Abstract
Fragile X Syndrome (FXS), the most common cause of inherited mental retardation and autism, is caused by transcriptional silencing of Fmr1, which encodes the translational repressor protein FMRP. FMRP and CPEB, an activator of translation, are present in neuronal dendrites, are predicted to bind many of the same mRNAs, and may mediate a translational homeostasis that, when imbalanced, results in FXS. Consistent with this possibility, Fmr1-/y Cpeb−/− double knockout mice displayed significant amelioration of biochemical, morphological, electrophysiological, and behavioral phenotypes associated with FXS. Acute depletion of CPEB in the hippocampus of Fmr1 -/y mice rescued working memory deficits, demonstrating reversal of this FXS phenotype in adults. Finally, we find that FMRP and CPEB balance translation at the level of polypeptide elongation. Our results suggest that disruption of translational homeostasis is causal for FXS, and that the maintenance of this homeostasis by FMRP and CPEB is necessary for normal neurologic function.
Fragile X Syndrome (FXS), the most common inherited form of mental retardation and syndromic autism1,2, is caused by inactivation of the Fmr1 gene3. FXS phenotypes include cognitive dysfunction, repetitive behaviors, anxiety, seizures and morphological abnormalities1. Fmr1 encodes the Fragile X Mental Retardation Protein (FMRP), a widely expressed RNA binding protein (RBP) that is found in neuronal soma and synapto-dendrites4. FMRP binds target mRNAs predominantly in coding regions and likely represses translation by tempering transit of polypeptide-elongating ribosomes5. FMRP responds to signaling from group I metabotropic glutamate receptors (mGluRs)6,7, which mediate its translation-repressing activity8. In Fmr1 -/y (Fmr1 KO) mice, protein synthesis is elevated by ~20%7,9–11, which most probably is responsible for the synapse dysmorphogenesis, aberrant synaptic plasticity7,12, and behavioral and cognitive dysfunctions displayed by FXS children and animal models. FMRP binds >1000 mRNAs in the brain5 and determining which contribute to FXS pathophysiology is a daunting task.
The cytoplasmic polyadenylation element binding protein (CPEB) also affects synapse function13–16; it binds the 3’ UTR cytoplasmic polyadenylation element (CPE) of target mRNAs and stimulates polyadenylation-induced translation13,17. In neurons, CPEB is localized to synapto-dendrites13,18 and activates translation in response to synaptic stimulation17,18. Cpeb −/− knockout (KO) mice display neurological phenotypes including perseverative hippocampal-dependent memory19 and defects in long-term potentiation15,underscoring the importance of CPEB in regulating translation and neural function.
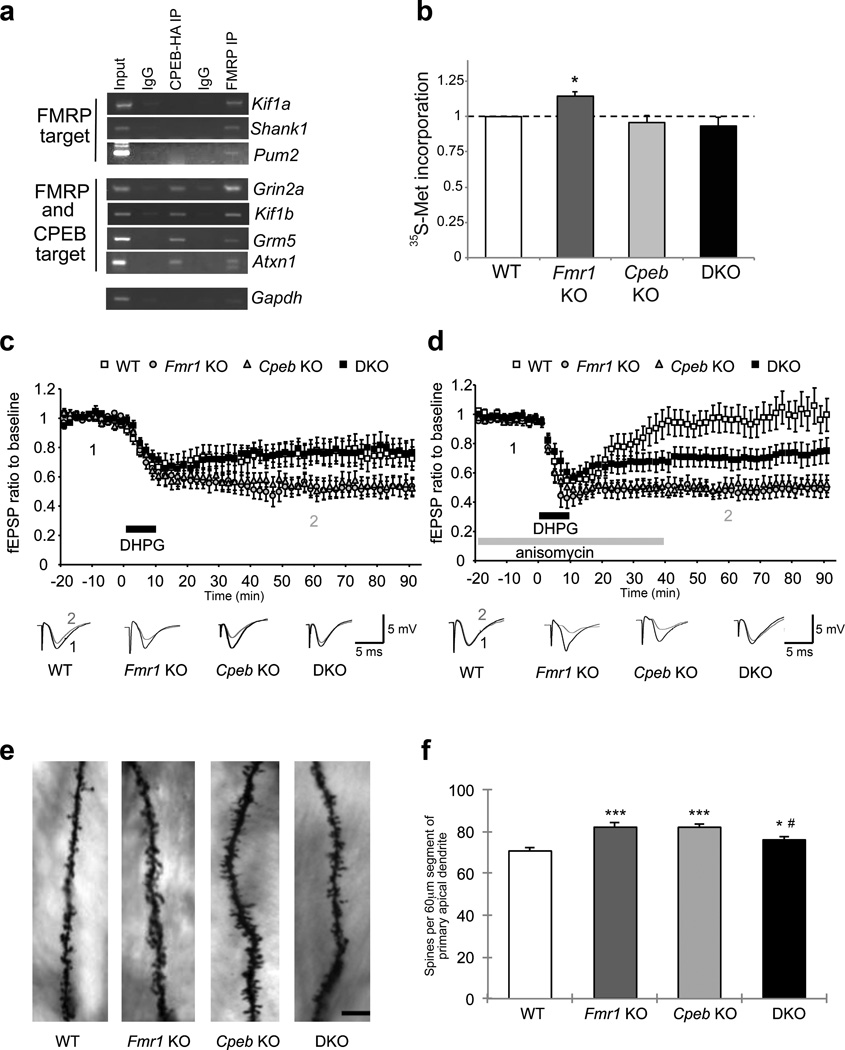
