Abstract
Increases in emergency room visits due to abuse of designer drugs, popularly known by the street names “K2” and “Spice,” are a cause for social, judicial, and clinical concerns. The psychoactive components in these herbal drugs mainly consist of different synthetic cannabinoids, and users of these street drugs are primarily within the age group of 12 to 20 years old. The abusive use of synthetic cannabinoids results in anxiety, nausea, vomiting, tachycardia, elevated blood pressure, tremors, seizures, hallucinations, and paranoid behavior, but the effects of maternal use of synthetic cannabinoids during pregnancy are ambiguous due to limited studies in humans and a relative short history of the drugs. In this review, we discuss the known and potential adverse effects of synthetic cannabinoids on human pregnancy using knowledge gathered from studies in mice and limited studies in humans. In mice, multiple sites and stages of pregnancy are potential targets of synthetic cannabinoids, including preimplantation embryo development, oviductal embryo transport, implantation, placentation, and parturition. It is anticipated that maternal use of synthetic cannabinoids would result in severely compromised female fertility and pregnancy outcome.
Keywords: cannabinoids, pregnancy, synthetic cannabinoids, female reproduction, ectopic pregnancy
Introduction
Marijuana is one of the world's most popular recreational drugs (SAMHSA, 2009), and the most commonly abused illicit drug in pregnant women in Western societies (UNODC, 2010). In 1964, the major psychoactive component of marijuana, Δ9-tetrahydrocannabinol (Δ9-THC), was discovered (Gaoni and Mechoulam, 1964). Later, endocannabinoids (endogenous cannabinoid-like compounds), were identified in vivo. While the studies in cannabinoids/endocannabinoids have been growing, hundreds of cannabinoid-mimics (synthetic cannabinoids) have been synthesized in vitro with similar effects to Δ9-THC. In recent years, synthetic cannabinoids are found in illicit “fake marijuana”, with different street names such as “Spice” or “K2”, and are usually sold as a mixture of herbs sprayed with a synthetic compound, typically including HU-210, HU-211, JWH-018, and JWH-073. As of March 2011, five synthetic cannabinoids were listed as Schedule I substances, making their possession and use illegal in the United States; nevertheless, it has been found that the popularity of synthetic cannabinoids is increasing among young people (ONDCP, 2012). The use of synthetic cannabinoids has been reported to cause agitation, anxiety, nausea, vomiting, tremors, seizures, hallucinations, and paranoid behavior (Fattore and Fratta, 2011). However, the toxicity of synthetic cannabinoids on human reproduction is not fully understood and appreciated. Although epidemiologic studies in humans showed controversial results on the adverse effect of maternal use of cannabis on pregnancy outcome (Fergusson et al., 2002, Shiono et al., 1995), the possibility that the newer generation of synthetic cannabinoids adversely impacts pregnancy outcome should not be neglected, since many synthetic cannabinoids have much higher affinity and potency to bind to cannabinoid receptors (Huffman and Padgett, 2005). Synthetic cannabinoids may have unexpected adverse side effects due to limited clinical data on these synthetic drugs and structural uniqueness of each drug. Here, we discuss the findings of various studies that have examined the effects of natural and synthetic cannabinoids in pregnant animal models and human tissues to determine the possibility and degree of toxic side effects on pregnant women and their developing embryos.
I. Endocannabinoid system
Research on cannabinoids blossomed after the discovery of the major psychoactive component of marijuana, Δ9-tetrahydrocannabinol (Δ9-THC) (Gaoni and Mechoulam, 1964). The two most studied endocannabinoids are anandamide (AEA) and 2 arachidonoylglycerol (2-AG) (Devane et al., 1992, Mechoulam et al., 1995, Sugiura et al., 1995). Δ9-THC and endocannabinoids exert their functions by targeting two major G protein-coupled cannabinoid receptors, brain-type CNR1, encoded by Cnr1 (Devane et al., 1988, Matsuda et al., 1990) and spleen-type CNR2, encoded by Cnr2 (Munro et al., 1993). In addition to CNR1 and CNR2, GPR55 (McPartland et al., 2006, Sawzdargo et al., 1999) and the transient receptor potential vanilloid 1 (TRPV1) (Van Der Stelt and Di Marzo, 2004) are also capable of mediating certain functions of cannabinoids/endocannabinoids.
Around the late 1980's, several labs worldwide began to make multiple compounds possessing similar effects to Δ9-THC for research purposes. Among others, the two most known series of synthetic cannabinoids are JWH and HU compounds. Hundreds of JWH compounds were generated by a group led by John W. Huffman from Clemson University, USA, and HU compounds were synthesized by a group led by Raphael Mechoulam at the Hebrew University, Israel. Many of the synthetic cannabinoids have a much higher affinity to bind cannabinoid receptors and are more potent compared to natural THC. Since the list of synthetic cannabinoids is rapidly growing, and only five of them are currently banned in the United States, there are many potential compounds which can be used as “fake marijuana” and sold legally.
