Abstract
BACKGROUND
Ethanol exposure prior to traumatic injury, such as a burn, elevates systemic and local inflammatory responses and increases morbidity and mortality. Adipose is a large tissue mass that is often inflamed during obesity or other stresses which disturbs metabolic homeostasis. To date, there has been little investigation into the inflammatory response of adipose tissue after combined ethanol exposure and burn injury.
METHODS
Two ethanol exposure regimens were utilized to examine the role of inflammation in adipose tissue after ethanol and burn injury. Mice were either given a single or episodic binge exposure to ethanol or saline followed by scald (burn) or sham injury 30 minutes later. Twenty-four hours post injury, serum and adipose tissue were collected for assessment of inflammatory mediators.
RESULTS
Single binge ethanol alone induced no inflammation in adipose when compared with sham vehicle treated mice. However, single binge ethanol followed by burn injury induced significant elevations in mRNA and protein concentrations of pro-inflammatory mediators interleukin-6 (IL-6), KC, and monocyte chemoattractant protein 1 (MCP-1) compared to either insult alone or sham vehicle group. Additionally, ethanol exposure and burn injury significantly blunted inducible nitric oxide synthase (iNOS), indicating a complex inflammatory response. Episodic binge ethanol exposure followed by burn injury exacerbated the post-burn adipose inflammatory response. The magnitude of the episodic binge-induced inflammatory parameters post-burn were 2- to 5- fold greater than the response detected after a single exposure of ethanol, indicating ethanol-induced potentiation of burn-induced inflammatory response. Finally, inflammatory loci and crown-like structures in adipose were significantly increased by episodic binge ethanol and burn injury.
CONCLUSIONS
This is the first report of binge and burn-induced crown-like structure formation. Evidence presented herein suggests an important role for alcohol and burn as an additional mediator of adipose inflammation in post-burn injury, a common complication in burn patients.
Keywords: alcohol, crown-like structure, trauma, cytokine, chemokine
INTRODUCTION
Burn-induced hyperglycemia, hepatosteatosis, and insulin resistance are common complications observed in the burn patient population and are associated with poor outcomes, contributing significantly to morbidity and mortality (Bonab et al., 2010, Silver et al., 2008). Insulin resistance in liver, skeletal muscle, and adipose can persist even 3 weeks after burn injury (Carter et al., 2004, Thorell et al., 1999, Cree and Wolfe, 2008). Burn injury also drives systemic inflammation with elevations in pro-inflammatory cytokines and suppressed cell-mediated immunity, leading to multi-organ dysfunction (Marshall, 2000). One mechanism of systemic insulin resistance may be through adipose dysfunction and inflammation (Johnson, 2012). Macrophages are known to infiltrate adipose during states of metabolic stress, such as in obesity, and contribute to inflammation, uncontrolled lipolysis, as well as local and systemic insulin resistance (Johnson, 2012).
Clinical and laboratory studies have demonstrated that ethanol exposure prior to traumatic injury, such as a burn, markedly elevates systemic and tissue-specific inflammatory responses (Bird and Kovacs, 2008, Jung et al., 2011) and is associated with poorer outcomes (McGill et al., 1995). In the United States, half of the patients with burn-related injuries have alcohol in their system at the time of admission and the vast majority of those subjects are binge drinkers rather than chronic alcoholics (Albright et al., 2009). It is well-established that alcohol increases the dysregulated inflammatory and immune response caused by burn in animal models and patients (reviewed in (Bird and Kovacs, 2008)). We and others have shown previously that neutrophils infiltrate the gut, lung, and site of injury after the combined insult (Faunce et al., 1999, Li et al., 2011, Zahs et al., 2012, Bird et al., 2010a, Zahs et al., 2013, Chen et al., 2013). While the primary role of neutrophils is to clear pathogens, they often cause damage due to production of enzymes such as elastase, reactive oxygen species, and pro-inflammatory cytokines including IL-6, IL-1β, and tumor necrosis factor alpha (TNFα). Serum cytokines IL-6 and TNFα and tissue levels of KC and IL-6, are elevated in response to the dual insult of ethanol and burn injury compared to either injury alone (Chen et al., 2013, Zahs et al., 2013, Li et al., 2011).
Chronic ethanol has been shown to drive macrophage infiltration into adipose tissue and it is associated with reduced fat mass due to upregulated lipolysis (Zhong et al., 2012, Kang et al., 2007). To date, it is not known how ethanol exposure combined with burn injury affects the adipose microenvironment. Using an established murine model of binge ethanol exposure and burn injury, we demonstrated that the combined insults drove systemic and adipose inflammation 24 hours post-injury. Furthermore, we found that employing an episodic multi-day binge ethanol exposure paradigm followed by burn injury potentiated adipose inflammation and induced macrophage infiltration, indicating that binge, and especially episodic binge ethanol exposure, followed by burn drives adipose inflammation that could contribute to systemic inflammation.
MATERIALS AND METHODS
Mice
Male C57BL/6 mice, 8 to 10 weeks old, were obtained from Jackson Laboratories (Bar Harbor, ME). They were housed in cages with food and water available ad libitum at the Loyola University Medical Center Animal Facility in rooms that were temperature and humidity controlled on a 12-h light-dark cycle. All animal studies described here were performed according to the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals, National Institutes of Health and were approved by the Loyola University IACUC. Before each experiment, mice were weighed and those weighing 22 to 27 grams were used in the studies.
Murine model of ethanol exposure and burn injury
The murine model of a single binge ethanol exposure and burn injury was employed as described previously (Messingham et al., 2000, Faunce et al., 1997) with minor modifications (Bird et al., 2010b). Briefly, mice were given 1) either a single binge dose of 150 μl of 20% (v/v) ethanol solution (1.12 g/kg) intraperitoneally (i.p.) that resulted in a blood ethanol level of 150 mg/dl at 30 minutes or saline vehicle (Murdoch et al., 2008) or 2) for episodic binge exposure, mice were given the same dose of ethanol (or saline) daily for 3 days consecutively, rested for 2-4 days (rest time did not alter outcome, data not shown), and then given 3 additional daily exposures. Thirty minutes after ethanol exposure (or the last ethanol exposure in the case of the episodic binge paradigm), mice were anesthetized with 100 mg/kg of Ketamaine and 10 mg/kg of Xylazine (Webster Veterinary, Sterling, MA), their dorsum shaved, and placed in a plastic template exposing 15% of the total body surface area of their back (Faunce et al., 1997) and subjected to a scald injury in a 90 to 92°C water bath for 8 seconds. As a control, sham animals were anesthetized, shaved, and immersed in room temperature water. The scald injury resulted in an insensate, full-thickness burn injury of approximately 15% total body surface area (Faunce et al., 1999). To compensate for fluid loss and prevent circulatory shock, all animals received 1 mL of body temperature saline i.p. immediately after burn injury and were allowed to recover on warming pads. No other therapeutic intervention was provided as administration of anti-inflammatory or analgesic medication may introduce confounding factors into the assessment of inflammatory responses. Twenty-four hours after burn injury, mice were sacrificed using carbon dioxide (CO2) inhalation and cervical dislocation. Blood was collected for serum isolation and measurement of cytokines. Epididymal white adipose tissue was removed. Tissue was either snap frozen in liquid nitrogen and stored at −80°C for mRNA isolation or immunologic analysis or fixed in paraformaldehyde, paraffin-embedded, and sectioned for immunohistochemistry.