We hypothesized that the translational imbalance and associated pathophysiologies in FXS animals might be rescued if the loss of FMRP was accompanied by ablation of a factor that stimulates rather than represses translation. In the normal condition, translation could be balanced by FMRP and factor(s) X; unbalanced translation due to the loss of FMRP yields FXS but perhaps ablation of the counter-balancing factor(s) could rebalance translation and restore normal neurologic function (Supplementary Fig. 1a). We surmised that factor X could be CPEB because about one-third of FMRP-bound mRNAs5 contain 3’UTR CPEs and thus are potential targets for CPEB (Supplementary Fig. 2b, Supplementary Table 1); indeed several mRNAs co-immunoprecipitate with both FMRP and CPEB (Fig. 1a). Moreover, CPEB and FMRP co-localize in dendrites of cultured hippocampal neurons (Supplementary Fig. 2a,b) and co-purify in neuroblastoma cells and in vitro (Supplementary Fig. 2c–f). Consequently, we generated Fmr1-/y Cpeb−/− double KO (DKO) mice (Supplementary Fig. 3) and examined protein synthesis in them. Although Fmr1 KO animals displayed a ~15% increase in protein synthesis in acute hippocampal slices, the DKO mice were similar to WT (Fig. 1b), suggesting that translational homeostasis was restored in these animals. There was no significant decrease in protein synthesis in the Cpeb KO animals.
Figure 1. Interplay between FMRP and CPEB in the brain.
a, Co-immunoprecipitation of RNAs with FMRP or epitope-taqged CPEB from neurons. IgG served as a negative control. b, Acute hippocampal slices were labeled with 35S-methionine and incorporation into protein was determined by scintillation counting (n=4; ANOVA, F(3,12)=5.458, p<0.05,). * p<0.05 as compared to wild-type (student’s t-test). Error bars are S.E.M. c, DHPG-induced LTD at Schaffer collateral-CA1 synapses was enhanced in the Fmr1 KO (n=15, ANOVA, p< 0.001) and Cpeb KO (n=19, ANOVA, p< 0.001) relative to WT (n=12), however, it was nearly identical to WT in the DKO (n=14, ANOVA, p> 0.05). b, Anisomycin inhibited the DHPG-induced LTD in WT (n=13), but not in the Fmr1 KO (n=8), Cpeb KO (n=13) (ANOVA, p< 0.001), or DKO (n=16) (ANOVA, p< 0.001). DHPG-induced LTD with anisomycin in the DKO slices was significantly different from the LTD observed in Fmr1, Cpeb and WT groups. (ANOVA, p<0.001). c, Representative images of Golgi-Cox stained dendritic segments of CA1 pyramidal neurons of the hippocampus from WT, Fmr1 KO, Cpeb KO, and DKO mice. Scale bar, 20 µm. d, Total number of spines were quantified along a 60-µm segment from the origin of primary apical dendritic branches of Golgi-Cox stained CA1 pyramidal neurons. n=26 dendritic segments, ***p<0.001, *p<0.05, as compared to WT (ANOVA). #p<0.05 by two-way ANOVA (Fmr1 KO vs. DKO). Error bars are S.E.M.
