During discussions of intraoperative monitoring with our anesthesiology residents, I like to describe the following clinical scenario involving a new patient and a rather unusual cardiologist. After waiting months for an appointment, Mrs. Jones is befuddled that her “heart doctor” performs only a neurologic examination and, on the basis of that examination, has ordered a stress test. She is flabbergasted to discover that this test consists of a brisk run on the treadmill followed by diagnostic evaluations with electroencephalography and brain scanning. Why is Mrs. Jones confused and upset? The residents—and you—can easily identify the problem: the cardiologist is making inferences about the cardiovascular system based on data from the nervous system, despite the fact that the target organ of cardiology is the heart. The good news, of course, is that real-life cardiology doesn’t work this way… the bad news is that real-life anesthesiology does. In operating rooms around the world, anesthesia providers are making inferences about the nervous system based on data from the cardiovascular system, despite the fact that—for general anesthetics—the target organ of anesthesiology is the brain. In this issue of Anesthesiology, Jordan and colleagues take multiple approaches to assessing brain function during propofol anesthesia in humans and pave the way for the neurobiology of consciousness to inform our clinical practice.1
In brief, Jordan and his coinvestigators found that propofol-induced unconsciousness is associated with a reduction of directed connectivity from anterior to posterior brain structures (see fig. 1 for summary). They derived this conclusion from the analysis of electroencephalography using the technique of symbolic transfer entropy, a method based in information theory. This has neurobiological significance, because such “top-down” or “feedback” processing from the prefrontal cortex is thought to be particularly important for human consciousness.2 Impairment of feedback connectivity from frontal to parietal areas of the brain has previously been associated with induction of unconsciousness using propofol, sevoflurane and ketamine in humans (see table 1 for review of relevant studies)3–9 as well as isoflurane in rats.10 The current study is unique because it extended investigation in two directions. First, in addition to using electroencephalography, Jordan and colleagues used functional magnetic resonance imaging to assess underlying brain events. Second, they conducted a separate analysis of their high-density electroencephalography recordings and identified selective changes in the frontal region that could be amenable to intraoperative monitoring. As such, they have helped bridge a translational gap and advanced our understanding of anesthetic mechanisms as well as anesthetic monitoring. We now know based on their neuroimaging findings that the loss of top-down (feedback) processing observed in prior studies is associated with functional disconnections between anterior and posterior brain structures, as opposed to some epiphenomenal marker identified by electroencephalographic analysis. This is important because of certain discrepancies among studies using only electroencephalography (see table 1) (although a past neuroimaging study also identified functional disconnections between anterior and posterior networks,9 it did not have simultaneous electroencephalography). Just as importantly, we now know that we might be able to capture a glimpse of that neurobiological picture with a more readily-calculated measure (permutation entropy) that can be used intraoperatively. Fortunately, the neurophysiologic relevance of the frontal lobes to consciousness and anesthesia fits well with the practical realities of the brain activity we can actually monitor in the operating room.
Figure 1.
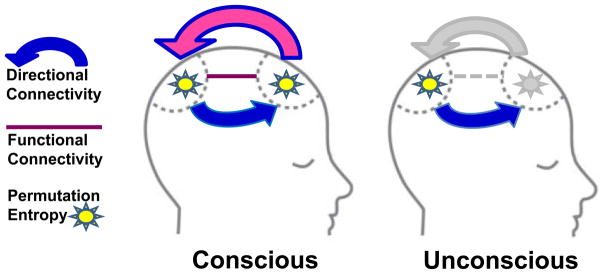
Table 1.