RNA isolation and analysis
QIAzol Lysis Reagent was used to isolate mRNA from adipose tissue with DNAse treatment (RNeasy Lipid Tissue Mini Kit mRNA; Qiagen, Valencia, CA). For quantitative PCR (qPCR) analysis, cDNA was synthesized using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Real-time qPCR was completed using TaqMan Assay on Demand primers/probe sets (Applied Biosystems, Carlsbad, CA) as previously described (Sampey et al., 2011). qPCR reactions were run using an Applied Biosystems thermocycler and SDS 2.4 software (Applied Biosystems, Carlsbad, CA).
Quantification of IL-6 in serum
Blood was obtained by cardiac puncture after sacrifice. Serum IL-6 levels were determined by multiplex according to manufacture instructions (Invitrogen, Carlsbad, CA), as previously described (Bird et al., 2010b).
Analysis of cytokine and chemokine protein levels
Adipose tissue was homogenized in 1 mL of BioPlex Cell Lysis Buffer according to manufacturer's protocol (BioRad, Hercules, CA). Homogenates were then filtered and analyzed for IL-6, MCP-1, KC, TNFα, IL-10 and IL-1β protein levels by multiplex (BioRad, Hercules, CA) and normalized to the total amount of protein in adipose tissue homogenate as described (Bird et al., 2010b).
Immunohistochemistry (IHC) examination of episodic binge exposed and burn-injured adipose
Paraffin-embedded tissues were sectioned at 5 microns and mounted for histological staining. Briefly, immunohistochemisty was carried out using anti-F4/80 primary antibody (Abcam ab562), similar to Sampey et al. (Sampey et al., 2011). All histological sections were digitally scanned on the Aperio ScanScope CS Ultra-Resolution Digital Scanner and analyzed by ScanScope Image Analysis Toolbox software (Aperio Inc., Vista, CA). Representative images for adipose tissue from each treatment group were selected. To determine the inflammatory state of the adipose tissue, ten randomly selected 10× fields per study animal were quantified for number of crown-like structures and inflammatory loci including F4/80+ macrophage staining. N=4-7 mice per group.
Statistical Analysis
Data are shown as means ± SEM. Analysis was performed using two-way analysis of variance (ANOVA). Post hoc comparisons were made with the Tukey post hoc test using JMP (SAS Institute, Cary, NC) and significant interactions are described. P < 0.05 was considered significant.
RESULTS
Single and episodic binge and burn injury drive inflammation in adipose tissue
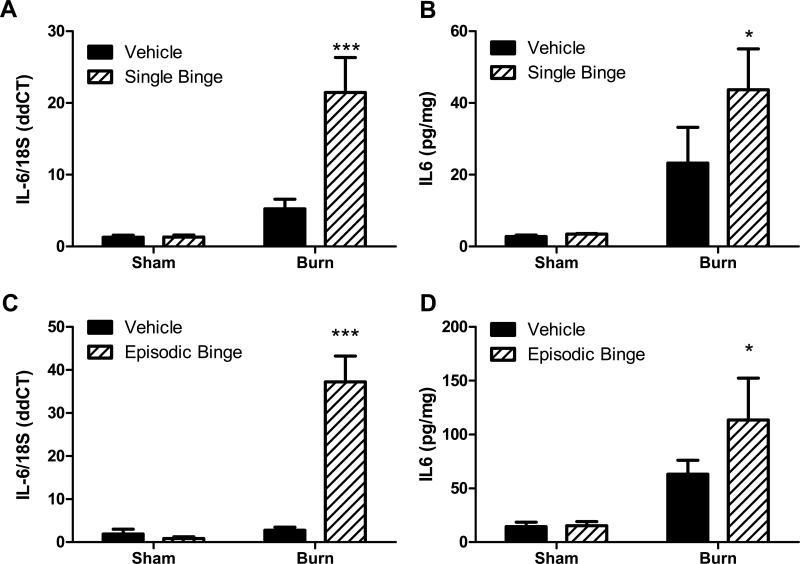
In our previous work, we demonstrated that serum IL-6 was higher at 24 hours after combined ethanol exposure and 15% total body surface area scald compared to either insult alone (Bird et al., 2010b). To determine if adipose tissue might contribute to the elevated systemic inflammation seen after ethanol and burn injury, we examined the expression of IL-6 and other inflammatory mediators in adipose tissue. As shown in Figure 1A, IL-6 mRNA levels were not altered by ethanol exposure alone and increased 4-fold with burn injury compared to sham mice. IL-6 mRNA was significantly upregulated after the combined insult when compared to all other groups (P<0.001). Likewise, the adipose tissue level of IL-6 protein was unchanged after ethanol exposure alone, and was 6-fold higher in adipose tissue from burn vehicle mice compared to sham mice (Figure 1B). Ethanol exposure doubled the burn-induced elevation in adipose IL-6 protein (Figure 1B, P<0.05 burn ethanol vs. sham groups).
Figure 1. Interleukin-6 (IL-6) levels increase after single and episodic binge ethanol exposure and burn injury.
Mice received single binge (A-B), episodic binge (C-D), or vehicle control. Thirty minutes after ethanol, mice were either subjected to burn or sham injury, sacrificed 24 hours later, and adipose tissue was isolated, as described in methods. A. IL-6 mRNA was measured by quantitative (q)PCR and normalized to 18S. N=16, 16, 18 and 19 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. There was a significant interaction between burn and ethanol (p<0.05). *** P<0.001 versus (vs.) all groups. B. Whole adipose tissue was homogenized and IL-6 protein levels were determined and normalized to the total amount of protein in the homogenate. N=4, 3, 5 and 5 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. No significant interaction was found between burn and ethanol. *P<0.05 vs. sham groups. C. After episodic binge and burn, IL-6 mRNA was measured by qPCR and normalized to 18S. N=7, 4, 7 and 10 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. There was a significant interaction between burn and ethanol (p<0.05). ***P<0.001 vs. all groups. D. IL-6 protein levels were detected after episodic binge in adipose tissue and were normalized to total protein as above. N=7, 5, 12 and 10 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. There was no significant interaction between burn and ethanol in episodic binge treatment.*P<0.05 vs. sham groups.
Further studies were conducted to determine if a more severe alcohol exposure paradigm, such as a multi-day episodic binge prior to burn would drive adipose inflammation when compared to a single binge followed by burn. To accomplish this, mice were given ethanol (or saline) for 3 days, rested 2-4 days, and exposed again for 3 days prior to burn or sham injury. Ethanol alone did not modulate IL-6 mRNA or protein expression (Figures 1C and D). After episodic binge ethanol exposure and burn, adipose tissue IL-6 mRNA levels were 18-, 42- and 12-fold higher than sham vehicle, sham alcohol and burn vehicle, respectively, (Figure 1C, P<0.001 all groups versus burn ethanol). Significant interactions existed between burn and ethanol in single binge treatment and in episodic binge for IL-6 mRNA expressions. No significant interactions were found in single and episodic binge for IL-6 protein expression. Moreover, adipose levels of IL-6 protein reached 110 pg/mg, a value which is approximately 2 times that of burn alone and 8-fold that of sham groups (Figure 1D, P<0.05). Of note, IL-6 protein levels in adipose tissue were 3 times greater in tissue obtained from episodic binge and burn injury mice compared to tissue from mice exposed to a single binge and burn (Figure 1B compared to Figure 1D).