Levels of endocannabinoids in vivo are tightly regulated by enzymes for synthesis and degradation of endocannabinoids. AEA is derived from the precursor N-acyl phosphatidylethanolamine (NAPE). Studies in knockout mice suggest that AEA can be generated via multiple enzymatic pathways (Leung et al., 2006, Simon and Cravatt, 2010), involving NAPE-hydrolyzing phospholipase D (Nape-pld) (Natarajan et al., 1982, Natarajan et al., 1984), α/β-hydrolase 4 (Abh4) (Simon and Cravatt, 2006), and a protein tyrosine phosphatase, Ptpn22 (Liu et al., 2006). However, degradation of AEA is mainly mediated by a membrane-bound fatty acid amide hydrolase (FAAH) to ethanolamine and arachidonic acid (AA) (Cravatt et al., 1996, Giang and Cravatt, 1997). A second enzyme displaying FAAH activity has been discovered in humans, but not in mice and rats (Wei et al., 2006). FAAH is critical for regulating both the magnitude and duration of AEA signaling (Cravatt and Lichtman, 2002). Besides FAAH, cyclooxygenase 2 (COX2) is also capable of inactivating AEA through an oxidative pathway (Rouzer and Marnett, 2008).
2-AG is produced by the cleavage of its precursor, diacylglycerol (DAG), via a membrane-bound sn1-diacylglycerol lipase (DAGL) (Moriyama et al., 1999), which exists in vivo in two different isoforms, DAGLα and DAGLβ. 2-AG is degraded to AA and glycerol by either FAAH or a membrane-associated serine hydrolase, monoacylglycerol lipase (MAGL) (Goparaju et al., 1999).
II. Toxicity of Endocannabinoids in female reproduction
Successful pregnancy depends on the appropriate orchestration of sequential pregnancy events, including fertilization, preimplantation embryo development, oviductal embryo transport, implantation, decidualization, placentation, and finally parturition; disturbances in any of these pregnancy events will have deleterious ripple effects on subsequent events, ultimately compromising pregnancy outcome (Cha et al., 2012). Cannabinoid signaling, which is conserved across species from sea urchins to humans, is involved in each step of pregnancy, thus suggesting that it has critical roles during pregnancy. Accumulating evidence suggests that a tightly controlled cannabinoid signaling tone is required for optimal progression of pregnancy events (Sun and Dey, 2012). During pregnancy, maternal use of synthetic cannabis is likely to offset the balance of cannabinoid signaling and compromise pregnancy outcome.
1. Higher cannabinoid signaling impairs preimplantation embryo development
The embryo is formed when an egg is fertilized by a sperm in the oviduct (Evans and Florman, 2002, Wassarman et al., 2001). In mice, embryos must develop into blastocysts within 4 days after fertilization to acquire the competency for implantation. Preimplantation embryo development involves several rounds of successive mitotic cell divisions— 2-cell, 8-cell, 16-cell and morula stages—ultimately forming blastocysts. A blastocyst consists of the outer layer of trophectoderm (TE) cells and the inner cell mass (ICM) (Rossant, 2004, Rossant and Tam, 2004).
The presence of cannabinoid receptors in preimplantation mouse embryos makes them a target of cannabinoid signaling. CNR1 is expressed in embryos from late 2-cell stage to blastocyst stage in which CNR1 is primarily localized in the trophectoderm. In contrast, CNR2 is expressed in embryos from 1-cell through the blastocyst stage, but in the latter stage CNR2 expression is primarily restricted to the ICM (Paria et al., 1995). In addition to the presence of these two receptors, FAAH is also present in the preimplantation embryos from the 2-cell stage to the blastocyst stage.
On-time preimplantation embryo development is critical to achieving an implantation competent blastocyst, and it requires finely tuned endocannabinoid signaling. In mice, preimplantation embryo development is derailed by increased levels of cannabinoid/endocannabinoid signaling. The development of 2-cell embryos to blastocysts was shown to be arrested by high levels of AEA, 2-AG, Δ9-THC, or a synthetic cannabinoid agonist WIN55212-2 (Paria et al., 1995, Paria et al., 1998). Moreover, blastocysts treated with high levels of AEA have a reduced number of TE cells and blastocyst zona-hatching becomes sluggish (Schmid et al., 1997, Yang et al., 1996). The adverse effects of higher levels of cannabinoid/endocannabinoid signaling is attenuated by SR141716A (a CNR1 selective antagonist), but not by SR144528 (a CNR2 specific antagonist), suggesting increased cannabinoid signaling via CNR1 inhibits normal mouse embryo development. The result is also supported by the observation that trophoblast outgrowth in vitro is promoted at a low level (7 nM), but becomes retarded at higher levels (28 nM) of AEA (Wang et al., 1999).
The generation of Cnr1, Cnr2, and Faah null mice provided useful model systems to study the effects of cannabinoid signaling in vivo. The null embryos, recovered from Cnr1−/− and Cnr1−/−/Cnr2−/− oviducts on day 3 and from uteri on day 4 of pregnancy, showed retarded growth compared with embryos derived from wild-type (WT) females (Paria et al., 2001). However, heterozygous embryos recovered from Cnr1−/− females mated with WT males showed normal embryo development (Wang et al., 2004). The results provide evidence that embryonic CNR1, but not maternal CNR1, directs appropriate embryo development (Wang et al., 2006a). In Faah−/− mice, significantly increased oviductal AEA levels are observed as compared with those in WT oviducts (Wang et al., 2006b). Interestingly, the development of preimplantation embryos in Faah−/− females is also delayed and become asynchronous (Wang et al., 2006b). This result not only suggests FAAH is indeed one of the primary enzymes that regulates anandamide turnover, but also indicates the oviductal environment in Faah−/− mouse may mimic that in long-term marijuana users in turn giving support to the theory that the maternal use of cannabis causes retarded development in preimplantation embryos.