We examined the levels of specific proteins in hippocampal lysates from mice of all four genotypes (Supplementary Table 2). The amounts of several proteins encoded by FMRP target mRNAs was increased in Fmr1 KO animals. Consistent with previous reports of CPEB function, translation of many mRNAs was decreased in Cpeb KO animals. In DKO animals, translation of FMRP targets were either similar to WT or decreased as in the Cpeb KO hippocampus. These results suggest that a “brake” is placed on the runaway translation of the Fmr1 KO brain in DKO animals. We also examined specific protein levels by Western blot in cortical samples, but found few significant differences between WT and Fmr1 KO animals (Supplementary Table 3).
Because several studies noted an increase in mTOR and ERK1/2 signaling in Fmr1 KO animals9,11, we examined the phosphorylation states and relative levels of several components of these signaling pathways. We did not observe a consistent pattern of enhanced signaling through either pathway. P70 ribosomal S6 kinase 1 (S6K1) lies downstream of both the ERK and mTOR pathways and while its phosphorylation was not significantly altered, phosphorylation of its immediate downstream target, ribosomal protein S6, was significantly increased in the Fmr1 KO hippocampus9. The levels of phospho-S6 were similar in Cpeb KO and DKO animals, indicating that signaling through S6K1 is restored in DKO animals. By analyzing the levels of specific proteins (Supplementary Table 2) as well as general protein synthesis (Fig. 1b), we demonstrate that CPEB and FMRP do indeed balance translation in the brain.
Metabotropic glutamate receptors (mGluRs) are hyper-activated in Fmr1 KO mice as evidenced by enhanced mGluR-dependent LTD at Schaffer collateral-CA1 synapses in the hippocampus7,12,20. Fmr1 KO and Cpeb KO mice both displayed exaggerated LTD in hippocampal slices treated with DHPG, a group I mGluR agonist12. LTD was restored to WT levels in slices derived from DKO animals (Fig. 1c). Basal synaptic transmission and presynaptic function were not affected in the DKO animals (Supplementary Fig. 4a, b). Because DHPG-induced LTD is a translation-dependent process12, we determined whether application of the protein synthesis inhibitor anisomycin attenuated LTD in hippocampal slices. Anisomycin inhibited LTD in WT slices but had no effect on Fmr1 KO, Cpeb KO, or DKO slices (Fig. 1d). These results suggest that the protein synthesis-dependency in the DKO slices occurs before mGluR stimulation, perhaps during synapse formation.
Long-term potentiation (LTP) is also affected in the Fmr1 KO hippocampus under certain stimulation protocols21. We reported a deficit in LTP induced by theta burst stimulation (TBS-LTP) in Cpeb KO mice15,16. We therefore examined TBS-LTP in Fmr1 KO and DKO animals (Supplementary Fig. 4c–e). Although Fmr1 KO animals displayed normal TBS-LTP, DKO animals also displayed normal TBS-LTP, suggesting the deficit in TBS-LTP previously seen in Cpeb KO animals is rescued by the absence of FMRP. However, when LTP is induced by 1mM glycine (Gly-LTP) which requires mGluR receptor function21, Fmr1 KO animals showed a significant deficit compared to WT animals, which was again rescued in the DKO. It therefore seems that both LTP and LTD defects in synaptic plasticity in the Fmr1 KO hippocampus are largely dependent upon mGluR signaling and are both rescued in DKO animals.
