Abstract
Thin filament associated proteins such as calponin, caldesmon, tropomyosin and smoothelin are thought to regulate acto-myosin interaction and thus, muscle contraction. However, the effect of inflammation on the expression of thin filament-associated proteins is not known. The aim of the present study is to determine the changes in the expression of calponin, caldesmon, tropomyosin, and smoothelin in colonic smooth muscle from TNBS- and DSS-induced colitis in mice. Expression of h-caldesmon, h2-calponin, α-tropomyosin and smoothelin-A was measured by qRT-PCR, and western blot. Contraction in response to acetylcholine in dispersed muscle cells was measured by scanning micrometry. mRNA and protein expression of α-actin, h2-calponin, h-caldesmon, smoothelin and α-tropomyosin in colonic muscle strips from mice with TNBS- or DSS-induced colitis was significantly increased compared to control animals. Contraction in response to acetylcholine was significantly decreased in muscle cells isolated from inflamed regions of TNBS- or DSS-treated mice compared to control mice. Our results show that increase in the expression of thin-filament associated contractile proteins, which inhibit acto-myosin interaction, could contribute to decrease in smooth muscle contraction in inflammation.
INTRODUCTION
The smooth muscle cells of the gastrointestinal tract are the final effectors of force development and work. The main contractile apparatus in the smooth muscle consists of two types of filaments: thin filaments and thick filaments [1–6]. Thin filaments consist of actin, a ~42 kDa protein which exists in vivo as filamentous actin (F-actin), and associated proteins such as caldesmon, calponin, tropomyosin and smoothelin. Thick filaments are aggregates of myosin molecules. The interaction of actin with myosin and subsequent hydrolysis of ATP is the fundamental reaction whereby chemical energy is converted into mechanical energy. An essential step in smooth muscle contraction is phosphorylation of the 20-kDa regulatory light chains (MLC20) at Ser19, which increases significantly the actin-activated myosin ATPase activity [1, 4]. Phosphorylation and dephosphorylation MLC20 are directly correlated to smooth muscle contraction and relaxation, respectively, and MLC20 phosphorylation levels are regulated by MLC kinase (MLCK) and MLC phosphatase (MLCP) activity. Thus, the amount of force depends on mechanisms that regulate MLC20 phosphorylation via MLCK and MLCP and/or the mechanisms that regulate acto-myosin interaction via thin-filament associated proteins [1–6].
Both in vivo and in vitro studies in patients and animal models of colitis support the idea that colitis is accompanied by an altered contractility from the inflamed area [7–9]. The mechanisms underlying the colonic dysmotility are complex and multiple and include: changes in enterochromaffin cell number and 5-HT release, enteric neurotransmission [10–14], afferent sensory input [15], interstitial cells of Cajal [16] and abnormalities of the effector smooth muscle itself [17–24]. The changes in the functional response of the smooth muscle are reported to be dependent on the cytokine pattern in response to inflammation [25–28]. Cytokines derived from T lymphocytes, among other things, drive the inflammatory response and the pattern of cytokines produced differs due to genetic background [29–35]. T helper (Th)1 cytokines (interferon (INF)-γ and interleukin (IL)-2) predominate in C57BL/6 mice, whereas Th2 cytokines (IL-4 and IL-5) predominate in Balb/c mice. Thus, C57BL/6 mice are regarded as Th1 dominant mouse strain, whereas Balb/c mice are regarded Th2-domnat mouse strain. Trinitrobenzene sulphonic acid (TNBS)- and dextran sodium sulphate (DSS)-induced colitis in animals are most used and chemically induced models. The immunological responses and clinical signs are different in these models. TNBS-induced induced colitis more closely resembles Crohn’s disease with exaggerated Th1-like responses, whereas DSS-induced colitis more closely resembles with exaggerated Th2-like responses [36–38]. The susceptibility of animals to inflammatory responses differs due to genetic background. Balb/c mice are susceptible to Leishmania major infection than C57BL/6 mice [39]. C57BL/6 mice are used before for acute colonic inflammation although they are less susceptible for TNBS-induce colitis than DSS-induced colitis [33].
Previous studies in animal models have shown that increase in Th1 and Th2 immune response is associated with hypocontractility and hypercontractility of smooth muscle, respectively (25–27). The changes in smooth muscle contraction was attributed to changes in the expression of receptors, Ca2+ channels and signaling molecules that regulate MLC20 phosphorylation [18, 40–49]. In the present study we used both TNBS- and DSS-induced colitis models in C57BL/c mice to test the hypothesis that expression of contractile proteins in colonic smooth muscle are altered during inflammation. Our results demonstrate that the expression of h2-calponin, h-caldesmon, smoothelin and α-tropomyosin is upregulated in colonic circular smooth muscle from TNBS- or DSS-treated mice.
MATERIALS AND METHODS
Induction of Colitis and Preparation of Tissue
The technique for induction of colitis with 2,4,6 trinitrobenzene sulphonic acid (TNBS) (Sigma, St. Louis, MO) was as described previously [21]. Briefly, 6- to 8-weeks old male adult C57BL/6J (Charles River, Wilmington, MA) mice were fasted for 24 h, lightly anesthesized with isoflurane, and 100 μl of TNBS solution (2.5% in 50% ethanol (v/v)) was injected via a catheter advanced to 3 cm proximal to the anus via 1 ml syringe fitted with a catheter. In order to distribute the TNBS within the colon, the mouse was kept in a vertical position with the head downwards for 1 min after the injection. Age-matched control mice were treated with vehicle. Mice were euthanized 3 days after the induction of inflammation. For dextran sulphate sodium salt (DSS) induced colitis, 6- to 8-weeks old male adults were administered 5% w/v DSS (MW = 36 000–50 000 Da, MP Biomedicals LLC, Solon, OH,) ad libitum in the drinking water for 5 days [19], and control mice received regular tap water. All mice were checked daily for loss of body weight, stool consistency and the presence of gross bleeding. Colonic tissue from mice treated with TNBS or DSS exhibited typical histological characteristics of colitis [19,21,44]. Macroscopic examination of the distal colon and rectum 3 days after TNBS administration or 5-days after DSS treatment revealed multiple mucosal erosions and ulcerations which were in sharp contrast with the appearance of the normal colon excised from control animals with intact smooth mucosa. Parameters such as weight loss (0 points = no weight loss to 5 points =more than 15% weight loss), stool consistency (0=normal to 5=watery diarrhoea) and bleeding (0=no bleeding, 2 points slight bleeding, 5 points gross bleeding) were assessed to calculate disease activity index as total of all three scores. DAI for TNBS was 6±2 for DSS was 8±3.