Collectively, normal preimplantation embryo development requires appropriate endocannabinoid signaling via CNR1. Either silenced or augmented CNR1 signaling leads to abnormal embryo development. In humans, the maternal use of synthetic cannabinoids which increases cannabinoid signaling may cause retarded embryo development. In mice which ovulated around 8–10 eggs as compared to only one or two mature eggs are ovulated in each cycle in humans. Therefore, any disturbance in embryo development may result in complete pregnancy failure. Although there are few medical records correlating maternal use of cannabis with abnormal preimplantation embryo development, it may be due to the fact that underdeveloped embryos fail to implant, thus causing no obvious clinical symptoms.
2. Higher cannabinoid signaling derails normal oviductal-uterine embryo transport
In both humans and mice, embryos develop within the oviduct until day 3 of pregnancy. At late morula to early blastocyst stage, the embryo starts migrating towards the uterine lumen. The normal passage through the oviduct into the uterus requires coordinated oscillation of the cilia on the oviductal epithelia and muscle contractions. Failure of this passage through the oviductal-uterine junction results in embryo retention in mouse oviducts or human Fallopian tubes. In humans, the entrapped embryos can implant in the Fallopian tube, causing ectopic pregnancy in women, which is fatal in many occasions (Farquhar, 2005, Pisarska et al., 1998).
In mice, endocannabinoids and their putative receptor CNR1 are present in the oviducts. Faah levels in the isthmus are lower than the levels in the ampullary region, whereas Nape-pld shows the reverse pattern (Guo et al., 2005, Wang et al., 2006b); this suggests a gradient of AEA levels in the oviduct.
Studies using knockout females mated with WT males show that Cnr1−/− and Cnr1−/−/Cnr2−/−, but not WT or Cnr2−/−, mice have oviductal retention of embryos, suggesting that normal oviductal transport requires CNR1, but not CNR2 (Paria et al., 2001, Wang et al., 2004). Reciprocal embryo transfer experiments further confirmed that maternal CNR1 is critical for appropriate oviductal transport of embryos, since only Cnr1−/− recipients displayed oviductal retention of embryos irrespective of embryonic genotypes (Wang et al., 2004). Higher cannabinoid signaling also affects oviductal embryo transport. WT mice exposed to Δ9-THC or methanandamide (a stable AEA analog) have similar oviductal retention of embryos, which is rescued by CNR1 antagonist treatment (Wang et al., 2006b). Studies using Faah−/− mice further confirm that higher oviductal AEA levels disrupt normal embryo passage into the mouse uterus. Research in women with ectopic pregnancy corroborates the discoveries in mice. In women with ectopic pregnancy, the Fallopian tubes and endometrium have lower levels of Cnr1 compared to those in women with normal pregnancies (Horne et al., 2008). AEA levels in ectopic Fallopian tubes are significantly higher than those in normal pregnancies (Gebeh et al., 2012), and increased AEA levels are associated with low FAAH activity in ectopic Fallopian tubes. In addition, treatment with oleoylethanolamide, an endocannabinoid, significantly decreases cilia beat frequency in human fallopian tube epithelial cells (Gebeh et al., 2013).
In conclusion, either higher or lower endocannabinoid signaling impairs normal wave movement through the oviduct, and consequently causes embryo retention in mouse oviducts. Since mouse oviducts cannot support implantation, the entrapped embryos in the oviduct ultimately degenerate. However, embryos are able to implant in human Fallopian tubes. Therefore, maternal consumption of cannabis during early pregnancy may cause tubal pregnancy in humans. In the developed countries, tubal pregnancy remains the major cause of maternal mortality in the first trimester of pregnancy (Cantwell et al., 2011).
3. Higher endocannabinoid signaling defers implantation timing
Implantation occurs only when the blastocyst becomes implantation competent and the uterus achieves the receptive phase (Paria et al., 1993). The window of uterine receptivity is transient and delayed implantation has an adverse ripple effect throughout the course of gestation, leading to a compromised pregnancy outcome.
Endocannabinoids AEA and 2-AG, as well as their putative receptor CNR1, are present in the mouse uterus. Higher levels of Nape-pld mRNA and NAPE-PLD activity are found in interimplantation sites, whereas both mRNA and protein levels are lower in implantation sites (Guo et al., 2005, Wang et al., 2007). In contrast, FAAH expression and activity are higher at the implantation sites. The expression patterns of NAPE-PLD and FAAH keep AEA levels low at the implantation sites. Similarly, DAGLα levels are higher in interimplantation sites on days 5 and 7 of pregnancy where MAGL levels are low. Conversely, DAGLα levels are lower in implantation sites with higher levels of MAGL (Wang et al., 2007). Therefore, 2-AG shows similar patterns as AEA with higher levels at the interimplantation site and lower levels at the implantation site. These results suggest that an appropriate endocannabinoid signaling is beneficial to implantation.