Dendritic spine density, which is commonly increased in FXS7,9 was enhanced in vivo in both Fmr1 KO and Cpeb KO mice on the primary apical dendritic branches of hippocampal CA1 pyramidal neurons (Fig. 1e,f; Supplementary Fig. 5a,b). Spine density was significantly reduced in the DKO neurons, demonstrating that FMRP and CPEB balance one another to control synapse number. We further compared synapse formation in hippocampal and cortical neuron cultures22, and found in both cases that a significant increase in synaptic puncta in Fmr1 KO neurons was partially restored in DKO neurons (Supplementary Fig. 6a–d). Total body weight, which is increased in adolescent Fmr1 KO animals, was reversed to WT level in DKO animals (Supplementary Fig. 5c), suggesting that the interplay between FMRP and CPEB that leads to phenotypic rescue is not limited to the brain.
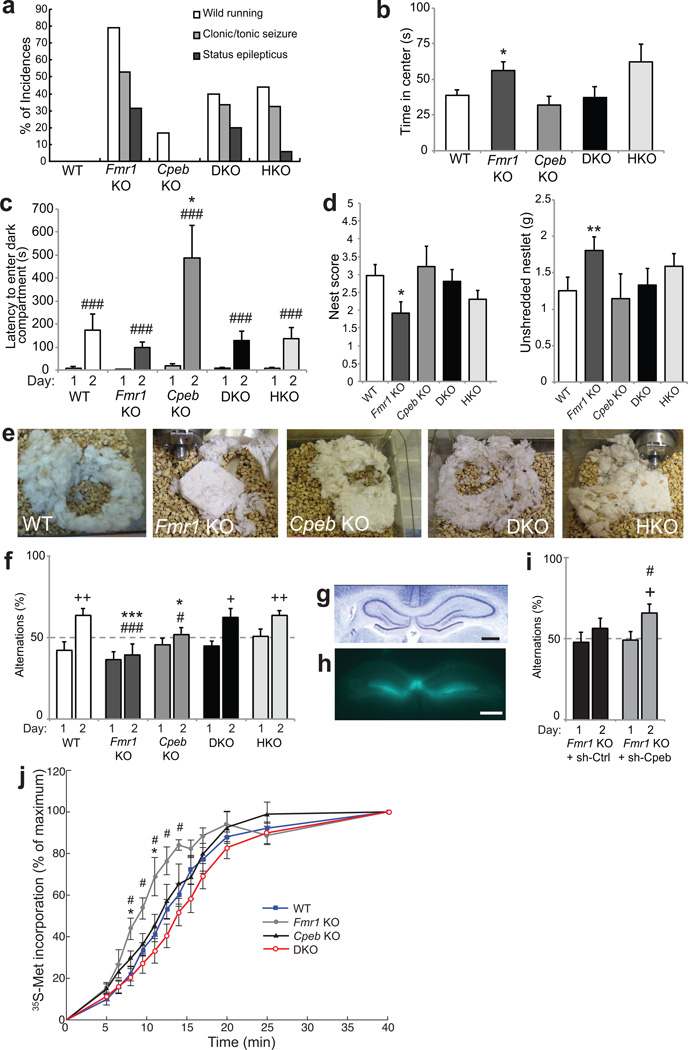
To examine whether higher neural function is rescued in the DKO mice, we tested animals for their sensitivity to acoustic stimulation, which in Fmr1 KO mice results in an initial period of wild running followed by clonic/tonic seizures, status epilepticus, and in some cases, death7,11. WT and Cpeb KO animals were little affected by the acoustic stimulus, yet the Fmr1 KO mice displayed the expected running and seizure responses (Fig. 2a). The seizure phenotype was reduced by ~50% in the DKO animals as well as in Fmr1 -/y, Cpeb +/− heterozygous (HKO) animals.