Two to three centimetre-long segments of the distal colon (starting from ~0.5 cm oral to the pelvic flexure) were obtained, opened along the mesenteric border, immediately examined for macroscopic lesions with the naked eye, and pinned flat in a petri dish with Sylgard base. The mucosal/submucosal layers were removed by microdissection and the muscularis propria was quick frozen in liquid nitrogen and homogenized with a chilled pestle for protein and RNA extractions.
Preparation of Dispersed Smooth Muscle Cells
Colonic circular muscle dissected free of mucosa as described above was incubated for 20 min in a smooth muscle buffer [NaCl 120 mM, KCl 4 mM, KH2PO4 2.6 mM, CaCl2 2.0 mM, MgCl2 0.6 mM, HEPES 25 mM, glucose 14 mM, and essential amino mixture 2.1% (pH 7.4)] at 31 °C containing 0.1% collagenase (300 U/ml) and 0.01% soybean trypsin inhibitor (w/v) (Worthington, Freehold, NJ) [18,50]. The partly digested tissues were washed twice with 50-ml of collagenase-free smooth muscle buffer and the muscle cells were allowed to disperse spontaneously for 30 min in collagenase-free medium. Cells were harvested by filtration through 500 μm Nitex and centrifuged twice at 350 g for 10 min to eliminate broken cells and organelles. The cells were counted in a hemocytometer and it is estimated that 95% of the cells excluded trypan blue. The experiments were done within 2–3 h of cell dispersion.
Quantitative Real-Time RT-PCR Analysis (qRT-PCR)
Total RNA was isolated from mucosa-free colonic muscle strips with TRIzol® reagent (Invitrogen, Grand Island, NY) and then treated with TURBO DNase (Ambion, Austin, TX). RNA from each preparation was reversely transcribed using the SuperScript™ II systemcontaining 50 mM Tris–HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol (DTT), 0.5 mM deoxynucleoside triphosphates (dNTP), 2.5 μM random hexamers and 200 units of reverse transcriptase in a 20 μl reaction volume. Quantitative RT-PCR was performed on cDNA samples using specific primers designed from known sequences in mouse and TaqMan gene expression master mix (Applied Biosystem, Grand Island, NY) or Quantitect™ SYBRgreen PCR Mastermix (Qiagen, Mississauga, ON). The target gene copy number is quantified by measuring threshold cycle parameter (Ct), defined as fractional cycle at which the fluorescence generated by cleavage of probe passes a fixed threshold above the base line, and by using a standard curve to determine the starting copy number. The primers are designed to satisfy the requirements for use of the 2−ΔΔCtquantification method and normalize to GAPDH expression [51]. Final results are expressed as fold difference in expression in TNBS- or DSS-treated samples relative to control samples. All PCR reactions were performed in an ABI StepOne Plus PCR (Applied Biosystems, Foster City, CA). The sequences of specific primers are listed in the results section. The ABI sequence-detection software and Microsoft Excel were used to calculate the quantities of the mRNAs [18,51].
Western Blot Analysis
Frozen mucosa-free colonic tissues (100 mg) were pulverized while immersed in liquid nitrogen using a mortar. The muscle powder was collected into a glass tube and homogenized in buffer containing 20% glycerol, 50 mM tris-HCl (pH 6.8), 0.5% (volume per volume) Tween-20 (BioRad, Hercules, CA) and protease inhibitors (0.5 mM phenylmethanesulfonyl fluoride, 2 μM pepstatin, 2 μM antipain and 0.1 mg/ml trypsin inhibitor). The homogenate was centrifuged and the supernatant was collected. Equal amounts (40 μg) of total protein from control, TNBS and DSS treated mice colonic muscle strips were loaded on 10% SDS-PAGE gels and transferred to nitrocellulose membranes (BioRad, Hercules, CA). Membranes were subsequently blocked in blocking buffer for 90 min at room temperature followed by incubation overnight at 4°C with primary antibodies [h-caldesmon (1:1000), h1-calponin (1:1000), α-tropomyosin (1:1000), and smoothelin (1:1000);] (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). After three 10-min washes with TBS-Tween 20, membranes were incubated with horseradish peroxidase-labelled secondary antibodies (1:5000) at room temperature for 90 min, followed by an additional three washes with 0.1% TBST. Bands were subsequently visualized on film using SuperSignal Femto maximum sensitivity substrate kit (Pierce, USA) and analysed by densitometry [18,50]. Densitometric values for protein expression are expressed in arbitrary units after normalization to β-actin.
Measurement of Contraction in Dispersed Smooth Muscle Cells
Contraction in freshly dispersed colonic circular smooth muscle cells was determined by scanning micrometry as previously described [18, 50]. An aliquots (0.4 ml) of cells containing approximately 104 cells/ml was treated with 100 μl of HEPES buffer containing different concentrations of acetylcholine (ACh; 10 pm to 1 μM) for 30 s and the reaction was terminated with 1% acrolein at a final concentration of 0.1%. The resting cell length was determined in control experiments in which muscle cells were incubated with 100 μl of buffer without the ACh. The mean lengths of 50 muscle cells treated with various agonists was measured by scanning micrometry and compared with the mean lengths of untreated cells. The contractile response was expressed as the percent decrease in mean cell length from control cell length.
Statistical Analysis
The results were expressed as means ± SE of n experiments and analyzed for statistical significance using Student’s t-test for paired and unpaired values. Each experiment was done on cells obtained from different animals. Five mice of each group were studied; qRT-PCR and western blot were performed in duplicate. A probability of p < 0.05 was considered significant.
RESULTS
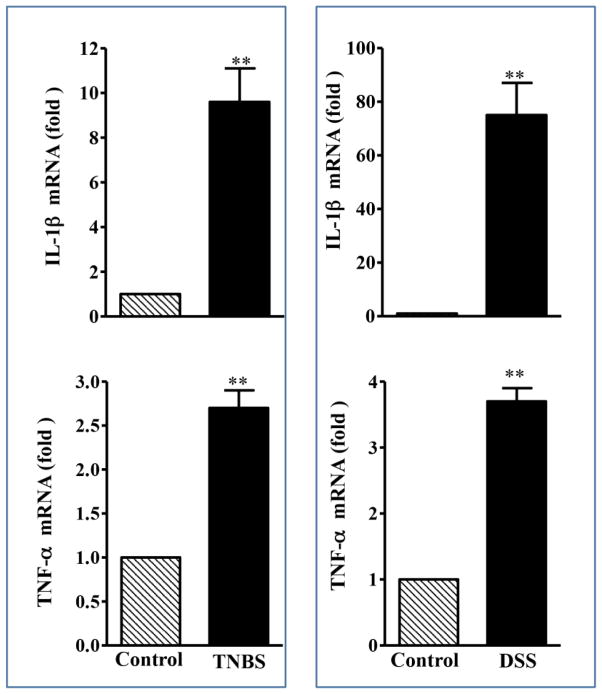
In the present study, we analyzed the changes in the expression of caldesmon, calponin, tropomyosin and smoothelin-A by qRT-PCR and western blot in smooth muscle of colon isolated from control mice and mice in which colitis was induced with TNBS- and DSS. IL-1β or TNF-α expression was measured by qRT-PCR using specific primers. Expression of IL-1β was significantly increased (IL1B:10-fold and 75-fold with TNBS and DSS, respectively, p<0.001, n=5; TNF-α: 3-fold and 4-fold with TNBS and DSS, respectively, p<0.01, n=5) in inflamed colonic tissue compared to control tissue (Fig. 1). These results strongly suggested that TNBS- or DSS-treatment results in the generation of proinflammatory cytokines.