Lower levels of cannabinoid signaling are critical in preparing embryos for implantation. Embryonic expression of CNR1 is downregulated in mouse blastocysts with approaching implantation (Paria et al., 2001). In the same vein, activated blastocysts have much lower CNR1 expression compared with dormant blastocysts (Paria et al., 2001, Wang et al., 2003). High levels of AEA or other synthetic cannabinoids inhibit blastocyst zona-hatching, while low levels of AEA accelerates trophoblast differentiation and outgrowth in culture (Paria et al., 1995, Paria et al., 1998, Schmid et al., 1997). In vivo, high levels of AEA in Faah−/− mice postpone implantation timing, leading to compromised pregnancy outcome (Wang et al., 2006b). This deferred implantation was substantially rescued by the selective CNR1 antagonist SR141716, suggesting CNR1 mediated AEA signaling derails on-time implantation. In humans, women with elevated peripheral AEA levels are associated with a higher rate of spontaneous pregnancy loss (Maccarrone et al., 2002, Maccarrone et al., 2000).
The mechanism by which cannabinoid signaling regulates blastocyst activation is still not clear. Some evidence indicates that AEA at a low concentration (7 nM) activates ERK signaling via CNR1, whereas AEA at a higher concentration (28 nM) fails to activate ERK, but instead inhibits Ca2+ mobilization (Wang et al., 2003). These results help explain how endocannabinoids at different concentrations differently modulate blastocyst activation.
Appropriate and regulated endocannabinoid signaling in both the blastocyst and uterus is required for the establishment of uterine receptivity and implantation competency of blastocysts. Gene expression studies and genetically engineered mouse models have shown that lower levels of endocannabinoid signaling are conducive to the process of implantation, and higher levels of cannabinoid signaling cause defer implantation, resulting in suboptimal pregnancy outcome.
4. Tightly regulated cannabinoid signaling is critical to normal placentation
In eutherians, the placenta is the only connection between the mother and fetus. Its functions include the exchange of oxygen, nutrients, and waste between the two entities. Most exchanges occur in the labyrinth layer of the placenta, which consists mainly of syncytiotrophoblasts. Adjunct to the labyrinth layer, the spongy layer, derived from EPC and extraembryonic ectoderm cells, confers structural support for the labyrinth layer. Most cells in the spongy layer are spongiotrophoblast cells (SPT); they are separated from maternal decidual cells by trophoblast giant cells (TGC) (Cross et al., 2003). All of the TGC, SPT, syncytiotrophoblast, and other trophoblast cells are derived from trophoblast stem (TS) cells originating from the trophectoderm. To exert its physiological function, the placenta requires an appropriate population and distribution of different trophoblast cells. Any aberration in trophoblast proliferation or differentiation compromises normal placentation.
Midgestational placentas are targets of endocannabinoid signaling. Two endocannabinoids, AEA and 2-AG, are detected in murine placentas. The placenta also has detectable levels of mRNAs for metabolic enzymes (Faah, Nape-pld, Magl, and Daglα) and cannabinoid receptors (Cnr1 and Trpv1). Immunohistochemistry results have provided evidence that FAAH and CNR1 are expressed in the EPC on day 10 of pregnancy and the SPT layer later on day 14 (Sun et al., 2010). CNR1 and FAAH were also identified in human placentas, including amniotic epithelial cells, chorionic cytotrophoblasts (Park et al., 2003), and syncytiotrophoblasts (Habayeb et al., 2008). Placentation was found to be compromised in Cnr1−/− females. Cnr1−/− females have a higher rate of embryo loss and lower placental weights on days 12 and 14 of pregnancy compared with WT mice. In Faah−/− females, the resorption rate starts to rise from day 14. These results suggest that both high and low endocannabinoid signaling compromises normal pregnancy events even at midgestation (Sun et al., 2010).
Aberrant cannabinoid signaling has adverse effects on the proliferation of trophoblast stem (TS) cells, which are derived from the trophectoderm in blastocysts. Proliferation of Cnr1−/−/Cnr2−/− and Faah−/− TS cells was remarkably sluggish compared to WT TS cells, because of reduced levels of activated AKT. Additionally, WT TS cells treated with methanandamide (7 nM) proliferated at a faster rate, but this was attenuated by a CNR1 selective antagonist, suggesting that CNR1 mediated endocannabinoid signaling regulates TS proliferation (Sun et al., 2010). The results also show that CNR1 mediated signaling via the PI3K-AKT pathway is important for appropriate TS cell proliferation in mice. Interestingly, AEA also prevented the proliferation of a human cell line, BeWo trophoblast cell, in a dose-dependent manner. Elevated plasma AEA levels also increased the risk for first trimester miscarriages (Habayeb et al., 2008).
Aberrant endocannabinoid signaling has an adverse impact on TS cell differentiation and development of the SPT layer. The differentiation of secondary TGCs from precursor cells in the SPT layer depends on the balance of two transcription factors: Mash2 prevents trophoblast precursor cells from differentiating into secondary TGCs (Guillemot et al., 1994), whereas Hand1 promotes differentiation into TGCs (Riley et al., 1998). Mash2 expression was shown to be much reduced in Faah−/− and Cnr1−/− placentas, indicating that Faah−/− and Cnr1−/− trophoblast precursor cells are more prone to differentiate into TGCs.
During placentation, trophoblast cells invade the maternal decidual zone and reshape the maternal blood vessels to direct appropriate rate of blood flow to support embryonic growth (Cross et al., 2002). Thus, this invasive function of trophoblast cells is critical for successful placentation in establishing maternal-fetal circulation and exchanges. The invasion of glycogen trophoblast cells (GTC) into the decidua basalis is an indicator of trophoblast invasiveness. In WT females, an abundant number of GTC invade into the decidual basalis; however only a sporadic number of them with shallow invasion was found beyond the TGC border in Cnr1−/− mice (Sun et al., 2010). These results suggest that the invasive capacity of trophoblast cells is compromised in the absence of CNR1.