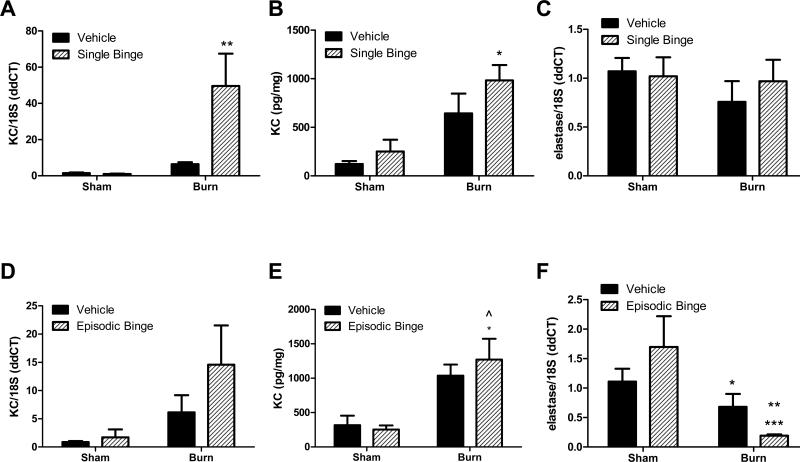
The presence of neutrophils in adipose tissue after either ethanol exposure or burn injury alone, or the combined insult was investigated, as was previously observed in gut and lung (Bird et al., 2010b, Li et al., 2011). Like IL-6, levels of the neutrophil chemokine KC were not increased after a single binge ethanol exposure, however, burn alone (in the absence of ethanol exposure) elevated KC mRNA and protein by approximately 5-fold, although this did not reach significance (Figures 2A and B). A statistically significant interaction between single binge and burn existed in the mRNA expression of KC (p<0.05), but this was lost at the protein level. Combined binge ethanol and burn exposure significantly elevated expression of KC 8-fold at the mRNA level compared to all other groups (Figure 2A, P<0.01), while ethanol plus burn increased KC protein levels 50% over burn alone (Figure 2B, P<0.05 versus sham groups). Based upon elevations in neutrophil chemokine, we next examined if there was neutrophil infiltration into adipose tissue in response to ethanol exposure and burn injury by measuring expression of elastase, an enzyme enriched in neutrophils and demonstrated to correlate with neutrophil infiltration into adipose (Talukdar et al., 2012). There were no significant changes in adipose tissue mRNA levels of neutrophil elastase following any treatment (Figure 2C). Although burn elevated KC mRNA levels compared to sham in episodic binge treatment, there were no statistically significant alterations between groups (Figure 2D). In contrast, while adipose tissue KC protein levels were not altered by ethanol alone, KC protein levels were nearly 4 times higher after burn alone compared to sham ethanol mice and were elevated 4-fold in ethanol plus burn injured mice compared to both sham groups (P<0.05, Figure 2E). There were no statistically significant interactions between burn and episodic binge in KC mRNA or protein expression. Relative to sham vehicle mice, mRNA levels of elastase were not significantly elevated after episodic binge exposure (Figure 2F). Burn alone was not different than sham vehicle, but was reduced to 40% the level of sham episodic binge (Figure 2F, P<0.05). However, episodic binge ethanol and burn injury dramatically blunted elastase expression 83% compared to sham vehicle (P<0.01) or by 89% versus sham ethanol treated mice (P<0.001, Figure 2F). This latter observation in episodic binge exposure differs from the adipose response to single ethanol exposure with and without injury where no significant differences were detected (Figures 2C and 2F). No interaction was found in elastase mRNA expression in single binge, but there was a significant interaction (p<0.05) between burn and ethanol in episodic binge.
Figure 2. Adipose expression of neutrophil chemokine KC after single and episodic binge ethanol and burn injury.
Mice received single (A-C) or episodic binge (D-F), or vehicle control and burn, as described above. A. Adipose tissue KC mRNA levels were measured by qPCR and normalized to 18S. N=20, 20, 27 and 21 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. There was a significant interaction between burn and ethanol (p<0.05). ** P<0.01 vs. all other groups. B. Whole adipose tissue homogenate levels of KC protein were determined and normalized to the total protein. N=4, 3, 5 and 4 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. No significant interaction was found between burn and ethanol. * P<0.05 vs. sham groups. C. Neutrophil elastase mRNA level was quantified by qPCR in adipose tissue and normalized to 18S. N=12, 11, 16 and 15 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. There was no significant interaction between burn and ethanol. D. After episodic binge and burn, adipose tissue KC mRNA levels were measured by qPCR and normalized to 18S. N=6, 4, 7 and 8 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. E. Whole adipose tissue KC protein levels were determined and normalized to total protein. N=7, 5, 12 and 10 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. There was no interaction between burn and ethanol (p<0.05) in episodic binge treatment. *P<0.05 vs. sham vehicle. ^P<0.05 vs. sham ethanol group. F. Neutrophil marker elastase mRNA level was quantified after episodic binge or burn by qPCR in adipose tissue. N=7, 4, 7 and 13 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. There was a significant interaction between burn and ethanol (p<0.05). *P<0.05 vs. sham ethanol. **P<0.001 vs. sham vehicle. ***P<0.001 vs. sham ethanol.
Macrophage infiltration is evident in adipose tissue after episodic binge exposure and burn injury
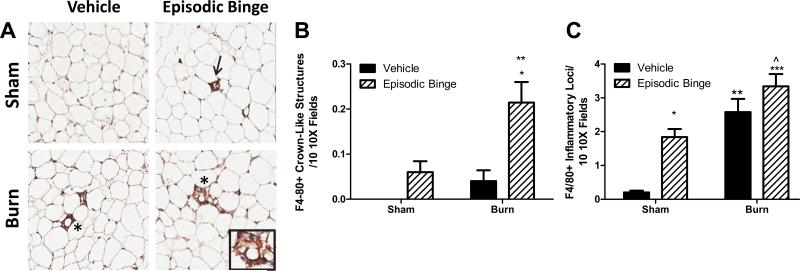
We next sought to examine if macrophages were responsible for the observed elevations in adipose IL-6 levels by investigating the degree of macrophage infiltration into adipose tissue after episodic binge ethanol exposure and burn injury. We and others have demonstrated that the formation of crown-like structures is a well-documented measure of adipose tissue inflammation that correlates with insulin resistance (Sampey et al., 2011, Kang et al., 2007, Johnson, 2012). Immunohistochemical (IHC) analysis was used to quantitate the number of F4/80-positive (F4/80+) crown-like structures. Representative images are shown in Figure 3A. No crown-like structures were detected in sham vehicle mice (Figure 3B). Mice exposed to ethanol or burn injury alone had detectable crown-like structures, but these measures did not reach statistical significance compared to sham vehicle mice (Figures 3B). However, the combined injury of episodic ethanol exposure and burn yielded a significant 2.5-and 4-fold increase in the crown-like structures compared to sham episodic binge, and burn vehicle, respectively (Figure 3B, P<0.01 both vehicle groups vs. burn ethanol, P<0.05 sham ethanol vs. burn ethanol). Sham ethanol treated adipose samples displayed an 8-fold elevation in F4/80+ inflammatory loci compared to sham vehicle (Figure 3C, P<0.05). There was a significant 12-fold increase in inflammatory loci in adipose tissue from burn vehicle, and a 16-fold increase from episodic binge and burn mice, over that of sham vehicle mice (Figure 3C, P<0.01 sham vehicle vs. burn vehicle and P<0.001 sham vehicle vs. burn ethanol). No interaction between burn and ethanol was found in episodic binge with the numbers of crown-like structures or inflammatory loci.