Fig. 1. Expression of IL-1β and TNF-α in the colonic muscle after TNBS or DSS treatment.
Total RNA was isolated from smooth muscle strips of control and TNBS or DSS treated mice using RNAqueous prep kits (Ambion, Austin, TX) and was reverse transcribed using 2 μg of total RNA using qScript cDNA prep kits (Quanta, Gaithersburg, MD). The cDNA was amplified with specific primers for IL-1β or TNF α. The sequences of specific primers are listed in Table 1 and 2. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to measure RNA levels of IL-1β or TNF α. For each cDNA sample, real-time PCR was conducted in a 20-μl reaction volume containing Quantitect™ SYBRgreen PCR Mastermix (Qiagen, Mississauga, ON). Real-time PCR reactions were performed in triplicate. Results are expressed as fold differences in IL-1β or TNF α gene expression in TNBS- or DSS-treated colon relative to that in vehicle-treated colon. Values represent the means ±SEM of 5 separate experiments. **p<0.001 versus control.
Changes in the Expression of Thin-Filament Associated Proteins in TNBS-Colitis Mice
Caldesmon
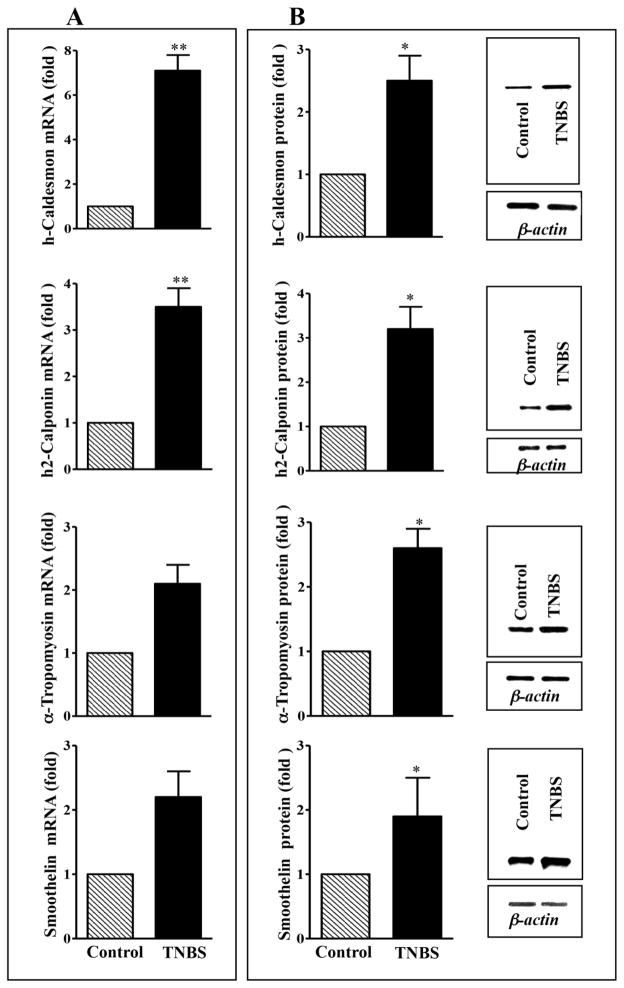
Two caldesmon isoforms (h-caldesmon and l-caldesmon) have been described. The h-caldesmon isoform is smooth muscle specific, whereas l-caldesmon is a non-muscle isoform [1, 4]. Expression of h-caldesmon mRNA was 7-fold higher (p<0.001) in colonic muscle cells from TNBS mice compared to control mice (Fig. 2A), whereas expression of h-caldesmon protein was 2.5-fold higher (p<0.05) in muscle from TNBS mice compared to control mice (Fig. 2B).
Fig. 2. Expression of h-caldesmon, h2-calponin α-tropomyosin and smoothelin in the colon of control and TNBS-treated mice.
(A) mRNA expression. Total RNA isolated from smooth muscle strips of control and inflamed colonic regions of TNBS-treated mice using RNAqueous prep kit and was reverse transcribed using 2 μg of total RNA using qScript cDNA prep kits. The cDNA was amplified with specific primers for h-caldesmon, h2-calponin, α-tropomyosin and smoothelin. The sequences of specific primers are listed in Table 1. For each cDNA sample, real-time PCR was conducted in a 20 μl reaction volume containing Taqman Gene expression PCR Mastermix. Real-time PCR reactions were performed in triplicate. Results are expressed as fold differences in h-caldesmon, h2-calponin, α-tropomyosin and smoothelin gene expression in TNBS-treated colon relative to that in control colon. Values represent the means ±SEM of 5 separate experiments. **p<0.001 versus control. (B) Protein expression. Colonic muscle tissue lysates containing equal amounts of total proteins were separated with SDS-PAGE and expression of h-caldesmon, h2-calponin, α-tropomyosin and smoothelin was analyzed using selective antibody. Densitometric values for protein expression are expressed in arbitrary units after normalization to β-actin. Results are expressed as fold differences in h-caldesmon, h2-calponin, α-tropomyosin and smoothelin protein expression in TNBS-treated colon relative to that in control colon. Values represent the means ±SEM of 5 separate experiments. *p<0.05 versus control.
Calponin
Three calponin isoforms (h1-acidic, h2-neutral and basic calponin) have been described. The h2-acidic is smooth muscle specific, whereas h2-neutral and basic calponin are non-muscle isoforms [52]. Expression of h2-calponin was measured in colonic muscle of control and TNBS-treated mice. Expression of h2-calponin mRNA was ~4-fold higher (p<0.001) in colonic muscle cells from TNBS mice compared to control mice (Fig. 2A), whereas expression of h2-calponin protein was 3-fold higher (p<0.05) in muscle from TNBS mice compared to control mice (Fig. 2B).