Figure 2. Cpeb deletion ameliorates FXS-related behavioral abnormalities.
a, Audiogenic seizure in WT (n=6), Fmr1 KO (n=19), Cpeb KO (n=6), DKO (n=15), and Fmr1 KO/Cpeb heterozygous (HKO) (n=34) P19–21 mice. Seizure reduction in the DKO (40%) and HKO (44%) compared to Fmr1 KO was significant (79%, p<0.05, Fisher exact test). b, Time spent in the center of an open field for 15 min (n=9–11, ANOVA, F(4,46)=3.28, p<0.05). c, Passive avoidance memory measured by latency to enter a dark compartment 24 hours after foot shock in the dark (n=8–11, ANOVA on effect of day F(1,44)=43.5, p<0.001; on genotype F(4,44)=5.04, p<0.01). d, Nest building scored on a scale of 1–542 (left) and weight of unused nesting 24 hours after 2.5g nestlet presentation (n=9–18, ANOVA, F(4,70)=2.93, p=0.058 and F(4,70)=1.63, p=0.17, respectively) (right). e, nests after 72 hours. f, T-maze assay of spontaneous alternations (n=8–11, ANOVA on genotype F(4,44)=4.48, p<0.01). g, Hematoxylin/eosin stained hippocampus. Bar = 1mm. h, hippocampus after bilateral injection with GFP/sh-RNA-expressing lentivirus. Bar = 1mm. i, T-maze assay after bilateral hippocampal injection of Fmr1 KO mice with lentivirus expressing sh-Ctrl or sh-Cpeb (n=8–11 per treatment/genotype). j, Ribosome transit rate (RTR) in brain lysates. Quantification of radiolabel incorporation was plotted as a percentage of the maximum (40 min.). WT vs. Fmr1 KO, *p<0.05; Fmr1 KO vs. DKO, #p<0.05 (ANOVA, followed by Bonferroni’s post-hoc test, n= 3–4 per genotype; Supplementary Table 5). For all panels, * and **, p<0.05 and 0.01, respectively compared to wild-type (Mann-Whitney U test); # and ###, p< 0.05 and 0.001, respectively compared to performance on day 1 (paired t-test); + and ++ p< 0.05 and 0.01, respectively (Wilcoxon signed-rank test to test the difference from chance level of 50%). Error bars are S.E.M.
Fmr1 KO mice displayed decreased anxiety as suggested by their tendency to remain in the center of an open experimental field 45% longer than WT animals, which preferentially moved about the periphery23 (Fig. 2b). Cpeb KO animals were comparable to WT with respect to time in the center field, although they moved slightly less than WT (Supplementary Fig. 7a). The DKO animals were indistinguishable from WT for time in the center and distance moved. Reducing the CPEB dose by one-half (HKO) did not ameliorate the anxiety phenotype.
We examined learning and memory by assessing passive avoidance. Mice placed into a light chamber of a two chamber test apparatus readily moved to the dark chamber where a foot shock was administered. After 24 hours, the test was repeated and in all genotypes there was increased latency in entering the dark chamber, indicating memory of the foot shock. Although we did not observe a statistical difference in the learning ability between WT and Fmr1 KO animals, the Cpeb KO animals displayed increased latency to move to the dark field, indicative of enhanced learning (Fig. 2c). This exaggerated learning was restored to WT levels in the DKO mice, demonstrating that the loss of FMRP corrected for the loss of CPEB. These results support the hypothesis that FMRP and CPEB maintain a functional homeostasis in the brain that is critical for normal cognition. Normal circadian activity was observed for all genotypes (Supplementary Fig. 7b).
Nest building is a social behavior in mice that is affected in another model of syndromic autism24. Squares of pressed cotton nesting material (Nestlet) presented to the animals were scored 24 and 72 hours later for shape; the amount of unused Nestlet was also measured. At 24 hours, nest quality of Fmr1 KO animals was poorer than those of WT (Fig. 2d). The Fmr1 KO mice also used less material in nest construction (Fig. 2d, e). The nest quality and Nestlet usage of both Cpeb KO and DKO animals were not distinguishable from WT. Nest quality of the four genotypes was not due to time required for construction as similar results were obtained after 72 hours (Supplementary Fig. 7c,d). Therefore, this autism-related paradigm is rescued by simultaneous depletion of FMRP and CPEB.