In humans, low FAAH and high CNR1 levels are associated with spontaneous miscarriage (Trabucco et al., 2009), suggesting higher cannabinoid signaling is harmful to the placenta during the first trimester. Similarly, preeclamptics placentas have significantly higher levels of NAPE-PLD and lower levels of FAAH (Aban et al., 2013). These observations are further confirmed by the results that higher plasma AEA levels are associated with non-viable pregnancies (Taylor et al., 2011).
Collectively, aberrant CNR1-mediated endocannabinoid signaling inhibits TS cell proliferation, differentiation into SPT cells, and trophoblast invasiveness, leading to defective placentation and pregnancy loss in the midgestational stage. Higher cannabinoid signaling is also associated with spontaneous miscarriage in women.
5. Endocannabinoid signaling plays a role in parturition
In humans, a pregnancy that lasts about 37 to 42 weeks is considered to be full term. A pregnancy with less than 37 weeks of gestational age is considered preterm, when a baby is not mature and competent for normal postnatal survival. Preterm birth accounts for 10% of neonatal mortality worldwide (CHRP, 1999). In developed countries, neonatal death decreases due to reduced infections and other causes of neonatal death, but the exact cause of preterm birth still remains unsolved. Therefore, preterm birth becomes the leading cause of neonatal mortality in developed countries and accounts for 25% of neonatal mortality in the U.S. (Mathews and MacDorman, 2006).
It was observed that genetic loss of Cnr1 leads to preterm birth and lower birth weight (Wang et al., 2008). Previous reports showed that P4 withdrawal and a decreased P4 versus estradiol (E2) ratio determines the parturition timing in rodents (Mesiano and Welsh, 2007). CNR1 deficiency induces preterm birth in mice by altering normal P4 and estrogen levels, suggesting cannabinoid signaling via CNR1 plays a role in mouse parturition. In humans, plasma levels of AEA are increased during labor (Habayeb et al., 2004). Endocannabinoids and the synthetic cannabinoid CP55,940 significantly increase PGE2 production in human amnions at term (Mitchell et al., 2008). It is known that prostaglandin (PG) production is associated with parturition, suggesting elevated cannabinoid signaling plays a role in human parturition. Thus, maternal use of cannabis during the third trimester of pregnancy may induce prostaglandin production, thus advancing the parturition process. In fact, evidence from a human epidemiological study indicates that regular use of cannabis throughout pregnancy is associated with statistically detectable decreases in birth weight (Fergusson et al., 2002), which is also associated with preterm birth.
III. Looking Forward
The use of marijuana and recent rise in the use of more potent synthetic and designer drugs raise serious concerns regarding higher incidence of psychosomatic disorders and reproductive consequences among young people. In addition, increasing use of cannabis products as medicinal antidotes against many health problems adds to the debate among proponents and opponents of the use of cannabis for medicinal purposes. Many studies in mice and epidemiological data on humans suggest a major regulatory role of cannabinoid signaling is in pregnancy. And maternal consumption of marijuana or synthetic cannabinoids is a potential risk factor for abnormal embryo development, tubal pregnancy, implantation failure, placental defects related to spontaneous abortion and preterm birth (Figure 1). Considering these social, political, judicial, and clinical concerns, it should be advocated that more invest be made in basic research to better understand the effects of marijuana products in health and diseases.
Figure 1.
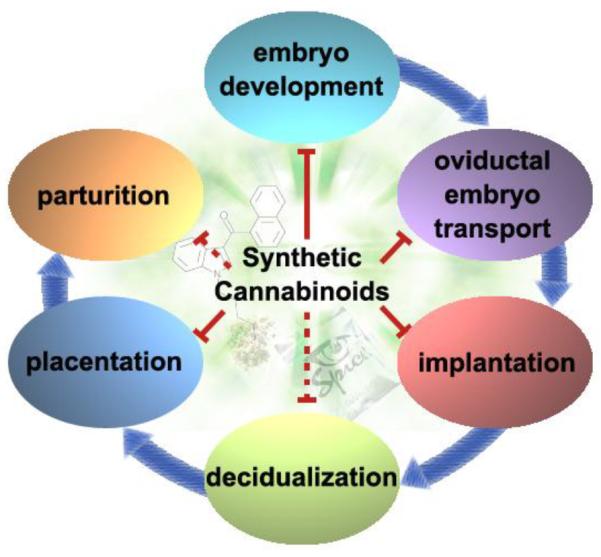
Cannabinoid/endocannabinoid signaling impacts various pregnancy events. Maternal use of natural and synthetic cannabinoids disturbs pregnancy events including preimplantation embryo development, oviductal embryo transport, implantation, and placentation. Although there is no direct evidence available to show that synthetic cannabinoids compromise decidualization or parturition, studies in CB1 knockout mice suggest both of them are potential targets of synthetic cannabinoids ((Wang et al., 2008) and unpublished data). The figure is adapted in modified form from REF (Sun and Dey, 2012).