Tropomyosin
Two tropomyosin isoforms (α- and β-tropomyosin) have been described [1, 4]. Expression of α-tropomyosin was measured in colonic muscle of control and TNBS-colitis mice. Expression of α-tropomyosin mRNA was ~2-fold higher in colonic muscle cells from TNBS mice compared to control mice (Fig. 2A), whereas expression of α-tropomyosin protein was 2.5-fold higher (p<0.05) in muscle from TNBS mice compared to control mice (Fig. 2B).
Smoothelin
Two smoothelin isoforms (Smoothelin-A and -B) have been described. Expression of smoothelin is specific to visceral smooth muscle, whereas smoothelin-B is specific to vascular smooth muscle [53]. Smoothelin was measured in colonic muscle of control and TNBS-treated mice using PCR primers and antibody that does not distinguish the isoforms. Expression of long isoform was not detected in colonic smooth muscle. Expression of smoothelin mRNA was ~2-fold higher in colonic muscle cells from TNBS mice compared to control mice (Fig. 2A), whereas expression of smoothelin protein was 2- fold higher (p<0.05) in muscle from TNBS mice compared to control mice (Fig. 2B).
Changes in the Expression of Thin-Filament Associated Proteins in DSS-Colitis Mice
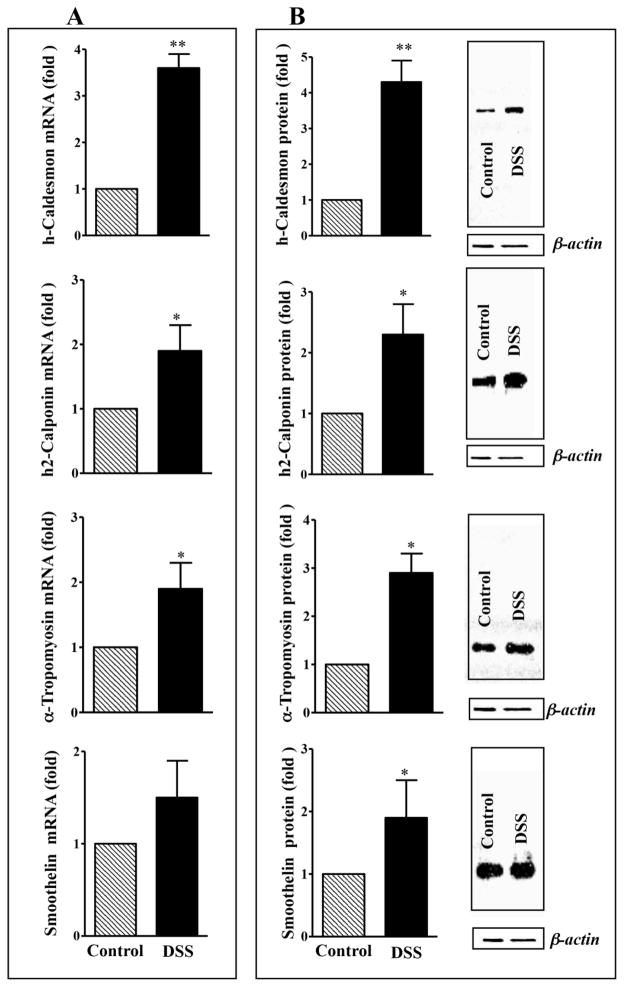
Analysis of mRNA expression in another model of colitis also revealed higher expression of h-caldesmon (~4-fold; p<0.001), h2-calponin (~2-fold; p<0.05), α-tropomyosin (2-fold; p<0.05), and smoothelin (~2-fold; p<0.05) in colonic muscle from inflamed regions of DSS treated mice compared to control mice (Fig. 3A). Similar increase in proteins expression was observed with h-caldesmon (4-fold; p<0.001), h2calponin (~2-fold; p<0.05), α-tropomyosin (~3-fold; p<0.05), and smoothelin (~2-fold; p<0.05) in colonic muscle from inflamed regions of DSS treated mice compared to control mice (Fig. 3B). These results suggest that inflammation induced by either TNBS or DSS treatment had similar effect on the expression of thin-filament associated proteins.
Fig. 3. Expression of h-caldesmon, h2-calponin α-tropomyosin and smoothelin in the colon of control and DSS-treated mice.
(A) mRNA expression. Total RNA isolated from smooth muscle strips of control and inflamed colonic regions of DSS-treated mice using RNAqueous prep kit and was reverse transcribed using 2 μg of total RNA using qScript cDNA prep kits. The cDNA was amplified with specific primers for h-caldesmon, h2-calponin, α-tropomyosin and smoothelin. The sequences of specific primers are listed in Table 1. For each cDNA sample, real-time PCR was conducted in a 20 μl reaction volume containing Taqman Gene expression PCR Mastermix. Real-time PCR reactions were performed in triplicate. Results are expressed as fold differences in h-caldesmon, h2-calponin, α-tropomyosin and smoothelin gene expression in DSS-treated colon relative to that in control colon. Values represent the means ±SEM of 5 separate experiments. **p<0.001 versus control. (B) Protein expression. Colonic muscle tissue lysates containing equal amounts of total proteins were separated with SDS-PAGE and expression of h-caldesmon, h2-calponin, α-tropomyosin and smoothelin was analyzed using selective antibody. Densitometric values for protein expression are expressed in arbitrary units after normalization to β-actin. Results are expressed as fold differences in h-caldesmon, h2-calponin, α-tropomyosin and smoothelin protein expression in DSS-treated colon relative to that in control colon. Values represent the means ±SEM of 5 separate experiments. *p<0.05 versus control.
Effect of Inflammation on Isolated Smooth Muscle Cell Contraction
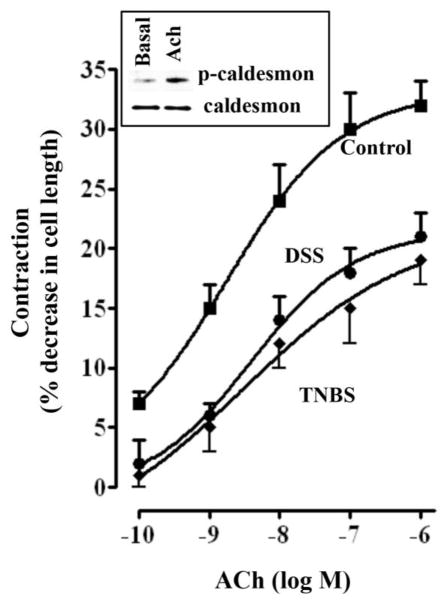
Previous studies have shown that acetylcholine-induced contraction of smooth muscle from rabbit rectosigmoid colon was associated with an increase in the phosphorylation of caldesmon at Ser789 [54]. Treatment of colonic muscle cells from mouse colon caused an increase in phosphorylation of caldesmon as determined by immunoblot using phosphor-specific antibody (abcam- ab58498) (Fig. 4). However, the contribution of caldesmon phosphorylation to acetylcholine-induced muscle contraction was not examined in this study.