We examined spatial working memory using a T-maze assay. Animals were placed in the start arm of a T-shaped maze and allowed to explore. Upon reaching the junction of the T arms, the animal must move right or left. Once the left/right choice was made, access to the non-chosen arm was denied by a door until the animal returned on its own to the start arm. The door was then re-opened and the paradigm repeated until the animal made 15 choices. WT mice chose right or left arms randomly on day 1 but chose alternating arms on day 2 suggesting they were aware of which arm they had most recently visited, a characteristic of working memory known as spontaneous alternation (SA) (Fig. 3f). Both Fmr1 KO and Cpeb KO animals had deficient SA on day 2; the DKO and HKO animals, however, displayed SA indistinguishable from WT, indicating a rescue of working memory. Working memory is thought to involve the hippocampus, particularly in adolescent mice25,26. To determine whether acute depletion of CPEB could rescue working memory, we injected lentivirus encoding GFP and shRNA targeting Cpeb (sh-Cpeb), or a non-targeting control (sh-Ctrl), bilaterally into the hippocampi of 30 day old Fmr1 KO animals (Fig. 3g,h). After allowing for efficient depletion (Supplementary Fig. 7e,f), injected animals were tested in the T-maze. Sh-Ctrl injected Fmr1 KO animals displayed SA on day 2 that was not statistically distinct from random chance (50%) (Fig. 3i). Amazingly, sh-Cpeb injected Fmr1 KO animals demonstrated significant improvement in SA on day 2 compared to the random chance level, demonstrating improvement in working memory upon acute depletion of CPEB.
FMRP predominantly binds the coding regions of mRNAs and may slow the translocation of ribosomes during polypeptide elongation5,27. In the absence of FMRP, ribosomes can translocate unimpeded and will produce more polypeptide from target mRNAs (Supplementary Fig 8a). Because general and specific protein synthesis is restored in DKO animals (Fig. 1b, Supplementary Table 2), we determined whether this rescue could be attributed to a restoration of ribosome translocation rate. We employed an assay to monitor the ribosome transit rate (RTR) in brain (less cerebellum and midbrain) lysates that is based on the run-off of ribosomes that are actively engaged in translation5. 35S-methoinine/cysteine was added to lysates from the four genotypes and the ribosomes allowed to elongate polypeptides without new initiation (Supplementary Fig. 8b, ref 7). When all active ribosomes have completed their elongation phase, the radioactive signal reaches saturation, which corresponds to the RTR28. Our results show that brain extracts derived from Fmr1 KO mice have an enhanced RTR compared to WT (Fig. 2j, Supplementary Table 4). The RTRs in Cpeb KO and DKO samples were indistinguishable from WT, demonstrating complete restoration of polypeptide elongation rate. These results identify the polypeptide elongation machinery as being essential for the translational homeostasis maintained by FMRP and CPEB in the brain.
Brain-associated FXS pathophysiologies are likely caused by excessive translation in neurons2,7,9,11, which may also occur in syndromic and idiopathic forms of autism29,30. We have restored the translational landscape that is altered in FXS by genetically ablating CPEB, which we surmised acts as an mRNA-specific translational counterweight to FMRP in the brain. When coupled to the loss of FMRP, the additional loss of CPEB restores several biochemical, morphological, electrophysiological, and behavioral phenotypes associated with FXS (Supplementary Table 5). Furthermore, our data, which confirm and extend the observations of several laboratories5,27 indicating that FMRP inhibits polypeptide elongation, demonstrate that ribosome transit times are excessive in FMRP KO brains but are mitigated with the concomitant loss of CPEB. This targeting of the ribosome transit machinery by FMRP and CPEB suggests that our original hypothesis, the balance of mRNA-specific translation to maintain normal neural function requires modification. Although these two proteins could act on common mRNAs that mediate polypeptide elongation such as eEF2 (Supplementary Table 2), they might also regulate distinct components to maintain elongation equilibrium. Indeed, one issue arising from our study is how the loss of CPEB, which alone has no detectable effect on ribosome transit, palliates this process upon loss of FMRP. Cytoplasmic polyadenylation-induced translation is a main CPEB activity that could regulate an elongation “sensor” that is involved in restoring protein synthesis (Supplementary Fig. 8c).