Figure 3. Episodic binge ethanol and burn injury drives crown like structure formation.
A. Representative immunohistochemical images of macrophage marker F4/80-positive staining in adipose tissue from vehicle, episodic binge, sham or burn injured mice (10X, N= 4,5, 5 and 7 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively). Inflammatory loci (arrow) and crown like structure (*, and inset) are indicated. Ten randomly selected 10× fields were assessed for F4/80+ crown like structures (B, P<0.01 vs. both vehicle groups, P<0.05 vs. sham ethanol) and inflammatory loci (C, *P<0.05, ** P<0.01, ***P<0.001 vs. sham vehicle mice and ^ P<0.05 vs. sham ethanol). No significant interaction was found between burn and ethanol.
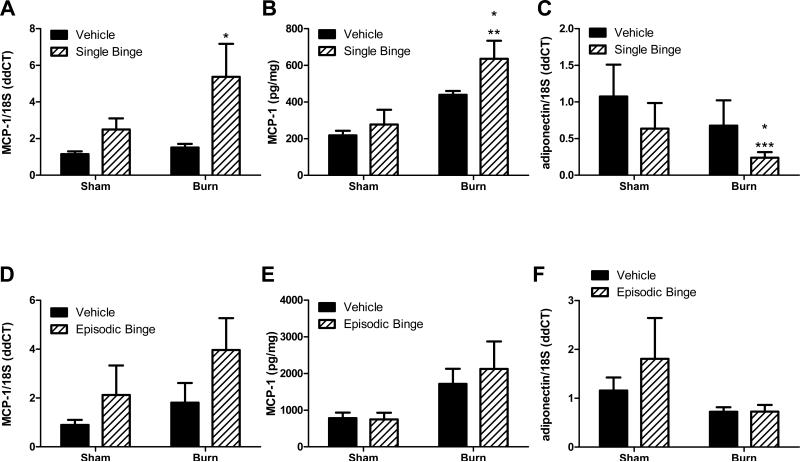
The expression of monocyte chemokine MCP-1 was next examined because it is a pro-inflammatory mediator demonstrated to be necessary and sufficient to drive macrophage infiltration into adipose tissue and induce crown-like structure formation (Johnson, 2012). In the absence of burn injury, mRNA and protein levels of MCP-1 were not altered by single binge ethanol alone (Figures 4A and B). The ethanol plus burn group displayed significant 5-fold increases in MCP-1 mRNA expression compared to both vehicle groups (Figure 4A, P<0.05). Burn injury alone doubled MCP-1 protein content in adipose versus sham vehicle (Figure 4B). MCP-1 protein was significantly increased in ethanol plus burn injured mice compared to sham treated groups (Figure 4B, P<0.01 sham vehicle versus burn ethanol, P<0.05 sham ethanol versus burn ethanol). Adiponectin is an anti-inflammatory adipokine that promotes insulin sensitivity (Johnson, 2012). Nagy et al. demonstrated down regulation of adiponectin mRNA associated with chronic ethanol-induced adipose inflammation (Kang et al., 2007). Single binge and burn significantly blunted adiponectin mRNA expression by 75% (Figure 4C, sham vehicle (P<0.001) or burn vehicle (P<0.05 vs. burn ethanol). Episodic binge did not change MCP-1 mRNA expression in mice, nor were levels significantly upregulated after burn (Figures 4D). MCP-1 protein levels were elevated more than 2-fold in mice exposed to episodic binge plus burn injury versus sham vehicle controls, although this increase was not statistically significant (Figure 4E). Importantly, MCP-1 protein levels were nearly 3 times as high in adipose tissue obtained from mice with episodic binge alcohol exposure prior to burn relative to single ethanol exposure plus burn injury (Figure 4B compared to Figure 4E). Episodic binge did not modulate adiponectin levels (Figure 4F). No significant interactions between burn and ethanol were found in MCP-1 or adiponectin mRNA or protein expression with single or episodic binge treatment.
Figure 4. Adipose chemokine levels of monocyte chemotactic protein-1 (MCP-1) and adiponectin were inversely regulated after single and episodic binge ethanol and burn injury.
Mice received single (A-C) or episodic binge (D-F), or vehicle control and burn. A. Adipose tissue MCP-1 and 18S mRNA levels were measured. N=16, 16, 20 and 19 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. * P<0.05 vs. vehicle. B. MCP-1 protein levels were examined and normalized to total protein. N=4, 3, 3 and 4 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. *P<0.05 vs. sham ethanol, **P<0.01 vs. sham vehicle. C. Adipose tissue adiponectin and 18S mRNA levels were measured. N=8, 8, 9 and 8 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. *P<0.05 vs. burn vehicle, ***P<0.001 vs. sham vehicle. D. In adipose tissue from mice exposed to episodic binge or burn, MCP-1 and 18S mRNA levels were measured. N=6, 4, 6 and 13 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. E. MCP-1 protein was normalized to the total amount of protein lysate. N=7, 4, 12 and 11 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. F. After episodic binge, adipose tissue adiponectin and 18S mRNA levels were measured. N=7, 4, 7 and 14 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. No significant interactions between burn and ethanol were found with single or episodic binge treatment for MCP-1 and adiponectin levels.
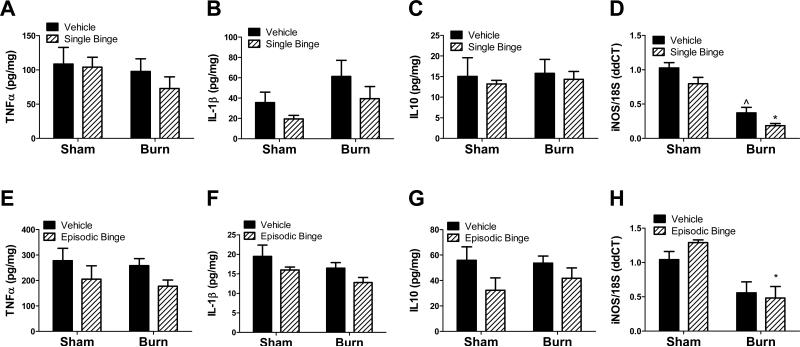
Protein levels of other pro-inflammatory (TNFα, IL-1β) or anti-inflammatory (IL-10) cytokines were not significantly different between each treatment group in single binge and burn studies (Figure 5 A-C). Interestingly, inducible nitric oxide synthases (iNOS) mRNA was not altered by ethanol exposure alone, but was significantly suppressed by 75% in both burn groups when compared to respective sham treated groups with greatest attenuation due to the combination of ethanol and burn (Figure 5D, P<0.001 burn treated groups vs. sham treated groups). Episodic binge followed by burn did not significantly change protein levels of TNFα, IL-1β and IL-10 (Figures 5 E- G). Similar to acute binge exposure and burn injury, the adipose iNOS mRNA level was not significantly altered by episodic ethanol, and was reduced by burn injury regardless of ethanol exposure. Compared to sham ethanol, burn ethanol iNOS mRNA was blunted 54% (Figure 5H, P<0.05). There were no significant interactions between burn and ethanol in any of the cytokines with single or episodic binge treatment.