Fig. 4. Effect of TNBS and DSS treatment on ACh-induced smooth muscle cell contraction.
Contraction of dispersed muscle cells from colon was measured by scanning micrometry in response to different concentrations of ACh. Cells were treated with ACh for 30s and contraction was expressed as percent decrease in cell length from basal cell length: basal length of muscle cells from control mice 95±4 μm; basal control length of muscle cells from TNBS-colitis mice 91±5 μm and from DSS-treated mice 89±6 μm. ACh caused contraction that was concentration-dependent in control mice that was significantly attenuated in cells from TNBS-or DSS-treated mice. The maximal response to 0.1 μM of ACh was 32±2% decrease in cell length in control mice, 20±3% decrease in cell length in TNBS-treated mice and 21±4% decrease in DSS-treated mice. Values represent the means ±SEM of 5 separate experiments. Inset: Phosphorylation of caldesmon. Cells were treated with Ach ((1 μM) for 30 s and phosphorylation of caldesmon was examined by immunoblot using phosphor-specific (Ser789) antibody.
To determine whether changes in the expression of thin-filament proteins was associated with changes in agonist-induced contraction, freshly dispersed colonic muscle cells from control mice and inflamed regions of TNBS- or DSS-treated mice were treated with different concentrations of acetylcholine and decrease in muscle cell length was measured by scanning micrometry. Basal colonic muscle cell lengths were similar in control (95±4 μm), TNBS-treated (91±5 μm) or DSS-treated (89±4 μm) mice. ACh caused contraction that was concentration-dependent in colonic muscle cells from control, TNBS- and DSS-treated mice (Fig. 4). However contraction in response to ACh was significantly lower in TNBS- or DSS-treated mice compared to control mice. The maximal response to 0.1 μM ACh was also significantly lower in TNBS-treated (20±32% decrease in cell length) or DSS-treated (21±3% decrease in cell length) compared to control mice (32±3% decrease in cell length).
DISCUSSION
Altered motility induced by colonic inflammation is associated with changes in the function of enteric nervous system (ENS), interstitial cells of Cajal (ICC) and smooth muscle cells [10–14, 17, 19, 20, 22–24]. The smooth muscle cells of the gastrointestinal tract are the final effectors of force development, and thus, it is important to understand the changes in expression of contractile proteins, which constitute the main contractile apparatus of smooth muscle. In the present study, we examined the effects of colonic inflammation induced by two well-characterized animal models of colitis, TNBS and DSS, on the expression of contractile proteins in colonic smooth muscle by two different approaches to measure the changes in the expression, such as qRT-PCR, and western blot. Biochemically, the colonic inflammation induced by TNBS or DSS was characterized by complex patterns of cytokine expression [29]. Acute DSS colitis is characterized by monokine (IL-6, TNF-α) and Th17 (IL-17) profile and by Th2 (IL-4 and IL-10) profile as the disease becomes chronic. Acute TNBS colitis was characterized by Th1 (IL-12, INF-γ) profile and with enhanced Th1/Th17 profile as the disease becomes chronic.
Our main findings are: 1) expression of thin-filament associated proteins such as α-tropomyosin, smoothelin, h2-calponin and h-caldesmon are increased, 2) contraction induced by acetylcholine in isolated smooth muscle cells is decreased by colitis, and 3) the changes in the expression of contractile proteins is observed at both protein and mRNA levels and the extent of changes in the expression of contractile proteins is similar in both TNBS- and DSS-induced colitic models. The present investigation of the response of the contractile proteins to inflammation may provide further insights into the mechanism by which inflammation modifies contractile proteins and suggests that inflammation may contribute to the regulation of the expression of genes responsible for synthesis of smooth muscle contraction proteins. Chronic inflammation of the colon also induces both hyperplasia and hypertrophy of the muscle leading to alterations in the cellular architecture. Increase in insulin-like growth factor 1 (IGF-1) and cell proliferation, decrease in apoptosis resulting in hyperplasia, hypertrophy and fibrosis was reported in Th1/Th17-dominated inflammation [21, 51].
Although TNBS and DSS are shown to induce inflammation via distinct inflammatory mediators, our studies demonstrated that in both models the changes in contractile protein expression and agonist-mediated colonic muscle contraction are similar. Previous studies have shown that Th1 and Th2 immune response elicit opposing effects on muscle contraction. Treatment of muscle trips or cells with TNF-α/IL-1β in vitro or increase in Th1-like cytokine in vivo cause decrease in muscle contraction, whereas treatment with IL-4/IL-13 or increase in Th2-cytokines in vivo cause increase in contraction. IL-13 increased muscarinic receptor affinity, whereas IFN-γ (Th1 cytokine) decreased the muscarinic affinity [40]. Decrease in contraction was attributed to down-regulation of L-type Ca2+ channels [19, 20, 23,24], CPI-17 [45], or regulator of G protein signaling 4 (RGS4) [18]. Increase in contraction was attributed to: 1) increase in the expression receptors such as muscarinic m3 receptors [40], protease-activated receptors 1 (PAR1) [41] and 5-HT2a [42] receptors via activation of signal transduces and activator of transcription 6 (STAT), and 2) increase in MAP kinase pathways [46]. Increase in IL-33, which increase Th2 immune responses and decrease Th1 immune responses, also causes increase in contraction in response to acetylcholine probably via STAT6 pathway [55]. The effect of inflammation on muscle contraction also appears to be tissue- and species-specific dependent. TNBS-induced inflammation in guinea pig caused hypercontraction in response to carbachol and histamine in longitudinal smooth muscle, whereas it caused hypocontraction in circular muscle [56].
Contraction of smooth muscle is determined by mechanisms that regulate the availability of actin to interact with myosin via the action of inhibitory actin binding proteins such as caldesmon and calponin [1, 4, 52]. These thin-filament associated proteins are capable of stabilizing actin filaments. Caldesmon is a highly conserved, actin and myosin binding protein which inhibits acto-myosin interaction; the inhibitory effect is reversed by binding of Ca2+/CaM or by phosphorylation via extracellular signal-regulated kinase1/2 (ERK1/2) [1, 4]. Calponin, an actin binding protein also inhibits acto-myosin ATPase activity and the inhibitory effect is reversed by phosphorylation via ERK1/2 or protein kinase C (PKC). Thus caldesmon and calponin are important regulators of smooth muscle contraction [1, 4, 52, 54]. Although studies suggest that tropomyosin is necessary for full inhibition of acto-myosin activity by caldesmon, the exact mechanism of action is unclear [1, 4]. The data presented in the current study reveal an association between increases in the expression of thin-filament associated proteins, especially, caldesmon, calponin and tropomyosin in response to inflammation and decreased muscle contraction. The cytoskeleton modifications due to increased expression of thin-filament associated proteins may also have an effect on smooth muscle contraction. Thus, our study in combination with previous studies proposes that in colitis, several mechanisms result in attenuated muscle contraction. Firstly, a lower expression and/or activity of L-type Ca2+ channels result in decrease in intracellular Ca2+ and inhibition of Ca2+/CaM-dependent MLCK activity [17, 19, 20, 24]. Secondly, a lower expression of CPI-17, an endogenous inhibitor of MLCP, results in stimulation of MLCP activity [18, 45, 57]. Thirdly, an increase in the expression of caldesmon and calponin results in attenuation of acto-myosin interaction. Further investigations might reveal whether the proposed mechanisms are dependent on each other or occur independently, and whether a singular mechanism is sufficient to produce altered muscle contraction.