Acknowledgement
We thank Serenity Curtis for her editorial assistance in preparing this manuscript. The work in the Dey lab is supported in part by grants from the National Institutes of Health (DA06668 and HD068524) and the March of Dimes. X.S. was supported by a Lalor Foundation postdoctoral fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aban C, Leguizamon GF, Cella M, Damiano A, Franchi AM, Farina MG. Differential expression of endocannabinoid system in normal and preeclamptic placentas: effects on nitric oxide synthesis. Placenta. 2013;34:67–74. doi: 10.1016/j.placenta.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving Mothers' Lives: Reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nature medicine. 2012;18:1754–67. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRP . Child Health Research Project Special Report: Reducing Perinatal and Neonatal Mortality. Baltimore, Maryland: 1999. [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–7. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Lichtman AH. The enzymatic inactivation of the fatty acid amide class of signaling lipids. Chemistry and physics of lipids. 2002;121:135–48. doi: 10.1016/s0009-3084(02)00147-0. [DOI] [PubMed] [Google Scholar]
- Cross JC, Baczyk D, Dobric N, Hemberger M, Hughes M, Simmons DG, et al. Genes, development and evolution of the placenta. Placenta. 2003;24:123–30. doi: 10.1053/plac.2002.0887. [DOI] [PubMed] [Google Scholar]
- Cross JC, Hemberger M, Lu Y, Nozaki T, Whiteley K, Masutani M, et al. Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol Cell Endocrinol. 2002;187:207–12. doi: 10.1016/s0303-7207(01)00703-1. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Molecular pharmacology. 1988;34:605–13. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science (New York, NY. 1992;258:1946–9. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Evans JP, Florman HM. The state of the union: the cell biology of fertilization. Nature cell biology. 2002;4(Suppl):s57–63. doi: 10.1038/ncb-nm-fertilityS57. [DOI] [PubMed] [Google Scholar]
- Farquhar CM. Ectopic pregnancy. Lancet. 2005;366:583–91. doi: 10.1016/S0140-6736(05)67103-6. [DOI] [PubMed] [Google Scholar]
- Fattore L, Fratta W. Beyond THC: The New Generation of Cannabinoid Designer Drugs. Frontiers in behavioral neuroscience. 2011;5:60. doi: 10.3389/fnbeh.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Northstone K. Maternal use of cannabis and pregnancy outcome. BJOG. 2002;109:21–7. doi: 10.1111/j.1471-0528.2002.01020.x. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. Journal of the American Chemical Society. 1964;86:1646–7. [Google Scholar]
- Gebeh AK, Willets JM, Bari M, Hirst RA, Marczylo TH, Taylor AH, et al. Elevated Anandamide and Related N-Acylethanolamine Levels Occur in the Peripheral Blood of Women With Ectopic Pregnancy and Are Mirrored by Changes in Peripheral Fatty Acid Amide Hydrolase Activity. J Clin Endocrinol Metab. 2013 doi: 10.1210/jc.2012-3390. [DOI] [PubMed] [Google Scholar]
- Gebeh AK, Willets JM, Marczylo EL, Taylor AH, Konje JC. Ectopic pregnancy is associated with high anandamide levels and aberrant expression of FAAH and CB1 in fallopian tubes. J Clin Endocrinol Metab. 2012;97:2827–35. doi: 10.1210/jc.2012-1780. [DOI] [PubMed] [Google Scholar]
- Giang DK, Cravatt BF. Molecular characterization of human and mouse fatty acid amide hydrolases. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2238–42. doi: 10.1073/pnas.94.6.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goparaju SK, Ueda N, Taniguchi K, Yamamoto S. Enzymes of porcine brain hydrolyzing 2-arachidonoylglycerol, an endogenous ligand of cannabinoid receptors. Biochemical pharmacology. 1999;57:417–23. doi: 10.1016/s0006-2952(98)00314-1. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Nagy A, Auerbach A, Rossant J, Joyner AL. Essential role of Mash-2 in extraembryonic development. Nature. 1994;371:333–6. doi: 10.1038/371333a0. [DOI] [PubMed] [Google Scholar]
- Guo Y, Wang H, Okamoto Y, Ueda N, Kingsley PJ, Marnett LJ, et al. N-acylphosphatidylethanolamine-hydrolyzing phospholipase D is an important determinant of uterine anandamide levels during implantation. The Journal of biological chemistry. 2005;280:23429–32. doi: 10.1074/jbc.C500168200. [DOI] [PubMed] [Google Scholar]
- Habayeb OM, Taylor AH, Bell SC, Taylor DJ, Konje JC. Expression of the endocannabinoid system in human first trimester placenta and its role in trophoblast proliferation. Endocrinology. 2008;149:5052–60. doi: 10.1210/en.2007-1799. [DOI] [PubMed] [Google Scholar]
- Habayeb OM, Taylor AH, Evans MD, Cooke MS, Taylor DJ, Bell SC, et al. Plasma levels of the endocannabinoid anandamide in women--a potential role in pregnancy maintenance and labor? J Clin Endocrinol Metab. 2004;89:5482–7. doi: 10.1210/jc.2004-0681. [DOI] [PubMed] [Google Scholar]
- Horne AW, Phillips JA, 3rd, Kane N, Lourenco PC, McDonald SE, Williams AR, et al. CB1 expression is attenuated in Fallopian tube and decidua of women with ectopic pregnancy. PLoS One. 2008;3:e3969. doi: 10.1371/journal.pone.0003969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JW, Padgett LW. Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indenes. Current medicinal chemistry. 2005;12:1395–411. doi: 10.2174/0929867054020864. [DOI] [PubMed] [Google Scholar]
