. [Google Scholar]
- 16.Mitobe K, Capi G, Nasu Y. A new control method for walking robots based on angular momentum. Mechatronics. 2004;14(2):163–174. [Google Scholar]
- 17.Wight DL, Kubica EG, Wang DWL. Introduction of the Foot Placement Estimator: A Dynamic Measure of Balance for Bipedal Robotics. Journal of Computational and Nonlinear Dynamics. 2008;3(1):011009. [Google Scholar]
- 18.Herr H, Popovic M. Angular momentum in human walking. J Exp Biol. 2008;211(4):467–481. doi: 10.1242/jeb.008573. [DOI] [PubMed] [Google Scholar]
- 19.Bruijn SM, et al. Coordination of leg swing, thorax rotations, and pelvis rotations during gait: The organisation of total body angular momentum. Gait & Posture. 2008;27(3):455–462. doi: 10.1016/j.gaitpost.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Harris-Love ML, et al. Improved hemiparetic muscle activation in treadmill versus overground walking. Neurorehabilitation and neural repair. 2004;18(3):154–60. doi: 10.1177/0888439004267678. [DOI] [PubMed] [Google Scholar]
- 21.Harris-Love ML, et al. Hemiparetic gait parameters in overground versus treadmill walking. Neurorehabilitation and neural repair. 2001;15(2):105–12. doi: 10.1177/154596830101500204. [DOI] [PubMed] [Google Scholar]
- 22.Kautz SA, et al. Comparison of motor control deficits during treadmill and overground walking poststroke. Neurorehabilitation and neural repair. 2011;25(8):756–65. doi: 10.1177/1545968311407515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitney S, Wrisley D, Furman J. Concurrent validity of the Berg Balance Scale and the Dynamic Gait Index in people with vestibular dysfunction. Physiotherapy Research International. 2003;8(4):178–186. doi: 10.1002/pri.288. [DOI] [PubMed] [Google Scholar]
- 24.Qutubuddin AA, et al. Validating the Berg Balance Scale for patients with Parkinson’s disease: A key to rehabilitation evaluation. Archives of Physical Medicine and Rehabilitation. 2005;86(4):789–792. doi: 10.1016/j.apmr.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Tyson SF, DeSouza LH. Reliability and validity of functional balance tests post stroke. Clinical Rehabilitation. 2004;18(8):916–923. doi: 10.1191/0269215504cr821oa. [DOI] [PubMed] [Google Scholar]
- 26.Mao H-F, et al. Analysis and Comparison of the Psychometric Properties of Three Balance Measures for Stroke Patients. Stroke. 2002;33(4):1022–1027. doi: 10.1161/01.str.0000012516.63191.c5. [DOI] [PubMed] [Google Scholar]
- 27.Harada N, et al. Screening for Balance and Mobility Impairment in Elderly Individuals Living in Residential Care Facilities. Physical therapy. 1995;75(6):462–469. doi: 10.1093/ptj/75.6.462. [DOI] [PubMed] [Google Scholar]
- 28.Scott V, et al. Multifactorial and functional mobility assessment tools for fall risk among older adults in community, home-support, long-term and acute care settings. Age and Ageing. 2007;36(2):130–139. doi: 10.1093/ageing/afl165. [DOI] [PubMed] [Google Scholar]
- 29.Whitney SL, Hudak MT, Marchetti GF. The dynamic gait index relates to self-reported fall history in individuals with vestibular dysfunction. Journal of Vestibular Research. 2000;10(2):99–105. [PubMed] [Google Scholar]
- 30.Day KV, et al. Foot placement variability as a walking balance mechanism post-spinal cord injury. Clinical biomechanics. 2012;27(2):145–50. doi: 10.1016/j.clinbiomech.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muir SW, et al. Use of the Berg Balance Scale for Predicting Multiple Falls in Community-Dwelling Elderly People: A Prospective Study. Physical therapy. 2008;88(4):449–459. doi: 10.2522/ptj.20070251. [DOI] [PubMed] [Google Scholar]
- 32.Silverman AK, Neptune RR. Differences in whole-body angular momentum between below-knee amputees and non-amputees across walking speeds. Journal of Biomechanics. 2011;44(3):379–385. doi: 10.1016/j.jbiomech.2010.10.027. [DOI] [PubMed] [Google Scholar]