Smoothelin is an actin binding protein and its expression is abundant in contractile smooth muscle cells and limited in non-contractile proliferative smooth muscle cells. Recent studies using transgenic mice suggest expression of smoothelin-A is important for slow wave activity and agonist-mediated contraction of gastrointestinal smooth muscle [53]. The increase in the expression of smoothelin in colitis suggests that it may be a compensatory mechanism to offset the inhibitory effects exerted by increased expression of caldesmon and calponin on smooth muscle contraction.
The results also suggest that up-regulation of thin-filament associated proteins is due to changes in the transcriptional regulation of these proteins, probably via the direct effect of proinflammatory cytokines on the transcription factors. Previous studies demonstrated that exposure of cultured muscle cells to IL-1β or TNF-α caused change in the transcriptional regulation of several proteins involved in smooth muscle contraction [18, 22, 23, 24]. The decrease in contraction on exposure of smooth muscle to IL-1β or TNF-α was reversed by an inhibitor of nuclear factor-kappa B (NF-κB), suggesting that the cytokine effects are mediated mainly by NF-κB. Future studies on the animal models combined with studies on the smooth muscle cultures exposed to proinflammatory cytokines should provide valuable information on the direct effects of cytokines on the smooth muscle contractile proteins. Our studies in isolated muscle cells also demonstrate that contraction induced by acetylcholine is decreased in both model of colitis. Clearly, changes in muscle contraction observed in vitro should be extrapolated into the in vivo situation with caution. Consideration must be given to the important roles mediated by enteric nerves, the ICC, and other endocrine, paracrine and autocrine factors.
CONCLUSION
The results of the present study demonstrated that colitis augmented the expression of h-caldesmon, h2-calponin, α-tropomyosin and smoothelin and that the decrease in the contraction of smooth muscle isolated from the colitic mouse colon is attributable to the increase in thin-filament associated proteins. Whether the changes in the expression of smooth muscle markers and the decrease of contraction are the cause or the consequence of disturbed GI tract motility remains to be determined. The changes in smooth muscle contractile proteins, assessed here in inflammation, associate to the functional alterations of the smooth muscle cell, and this was not studied before. Clearly, a comprehensive appraisal of the enteric muscle contractile proteins and signal transduction pathways, in addition to the evaluation of enteric neurons and ICC, may contribute to further characterization of mechanisms underlying gastrointestinal motility disorders.
Table 1.
RT-PCR TaqMan primers for the amplification of thin-filaments associated proteins
| Primer set | Assay ID | Company name | Product size |
|---|---|---|---|
| Cnn1 | Mm00487032- m1 | Applied Biosystems | 59 bp |
| Cald1 | Mm00513995- m1 | Applied Biosystems | 102 bp |
| Tpm2 | Mm00437172- g1 | Applied Biosystems | 97 bp |
| Smtn | Mm00449973- m1 | Applied Biosystems | 66 bp |
| GAPDH | NM-008084.2 | Applied Biosystems | 107 bp |
Table 2.
RT-PCR SYBRgreen primers for the amplification of thin-filaments associated proteins
| Primer set | Forward 5′→3′ | Reverse 3′→5′ | Product size |
|---|---|---|---|
| IL-1β | TACCTGTGGCCTTGGGCCTCAA | GCTTGGGATCCACACTCTCCAGCT | 99 bp |
| TNF-α | ATGGCCCAGACCCTCACACTCAG | TTGGTGGTTTGCTACGACGTGGG | 81 bp |
| GAPDH | AGAAACCTGCCAAGTATGATG | GGAGTTGCTGTTGAAGTCG | 122 bp |
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants (DK28300 and DK15564 to KS Murthy).
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- 1.Haeberle JR. Thin-filament linked regulation of smooth muscle myosin. J Muscle Res Cell Motil. 1999;20:363–370. doi: 10.1023/a:1005408402323. [DOI] [PubMed] [Google Scholar]
- 2.Hartshorne DJ, Ito M, Erdödi F. Role of protein phosphatase type 1 in contractile functions: myosin phosphatase. J Biol Chem. 2004;279:37211–37214. doi: 10.1074/jbc.R400018200. [DOI] [PubMed] [Google Scholar]
- 3.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001;276:4527–4530. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- 4.Morgan KG, Gangopadhyay SS. Invited review: cross-bridge regulation by thin filament-associated proteins. J Appl Physiol. 2001;91:953–962. doi: 10.1152/jappl.2001.91.2.953. [DOI] [PubMed] [Google Scholar]
- 5.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–374. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- 6.Somlyo AP, Somlyo AV. Signal transduction through the RhoA/Rho-kinase pathway in smooth muscle. J Muscle Res Cell Motil. 2004;25:613–615. doi: 10.1007/s10974-004-3146-1. [DOI] [PubMed] [Google Scholar]
- 7.Mawe GM, Collins SM, Shea-Donohue T. Changes in enteric neural circuitry and smooth muscle in the inflamed and infected gut. Neurogastroenterol Motil. 2004;16:133–136. doi: 10.1111/j.1743-3150.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- 8.Neunlist M, Aubert P, Toquet C, Oreshkova T, Barouk J, Lehur PA, Schemann M, Galmiche JP. Changes in chemical coding of myenteric neurones in ulcerative colitis. Gut. 2003;52:84–90. doi: 10.1136/gut.52.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Hara JR, Lomax AE, Mawe GM, Sharkey KA. Ileitis alters neuronal and enteroendocrine signalling in guinea pig distal colon. Gut. 2007;56:186–194. doi: 10.1136/gut.2006.102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costedio MM, Hyman N, Mawe GM. Serotonin and its role in colonic function and in gastrointestinal disorders. Dis Colon Rectum. 2007;50:376–388. doi: 10.1007/s10350-006-0763-3. [DOI] [PubMed] [Google Scholar]
- 11.Frieling T, Palmer JM, Cooke HJ, Wood JD. Neuroimmune communication in the submucous plexus of guinea pig colon after infection with Trichinella spiralis. Gastroenterology. 1994;107:1602–1609. doi: 10.1016/0016-5085(94)90798-6. [DOI] [PubMed] [Google Scholar]