Because the Fmr1 KO and Cpeb KO mice display enhanced DHPG-induced LTD, it is not intuitively obvious how this measure of synaptic efficacy is rescued in the DKO. FMRP and CPEB are both downstream of Group I mGluRs because the KO of each has a deficient response to DHPG treatment. These proteins are unlikely to be completely epistatic to one another and could reside at least partially in different regulatory pathways such that biologic responses to null mutations in each gene could be cancelled by null mutations in both genes. If such pathways include positive and negative influences on LTD, then one could envision how two wrongs (e.g., immature dendritic spines) make a right (i.e., near WT spines in the DKO).
We rescued working memory, an FXS-associated pathology in adult Fmr1 KO mice by injecting lentivirus expressing shRNA for CPEB bilaterally into the hippocampus. Working memory requires the hippocampus as well as the prefrontal cortex but is generally thought to be independent of protein synthesis26. Although protein synthesis is not required for the short working memory time (several minutes), the CPEB shRNA was injected 10 days prior to the memory test, sufficient time for it to alter translation homeostasis. These experiments demonstrate that at least this one characteristic of FXS, which is generally thought to be a developmental disease, can be reversed in the adult. Therefore, therapies that target CPEB and/or the translational apparatus, particularly the polypeptide elongation machinery, might be efficacious in ameliorating other features of FXS.
Supplementary Material
Acknowledgements
We thank N. Dawra for technical assistance, P. Lombroso and C. Proud for kind gifts of antibodies, J. Pelletier for the kind gift of hippuristanol, and members of the Richter lab for helpful discussions. T.U. and N.G.F. gratefully acknowledge fellowships from the FRAXA Research Foundation. J.A.H. was supported by NIH-NRSA Postdoctoral Fellowship F32GM095060. This work was supported by NIH grants GM46779 and NS079415 to J.D.R. and MH086509 to S. Akbarian.
Footnotes
Author Contributions:
T.U. and J.D.R. conceived of the initial project with much input from L.J.L. T.U., N.G.F., and M.J. designed and performed the majority of the experiments. H.K. and E.K. performed the electrophysiology experiments in Figure 1c,d and Supplementary Fig. 4a–b. J.M.A. performed the electrophysiology experiments in Supplementary Fig. 4c–e. S. Anilkumar and S.C. performed spine density analysis in Fig. 1e,f and Supplementary Fig. 5a–b. M.I. created and tested the CPEB antibody used in Supplementary Fig 2f. J.A.H performed the bioinformatics analysis in Supplementary Table 1. V.C.N. and G.J.B. performed the immunocytochemistry analysis in Supplementary Fig. 2a–b. N.G.F. and J.D.R. wrote the manuscript. All authors contributed to interpretation and discussion of results and to editing of the manuscript.
The authors declare no competing financial interests.