Figure 5. Single and episodic binge followed by burn injury failed to upregulate pro-inflammatory mediators.
Mice received single (A-D) or episodic binge (E-H), or vehicle control and burn. A-C. Adipose tissue TNFα, IL-1β, or IL-10 was measured and normalized to total protein. N=4, 3, 5 and 5 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. D. Adipose tissue iNOS mRNA level was measured by qPCR . N=12, 12, 15 and 15 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. ***P<0.001 vs. sham groups. ^^^P<0.001 vs. sham groups. E-G. After episodic binge and burn, adipose tissue levels of TNFα, IL-1β, or IL-10 were measured and normalized to total protein. N=6, 5, 12 and 11 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. H. Adipose tissue iNOS mRNA level was measured after episodic binge by qPCR as above. N=7, 4, 7 and 13 for sham vehicle, sham ethanol, burn vehicle and burn ethanol, respectively. *P<0.05 vs. sham ethanol. There were no significant interactions between burn and ethanol detected.
DISCUSSION
Half of burn patients admitted to the hospital consumed alcohol prior to sustaining their injuries (Albright et al., 2009, Nelson and Kolls, 2002, Thai et al., 1985) . The vast majority of inebriated burn patients are not chronic alcohol abusers, but rather binge drinkers. Recent evidence suggests that binge drinking is on the rise with 1 in 6 Americans reporting an average of four binge drinking episodes per month. Thus, gaining a better understanding about the effects of episodic binge alcohol exposure on post burn morbidity and mortality is of immediate public health relevance. Burn injury induces dramatic insulin resistance, hyperlipidemia, and hyperglycemia, contributing to elevated morbidity and mortality (Bonab et al., 2010, Carter et al., 2004). Ethanol exacerbates burn-induced inflammation and impairs the immune response, thus increasing a patient's susceptibility to infection (Bird and Kovacs, 2008).
Previous work by our lab has demonstrated that IL-6 is elevated in serum, lungs and gut of animals exposed to ethanol and burn (Zahs et al., 2013, Chen et al., 2013, Li et al., 2011). We provide evidence herein that cytokines and chemokines are also elevated in adipose tissue suggesting that this tissue may be an additional source of circulating inflammatory mediators. Adipose tissue plays a critical role in maintaining systemic metabolic homeostasis, and adipose inflammation leads to metabolic dysregulation characterized by insulin resistance, hyperlipidemia and hyperglycemia – all of which commonly occur in burn patients. We set out to examine the role of adipose inflammation in response to the combined insult of ethanol exposure and burn injury. We have demonstrated that mice singly or episodically exposed to ethanol alone did not mount a dramatic inflammatory response in adipose tissue in the absence of a secondary insult such as burn. However, in mice administered a single ethanol binge and burn injury, there was a dramatic elevation in the pro-inflammatory response in adipose tissue indicated by elevations of cytokine and chemokines IL-6, KC, and MCP-1, along with a blunting of adiponectin, an anti-inflammatory adipokine. IL-6 is primarily secreted by macrophages and T cells to stimulate the immune response, e.g. during infection and after burn (Bird and Kovacs, 2008), and elevated serum IL-6 in injured patients is correlated with negative outcomes (Biffl et al., 1996). While we detected elevations in KC, we found that there was no change in neutrophil elastase in adipose tissue after single binge ethanol exposure, burn injury, or the combined insults. It is likely that 24 hours post-injury may not be the correct time point for the neutrophil composition of adipose to be dramatically regulated, unlike the neutrophil response in lung, skin, or gut. Furthermore, Nagy et al. reported down regulation of the anti-inflammatory adipokine adiponectin in chronic ethanol-induced adipose inflammation, while Xu et al. showed that increasing adiponectin improves alcoholic fatty liver disease (Kang et al., 2007, Xu et al., 2003). We demonstrate dramatic blunting of adiponectin after single binge and burn, which may aid in driving the pro-inflammatory milieu. Finally, iNOS was down-regulated by single binge ethanol and burn injury. Interestingly, Syapin et al. have demonstrated that iNOS is inhibited by ethanol in glial cells (Sanchez et al., 2007). In other tissues, burn has been shown to elevate iNOS expression or have no effect (Oppeltz et al., 2012, Babcock et al., 2012). Hence, iNOS regulation by ethanol and burn in adipose needs further investigation. Taken together, our data support the findings that adipose tissue may be a source of some circulating cytokines after single binge ethanol and burn injury.
Binge drinking is not usually a single acute event; binge drinkers tend to consume alcohol in multiple binge episodes. Using an episodic binge model, we demonstrated that burn-induced inflammation was markedly elevated in mice given multiple exposures to binge levels of ethanol and that this occurred to a greater extent than following a single binge exposure. Similar to single binge, after episodic binge and subsequent burn, IL-6 and KC were elevated compared to either insult alone or sham vehicle controls. Despite elevations in KC protein in combined injury, neutrophil elastase mRNA level was dramatically reduced in burn ethanol groups relative to either insult alone. This was a surprising finding because other insults such as a 3 day exposure to high fat diet have been shown to induce neutrophil infiltration into adipose tissue (Elgazar-Carmon et al., 2008, Talukdar et al., 2012), which persisted for up to 90 days (Talukdar et al., 2012). Perhaps a more detailed study of episodic binge and burn injury over a time course might capture neutrophil infiltration in response to high levels of KC. Even with dramatic increases in IL-6, KC, and MCP-1 associated with episodic binge ethanol exposure, iNOS was blunted similar to a single binge. It is evident that while some production of pro-inflammatory cytokines or chemokines occurs in response to burn and ethanol, some immunosuppression is concurrent. Future studies will include examining the role of IL-6 in adipose inflammation as it has been shown to be both pro-inflammatory and anti-inflammatory in binge and burn injured animals, and may mediate some of the divergent effects demonstrated.
Finally, we report for the first time that F4/80+ macrophages were detected in adipose crown-like structures and inflammatory loci resulting from episodic binge ethanol exposure and burn injury, likely due to elevations in MCP-1. MCP-1 and its receptor CCR2 have been shown to mediate macrophage infiltration into adipose tissue in response to obesity (Johnson, 2012), and these infiltrating macrophages often surround dying adipocytes forming crown-like structures (Johnson, 2012). Kang et al. have demonstrated infiltration of macrophages and production of pro-inflammatory cytokines, IL-6, MCP-1and TNFα, in adipose tissue after chronic ethanol exposure for 4 weeks (Kang et al., 2007). Our data demonstrate that macrophage infiltration occurs within days after burn and ethanol exposure.
From our studies it is evident that burn injury primarily drives inflammation in adipose tissue, and ethanol exposure prior to burn potentiates this response. The dramatic increase in magnitude of response between single and episodic binge exposure suggests that the driving factor in the inflammatory response is the frequency of the ethanol exposure, since the burn injury and time of sacrifice is identical in each group. Our findings support the relevance of adipose inflammatory response to ethanol and burn insult that warrants further investigation. Like ethanol, obesity is also associated with a prolonged increase in pro-inflammatory mediators, such as IL-6 and MCP-1, an impaired immune response, and an increased susceptibility to bacterial infection (Johnson, 2012, Milner and Beck, 2012). Work from our group and others over the past decade has linked adipose inflammation to obesity and insulin resistance (Johnson, 2012, Sampey, 2012, Sampey et al., 2011).
