Abstract
This study characterized human cerebellar activity during eyeblink classical conditioning (EBC) in children and adults using functional magnetic resonance imaging (fMRI). During fMRI, participants were administered delay conditioning trials, in which the conditioned stimulus (a tone) precedes, overlaps, and coterminates with the unconditioned stimulus (a corneal airpuff). Behavioral eyeblink responses and brain activation were measured concurrently during two phases: pseudoconditioning, involving presentations of tone alone and airpuff alone, and conditioning, during which the tone and airpuff were paired. Although all participants demonstrated significant conditioning, the adults produced more conditioned responses (CRs) than the children. When brain activations during pseudoconditioning were subtracted from those elicited during conditioning, significant activity was distributed throughout the cerebellar cortex (Crus I‐II, lateral lobules IV‐IX, and vermis IV–VI) in all participants, suggesting multiple sites of associative learning‐related plasticity. Despite their less optimal behavioral performance, the children showed greater responding in the pons, lateral lobules VIII, IX, and Crus I, and vermis VI, suggesting that they may require greater activation and/or the recruitment of supplementary structures to achieve successful conditioning. Correlation analyses relating brain activations to behavioral CRs showed a positive association of activity in cerebellar deep nuclei (including dentate, fastigial, and interposed nuclei) and vermis VI with CRs in the children. This is the first study to compare cerebellar cortical and deep nuclei activations in children versus adults during EBC. Hum Brain Mapp 35:1390–1403, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: cerebellum, development, learning, memory, neuroimaging
INTRODUCTION
Eyeblink classical conditioning (EBC) is a widely used model of learning, in which a neutral conditioned stimulus (CS; e.g., a tone) and an unconditioned stimulus (US; e.g., a corneal airpuff) are temporally paired. One version of this procedure is delay conditioning, in which the CS precedes, overlaps, and coterminates with the US. Following repeated CS–US presentations, the CS alone reliably elicits a conditioned response (CR; e.g., an eyeblink) in anticipation of the US presentation, indicating that an association between the CS and US has been learned.
This well‐characterized model system provides a powerful tool for studying the neural correlates of learning and memory. Through extensive laboratory animal investigations, the neural circuitry supporting EBC has been mapped in great detail [for review, see Christian and Thompson, 2003]. This line of research has produced overwhelming evidence that the cerebellum and associated structures are critically important for eyeblink conditioning. Specifically, contributions from the cerebellar cortex [Yeo, et al., 1984, 1985; Yeo and Hardiman, 1992], particularly in lateral lobule VI, and cerebellar deep nuclei [Lavond et al., 1984; McCormick and Thompson, 1984a, 1984b; Lavond et al., 1985] have been well‐documented.
EBC has also been used to investigate cerebellar development and the ontogeny of learning. Developmental studies of EBC have shown that older (24 days) rat pups conditioned at a faster rate compared to younger (17 days) rat pups [Stanton et al., 1992; Stanton, et al., 1998], reflecting specific ontogenetic changes in cerebellar circuitry [Freeman et al., 1995a; Freeman, 2010].
Developmental EBC studies in humans ranging from infants to older adults have focused mainly on behavioral measures of conditioning [Woodruff‐Pak and Thompson, 1988; Knuttinen et al., 2001; Claflin et al., 2002; Herbert et al., 2003; Jacobson et al., 2008; Cheng et al., 2010; Stanton et al., 2010; Jacobson et al., 2011]. Although these studies offer significant insight on how development affects behavioral performance during eyeblink conditioning, little is known about how development affects cerebellar activity mediating human EBC. To date, investigations of cerebellar function in humans during EBC have been limited to studies of adult patients with cerebellar lesions [Gerwig et al., 2003, 2010] and adult neuroimaging studies [Molchan et al., 1994; Logan and Grafton, 1995; Blaxton et al., 1996; Schreurs et al., 1997; Ramnani, et al., 2000; Schreurs et al., 2001; Knuttinen et al., 2002; Cheng et al., 2008; Parker et al., 2012]. Findings from these studies support the premise that the adult cerebellum is critically important for EBC. Functional magnetic resonance imaging (fMRI) studies in adults report activations in cerebellar lobule VI during EBC [Ramnani et al., 2000; Cheng et al., 2008]. In a PET study of older and younger adults [Schreurs et al., 2001], older adults showed decreased regional cerebral blood flow, but both populations demonstrated learning‐related changes in the cerebellum. However, to date, no study has directly examined cerebellar activation patterns in children during EBC.
The lack of information relating to cerebellar function in children during EBC represents a significant gap in knowledge. By comparing activity patterns between the developing and adult brain, we can begin to address important questions concerning how and when this fundamental form of learning occurs. This study investigated how development affects cerebellar cortical and deep nuclear activity during delay eyeblink conditioning in children and adults using fMRI.
MATERIALS AND METHODS
Participants
Fourteen children and 16 adults participated in this study, which was conducted at the Cape Universities Brain Imaging Centre (CUBIC) in Cape Town, South Africa. Two children were excluded for failure to respond to the US, and 11 of the remaining 12 (91.7%) met criterion for conditioning. Three adults were excluded for failure to respond to the US and one for excessive spontaneous blinks during baseline. Of the remaining 12, nine (75.0%) met criterion for conditioning. The criteria for conditioning were one or more conditioning sessions with at least 25% CRs and 15% CRs above the level of blinking during pseudoconditioning. These exclusion criteria were modified from those we have used previously [Jacobson et al., 2008] to reflect the general reduction in % CRs observed in the scanner environment and were defined based on visual inspection of the behavioral data before any imaging analyses were performed. Exclusion rates did not differ for the children versus the adults (χ2 = 1.20, P > 0.20).
Data from the 11 children (seven male, four female; mean age = 11.5 years; range = 9.3–13.8) and nine adults (five male, four female; mean age = 24.9 years; range = 19.0–29.7) who met criteria for conditioning were included in the analyses. Neuroimaging data from one child's last two conditioning sessions were lost due to technical difficulties. None of the children or adults had previously participated in any eyeblink conditioning studies. All procedures were approved by the Human Investigation Committee at Wayne State University School of Medicine and Faculty of Health Sciences Human Research Ethics Committee at the University of Cape Town.
Procedure
Behavioral apparatus
Stimulus presentation was controlled and behavioral data were recorded using a laptop computer interfaced to an NI USB‐6218 data acquisition module running custom software developed under LabView version 7.1 (National Instruments, Austin, TX). Auditory stimuli were presented through the standard Siemens MR scanner headphones. A video (The Adventures of Milo and Otis) was projected without its soundtrack through a waveguide in‐line with the bore of the magnet onto a rear projection screen positioned behind the bore of the magnet and viewed using an adjustable mirror attached to the single channel head coil. Standard laboratory safety goggles were modified by attaching the end of a polyethylene tube (Nalgene, Rochester, NY), which delivered an airpuff, and an MRI‐compatible infrared sensor, which recorded eyeblinks. Airpuff delivery was controlled by a solenoid valve (Asco, Florham Park, NJ), and a fiber‐optic probe (RoMack, Williamsburg, VA) measured the reflectance of infrared light from the left eye [Miller et al., 2005; Cheng et al., 2008].
Imaging apparatus
The MR scans were acquired on a 3T Allegra (Siemens, Erlangen, Germany) MRI scanner at the CUBIC. All children were prepared for scanning in a mock scanner where they listened to a recording of the scanner noises.