References
- 1.Penagarikano O, Mulle JG, Warren ST. The pathophysiology of fragile x syndrome. Annu Rev Genomics Hum Genet. 2007;8:109–129. doi: 10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- 2.Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu Rev Pathol. 2012;7:219–245. doi: 10.1146/annurev-pathol-011811-132457. [DOI] [PubMed] [Google Scholar]
- 3.Verkerk AJ, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 4.Feng Y, et al. Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci. 1997;17:1539–1547. doi: 10.1523/JNEUROSCI.17-05-01539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darnell JC, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Dolen G, et al. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharya A, et al. Genetic Removal of p70 S6 Kinase 1 Corrects Molecular, Synaptic, and Behavioral Phenotypes in Fragile X Syndrome Mice. Neuron. 2012;76:325–337. doi: 10.1016/j.neuron.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin M, Kang J, Burlin TV, Jiang C, Smith CB. Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the FMR1 null mouse. J Neurosci. 2005;25:5087–5095. doi: 10.1523/JNEUROSCI.0093-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J Neurosci. 2010;30:15616–15627. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 13.Wu L, et al. CPEB-mediated cytoplasmic polyadenylation and the regulation of experience-dependent translation of alpha-CaMKII mRNA at synapses. Neuron. 1998;21:1129–1139. doi: 10.1016/s0896-6273(00)80630-3. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Yario TA, Steitz JA. A molecular link between SR protein dephosphorylation and mRNA export. Proc Natl Acad Sci U S A. 2004;101:9666–9670. doi: 10.1073/pnas.0403533101. Epub 2004 Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alarcon JM, et al. Selective modulation of some forms of schaffer collateral-CA1 synaptic plasticity in mice with a disruption of the CPEB-1 gene. Learn Mem. 2004;11:318–327. doi: 10.1101/lm.72704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zearfoss NR, Alarcon JM, Trifilieff P, Kandel E, Richter JD. A molecular circuit composed of CPEB-1 and c-Jun controls growth hormone-mediated synaptic plasticity in the mouse hippocampus. J Neurosci. 2008;28:8502–8509. doi: 10.1523/JNEUROSCI.1756-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang YS, Jung MY, Sarkissian M, Richter JD. N-methyl-D-aspartate receptor signaling results in Aurora kinase-catalyzed CPEB phosphorylation and alpha CaMKII mRNA polyadenylation at synapses. EMBO J. 2002;21:2139–2148. doi: 10.1093/emboj/21.9.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Udagawa T, et al. Bidirectional control of mRNA translation and synaptic plasticity by the cytoplasmic polyadenylation complex. Mol Cell. 2012;47:253–266. doi: 10.1016/j.molcel.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger-Sweeney J, Zearfoss NR, Richter JD. Reduced extinction of hippocampal-dependent memories in CPEB knockout mice. Learn Mem. 2006;13:4–7. doi: 10.1101/lm.73706. [DOI] [PubMed] [Google Scholar]
- 20.Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang Y, et al. Fragile X mental retardation protein is required for chemically-induced long-term potentiation of the hippocampus in adult mice. J Neurochem. 2009;111:635–646. doi: 10.1111/j.1471-4159.2009.06314.x. [DOI] [PubMed] [Google Scholar]
- 22.Pfeiffer BE, Huber KM. Fragile X mental retardation protein induces synapse loss through acute postsynaptic translational regulation. J Neurosci. 2007;27:3120–3130. doi: 10.1523/JNEUROSCI.0054-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avgustinovich DF, Lipina TV, Bondar NP, Alekseyenko OV, Kudryavtseva NN. Features of the genetically defined anxiety in mice. Behav Genet. 2000;30:101–109. doi: 10.1023/a:1001999020138. [DOI] [PubMed] [Google Scholar]
- 24.Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- 25.Laroche S, Davis S, Jay TM. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10:438–446. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 27.Feng Y, et al. FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol Cell. 1997;1:109–118. doi: 10.1016/s1097-2765(00)80012-x. [DOI] [PubMed] [Google Scholar]
- 28.Richter JD, Wasserman WJ, Smith LD. The mechanism for increased protein synthesis during Xenopus oocyte maturation. Dev Biol. 1982;89:159–167. doi: 10.1016/0012-1606(82)90304-9. [DOI] [PubMed] [Google Scholar]
- 29.Santini E, et al. Exaggerated translation causes synaptic and behavioural aberrations associated with autism. Nature. 2013;493:411–415. doi: 10.1038/nature11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gkogkas CG, et al. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature. 2013;493:371–377. doi: 10.1038/nature11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.