- 12.Grider JR. Interleukin-1 beta selectively increases substance P release and augments the ascending phase of the peristaltic reflex. Neurogastroenterol Motil. 2003;15:607–615. doi: 10.1046/j.1350-1925.2003.00445.x. [DOI] [PubMed] [Google Scholar]
- 13.Mawe GM, Strong DS, Sharkey KA. Plasticity of enteric nerve functions in the inflamed and postinflamed gut. Neurogastroenterol Motil. 2009;21:481–491. doi: 10.1111/j.1365-2982.2009.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poli E, Lazzaretti M, Grandi D, Pozzoli C, Coruzzi G. Morphological and functional alterations of the myenteric plexus in rats with TNBS-induced colitis. Neurochem Res. 2001;26:1085–1093. doi: 10.1023/a:1012313424144. [DOI] [PubMed] [Google Scholar]
- 15.Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis JB, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24:4300–4312. doi: 10.1523/JNEUROSCI.5679-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rumessen JJ. Ultrastructure of interstitial cells of Cajal at the colonic submuscular border in patients with ulcerative colitis. Gastroenterol. 1996;111:1447–1455. doi: 10.1016/s0016-5085(96)70005-7. [DOI] [PubMed] [Google Scholar]
- 17.Akbarali HI, Hawkins GE, Ross GR, Kang M. Ion channel remodeling in gastrointestinal inflammation. Neurogastroenterol Motil. 2010;22:1045–1055. doi: 10.1111/j.1365-2982.2010.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu W, Li F, Mahavadi S, Murthy KS. Interleukin-1beta up-regulates RGS4 through the canonical IKK2/IkappaBalpha/NF-kappaB pathway in rabbit colonic smooth muscle. Biochem J. 2008;412:35–43. doi: 10.1042/BJ20080042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang M, Morsy N, Jin X, et al. Protein and gene expression of Ca2+ channel isoforms in murine colon: effect of inflammation. Pflugers Arch. 2004;449:288–297. doi: 10.1007/s00424-004-1339-5. [DOI] [PubMed] [Google Scholar]
- 20.Kang M, Ross GR, Akbarali HI. The effect of tyrosine nitration of L-type Ca2+ channels on excitation-transcription coupling in colonic inflammation. Br J Pharmacol. 2010;159:1226–1235. doi: 10.1111/j.1476-5381.2009.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hazelgrove KB, Flynn RS, Qiao LY, Grider JR, Kuemmerle JF. Endogenous IGF-I and alpha v beta3 integrin ligands regulate increased smooth muscle growth in TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1230–G1237. doi: 10.1152/ajpgi.90508.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi XZ, Lindholm PF, Sarna SK. NF-kappa B activation by oxidative stress and inflammation suppresses contractility in colonic circular smooth muscle cells. Gastroenterol. 2003;124:1369–1380. doi: 10.1016/s0016-5085(03)00263-4. [DOI] [PubMed] [Google Scholar]
- 23.Shi XZ, Pazdrak K, Saada N, Dai B, Palade P, Sarna SK. Negative transcriptional regulation of human colonic smooth muscle Cav1.2 channels by p50 and p65 subunits of nuclear factor-kappaB. Gastroenterol. 2005;129:1518–1532. doi: 10.1053/j.gastro.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 24.Shi XZ, Sarna SK. Transcriptional regulation of inflammatory mediators secreted by human colonic circular smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G274–G284. doi: 10.1152/ajpgi.00512.2004. [DOI] [PubMed] [Google Scholar]
- 25.Khan WI, Collins SM. Gut motor function: immunological control in enteric infection and inflammation. Clin Exp Immunol. 2006;143:389–397. doi: 10.1111/j.1365-2249.2005.02979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shea-Donohue T, Notari L, Sunan R, Zhao A. Mechanisms of smooth muscle responses to inflammation. Neurogastroenterol Motil. 2012;24:802–811. doi: 10.1111/j.1365-2982.2012.01986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akiho H, Ihara E, Motomura Y, Nakamura K. Cytokine-induced alterations of gastrointestinal motility in gastrointestinal disorders. World J Gastrointest Pathophysiol. 2011;2:72–81. doi: 10.4291/wjgp.v2.i5.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hakonarson H, Maskeri N, Carter C, Grunstein MM. Regulation of TH1- and TH2-type cytokine expression and action in atopic asthmatic sensitized airway smooth muscle. J Clin Invest. 1999;103:1077–1087. doi: 10.1172/JCI5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341–352. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock. 2004;22:460–466. doi: 10.1097/01.shk.0000142249.08135.e9. [DOI] [PubMed] [Google Scholar]
- 31.De Vooght V, Vanoirbeek JA, Luyts K, Haenen S, Nemery B, Hoet PHM. Choice of mouse strain influences the outcome in a mouse model of chemical-induced asthma. PLoS One. 2010;5:e12581. doi: 10.1371/journal.pone.0012581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taherzadeh Z, VanBavel E, de Vos J, Matlung HL, van Montfrans G, Brewster LM, Seghers L, Quax PHA, Bakker ENTP. Strain-dependent susceptibility for hypertension in mice resides in the natural killer gene complex. Am J Physiol Heart Circ Physiol. 2010;298:H1273–1282. doi: 10.1152/ajpheart.00508.2009. [DOI] [PubMed] [Google Scholar]
- 33.Bushell KN, Leeman SE, Gillespie E, Gower AC, Reed KL, Stucchi AF, Backer JM, Amar S. LITAF mediation of increased TNF-alpha secretion from inflamed colonic lamina propria macrophages. PLoS One. 2011;6:e25849. doi: 10.1371/journal.pone.0025849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen ES, Greenlee BM, Wills-Karp M, Moller DR. Attenuation of lung inflammation and fibrosis in interferon-gamma-deficient mice after intratracheal bleomycin. Am J Respir Cell Mol Biol. 2001;24:545–555. doi: 10.1165/ajrcmb.24.5.4064. [DOI] [PubMed] [Google Scholar]
- 35.Melgar S, Karlsson A, Michaelsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1328–1338. doi: 10.1152/ajpgi.00467.2004. [DOI] [PubMed] [Google Scholar]