High‐resolution T1‐weighted structural MR images were acquired using a three‐dimensional (3D) echo planar imaging (EPI)‐navigated [Tisdall et al., 2012] multiecho MPRAGE [van der Kouwe et al., 2008] sequence that had been optimized for morphometric analyses using FreeSurfer software. Imaging parameters were: field of view (FOV): 256 × 256 mm2; 128 sagittal slices, repetition time (TR): 2,530 ms; echo time (TE): 1.53/3.21/4.89/6.57 ms; inversion time: 1,100 ms; Flip angle: 7°; voxel size: 1.3 × 1.0 × 1.3 mm3. The 3D EPI navigator provided real‐time motion tracking and correction [Tisdall et al., 2012], which served to substantially reduce the presence of any motion artifacts in the structural imaging data.
A T 2 *‐weighted gradient echo, EPI pulse sequence was used to collect 205 whole brain functional volumes (6:36 min) sensitive to blood oxygen level dependent contrast (TR 2,000 ms, TE 30 ms, 34 interleaved slices, slice thickness 3 mm, gap 1.5 mm, FOV 200 × 200 mm2, in‐plane resolution 3.125 × 3.125 mm2) during the EBC acquisitions.
Presentation of stimuli
We used a conditioning procedure that produced significant learning in young adults [Cheng et al., 2008, 2010]. The CS tone was a binaural 1,000 Hz tone (95 dB), lasting 750 ms, that co‐terminated with a 100 ms corneal airpuff to the left eye (10 psi measured at the delivery site). Trials were grouped into blocks: nine trials/block, 2 s/trial, 4 s inter‐trial interval (ITI), such that each block lasted 34 s. Each session consisted of eight 9‐trial blocks. Pseudoconditioning consisted of four sets of alternating tone alone and airpuff alone blocks. Conditioning blocks consisted of eight paired CS–US trials, plus a ninth CS‐alone test trial. Blocks were separated by 16 s rest periods (Fig. 1A). The ITI in the current paradigm is similar to the ITI we used previously [Cheng et al., 2008] and was selected to ensure a sufficient number of trials to permit analysis of the fMRI data. These temporal parameters were also selected to provide enough trials to permit conditioning within a limited time period and to ensure participant comfort. For children, we performed five scans, one pseudoconditioning and four conditioning sessions. For adults, we performed three scans, one pseudoconditioning and two conditioning sessions. Children were administered two additional conditioning sessions to determine whether, with additional training, their conditioning performance would reach the level of the adults.
Figure 1.
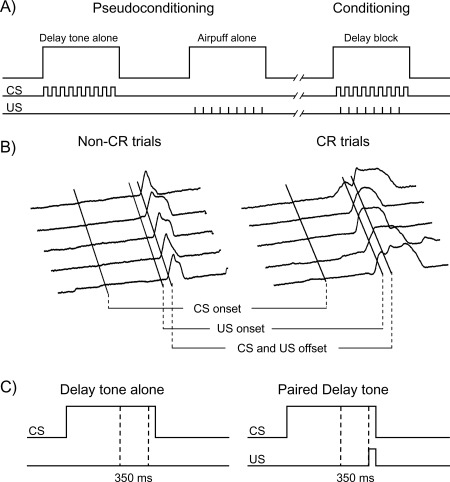
Study design/analysis and sample topography of eyeblink responses measured in the MRI scanner. (A) Pseudoconditioning consisted of alternating four delay tone alone and four airpuff alone blocks. Conditioning sessions consisted of eight blocks of paired CS‐US trials. Adults received three separate sessions (1 pseudoconditioning + 2 conditioning) and children received five separate sessions (1 pseudoconditioning + 4 conditioning). (B) Typical response profiles are shown for non‐CR and CR trials. Peak amplitude responses indicate maximal eye closure. (C) Dotted lines indicate the time window from which eyeblink responses were sampled.
Once participants were fitted with the conditioning goggles and positioned in the magnet, they were asked to lie still and watch the video. They were instructed to pay attention to the video as best they could while distracting tones and airpuffs were presented. The children were given a 2‐h lunch break between the second and third conditioning sessions.
Data Analysis
The topography of typical behavioral eyeblink responses collected inside the magnet is shown in Figure 1B. The 300‐ms time period prior to presentation of the tone was used as a baseline. To determine trials during which CRs occurred, blink amplitudes during this baseline period were compared with the maximum blink amplitude during the 350 ms preceding the airpuff (Fig. 1C). The time window of 350‐ms pre‐US presentation was selected, in accordance with common conventions in the literature [Finkbiner and Woodruff‐Pak, 1991; Jacobson et al., 2011, 2008], to exclude voluntary and alpha blink responses as CRs [Spence and Ross, 1959; Gormezano, 1966]. To qualify as a CR, the difference between the maximum blink amplitude during this 350‐ms time window and the mean response amplitude during the baseline had to exceed three times the standard deviation of the mean during the 300‐ms baseline period. Performance was expressed as the percentage of trials with valid CRs (% CR). This measure of learning was examined in a session (pseudoconditioning/session 1/session 2) by age (child/adult) repeated measures analysis of variance (ANOVA). Latency of the peak amplitude of the blink response (relative to onset of the 350 ms CR time window) was averaged across the CS tone alone trials (ninth trial in each conditioning block; to avoid UR contamination) to examine age‐related differences in the timing of eyeblink responses. For pseudoconditioning, all nine tone alone trials from the four tone alone blocks were used in the peak latency analysis.
Structural and functional imaging preprocessing and statistical analyses were performed with Statistical Parametric Mapping (SPM2 and SPM8) software (Wellcome Department of Cognitive Neurology, London, UK). Preprocessing included motion correction, structural data coregistration, normalization, and smoothing. EPI functional images were realigned and resliced correcting for minor motion artifacts, and structural images were co‐registered to the mean motion‐corrected functional image for each participant. Cerebellar structural and functional data were isolated and normalized into standard stereotaxic space using the spatially unbiased atlas template (SUIT) of the human cerebellum and brainstem [Diedrichsen, 2006], and the functional images were smoothed with a Gaussian filter (full‐width half‐maximum 5 mm). Given the children's age range, all structural and functional data were carefully inspected to ensure proper normalization and every child was found to be acceptable. Following SUIT transformation, voxel dimension was 2 mm3. The general linear model was used to estimate individual subject activations and a random‐effects analysis was conducted on all subjects.
Contrasts were designed to investigate brain activity changes as a function of associative learning (conditioning versus pseudoconditioning) as well as age‐related differences (children versus adults). To control for Type I error, Monte Carlo simulations [Forman et al., 1995] were performed, which indicated that activation clusters of at least 10 voxels were significant at a P < 0.01 (corrected) level. Activation clusters within the cerebellum surviving this threshold and also previously reported to be involved during EBC were used in region‐of‐interest (ROI) analyses. These included a region in lateral lobule VI [Yeo et al., 1984; Ramnani et al., 2000; Cheng et al., 2008] and vermis VI. The latter was selected due to its involvement during event timing [Spencer et al., 2007] and the role of timing during CR expression [Gerwig et al., 2005]. Exploratory analyses were performed to examine the relations of additional large clusters of activity in left Crus II and right lobule VIII to learning [Gerwig et al., 2003; Plakke et al., 2007]. Coordinates for the peak response within each ROI were identified at the group level and served as the center of a 3D sphere (4 mm radius) that was created to sample individual subject mean brain activity in that ROI. This radius was chosen to restrict functional activity to within the anatomical boundaries of these regions. In addition to this functional ROI approach, anatomically derived ROIs were generated for the deep nuclei (which were subsequently isolated into the dentate, fastigial, and interposed nuclei) and the hippocampus using probabilistic maps [Amunts et al., 2005; Dimitrova et al., 2006; Diedrichsen et al., 2011], in light of the extensive evidence of the involvement of these regions in eyeblink conditioning [Berger et al., 1976; McCormick and Thompson, 1984a; Christian and Thompson, 2003]. Estimated mean and peak beta weights generated from ROI analyses were used as indices of brain activation. Mean and peak responses within the ROIs were examined in relation to behavioral performance to assess the relation between neural activation and behavioral CRs.