- Leung D, Saghatelian A, Simon GM, Cravatt BF. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry. 2006;45:4720–6. doi: 10.1021/bi060163l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, et al. A biosynthetic pathway for anandamide. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13345–50. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Bisogno T, Valensise H, Lazzarin N, Fezza F, Manna C, et al. Low fatty acid amide hydrolase and high anandamide levels are associated with failure to achieve an ongoing pregnancy after IVF and embryo transfer. Molecular human reproduction. 2002;8:188–95. doi: 10.1093/molehr/8.2.188. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Valensise H, Bari M, Lazzarin N, Romanini C, Finazzi-Agro A. Relation between decreased anandamide hydrolase concentrations in human lymphocytes and miscarriage. Lancet. 2000;355:1326–9. doi: 10.1016/S0140-6736(00)02115-2. [DOI] [PubMed] [Google Scholar]
- Mathews TJ, MacDorman MF. Infant mortality statistics from the 2003 period linked birth/infant death data set. Natl Vital Stat Rep. 2006;54:1–29. [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–4. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Matias I, Di Marzo V, Glass M. Evolutionary origins of the endocannabinoid system. Gene. 2006;370:64–74. doi: 10.1016/j.gene.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochemical pharmacology. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Welsh TN. Steroid hormone control of myometrial contractility and parturition. Seminars in cell & developmental biology. 2007;18:321–31. doi: 10.1016/j.semcdb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Mitchell MD, Sato TA, Wang A, Keelan JA, Ponnampalam AP, Glass M. Cannabinoids stimulate prostaglandin production by human gestational tissues through a tissue- and CB1-receptor-specific mechanism. Am J Physiol Endocrinol Metab. 2008;294:E352–6. doi: 10.1152/ajpendo.00495.2007. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Urade R, Kito M. Purification and characterization of diacylglycerol lipase from human platelets. Journal of biochemistry. 1999;125:1077–85. doi: 10.1093/oxfordjournals.jbchem.a022389. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–5. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Natarajan V, Reddy PV, Schmid PC, Schmid HH. N-Acylation of ethanolamine phospholipids in canine myocardium. Biochimica et biophysica acta. 1982;712:342–55. doi: 10.1016/0005-2760(82)90352-6. [DOI] [PubMed] [Google Scholar]
- Natarajan V, Schmid PC, Reddy PV, Schmid HH. Catabolism of N-acylethanolamine phospholipids by dog brain preparations. Journal of neurochemistry. 1984;42:1613–9. doi: 10.1111/j.1471-4159.1984.tb12750.x. [DOI] [PubMed] [Google Scholar]
- ONDCP . Synthetic Drugs (a.k.a. K2, Spice, Bath Salts, etc.) Office of National Drug Control Policy; 2012. [Google Scholar]
- Paria BC, Das SK, Dey SK. The preimplantation mouse embryo is a target for cannabinoid ligand-receptor signaling. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9460–4. doi: 10.1073/pnas.92.21.9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Huet-Hudson YM, Dey SK. Blastocyst's state of activity determines the “window” of implantation in the receptive mouse uterus. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:10159–62. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Ma W, Andrenyak DM, Schmid PC, Schmid HH, Moody DE, et al. Effects of cannabinoids on preimplantation mouse embryo development and implantation are mediated by brain-type cannabinoid receptors. Biology of reproduction. 1998;58:1490–5. doi: 10.1095/biolreprod58.6.1490. [DOI] [PubMed] [Google Scholar]
- Paria BC, Song H, Wang X, Schmid PC, Krebsbach RJ, Schmid HH, et al. Dysregulated cannabinoid signaling disrupts uterine receptivity for embryo implantation. The Journal of biological chemistry. 2001;276:20523–8. doi: 10.1074/jbc.M100679200. [DOI] [PubMed] [Google Scholar]
- Park B, Gibbons HM, Mitchell MD, Glass M. Identification of the CB1 cannabinoid receptor and fatty acid amide hydrolase (FAAH) in the human placenta. Placenta. 2003;24:990–5. doi: 10.1053/plac.2002.0926. [DOI] [PubMed] [Google Scholar]
- Pisarska MD, Carson SA, Buster JE. Ectopic pregnancy. Lancet. 1998;351:1115–20. doi: 10.1016/S0140-6736(97)11476-3. [DOI] [PubMed] [Google Scholar]
- Riley P, Anson-Cartwright L, Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat Genet. 1998;18:271–5. doi: 10.1038/ng0398-271. [DOI] [PubMed] [Google Scholar]
- Rossant J. Lineage development and polar asymmetries in the peri-implantation mouse blastocyst. Seminars in cell & developmental biology. 2004;15:573–81. doi: 10.1016/j.semcdb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Rossant J, Tam PP. Emerging asymmetry and embryonic patterning in early mouse development. Developmental cell. 2004;7:155–64. doi: 10.1016/j.devcel.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Rouzer CA, Marnett LJ. Non-redundant functions of cyclooxygenases: oxygenation of endocannabinoids. The Journal of biological chemistry. 2008;283:8065–9. doi: 10.1074/jbc.R800005200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA . The NSDUH Report. 2009. Substance Abuse and Mental Health Services Administration: Substance Use among Women During Pregnancy and Following Childbirth. [Google Scholar]