One mechanism linking both obesity-induced inflammation and ethanol exposure that could drive insulin resistance is an increase in gut permeability, resulting in bacterial translocation into tissues to induce both organ-specific and systemic inflammation. We have previously reported on increased gut permeability and bacterial translocation in ethanol and burn models (Zahs et al., 2013, Rendon et al., 2013, Zahs et al., 2012, Kavanaugh et al., 2005). In humans and murine models, elevated morbidity and mortality result after burn injury due to inflammation secondary to intestinal permeability and septicemia (Zahs et al., 2013, Zahs et al., 2012, Magnotti and Deitch, 2005, Kavanaugh et al., 2005, Messingham et al., 2002), which is often followed by an exaggerated alcohol-induced suppression of immune function through a lower delayed-type hypersensitivity (DTH) response and blunted lymphocyte proliferation (Messingham et al., 2002, Choudhry et al., 2000, Messingham et al., 2000, Faunce et al., 1998). At 6 and 24 hours post ethanol plus burn injury, gut permeability is compromised, which could account for elevated leukocyte infiltration, and IL-1β and IL-6 in mice exposed to ethanol then burn as compared to either insult alone (Zahs et al., 2013, Zahs et al., 2012). Gut bacteria also regulate obesity susceptibility and systemic inflammation in response to high fat diet (Turnbaugh et al., 2006). Additionally, we have previously demonstrated the dependence of toll-like receptor 4 (TLR4, but not TLR2) in ethanol and burn-induced lung pathology and pro-inflammatory response (Bird et al., 2010b). TLR4 has also been shown to be necessary for obesity-induced inflammation (Shi et al., 2006). Elevated gut permeability and bacterial translocation after the combined insult of ethanol and burn may be responsible for the rise in lipopolysaccharide (LPS, endotoxin) burden and a TLR4 dependent inflammatory response in adipose, similar to obesity.
A second mechanism regulating ethanol and burn-induced alterations in adipose inflammation is lipolysis and adipocyte apoptosis, which would release free fatty acids into the local microenvironment and circulation. In humans, chronic alcohol use correlated with reduced fat mass (Addolorato et al., 1997). Burn injury also results in loss of fat mass through apoptotic cell loss (Yasuhara et al., 2006). Duffy et al. (2009) demonstrated that insulin and glucose are elevated in patients post-burn compared to healthy controls, with burn-induced increases in cytokine release from adipose tissue macrophages and circulating monocytes, which could interfere with insulin signaling (Duffy et al., 2009). In rodents, single binge and burn drive a transient microvesicular steatosis, while chronic alcohol exposure also leads to reduced fat mass and adipocyte size, along with an increase in hepatosteatosis and induction of systemic insulin resistance (Zhong et al., 2012, Emanuele et al., 2009). It has been shown that alcohol-driven lipolysis is not catecholamine-mediated (Kang and Nagy, 2006). Ethanol has been shown to increase levels of phosphatase and tensin homolog (PTEN) and suppressor of cytokine signaling (SOCS3), which are important negative regulators of insulin signaling in both liver and adipose and can lead to elevated lipolysis and cytotoxicity (Zhong et al., 2012, Shulga et al., 2005). It is possible that saturated free fatty acids liberated by lipolysis, which are known to signal through TLRs during obesity-induced inflammation and insulin resistance (Suganami et al., 2007), may mediate a pro-inflammatory response after ethanol and burn exposure. Additionally, reduction in fat mass through apoptosis or lipolysis contributes to reduced insulin sensitivity because fat is redistributed from adipose to other metabolically sensitive tissues, such as the liver where hepatosteatosis ensues (Johnson, 2012). Liver IL-6 and other pro-inflammatory mediators increase with ethanol and/or burn exposure (Colantoni et al., 2000, Li et al., 2011). Emanuele et al. demonstrated single and/or combined injury increased hepatic ICAM-1, IL-1β, TNFα, and nuclear NF-KB (Emanuele et al., 2007) which can lead to insulin resistance. Indeed, we have previously reported that insulin administration to rodents after ethanol and burn, improves liver inflammation and microvesicular steatosis, demonstrating further evidence of links between metabolic homeostasis and inflammatory response (Emanuele et al., 2007).
Finally, a third mechanism linking ethanol intake to exacerbated inflammation is oxidative stress resulting from alcohol metabolism. Besides alcohol dehydrogenase (ADH), ethanol can also be metabolized through the microsomal ethanol oxidizing system (MEOS) by cytochrome P4502E1 (CYP2E1), which has been shown to lead to increased oxidative stress (Nagy, 2004). CYP2E1 is mainly expressed in the liver, but also found in the white adipose tissues (Tang et al., 2012). Sebastian et al. demonstrated that CYP2E1 protein levels in adipose tissue were increased after chronic ethanol feeding (Sebastian et al., 2011). In our study, we failed to find a significant increase in mRNA or protein expression of CYP2E1 in adipose tissue of episodic binge, burned mice, or the combined exposure (data not shown). This may due to the type or length of exposure, as our treatment was shorter with either a one-dose single binge or episodic binge constituting a total of 6 days of ethanol exposure.
Taken together, we report for the first time that there is an inflammatory response in adipose tissue after the combined insult of ethanol and burn injury, and that this response is augmented after episodic binge relative to a single ethanol exposure. While binge drinking leads to unintentional injuries such as falls, crashes and burns, it may also lead to more insidious tissue inflammation as a co-morbidity with obesity leading to insulin resistance. Future studies in lean versus obese rodents could yield further mechanistic insight into burn and ethanol-induced effects on local and systemic inflammation.
Acknowledgments
FUNDING
LM is supported by UNC University Cancer Research Fund, NIH NIAAA AA017376; NIH NIEHS/NCI ES019472; NIH NIDDK P30DK056350 and P30DK034987. YQ is supported by Sanofi. EJK supported by NIH R01 AA012034, NIH T32AA013527, and the Dr. Ralph and Marian C Falk Medical Research Trust. AZ was supported by NIH NRSA F31 AA019913 and MDB by NIH F32 AA018068.