RESULTS
Behavioral Findings
Significant across session differences in eyeblink CRs (% CRs) were observed in both children and adults (Fig. 2). A repeated‐measures ANOVA showed main effects of session, F(1,18) = 42.63, P < 4.0 × 10−6 and age, F(1,18) = 7.94, P < 0.02. The session x age interaction fell short of statistical significance F(1,18) = 3.52, P < 0.08. Post hoc comparisons showed that child (M ± SD = 12.88 ± 12.00% CR) and adult (18.16 ± 6.31) performance did not differ during pseudoconditioning, t(18) = 1.19, P > 0.20, but the adults produced a significantly greater percentage of CRs than the children during both conditioning session 1 (49.77 ± 19.37 and 27.78 ± 13.00, respectively), t(18) = 3.03, P < 0.008, and session 2 (55.81 ± 24.52 and 33.71 ± 17.15, respectively), t(18) = 2.37, P < 0.03.
Figure 2.
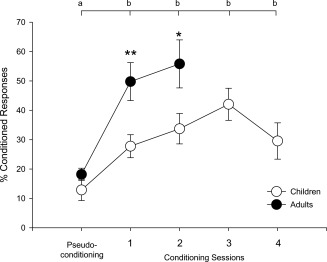
Behavioral findings in children and adults. A significantly greater percentage of CRs was exhibited during conditioning relative to pseudoconditioning, indicating that both groups learned the CS‐US association. Between session contrasts: % CRs in each session labeled “b” was significantly greater (at P < 0.05) than during pseudoconditioning, which is labeled “a.” Between group contrasts: adults produced significantly more CRs than children during sessions 1 and 2 (**P < 0.01; *P < 0.05).
Within‐group ANOVAs showed main effects of session for both the children, F(1,10) = 6.19, P < 0.04, and the adults, F(1,8) = 17.97, P < 0.003. Post hoc comparisons showed that both the children (P's < 0.05) and the adults (P's < 0.004) produced significantly more CRs during conditioning relative to pseudoconditioning. Importantly, there were no significant differences in percent unconditioned responses across sessions (pseudoconditioning: M ± SD = 87.44 ± 4.75, conditioning 1: 87.74 ± 2.01, conditioning 2: 84.71 ± 2.42), F(1,18) = 0.24, P > 0.20, or between children (87.55 ± 2.16), and adults (85.52 ± 4.70), F(1,18) = 0.18, P > 0.20. Thus, these behavioral findings indicate that, although all participants met criteria for conditioning and produced similar rates of unconditioned responses, the adults produced more CRs than the children.
Repeated‐measures ANOVA on latency of peak responses on CS alone trials showed a main effect of session, F(1,17) = 54.83, P <.001 but not age, F(1,17) = 1.31, P > 0.20. The session × age interaction fell short of statistical significance, F(1,17) = 3.87 P < 0.07. Post hoc comparisons showed that child and adult performance did not differ during pseudoconditioning, t(17) = 1.36, P > 0.19, but the children's peak latency (715.91 ± 81.63 ms) was significantly longer than the adults (458.56 ± 69.27 ms) during conditioning session 1, t(18) = 2.34, P < 0.04, but not during session 2, t(18) = 0.86, P > 0.20.
Neuroimaging Findings
Initial imaging analyses focused primarily on differences in brain activity between pseudoconditioning and conditioning session 1, because that was when initial learning occurred (Fig. 2). Whole cerebellar analyses of children and adults revealed structures that demonstrated significantly (P < 0.01, corrected) greater responses during conditioning session 1 relative to pseudoconditioning (Table 1). Significant cerebellar areas of activation in children (Fig. 3) included lateral lobules IV, V, VI, VIII, IX, Crus I and II, and vermis IV, V, and VI. Although the adults activated fewer cerebellar areas, most of those overlapped with areas activated in the children (e.g., lobule VIII, Crus I).
Table 1.
Significant activations during Session 1 relative to Pseudoconditioning
| a) Children | ||||||
|---|---|---|---|---|---|---|
| Hemisphere | X | Y | Z | SPM {Z} | N Vox | Brain region |
| Right | 26 | −48 | −48 | 4.8 | 572 | Lobule VIII |
| 26 | −52 | −58 | 3.7 | Lobule VIII | ||
| 18 | −42 | −54 | 3.5 | Lobule IX | ||
| 6 | −64 | −30 | 3.3 | 33 | Lobule VIII | |
| 20 | −86 | −24 | 3.4 | 29 | Crus I | |
| 18 | −68 | −42 | 3.2 | 26 | Lobule VIII | |
| 44 | −58 | −30 | 2.9 | 24 | Crus I | |
| 26 | −56 | −30 | 3.3 | 21 | Lobule VI | |
| 18 | −58 | −48 | 2.9 | 10 | Lobule VIII | |
| Left | −42 | −58 | −46 | 3.7 | 65 | Crus II |
| −30 | −38 | −34 | 4.1 | 50 | Lobule VI | |
| −24 | −68 | −28 | 3.9 | 42 | Lobule VI | |
| −2 | −48 | −16 | 3.1 | 28 | Vermis IV,V | |
| −18 | −42 | −50 | 3.2 | 24 | Lobule IX | |
| −22 | −30 | −28 | 3 | 21 | Lobule IV, V | |
| −44 | −60 | −24 | 3.3 | 20 | Crus I | |
| −16 | −44 | −46 | 2.8 | 13 | Lobule IX | |
| 2 | −66 | −18 | 3 | 12 | Vermis VI | |
| −12 | −50 | −38 | 3 | 10 | Lobule IX | |
| −6 | −44 | −54 | 2.9 | 10 | Lobule IX | |
| b) Adults | ||||||
| Right | 44 | −44 | −54 | 3.1 | 45 | Lobule VIIB |
| 30 | −40 | −54 | 2.9 | 17 | Lobule VIII | |
| 52 | −56 | −28 | 2.7 | 16 | Crus I | |
| 16 | −76 | −38 | 3.3 | 13 | Crus II | |
| 46 | −58 | −42 | 3.1 | 12 | Crus II | |
| Left | −16 | −34 | −18 | 2.9 | 21 | Lobule IV, V |
MNI coordinates of activation maxima in the cerebellum (Schmahmann et al., 2000) in children (a) and adults (b). Regions listed were thresholded at a minimum cluster size of 10 voxels and z‐scores of P < 0.01 (corrected). Indented entries represent local maxima within the main cluster.
Figure 3.
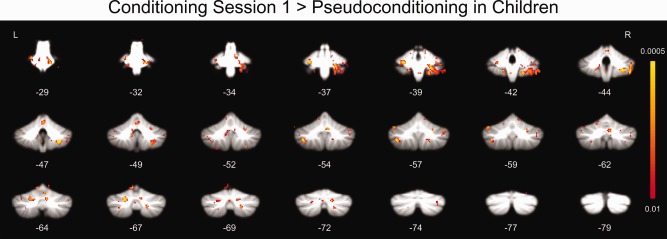
Whole cerebellum analyses showing regions with significantly greater responses during session 1 of conditioning relative to pseudoconditioning in children.