- Sawzdargo M, Nguyen T, Lee DK, Lynch KR, Cheng R, Heng HH, et al. Identification and cloning of three novel human G protein-coupled receptor genes GPR52, PsiGPR53 and GPR55: GPR55 is extensively expressed in human brain. Brain Res Mol Brain Res. 1999;64:193–8. doi: 10.1016/s0169-328x(98)00277-0. [DOI] [PubMed] [Google Scholar]
- Schmid PC, Paria BC, Krebsbach RJ, Schmid HH, Dey SK. Changes in anandamide levels in mouse uterus are associated with uterine receptivity for embryo implantation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4188–92. doi: 10.1073/pnas.94.8.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiono PH, Klebanoff MA, Nugent RP, Cotch MF, Wilkins DG, Rollins DE, et al. The impact of cocaine and marijuana use on low birth weight and preterm birth: a multicenter study. American journal of obstetrics and gynecology. 1995;172:19–27. doi: 10.1016/0002-9378(95)90078-0. [DOI] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. The Journal of biological chemistry. 2006;281:26465–72. doi: 10.1074/jbc.M604660200. [DOI] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Characterization of mice lacking candidate N-acyl ethanolamine biosynthetic enzymes provides evidence for multiple pathways that contribute to endocannabinoid production in vivo. Mol Biosyst. 2010 doi: 10.1039/c000237b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kudo N, Ojima T, Mabuchi-Itoh K, Yamashita A, Waku K. Coenzyme A-dependent cleavage of membrane phospholipids in several rat tissues: ATP-independent acyl-CoA synthesis and the generation of lysophospholipids. Biochimica et biophysica acta. 1995;1255:167–76. doi: 10.1016/0005-2760(94)00237-s. [DOI] [PubMed] [Google Scholar]
- Sun X, Dey SK. Endocannabinoid signaling in female reproduction. ACS Chem Neurosci. 2012;3:349–55. doi: 10.1021/cn300014e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Xie H, Yang J, Wang H, Bradshaw HB, Dey SK. Endocannabinoid signaling directs differentiation of trophoblast cell lineages and placentation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16887–92. doi: 10.1073/pnas.1010892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AH, Finney M, Lam PM, Konje JC. Modulation of the endocannabinoid system in viable and non-viable first trimester pregnancies by pregnancy-related hormones. Reprod Biol Endocrinol. 2011;9:152. doi: 10.1186/1477-7827-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabucco E, Acone G, Marenna A, Pierantoni R, Cacciola G, Chioccarelli T, et al. Endocannabinoid system in first trimester placenta: low FAAH and high CB1 expression characterize spontaneous miscarriage. Placenta. 2009;30:516–22. doi: 10.1016/j.placenta.2009.03.015. [DOI] [PubMed] [Google Scholar]
- UNODC . United Nations Office on Drugs and Crime (UNODC), World Drug Report. 2010. [Google Scholar]
- Van Der Stelt M, Di Marzo V. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. European journal of biochemistry / FEBS. 2004;271:1827–34. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK, Maccarrone M. Jekyll and hyde: two faces of cannabinoid signaling in male and female fertility. Endocrine reviews. 2006a;27:427–48. doi: 10.1210/er.2006-0006. [DOI] [PubMed] [Google Scholar]
- Wang H, Guo Y, Wang D, Kingsley PJ, Marnett LJ, Das SK, et al. Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nature medicine. 2004;10:1074–80. doi: 10.1038/nm1104. [DOI] [PubMed] [Google Scholar]
- Wang H, Matsumoto H, Guo Y, Paria BC, Roberts RL, Dey SK. Differential G protein-coupled cannabinoid receptor signaling by anandamide directs blastocyst activation for implantation. Proc Natl Acad Sci U S A. 2003;100:14914–9. doi: 10.1073/pnas.2436379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xie H, Dey SK. Loss of cannabinoid receptor CB1 induces preterm birth. PLoS ONE. 2008;3:e3320. doi: 10.1371/journal.pone.0003320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xie H, Guo Y, Zhang H, Takahashi T, Kingsley PJ, et al. Fatty acid amide hydrolase deficiency limits early pregnancy events. The Journal of clinical investigation. 2006b;116:2122–31. doi: 10.1172/JCI28621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xie H, Sun X, Kingsley PJ, Marnett LJ, Cravatt BF, et al. Differential regulation of endocannabinoid synthesis and degradation in the uterus during embryo implantation. Prostaglandins & other lipid mediators. 2007;83:62–74. doi: 10.1016/j.prostaglandins.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Paria BC, Dey SK, Armant DR. Stage-specific excitation of cannabinoid receptor exhibits differential effects on mouse embryonic development. Biol Reprod. 1999;60:839–44. doi: 10.1095/biolreprod60.4.839. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Jovine L, Litscher ES. A profile of fertilization in mammals. Nature cell biology. 2001;3:E59–64. doi: 10.1038/35055178. [DOI] [PubMed] [Google Scholar]
- Wei BQ, Mikkelsen TS, McKinney MK, Lander ES, Cravatt BF. A second fatty acid amide hydrolase with variable distribution among placental mammals. The Journal of biological chemistry. 2006;281:36569–78. doi: 10.1074/jbc.M606646200. [DOI] [PubMed] [Google Scholar]
- Yang ZM, Paria BC, Dey SK. Activation of brain-type cannabinoid receptors interferes with preimplantation mouse embryo development. Biology of reproduction. 1996;55:756–61. doi: 10.1095/biolreprod55.4.756. [DOI] [PubMed] [Google Scholar]