Footnotes
DISCLOSURES- No conflicts of interest to disclose
References
- ADDOLORATO G, CAPRISTO E, GRECO AV, STEFANINI GF, GASBARRINI G. Energy expenditure, substrate oxidation, and body composition in subjects with chronic alcoholism: new findings from metabolic assessment. Alcohol Clin Exp Res. 1997;21:962–7. [PubMed] [Google Scholar]
- ALBRIGHT JM, KOVACS EJ, GAMELLI RL, SCHERMER CR. Implications of formal alcohol screening in burn patients. J Burn Care Res. 2009;30:62–9. doi: 10.1097/BCR.0b013e3181921f31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BABCOCK GF, HERNANDEZ L, YADAV E, SCHWEMBERGER S, DUGAN A. The burn wound inflammatory response is influenced by midazolam. Inflammation. 2012;35:259–70. doi: 10.1007/s10753-011-9313-9. [DOI] [PubMed] [Google Scholar]
- BIFFL WL, MOORE EE, MOORE FA, PETERSON VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224:647–64. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRD MD, KOVACS EJ. Organ-specific inflammation following acute ethanol and burn injury. J Leukoc Biol. 2008;84:607–13. doi: 10.1189/jlb.1107766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRD MD, MORGAN MO, RAMIREZ L, YONG S, KOVACS EJ. Decreased pulmonary inflammation after ethanol exposure and burn injury in intercellular adhesion molecule-1 knockout mice. J Burn Care Res. 2010a;31:652–60. doi: 10.1097/BCR.0b013e3181e4c58c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRD MD, ZAHS A, DEBURGHGRAEVE C, RAMIREZ L, CHOUDHRY MA, KOVACS EJ. Decreased pulmonary inflammation following ethanol and burn injury in mice deficient in TLR4 but not TLR2 signaling. Alcohol Clin Exp Res. 2010b;34:1733–41. doi: 10.1111/j.1530-0277.2010.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONAB AA, CARTER EA, PAUL K, KANEKI M, YU YM, TOMPKINS RG, FISCHMAN AJ. Effect of simvastatin on burn-induced alterations in tissue specific glucose metabolism: implications for burn associated insulin resistance. Int J Mol Med. 2010;26:311–6. [PMC free article] [PubMed] [Google Scholar]
- CARTER EA, BURKS D, FISCHMAN AJ, WHITE M, TOMPKINS RG. Insulin resistance in thermally-injured rats is associated with post-receptor alterations in skeletal muscle, liver and adipose tissue. Int J Mol Med. 2004;14:653–8. [PubMed] [Google Scholar]
- CHEN MM, BIRD MD, ZAHS A, DEBURGHGRAEVE C, POSNIK B, DAVIS CS, KOVACS EJ. Pulmonary inflammation after ethanol exposure and burn injury is attenuated in the absence of IL-6. Alcohol. 2013;47:223–9. doi: 10.1016/j.alcohol.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOUDHRY MA, MESSINGHAM KA, NAMAK S, COLANTONI A, FONTANILLA CV, DUFFNER LA, SAYEED MM, KOVACS EJ. Ethanol exacerbates T cell dysfunction after thermal injury. Alcohol. 2000;21:239–43. doi: 10.1016/s0741-8329(00)00093-8. [DOI] [PubMed] [Google Scholar]
- COLANTONI A, DUFFNER LA, DE MARIA N, FONTANILLA CV, MESSINGHAM KA, VAN THIEL DH, KOVACS EJ. Dose-dependent effect of ethanol on hepatic oxidative stress and interleukin-6 production after burn injury in the mouse. Alcohol Clin Exp Res. 2000;24:1443–8. [PubMed] [Google Scholar]
- CREE MG, WOLFE RR. Postburn trauma insulin resistance and fat metabolism. Am J Physiol Endocrinol Metab. 2008;294:E1–9. doi: 10.1152/ajpendo.00562.2007. [DOI] [PubMed] [Google Scholar]
- DUFFY SL, LAGRONE L, HERNDON DN, MILESKI WJ. Resistin and postburn insulin dysfunction. J Trauma. 2009;66:250–4. doi: 10.1097/TA.0b013e31815ebad4. [DOI] [PubMed] [Google Scholar]
- ELGAZAR-CARMON V, RUDICH A, HADAD N, LEVY R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- EMANUELE MA, EMANUELE NV, GAMELLI RL, KOVACS EJ, LAPAGLIA N. Effects of insulin on hepatic inflammation induced by ethanol and burn injury in a murine model of critical illness. J Burn Care Res. 2007;28:490–9. doi: 10.1097/BCR.0B013E318053DAED. [DOI] [PubMed] [Google Scholar]
- EMANUELE NV, EMANUELE MA, MORGAN MO, SULO D, YONG S, KOVACS EJ, HIMES RD, CALLACI JJ. Ethanol potentiates the acute fatty infiltration of liver caused by burn injury: prevention by insulin treatment. J Burn Care Res. 2009;30:482–8. doi: 10.1097/BCR.0b013e3181a28df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAUNCE DE, GREGORY MS, KOVACS EJ. Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. J Leukoc Biol. 1997;62:733–40. doi: 10.1002/jlb.62.6.733. [DOI] [PubMed] [Google Scholar]
- FAUNCE DE, GREGORY MS, KOVACS EJ. Acute ethanol exposure prior to thermal injury results in decreased T-cell responses mediated in part by increased production of IL-6. Shock. 1998;10:135–40. doi: 10.1097/00024382-199808000-00009. [DOI] [PubMed] [Google Scholar]
- FAUNCE DE, LLANAS JN, PATEL PJ, GREGORY MS, DUFFNER LA, KOVACS EJ. Neutrophil chemokine production in the skin following scald injury. Burns. 1999;25:403–10. doi: 10.1016/s0305-4179(99)00014-5. [DOI] [PubMed] [Google Scholar]
- JOHNSON AR, MILNER JJ, MAKOWSKI L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunologic Reviews. 2012;249:218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNG MK, CALLACI JJ, LAUING KL, OTIS JS, RADEK KA, JONES MK, KOVACS EJ. Alcohol exposure and mechanisms of tissue injury and repair. Alcohol Clin Exp Res. 2011;35:392–9. doi: 10.1111/j.1530-0277.2010.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANG L, NAGY LE. Chronic ethanol feeding suppresses beta-adrenergic receptor-stimulated lipolysis in adipocytes isolated from epididymal fat. Endocrinology. 2006;147:4330–8. doi: 10.1210/en.2006-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANG L, SEBASTIAN BM, PRITCHARD MT, PRATT BT, PREVIS SF, NAGY LE. Chronic ethanol-induced insulin resistance is associated with macrophage infiltration into adipose tissue and altered expression of adipocytokines. Alcohol Clin Exp Res. 2007;31:1581–8. doi: 10.1111/j.1530-0277.2007.00452.x. [DOI] [PubMed] [Google Scholar]
- KAVANAUGH MJ, CLARK C, GOTO M, KOVACS EJ, GAMELLI RL, SAYEED MM, CHOUDHRY MA. Effect of acute alcohol ingestion prior to burn injury on intestinal bacterial growth and barrier function. Burns. 2005;31:290–6. doi: 10.1016/j.burns.2004.09.021. [DOI] [PubMed] [Google Scholar]
- LI X, AKHTAR S, KOVACS EJ, GAMELLI RL, CHOUDHRY MA. Inflammatory response in multiple organs in a mouse model of acute alcohol intoxication and burn injury. J Burn Care Res. 2011;32:489–97. doi: 10.1097/BCR.0b013e3182223c9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGNOTTI LJ, DEITCH EA. Burns, bacterial translocation, gut barrier function, and failure. J Burn Care Rehabil. 2005;26:383–91. doi: 10.1097/01.bcr.0000176878.79267.e8. [DOI] [PubMed] [Google Scholar]