Between‐group comparisons between children and adults also showed significant age‐related differences in cerebellar activations (Fig. 4). Although the adults produced more CRs, the children showed significantly (P < 0.01, corrected) greater activation in the pons, cerebellar lateral lobule VIII, lateral lobule IX, Crus I, and vermis VI compared to adults (Table 2). By contrast, the adults did not show any areas of activation that were significantly greater than the children's during conditioning session 1.
Figure 4.
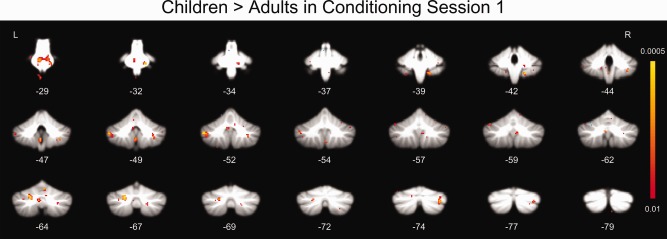
Whole cerebellum analyses showing regions with significantly greater response in children relative to adults during session 1 of conditioning (minus pseudoconditioning).
Table 2.
Greater activations in children relative to adults during Session 1
| Hemisphere | X | Y | Z | SPM {Z} | N Vox | Brain region |
|---|---|---|---|---|---|---|
| Right | 8 | −26 | −30 | 3.9 | 166 | Pons |
| 32 | −46 | −48 | 3.2 | 27 | Lobule VIII | |
| 34 | −76 | −36 | 3.3 | 25 | Crus I | |
| 16 | −42 | −54 | 3.4 | 19 | Lobule IX | |
| 2 | −66 | −18 | 3.1 | 13 | Vermis VI | |
| Left | −50 | −52 | −40 | 3.8 | 62 | Crus I |
| −22 | −68 | −32 | 3.7 | 41 | Crus I | |
| −4 | −48 | −50 | 3.3 | 25 | Lobule IX | |
| −8 | −64 | −36 | 3.2 | 23 | Lobule VIII | |
| −6 | −52 | −32 | 2.7 | 12 | Lobule IX |
MNI coordinates of activation maxima in the cerebellum (Schmahmann et al., 2000) in children relative to adults. Regions listed were thresholded at a minimum cluster size of 10 voxels and z‐scores of P < 0.01 (corrected).
Relation of Neuroimaging Data to Behavior
Correlation analyses relating brain activity to behavior were performed to identify regions that may contribute to the expression of CRs. Mean activation within two functionally (lateral lobule VI and vermis VI) and five anatomically (cerebellar deep nuclei, dentate nucleus, fastigial nucleus, interposed nuclei, and hippocampus) defined ROIs was examined in relation to % CRs within each session (Table 3), and mean activity within these ROIs during session 1 was also examined in relation to % CRs during each of the subsequent conditioning sessions (Table 4).
Table 3.
Relation of mean brain activity to percent conditioned responses within each session
| a) Children | Structure | ||||||
|---|---|---|---|---|---|---|---|
| Session | Left Deep Nuclei | Right Deep Nuclei | Left Lobule VI | Right Lobule VI | Vermis VI | Left Hippocampus | Right Hippocampus |
| 1 | 0.78 c | 0.65 b | 0.38 | 0.08 | 0.88 d | 0.37 | 0.20 |
| 2 | 0.48 | 0.47 | −0.04 | 0.11 | 0.27 | 0.30 | 0.34 |
| 3 | 0.37 | 0.36 | 0.41 | 0.49 | 0.40 | 0.57 a | 0.35 |
| 4 | −0.17 | 0.08 | −0.02 | 0.10 | 0.03 | −0.15 | −0.07 |
| Session | Left Dentate | Left Fastigial | Left Interposed | Right Dentate | Right Fastigial | Right Interposed | |
| 1 | 0.73 b | 0.77 c | 0.67 b | 0.65 b | 0.67 b | 0.79 c | |
| b) Adults | Structure | ||||||
| Session | Left Deep Nuclei | Right Deep Nuclei | Left Lobule VI | Right Lobule VI | Vermis VI | Left Hippocampus | Right Hippocampus |
| 1 | 0.53 | 0.02 | 0.08 | 0.53 | 0.31 | 0.51 | −0.20 |
| 2 | −0.03 | 0.05 | 0.07 | 0.36 | 0.32 | 0.40 | 0.18 |
| Session | Left Dentate | Left Fastigial | Left Interposed | Right Dentate | Right Fastigial | Right Interposed | |
| 1 | 0.73 b | −0.13 | 0.13 | 0.09 | 0.06 | 0.18 | |
Pearson correlation values between mean brain activity and behavioral performance within each session. Mean activity in the deep nuclei and vermis VI significantly correlated with conditioned responses during session 1 in children. Activity in the sub‐nuclei (dentate, fastigial, and interposed) were also signficantly correlated during session 1 in children.
P < 0.10,
P < 0.05,
P < 0.01,
P < 0.001
Bold values indicate correlation coefficients that were significant or approached significance.
Table 4.
Relation of mean brain activity in session 1 to percent conditioned responses in each of the sessions
| a) Children | Structure | ||||||
|---|---|---|---|---|---|---|---|
| Session | Left Deep Nuclei | Right Deep Nuclei | Left Lobule VI | Right Lobule VI | Vermis VI | Left Hippocampus | Right Hippocampus |
| 1 | 0.78 c | 0.65 b | 0.38 | 0.08 | 0.88 d | 0.37 | 0.20 |
| 2 | 0.67 b | 0.57 a | 0.48 | 0.37 | 0.66 b | 0.45 | 0.32 |
| 3 | 0.40 | 0.42 | 0.70 b | 0.13 | 0.29 | 0.42 | 0.22 |
| 4 | 0.05 | 0.09 | 0.21 | −0.11 | 0.03 | −0.01 | −0.07 |
| Session | Left Dentate | Left Fastigial | Left Interposed | Right Dentate | Right Fastigial | Right Interposed | |
| 1 | 0.73 b | 0.77 c | 0.67 b | 0.65 b | 0.67 b | 0.79 c | |
| 2 | 0.65 b | 0.26 | 0.54 a | 0.58 a | 0.36 | 0.65 b | |
| b) Adults | Structure | ||||||
| Session | Left Deep Nuclei | Right Deep Nuclei | Left Lobule VI | Right Lobule VI | Vermis VI | Left Hippocampus | Right Hippocampus |
| 1 | 0.53 | 0.02 | 0.08 | 0.53 | 0.31 | 0.51 | −0.20 |
| 2 | 0.31 | −0.11 | 0.51 | 0.50 | 0.38 | 0.53 | −0.03 |
| Session | Left Dentate | Left Fastigial | Left Interposed | Right Dentate | Right Fastigial | Right Interposed | |
| 1 | 0.73 b | −0.13 | 0.13 | 0.09 | 0.06 | 0.18 | |
| 2 | 0.53 | −0.37 | −0.22 | −0.03 | −0.13 | −0.11 | |
Pearson correlation values between mean brain activity during session 1 and behavioral performance in each of the sessions. Session 1 activity in the left deep nuclei and vermis VI signficantly correlated with conditioned responses during sessions 1 and 2 in children.
P < 0.10,
P < 0.05,
P < 0.01,
P < 0.001
Bold values indicate correlation coefficients that were significant or approached significance.