- 36.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 37.Gaudio E, Taddei G, Vetuschi A, Sferra R, Frieri G, Ricciardi G, Caprilli R. Dextran sulfate sodium (DSS) colitis in rats: clinical, structural, and ultrastructural aspects. Dig Dis Sci. 1999;44:1458–1475. doi: 10.1023/a:1026620322859. [DOI] [PubMed] [Google Scholar]
- 38.Strober W, I, Fuss J, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 39.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 40.Akiho H, Khan WI, Al-Kaabi A, Blennerhassett P, Deng Y, Collins SM. Cytokine modulation of muscarinic receptors in the murine intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G250–255. doi: 10.1152/ajpgi.00545.2006. [DOI] [PubMed] [Google Scholar]
- 41.Zhao A, Morimoto M, Dawson H, Elfrey JE, Madden KB, Gause WC, Min B, Finkelman FD, Urban JF, Jr, Shea-Donohue T. Immune regulation of protease-activated receptor-1 expression in murine small intestine during Nippostrongylus brasiliensis infection. J Immunol. 2005;175:2563–2569. doi: 10.4049/jimmunol.175.4.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao A, Urban JF, Jr, Morimoto M, Elfrey JE, Madden KB, Finkelman FD, Shea-Donohue T. Contribution of 5-HT2A receptor in nematode infection-induced murine intestinal smooth muscle hypercontractility. Gastroenterology. 2006;131:568–578. doi: 10.1053/j.gastro.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Jin X, Malykhina AP, Lupu F, Akbarali HI. Altered gene expression and increased bursting activity of colonic smooth muscle ATP-sensitive K+ channels in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G274–G285. doi: 10.1152/ajpgi.00472.2003. [DOI] [PubMed] [Google Scholar]
- 44.Ross GR, Kang M, Shirwany N, Malykhina AP, Drozd M, Akbarali HI. Nitrotyrosylation of Ca2+ channels prevents c-Src kinase regulation of colonic smooth muscle contractility in experimental colitis. J Pharmacol Exp Ther. 2007;322:948–956. doi: 10.1124/jpet.107.123075. [DOI] [PubMed] [Google Scholar]
- 45.Sato K, Ohkura S, Kitahara Y, Ohama T, Hori M, Sato M, Kobayashi S, Sasaki Y, Hayashi T, Nasu T, Ozaki H. Involvement of CPI-17 downregulation in the dysmotility of the colon from dextran sodium sulphate-induced experimental colitis in a mouse model. Neurogastroenterol Motil. 2007;19:504–514. doi: 10.1111/j.1365-2982.2007.00911.x. [DOI] [PubMed] [Google Scholar]
- 46.Ihara E, Beck PL, Chappellaz M, Wong J, Medlicott SA, MacDonald JA. Mitogen-activated protein kinase pathways contribute to hypercontractility and increased Ca2+ sensitization in murine experimental colitis. Mol Pharmacol. 2009;75:1031–1041. doi: 10.1124/mol.108.049858. [DOI] [PubMed] [Google Scholar]
- 47.Ihara E, Chappellaz M, Turner SR, MacDonald JA. The contribution of protein kinase C and CPI-17 signaling pathways to hypercontractility in murine experimental colitis. Neurogastroenterol Motil. 2012;24:e15–26. doi: 10.1111/j.1365-2982.2011.01821.x. [DOI] [PubMed] [Google Scholar]
- 48.Akiho H, Blennerhassett P, Deng Y, Collins SM. Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G226–232. doi: 10.1152/ajpgi.2002.282.2.G226. [DOI] [PubMed] [Google Scholar]
- 49.Cao W, Vrees MD, Potenti FM, Harnett KM, Fiocchi C, Pricolo VE. Interleukin 1 beta-induced production of H2O2 contributes to reduced sigmoid colonic circular smooth muscle contractility in ulcerative colitis. J Pharmacol Exp Ther. 2004;311:60–70. doi: 10.1124/jpet.104.068023. [DOI] [PubMed] [Google Scholar]
- 50.Murthy KS, Zhou H, Grider JR, Brautigan DL, Eto M, Makhlouf GM. Differential signalling by muscarinic receptors in smooth muscle: m2-mediated inactivation of myosin light chain kinase via Gi3, Cdc42/Rac1 and p21-activated kinase 1 pathway, and m3-mediated MLC20 (20 kDa regulatory light chain of myosin II) phosphorylation via Rho-associated kinase/myosin phosphatase targeting subunit 1 and protein kinase C/CPI-17 pathway. Biochem J. 2003;374:145–155. doi: 10.1042/BJ20021274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flynn RS, Mahavadi S, Murthy KS, Grider JR, Kellum JM, Akbari H, Kuemmerle JF. Endogenous IGFBP-3 regulates excess collagen expression in intestinal smooth muscle cells of crohn’s disease strictures. Inflamm Bowel Dis. 2011;17:193–201. doi: 10.1002/ibd.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Babu GJ, Celia G, Rhee AY, Yamamura H, Takahashi K, Brozovich FV, Osol G, Periasamy M. Effects of h1-calponin ablation on the contractile properties of bladder versus vascular smooth muscle in mice lacking SM-B myosin. J Physiol. 2006;577:1033–1042. doi: 10.1113/jphysiol.2006.118828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niessen P, Rensen S, van Deursen J, De Man J, De Laet A, Vanderwinden JM, Wedel T, Baker D, Doevendans P, Hofker M, Gijbels M, van Eys G. Smoothelin-A is essential for functional intestinal smooth muscle contractility in mice. Gastroenterology. 2005;129:1592–1601. doi: 10.1053/j.gastro.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 54.Somara S, Bitar KN. Phosphorylated HSP27 modulates the association of phosphorylated caldesmon with tropomyosin in colonic smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2006;291:G630–639. doi: 10.1152/ajpgi.00350.2005. [DOI] [PubMed] [Google Scholar]
- 55.Yang Z, Sun R, Grinchuk V, Blanco JA, Notari L, Bohl JA, McLean LP, Ramalingam TR, Wynn TA, Urban JF, Jr, Vogel SN, Shea-Donohue T, Zhao A. IL-33-induced alterations in murine intestinal function and cytokine responses are MyD88, STAT6, and IL-13 dependent. Am J Physiol Gastrointest Liver Physiol. 2013;304:G381–389. doi: 10.1152/ajpgi.00357.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinolle JP, Garcia-Villar R, Fioramonti J, Bueno L. Altered contractility of circular and longitudinal muscle in TNBS-inflamed guinea pig ileum. 1997;272:G1258–1267. doi: 10.1152/ajpgi.1997.272.5.G1258. [DOI] [PubMed] [Google Scholar]
- 57.Ohama T, Hori M, Sato K, Ozaki H, Karaki H. Chronic treatment with interleukin-1 beta attenuates contractions by decreasing the activities of CPI-17 and MYPT1 in intestinal smooth muscle. J Biol Chem. 2003;278:48794–48804. doi: 10.1074/jbc.M310166200. [DOI] [PubMed] [Google Scholar]