- MARSHALL JC. Complexity, chaos, and incomprehensibility: parsing the biology of critical illness. Crit Care Med. 2000;28:2646–8. doi: 10.1097/00003246-200007000-00080. [DOI] [PubMed] [Google Scholar]
- MCGILL V, KOWAL-VERN A, FISHER SG, KAHN S, GAMELLI RL. The impact of substance use on mortality and morbidity from thermal injury. J Trauma. 1995;38:931–4. doi: 10.1097/00005373-199506000-00019. [DOI] [PubMed] [Google Scholar]
- MESSINGHAM KA, FAUNCE DE, KOVACS EJ. Alcohol, injury, and cellular immunity. Alcohol. 2002;28:137–49. doi: 10.1016/s0741-8329(02)00278-1. [DOI] [PubMed] [Google Scholar]
- MESSINGHAM KA, FONTANILLA CV, COLANTONI A, DUFFNER LA, KOVACS EJ. Cellular immunity after ethanol exposure and burn injury: dose and time dependence. Alcohol. 2000;22:35–44. doi: 10.1016/s0741-8329(00)00100-2. [DOI] [PubMed] [Google Scholar]
- MILNER JJ, BECK MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012:1–9. doi: 10.1017/S0029665112000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURDOCH EL, BROWN HG, GAMELLI RL, KOVACS EJ. Effects of ethanol on pulmonary inflammation in postburn intratracheal infection. J Burn Care Res. 2008;29:323–30. doi: 10.1097/BCR.0b013e3181667599. [DOI] [PubMed] [Google Scholar]
- NAGY LE. Molecular aspects of alcohol metabolism: transcription factors involved in early ethanol-induced liver injury. Annu Rev Nutr. 2004;24:55–78. doi: 10.1146/annurev.nutr.24.012003.132258. [DOI] [PubMed] [Google Scholar]
- NELSON S, KOLLS JK. Alcohol, host defence and society. Nat Rev Immunol. 2002;2:205–9. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- OPPELTZ RF, RANI M, ZHANG Q, SCHWACHA MG. Gamma delta (gammadelta) T-cells are critical in the up-regulation of inducible nitric oxide synthase at the burn wound site. Cytokine. 2012 doi: 10.1016/j.cyto.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENDON JL, LI X, AKHTAR S, CHOUDHRY MA. Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury. Shock. 2013;39:11–8. doi: 10.1097/SHK.0b013e3182749f96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMPEY BP, FREEMERMAN AJ, ZHANG J, KUAN P-F, GALANAKI JA, O'CONNELL TM, ILKAYEVA OR, MUEHLBAUER MJ, STEVENS RD, NEWGARD CB, BRAUER HA, TROESTER MA, MAKOWSKI L. Metabolomic Profiling Reveals Mitochondrial-Derived Lipid Biomarkers that Drive Obesity-Associated Inflammation. PLoS One. 2012 doi: 10.1371/journal.pone.0038812. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMPEY BP, VANHOOSE AM, WINFIELD HM, FREEMERMAN AJ, MUEHLBAUER MJ, FUEGER PT, NEWGARD CB, MAKOWSKI L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity (Silver Spring) 2011;19:1109–17. doi: 10.1038/oby.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCHEZ AC, DAVIS RL, SYAPIN PJ. The Oct DNA motif participates in the alcohol inhibition of the inducible nitric oxide synthase gene promoter in rat C6 glioma cells. Brain Res. 2007;1179:16–27. doi: 10.1016/j.brainres.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEBASTIAN BM, ROYCHOWDHURY S, TANG H, HILLIAN AD, FELDSTEIN AE, STAHL GL, TAKAHASHI K, NAGY LE. Identification of a cytochrome P4502E1/Bid/C1q-dependent axis mediating inflammation in adipose tissue after chronic ethanol feeding to mice. J Biol Chem. 2011;286:35989–97. doi: 10.1074/jbc.M111.254201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHI H, KOKOEVA MV, INOUYE K, TZAMELI I, YIN H, FLIER JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHULGA N, HOEK JB, PASTORINO JG. Elevated PTEN levels account for the increased sensitivity of ethanol-exposed cells to tumor necrosis factor-induced cytotoxicity. J Biol Chem. 2005;280:9416–24. doi: 10.1074/jbc.M409505200. [DOI] [PubMed] [Google Scholar]
- SILVER GM, ALBRIGHT JM, SCHERMER CR, HALERZ M, CONRAD P, ACKERMAN PD, LAU L, EMANUELE MA, KOVACS EJ, GAMELLI RL. Adverse clinical outcomes associated with elevated blood alcohol levels at the time of burn injury. J Burn Care Res. 2008;29:784–9. doi: 10.1097/BCR.0b013e31818481bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGANAMI T, TANIMOTO-KOYAMA K, NISHIDA J, ITOH M, YUAN X, MIZUARAI S, KOTANI H, YAMAOKA S, MIYAKE K, AOE S, KAMEI Y, OGAWA Y. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- TALUKDAR S, OH DY, BANDYOPADHYAY G, LI D, XU J, MCNELIS J, LU M, LI P, YAN Q, ZHU Y, OFRECIO J, LIN M, BRENNER MB, OLEFSKY JM. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012 doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANG H, SEBASTIAN BM, AXHEMI A, CHEN X, HILLIAN AD, JACOBSEN DW, NAGY LE. Ethanol-induced oxidative stress via the CYP2E1 pathway disrupts adiponectin secretion from adipocytes. Alcohol Clin Exp Res. 2012;36:214–22. doi: 10.1111/j.1530-0277.2011.01607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THAI AC, YEO PP, CHEAH JS. Hypoglycaemia in diabetes mellitus. Ann Acad Med Singapore. 1985;14:354–9. [PubMed] [Google Scholar]
- THORELL A, NYGREN J, LJUNGQVIST O. Insulin resistance: a marker of surgical stress. Curr Opin Clin Nutr Metab Care. 1999;2:69–78. doi: 10.1097/00075197-199901000-00012. [DOI] [PubMed] [Google Scholar]
- TURNBAUGH PJ, LEY RE, MAHOWALD MA, MAGRINI V, MARDIS ER, GORDON JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- XU A, WANG Y, KESHAW H, XU LY, LAM KS, COOPER GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YASUHARA S, KANEKI M, SUGITA H, SUGITA M, ASAI A, SAHANI N, CHON JY, TOMPKINS RG, MARTYN JA. Adipocyte apoptosis after burn injury is associated with altered fat metabolism. J Burn Care Res. 2006;27:367–76. doi: 10.1097/01.BCR.0000216777.94365.47. [DOI] [PubMed] [Google Scholar]
- ZAHS A, BIRD MD, RAMIREZ L, CHOUDHRY MA, KOVACS EJ. Anti-IL-6 Antibody Treatment but Not IL-6 Knockout Improves Intestinal Barrier Function and Reduces Inflammation After Binge Ethanol Exposure and Burn Injury. Shock. 2013;39:373–9. doi: 10.1097/SHK.0b013e318289d6c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAHS A, BIRD MD, RAMIREZ L, TURNER JR, CHOUDHRY MA, KOVACS EJ. Inhibition of Long Myosin Light Chain Kinase Activation Alleviates Intestinal Damage after Binge Ethanol Exposure and Burn Injury. Am J Physiol Gastrointest Liver Physiol. 2012 doi: 10.1152/ajpgi.00157.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHONG W, ZHAO Y, TANG Y, WEI X, SHI X, SUN W, SUN X, YIN X, KIM S, MCCLAIN CJ, ZHANG X, ZHOU Z. Chronic Alcohol Exposure Stimulates Adipose Tissue Lipolysis in Mice Role of Reverse Triglyceride Transport in the Pathogenesis of Alcoholic Steatosis. Am J Pathol. 2012 doi: 10.1016/j.ajpath.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]