Mean activity in left and right deep nuclei and vermis VI in session 1 was significantly correlated with CRs during conditioning session 1 in the children (Table 3, Figs. 5 and 6). Mean activity within each of the cerebellar subnuclei (dentate, fastigial, and interposed) was also significantly correlated with CRs during session 1 in children. Scatter plots with regression lines show the strong, positive relation of mean activity in left cerebellar deep nuclei and each of the subnuclei to % CRs during session 1 (Fig. 5). When session 1 brain activity was examined in relation to subsequent behavioral performance (sessions 2–4), activity in the left deep nuclei and vermis VI was also significantly correlated with CRs during session 2 in the children (Table 4). Significant correlations between CRs during session 2 and cerebellar subnuclei activity in session 1 were only detected in the left dentate and right interposed nuclei while correlations with the left interposed and right dentate nuclei approached significance.
Figure 5.
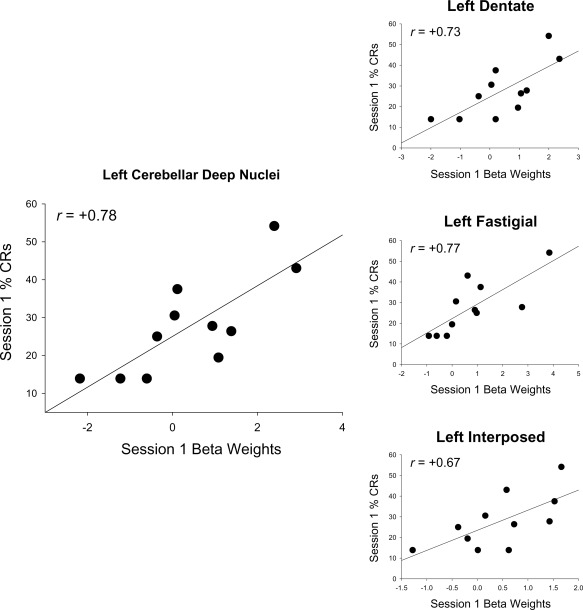
Relation of mean activation in left cerebellar deep nuclei to CRs in children. Left panel shows that activity in the left cerebellar deep nuclei was positively correlated with CRs in session 1. Smaller graphs on the right show this region separated into dentate, fastigial, and interposed nuclei and show that session 1 activity within these subnuclei also correlated with CRs.
Figure 6.
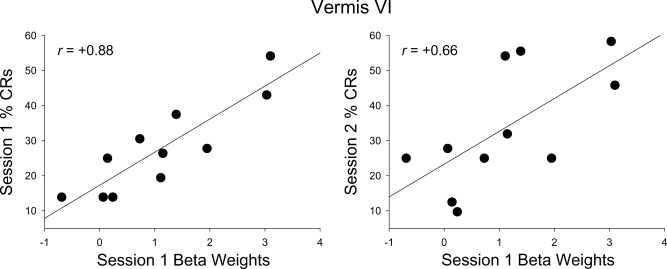
Relation of mean activation in vermis to CRs in children. Activity in the vermis was positively correlated with CRs in sessions 1 and 2.
In adults, mean activity in the left dentate nucleus was significantly correlated with CRs during session 1 but the correlation for all other regions fell short of statistical significance (Table 3). Finally, age of children and adults was not significantly correlated with either behavioral CRs or brain beta weights (r's ranged from −0.06 to +0.25 all P's > 0.20).
DISCUSSION
This is the first fMRI study to examine the neural substrates underlying EBC in children, compared with adults. Both children and adults demonstrated successful conditioning in the MRI environment. Although both groups produced similar rates of unconditioned responses, the adults produced more CRs than the children. Significant learning‐related activity was distributed throughout the cerebellar cortex in all participants, suggesting multiple sites of plasticity. Despite higher levels of behavioral conditioning in the adults, the children showed greater activity in multiple areas within the cerebellar cortex, suggesting that they may require greater activation and/or the recruitment of supplementary structures to achieve successful conditioning. The strong correlations between the imaging data and the learned behavioral responses further emphasized the importance of the cerebellar deep nuclei and vermis VI in the expression of conditioned eyeblink responses.
Behavior
Behavioral EBC studies have demonstrated that humans ranging from infants as young as 5 months to older adults show successful conditioning [Woodruff‐Pak and Thompson, 1988; Knuttinen et al., 2001; Claflin et al., 2002; Herbert et al., 2003; Jacobson et al., 2008; Cheng et al., 2010; Stanton et al., 2010; Jacobson et al., 2011]. In the current study, children and adults demonstrated significantly greater % CRs during conditioning compared to pseudoconditioning, suggesting both groups learned the CS–US association. Adults showed higher levels of conditioning than children, even though the children received twice as many trials, providing evidence of continued developmental change from child to adulthood in this domain. Importantly, although their learning was not as robust as adults, children showed significant conditioning inside the MRI environment.
The current behavioral findings, coupled with results from other studies on how aging affects conditioning [Woodruff‐Pak and Thompson, 1988; Knuttinen et al., 2001; Claflin et al., 2002; Herbert et al., 2003; Jacobson et al., 2008; Cheng et al., 2010; Stanton et al., 2010; Jacobson et al., 2011], suggest that EBC performance is optimal during young adulthood and less robust during childhood and in older populations. The present findings in children extend our knowledge on EBC performance over the human lifespan.
Learning‐Related Changes in Brain Activity
Laboratory animal work has identified the cerebellar cortex as one important site of plasticity for EBC, particularly lateral lobule VI [Yeo et al., 1984, 1985; Lavond and Steinmetz, 1989; Yeo and Hardiman, 1992; Harvey et al., 1993; Nolan and Freeman, 2006]. Rats with damage to Purkinje cells in the cerebellar cortex were impaired in the acquisition and extinction of eyeblink conditioning [Nolan and Freeman, 2006], and aspirations of lateral lobule VI were found to impair normal acquisition of eyeblink conditioning in rabbits [Yeo et al., 1984, 1985). In humans, conditioning levels in patients with damage to lateral lobule VI were reduced compared to controls [Gerwig et al., 2003], and fMRI findings showed that this area of cortex responds during delay EBC [Ramnani et al., 2000; Cheng et al., 2008]. The present data revealed two distinct foci of activation within left lateral lobule VI for the children (Table 1), suggesting multiple regions of plasticity within this lobule. This finding is consistent with evidence that partial lesions to this structure may not be sufficient to eliminate conditioning completely [Yeo and Hardiman, 1992; Harvey et al., 1993].
Differential responding in the adult cerebellar cortex has been reported in several neuroimaging studies on human EBC [Molchan et al., 1994; Logan and Grafton, 1995; Blaxton et al., 1996; Schreurs et al., 1997; Ramnani et al., 2000; Knuttinen et al., 2002; Cheng et al., 2008; Parker et al., 2012], although PET studies have produced varying results—decreases in one study [Molchan et al., 1994] and increases in another [Logan and Grafton, 1995]. Using fMRI and the cerebellar SUIT template [Diedrichsen, 2006], the present study was able to improve localization and report the spatial extent of activation within the cerebellar cortex (Tables 1 and 2). Although Ramnani et al. 2000 showed increased fMRI activation in lateral lobule VI in adults, the adults in the current study did not show learning‐related activity in this area. This lack of differential activity may reflect a more rapid acquisition due to the relatively simple conditioning protocol. The present study used a single‐cue conditioning protocol and reinforced 8/9 trials within each block, whereas Ramnani et al. 2000 used a differential conditioning protocol and a 50% reinforcement rate. The greater activation levels in children, who do not learn as well as adults, may reflect an active learning process and are consistent with previous fMRI studies using more complex designs [Ramnani et al., 2000; Cheng et al., 2008].
Gerwig et al. 2010 reported that patients with focal and degenerative lesions to lateral lobule VI showed deficits in CR acquisition even after multiple training sessions, indicating that this region is involved in the learning of the CS–US association. The rapid acquisition by the adults in the current study makes it difficult to detect changes in the fMRI signal related to an active learning process. The lack of lateral lobule VI activation in the adults, therefore, does not rule out an important role for this area during acquisition but rather suggests that CR expression, in well‐trained participants, does not critically rely on this area. It is possible that if the adult's acquisition rate were reduced (similar to that of the children), acquisition‐related activity would have been detected in this area.
Large, significant learning‐related activations in right lobule VIII were found in all participants, and age‐related comparisons showed that children activated this region bilaterally more than adults. Gerwig et al. 2003 found that patients with lesions in the inferior cerebellar cortex (e.g., lobule VIII) were not as impaired during EBC as patients with lesions to the superior cerebellar cortex (e.g., lobule VI). Thus, lobule VIII may play a supportive, but not critical role during EBC. These authors also found that relative to unilateral cerebellar cortical lesions, bilateral lesions produced modest (but not statistically significant) impairments during EBC, and animal studies suggest that contralateral cerebellar cortical lesions do not affect eyeblink conditioning [Freeman, et al., 1995b]. In this study, bilateral cerebellar cortical activations in children were detected in lobules VI, IX, and Crus I (Table 1). In conjunction with lesion data [Freeman et al., 1995b; Gerwig et al., 2003], bilateral cerebellar activation in PET work [Logan and Grafton, 1995; Blaxton et al., 1996], and the presence of bilateral eyeblink CRs [Disterhoft et al., 1977; Campolattaro and Freeman, 2009], the present neuroimaging findings support the idea that contralateral regions of the cerebellar cortex may play a modulatory but not necessary role during EBC. The widespread fMRI activations in the current study suggest that delay EBC engaged multiple structures throughout the cerebellar cortex [Plakke et al., 2007; Gerwig et al., 2010].
Differential activation within the cerebellar deep nuclei was not seen in any conditioning versus pseudoconditioning comparisons in the present study. This is consistent with the majority of neuroimaging investigations of human eyeblink conditioning [Molchan et al., 1994; Blaxton et al., 1996; Schreurs et al., 1997; Ramnani et al., 2000; Schreurs et al., 2001; Knuttinen et al., 2002; Cheng et al., 2008; Parker et al., 2012]. Given that the only study to report cerebellar deep nuclear activation during human EBC used PET [Logan and Grafton, 1995], it is possible that current fMRI techniques and analytic approaches may not be optimal for characterizing activity in these regions. The lack of differential activity in the present study may also be due to significant elevated responding in the deep nuclei elicited during pseudoconditioning. Unpaired CS–US presentations appear to be sufficient to activate the deep nuclei due to stimulation of the afferent mossy fiber (pons) and climbing fiber (inferior olive) pathways that transmit the CS and US, respectively [Mauk et al., 1986; Steinmetz et al., 1986]. Alternatively, although the deep nuclei have been identified as a critical site of plasticity in EBC in laboratory animals [Lavond et al., 1984; McCormick and Thompson, 1984a, 1984b; Lavond et al., 1985; Christian and Thompson, 2003], it is possible that the relative role of the deep nuclei in the circuitry subserving this form of learning differs between species.
Age‐Related Changes in Brain Activity
As indicated earlier, despite adults' producing more CRs, children showed greater activation in several regions, including the pons and cerebellar cortical structures (Fig. 4 and Table 2). Conversely, adults did not show greater activation in any cerebellar region. The pons are part of the essential neural circuitry underlying EBC and serve as a pathway for auditory CS afferents [Christian and Thompson, 2003]. The increased cerebellar activation exhibited by children may represent a more active learning process given their lower conditioning levels as compared to adults. The children's more distributed activation maps compared to adults (Tables 1 and 2) support the idea that children may recruit supplementary structures to achieve successful conditioning, which is consistent with studies showing that children exhibit more extensive and diffuse activations to achieve the same level of performance as adults in other neuroimaging tasks as well [Rivera et al., 2005; Meintjes et al., 2010; De Guio et al., 2012].
Behavioral analyses of the latency of peak responses showed that relative to children, adults' peak responses occurred earlier; that is, closer to when the US would have been presented on the nonreinforced CS tone alone trials during conditioning session 1. Despite their poorer timing, children showed greater activation in vermis VI (Table 2), which has been linked to cerebellar‐mediated timing in finger tapping studies [Spencer et al., 2007; Stoodley et al., 2010]. Timing of the blink response is critically important in EBC, as poorly timed responses do not fully protect the eye from the airpuff. The significant correlations between activity in vermis VI and CRs during sessions 1 and 2 further confirm the importance of the cerebellar cortex in event timing [Perrett, et al., 1993; Ivry et al., 2002; Gerwig et al., 2005; Spencer et al., 2007] in EBC (Fig. 6). Left anterior lobules IV and V were also more active in all participants. The anterior cerebellar cortex in adult rabbits has been implicated in CR timing [Perrett et al., 1993], which becomes more accurate between adolescence and adulthood in the rat [Brown et al., 2006].
Neurodevelopmental changes presumably mediate the age‐related changes in brain activation during EBC seen in this study. Whole brain volume is not likely to account for these changes because children (7–11 years) have approximately 95% of the volume of the adult brain [Caviness et al., 1996]. However, nonlinear changes in cerebral gray and white matter volume continue throughout the lifespan [Sowell et al., 2003]. Although the greatest changes in myelination in the cerebellar peduncles occur during the first 36 months of human development, a gradual increase in myelination continues through age 11 years [Saksena et al., 2008]. The mechanisms mediating the developmental changes seen here require further investigation.
Behavior and Neuroimaging
The analysis examining the relation between neuroimaging data and behavioral CRs highlighted several regions. Activity in the lateral cerebellar cortex was related to learning rates in children and adults, but these correlations fell short of statistical significance in this small sample. For example, moderate correlations were seen between activation in left lobule VI and session 1 performance in children (Table 3) and between right lobule VI activation and session 1 performance in adults (Table 3). Other large clusters of activity that were examined in relation to children's CR performance but did not reach statistical significance in this small sample were left Crus II (r = +0.24, P = 0.49) and right lobule VIII (r = +0.35, P = 0.29).
Children's mean activity in the cerebellar deep nuclei in session 1 was significantly correlated with CRs in sessions 1 and 2 (Table 4). The relation between brain activity during session 1 and behavioral CRs during session 2 suggests that brain processes associated with initial CS–US learning may be predictive of future performance. Deep nuclear activity is critically involved in the acquisition and expression of the behavioral conditioned eyeblink response in laboratory animals [McCormick and Thompson, 1984a, b]. Although the interposed nuclei have been identified as a critical site of plasticity in animal EBC studies [Lavond et al., 1985], activity within all three subnuclei (dentate, fastigial, and interposed) was significantly correlated with conditioned responding in children in this study (Fig. 5). Significant learning‐related activation was not detected in the interposed nuclei in adults in this and previous fMRI eyeblink conditioning studies [Ramnani et al., 2000; Knuttinen et al., 2002; Cheng et al., 2008], possibly due to iron accumulation in this region associated with normal development. The MRI signal within the deep cerebellar nuclei is susceptible to iron deposits, and the variability of mean signal intensities in this region also significantly increases with age [Maschke et al., 2004]. An fMRI study in rabbits showed increased activation in the anterior interposed nuclei during eyeblink conditioning [Miller et al., 2003], and human cerebellar deep nuclei activity, as assessed with PET, was correlated with conditioned eyeblink responses [Logan and Grafton, 1995]. This is the first fMRI study to show that activity in the interposed nuclei was significantly correlated with human eyeblink conditioned responding.
Activity in only the left dentate nucleus was significantly correlated with CRs in adults. A secondary analysis revealed that if peak responses within the ROI are used as the index of brain activity, strong positive correlations with behavior are found in the left (r = +0.86, P < 0.003), but not right (r = +0.20, P > 0.20), deep nuclei. These correlations seem to be largely driven by peak activity within the left dentate nucleus (r = +0.73, P < 0.03). It was somewhat unexpected that the dentate and not interposed nuclei showed activity correlated with CRs. Although this specific nucleus is not thought to be crucial for EBC, animal recordings from and lesions to the dentate‐interposed nuclear complex [Bracha et al., 1994; McCormick and Thompson, 1984a] suggest that the dentate activity detected in the present study may contribute to, but is probably not necessary for, EBC.
Limitations
Limitations of the present study include the relatively small sample. Certain correlations reported in Tables 3 and 4 might have reached statistical significance given more power. Another limitation is that our children's age range (9.3–13.8 years), although narrow, may have encompassed more than one stage of neurodevelopment. Finally, the MRI environment, including loud noise and discomfort associated with lying in the bore of the scanner during learning, may have contributed to the relatively low levels of conditioning in this study.
Summary
This is the first fMRI investigation of the neural substrates underlying EBC to compare responses in children and adults. Thus, it is the first study to begin to address important questions regarding the ontogeny of eyeblink conditioning, including how the developing brain processes this fundamental learning task. Additional studies are needed to determine when during development brain activity and behavioral responses begin to resemble more mature brain function. These questions may further aid in the diagnoses and treatment of neurodevelopmental disorders known to affect EBC, such as fetal alcohol syndrome [Jacobson et al., 2011, 2008].
ACKNOWLEDGMENTS
The authors thank Maggie September and Moira Raatz from the University of Cape Town Child Development Research Laboratory and the staff at the Cape Universities Brain Imaging Centre for their contributions to the study.
REFERENCES
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K (2005): Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol (Berl),210:343–352. [DOI] [PubMed] [Google Scholar]
- Berger TW, Alger B, Thompson RF (1976): Neuronal substrate of classical conditioning in the hippocampus. Science 192:483–485. [DOI] [PubMed] [Google Scholar]
- Blaxton TA, Zeffiro TA, Gabrieli JD, Bookheimer SY, Carrillo MC, Theodore WH, Disterhoft JF (1996): Functional mapping of human learning: A positron emission tomography activation study of eyeblink conditioning. J Neurosci 16:4032–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha V, Webster ML, Winters NK, Irwin KB, Bloedel JR (1994): Effects of muscimol inactivation of the cerebellar interposed‐dentate nuclear complex on the performance of the nictitating membrane response in the rabbit. Exp Brain Res 100:453–468. [DOI] [PubMed] [Google Scholar]
- Brown KL, Pagani JH, Stanton ME (2006): The ontogeny of interstimulus interval (ISI): Discrimination of the conditioned eyeblink response in rats. Behav Neurosci 120:1057–1070. [DOI] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JH (2009): Examination of bilateral eyeblink conditioning in rats. Behav Neurosci 123:1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS Jr, Kennedy DN, Richelme C, Rademacher J, Filipek PA (1996): The human brain age 7–11 years: A volumetric analysis based on magnetic resonance images. Cereb Cortex 6:726–736. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Disterhoft JF, Power JM, Ellis DA, Desmond JE (2008): Neural substrates underlying human delay and trace eyeblink conditioning. Proc Natl Acad Sci USA 105:8108–8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Faulkner ML, Disterhoft JF, Desmond JE (2010): The effects of aging in delay and trace human eyeblink conditioning. Psychol Aging 25:684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Thompson RF (2003): Neural substrates of eyeblink conditioning: Acquisition and retention. Learn Mem 10:427–455. [DOI] [PubMed] [Google Scholar]
- Claflin DI, Stanton ME, Herbert J, Greer J, Eckerman CO (2002): Effect of delay interval on classical eyeblink conditioning in 5‐month‐old human infants. Dev Psychobiol 41:329–340. [DOI] [PubMed] [Google Scholar]
- De Guio F, Jacobson SW, Molteno CD, Jacobson JL, Meintjes EM (2012): Functional magnetic resonance imaging study comparing rhythmic finger tapping in children and adults. Pediatr Neurol 46:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J (2006): A spatially unbiased atlas template of the human cerebellum. Neuroimage 33:127–138. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Maderwald S, Kuper M, Thurling M, Rabe K, Gizewski ER, Ladd ME, Timmann D (2011): Imaging the deep cerebellar nuclei: A probabilistic atlas and normalization procedure. Neuroimage 54:1786–1794. [DOI] [PubMed] [Google Scholar]
- Dimitrova A, Zeljko D, Schwarze F, Maschke M, Gerwig M, Frings M, Beck A, Aurich V, Forsting M, Timmann D (2006): Probabilistic 3D MRI atlas of the human cerebellar dentate/interposed nuclei. Neuroimage 30:12–25. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Kwan HH, Lo WD (1977): Nictitating membrane conditioning to tone in the immobilized albino rabbit. Brain Res 137:127–143. [DOI] [PubMed] [Google Scholar]
- Finkbiner RG, Woodruff‐Pak DS (1991): Classical eyeblink conditioning in adulthood: Effects of age and interstimulus interval on acquisition in the trace paradigm. Psychol Aging 6:109–117. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33:636–647. [DOI] [PubMed] [Google Scholar]
- Freeman JH (2010): Developmental neurobiology of cerebellar learning In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience.New York:Oxford University Press; pp546–572. [Google Scholar]
- Freeman JH Jr, Barone S Jr, Stanton ME (1995a): Disruption of cerebellar maturation by an antimitotic agent impairs the ontogeny of eyeblink conditioning in rats. J Neurosci 15:7301–7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH Jr, Carter CS, Stanton ME (1995b): Early cerebellar lesions impair eyeblink conditioning in developing rats: Differential effects of unilateral lesions on postnatal day 10 or 20. Behav Neurosci 109:893–902. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Dimitrova A, Kolb FP, Maschke M, Brol B, Kunnel A, Boring D, Thilmann AF, Forsting M, Diener HC, Timmann D (2003): Comparison of eyeblink conditioning in patients with superior and posterior inferior cerebellar lesions. Brain 126:71–94. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Guberina H, Esser AC, Siebler M, Schoch B, Frings M, Kolb FP, Aurich V, Beck A, Forsting M, Timmann D (2010): Evaluation of multiple‐session delay eyeblink conditioning comparing patients with focal cerebellar lesions and cerebellar degeneration. Behav Brain Res 212:143–151. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Hajjar K, Dimitrova A, Maschke M, Kolb FP, Frings M, Thilmann AF, Forsting M, Diener HC, Timmann D (2005): Timing of conditioned eyeblink responses is impaired in cerebellar patients. J Neurosci 25:3919–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormezano I (1966): Classical conditioning In: Sidowski JB, editor. Experimental Methods and Instrumentation in Psychology.New York:McGraw‐Hill Book Co; pp385–420. [Google Scholar]
- Harvey JA, Welsh JP, Yeo CH, Romano AG (1993): Recoverable and nonrecoverable deficits in conditioned responses after cerebellar cortical lesions. J Neurosci 13:1624–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert JS, Eckerman CO, Stanton ME (2003): The ontogeny of human learning in delay, long‐delay, and trace eyeblink conditioning. Behav Neurosci 117:1196–1210. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Spencer RM, Zelaznik HN, Diedrichsen J (2002): The cerebellum and event timing. Ann N Y Acad Sci 978:302–317. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N, Jacobson JL (2011): Impaired delay and trace eyeblink conditioning in school‐age children with fetal alcohol syndrome. Alcohol Clin Exp Res 35:250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL (2008): Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res 32:365–372. [DOI] [PubMed] [Google Scholar]
- Knuttinen MG, Power JM, Preston AR, Disterhoft JF (2001): Awareness in classical differential eyeblink conditioning in young and aging humans. Behav Neurosci 115:747–757. [DOI] [PubMed] [Google Scholar]
- Knuttinen MG, Parrish TB, Weiss C, LaBar KS, Gitelman DR, Power JM, Mesulam MM, Disterhoft JF (2002): Electromyography as a recording system for eyeblink conditioning with functional magnetic resonance imaging. Neuroimage 17:977–987. [PubMed] [Google Scholar]
- Lavond DG, Hembree TL, Thompson RF (1985): Effect of kainic acid lesions of the cerebellar interpositus nucleus on eyelid conditioning in the rabbit. Brain Res 326:179–182. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Lincoln JS, McCormick DA, Thompson RF (1984): Effect of bilateral lesions of the dentate and interpositus cerebellar nuclei on conditioning of heart‐rate and nictitating membrane/eyelid responses in the rabbit. Brain Res 305:323–330. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Steinmetz JE (1989): Acquisition of classical conditioning without cerebellar cortex. Behav Brain Res 33:113–164. [DOI] [PubMed] [Google Scholar]
- Logan CG, Grafton ST (1995): Functional anatomy of human eyeblink conditioning determined with regional cerebral glucose metabolism and positron‐emission tomography. Proc Natl Acad Sci USA 92:7500–7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschke M, Weber J, Dimitrova A, Bonnet U, Bohrenkamper J, Sturm S, Kindsvater K, Muller BW, Gastpar M, Diener HC, Forsting M, Timmann D (2004): Age‐related changes of the dentate nuclei in normal adults as revealed by 3D fast low angle shot (FLASH) echo sequence magnetic resonance imaging. J Neurol 251:740–746. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Steinmetz JE, Thompson RF (1986): Classical conditioning using stimulation of the inferior olive as the unconditioned stimulus. Proc Natl Acad Sci USA 83:5349–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF (1984a): Cerebellum: Essential involvement in the classically conditioned eyelid response. Science 223:296–299. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF (1984b): Neuronal responses of the rabbit cerebellum during acquisition and performance of a classically conditioned nictitating membrane‐eyelid response. J Neurosci 4:2811–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes EM, Jacobson JL, Molteno CD, Gatenby JC, Warton C, Cannistraci CJ, Hoyme HE, Robinson LK, Khaole N, Gore JC, Jacobson SW (2010): An FMRI study of number processing in children with fetal alcohol syndrome. Alcohol Clin Exp Res 34:1450–1464. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Chen NK, Li L, Tom B, Weiss C, Disterhoft JF, Wyrwicz AM (2003): fMRI of the conscious rabbit during unilateral classical eyeblink conditioning reveals bilateral cerebellar activation. J Neurosci 23:11753–11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Li L, Weiss C, Disterhoft JF, Wyrwicz AM (2005): A fiber optic‐based system for behavioral eyeblink measurement in a MRI environment. J Neurosci Methods 141:83–87. [DOI] [PubMed] [Google Scholar]
- Molchan SE, Sunderland T, McIntosh AR, Herscovitch P, Schreurs BG (1994): A functional anatomical study of associative learning in humans. Proc Natl Acad Sci USA 91:8122–8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan BC, Freeman JH (2006): Purkinje cell loss by OX7‐saporin impairs acquisition and extinction of eyeblink conditioning. Learn Mem 13:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Andreasen NC, Liu D, Freeman JH, Ponto LL, O'Leary DS (2012): Eyeblink conditioning in healthy adults: A positron emission tomography study. Cerebellum 11:946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD (1993): Cerebellar cortex lesions disrupt learning‐dependent timing of conditioned eyelid responses. J Neurosci 13:1708–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plakke B, Freeman JH, Poremba A (2007): Metabolic mapping of the rat cerebellum during delay and trace eyeblink conditioning. Neurobiol Learn Mem 88:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Toni I, Josephs O, Ashburner J, Passingham RE (2000): Learning‐ and expectation‐related changes in the human brain during motor learning. J Neurophysiol 84:3026–3035. [DOI] [PubMed] [Google Scholar]
- Rivera SM, Reiss AL, Eckert MA, Menon V (2005): Developmental changes in mental arithmetic: Evidence for increased functional specialization in the left inferior parietal cortex. Cereb Cortex 15:1779–1790. [DOI] [PubMed] [Google Scholar]
- Saksena S, Husain N, Malik GK, Trivedi R, Sarma M, Rathore RS, Pandey CM, Gupta RK (2008): Comparative evaluation of the cerebral and cerebellar white matter development in pediatric age group using quantitative diffusion tensor imaging. Cerebellum 7:392–400. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Bahro M, Molchan SE, Sunderland T, McIntosh AR (2001): Interactions of prefrontal cortex during eyeblink conditioning as a function of age. Neurobiol Aging 22:237–246. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, McIntosh AR, Bahro M, Herscovitch P, Sunderland T, Molchan SE (1997): Lateralization and behavioral correlation of changes in regional cerebral blood flow with classical conditioning of the human eyeblink response. J Neurophysiol 77:2153–2163. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (2003): Mapping cortical change across the human life span. Nat Neurosci 6:309–315. [DOI] [PubMed] [Google Scholar]
- Spence KW, Ross LE (1959): A methodological study of the form and latency of eyelid responses in conditioning. J Exp Psychol 58:376–381. [DOI] [PubMed] [Google Scholar]
- Spencer RM, Verstynen T, Brett M, Ivry R (2007): Cerebellar activation during discrete and not continuous timed movements: An fMRI study. Neuroimage 36:378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ME, Claflin DI, Herbert J (2010): Ontogeny of multiple memory systems: eyeblink conditioning in rodents and humans In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience.New York:Oxford University Press; pp501–526. [Google Scholar]
- Stanton ME, Fox GD, Carter CS (1998): Ontogeny of the conditioned eyeblink response in rats: Acquisition or expression? Neuropharmacology 37:623–632. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Freeman JH Jr, Skelton RW (1992): Eyeblink conditioning in the developing rat. Behav Neurosci 106:657–665. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Rosen DJ, Chapman PF, Lavond DG, Thompson RF (1986): Classical conditioning of the rabbit eyelid response with a mossy‐fiber stimulation CS: I. Pontine nuclei and middle cerebellar peduncle stimulation. Behav Neurosci 100:878–887. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD (2010): An fMRI study of intra‐individual functional topography in the human cerebellum. Behav Neurol 23:65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJ (2012): Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med 68:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kouwe AJ, Benner T, Salat DH, Fischl B (2008): Brain morphometry with multiecho MPRAGE. Neuroimage 40:559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff‐Pak DS, Thompson RF (1988): Classical conditioning of the eyeblink response in the delay paradigm in adults aged 18–83 years. Psychol Aging 3:219–229. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ (1992): Cerebellar cortex and eyeblink conditioning: A reexamination. Exp Brain Res 88:623–638. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ, Glickstein M (1984): Discrete lesions of the cerebellar cortex abolish the classically conditioned nictitating membrane response of the rabbit. Behav Brain Res 13:261–266. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ, Glickstein M (1985): Classical conditioning of the nictitating membrane response of the rabbit. II. Lesions of the cerebellar cortex. Exp Brain Res 60:99–113. [DOI] [PubMed] [Google Scholar]