Electroencephalographic Studies of Directional (Anterior-Posterior) Brain Connectivity during Consciousness and Anesthesia in Humans
| Study | Participants | Anesthetic | Analytic Technique | Comments |
|---|---|---|---|---|
| Lee et al, 2009 3 | Healthy Volunteers | Propofol | Evolution Map Approach | Disrupted frontoparietal feedback connectivity |
| Ku et al, 2011 4 | Surgical Patients | Propofol, Sevoflurane | Evolution Map Approach, Symbolic Transfer Entropy | Disrupted frontoparietal feedback connectivity, return of feedback during recovery of consciousness |
| Barrett et al, 2012 5 | Healthy Volunteers | Propofol | Granger Causality | Increased bidirectional connectivity |
| Nicolaou et al, 2012 6 | Surgical Patients | Routine surgical anesthetic regimens | Granger Causality | Increased frontoparietal connectivity, return to baseline with recovery of consciousness |
| Boly et al, 2012 7 | Healthy Volunteers | Propofol | Dynamic Causal Modeling | Disrupted frontoparietal feedback connectivity |
| Lee et al, 2013 8 | Surgical Patients | Ketamine, Propofol, Sevoflurane | Normalized Symbolic Transfer Entropy | Disrupted frontoparietal feedback connectivity |
| Jordan et al, 2013 1 | Healthy Volunteers | Propofol | Symbolic Transfer Entropy | Disrupted anterior- posterior feedback connectivity; confirmation with simultaneous fMRI |
Note that the two studies highlighted in blue, which used Granger causality as a measure of directional connectivity, had opposite results compared with the other investigations. The use of simultaneous functional magnetic resonance imaging (fMRI) by Jordan et al 1 supports the reduction of frontal-parietal connectivity found in the majority of electroencephalographic studies, but further work is required. The magnetic resonance imaging results are consistent with those of Boveroux et al.9
Despite the compelling findings of this study, there are many questions that have yet to be answered. On the mechanistic level, why should there be a preferential interruption of top-down information processing in the brain? How do molecularly and pharmacologically diverse anesthetics achieve this potentially common endpoint?8 How do we link the events at the “top” of the brain (prefrontal cortex) with the influence of subcortical structures (brainstem, diencephalon) controlling arousal states and mediating anesthetic endpoints?11 In terms of the implications for monitoring, we know that this loss of top-down/feedback connectivity from frontal to parietal areas consistently occurs with loss of consciousness, but what about the recovery phase? Does feedback connectivity return in advance of or in association with returning consciousness? If so, will we be able to detect it in real time in the real-world intraoperative setting? The data presented by Jordan et al. encourage future research into both anesthetic mechanism and anesthetic monitoring.
The current study is among a handful of emerging investigations that are combining magnetic resonance imaging with electroencephalography. Although no one would suggest that real-time neuroimaging is practical for the operating room, the data derived from these studies will help establish a scientifically-grounded basis for new metrics of anesthetic depth. These technological advances, in conjunction with hypotheses based on the cognitive neuroscience of consciousness, will hopefully usher in an era of brain monitoring techniques that have a clear neurobiological basis. Without such a basis, it is unlikely that we will ever adopt a standard monitor for the brain… this is because anesthesiologists are compelled not only by the numbers on their monitors, but more fundamentally by the underlying physiology. Studies like that of Jordan et al will help advance the field toward routine monitoring of a key target of general anesthetics by making the assessment of consciousness a practical and mechanistically-grounded tool for the 21st century operating room.
Footnotes
Disclosure: Dr. Mashour is supported by National Institutes of Health (Bethesda, Maryland) grant 1RO1GM098578 and the James S. McDonnell Foundation (St. Louis, Missouri). He has a patent pending (with UnCheol Lee and the University of Michigan) on measures of directional connectivity for brain monitoring (Application No.: 13/804,706, Filed March 14, 2013, “System and Method to Assess Causal Signaling in the Brain during States of Consciousness”).
References
- 1.Jordan D, Ilg R, Riedl V, Schorer A, Grimberg S, Neufang S, Omerovic A, Begrer S, Untergehrer G, Preibisch C, Schulz E, Schuster T, Schröter M, Spoormaker V, Zimmer C, Hemmer B, Wohlschläger A, Kochs EF, Schneider G. Simultaneous electroencephalographic and functional magnetic resonance imaging indicate impaired top-down access to sensory processes as mechanism of brain connectivity changes during anesthesia-induced unconsciousness. Anesthesiology. 2013;XXX:XXX–XXX. doi: 10.1097/ALN.0b013e3182a7ca92. [DOI] [PubMed] [Google Scholar]
- 2.Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70:200–27. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Lee U, Kim S, Noh GJ, Choi BM, Hwang E, Mashour GA. The directionality and functional organization of frontoparietal connectivity during consciousness and anesthesia in humans. Conscious Cogn. 2009;18:1069–78. doi: 10.1016/j.concog.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Ku SW, Lee U, Noh GJ, Jun IG, Mashour GA. Preferential inhibition of frontal-to-parietal feedback connectivity is a neurophysiologic correlate of general anesthesia in surgical patients. PLoS One. 2011;6:e25155. doi: 10.1371/journal.pone.0025155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett AB, Murphy M, Bruno MA, Noirhomme Q, Boly M, Laureys S, Seth AK. Granger causality analysis of steady-state electroencephalographic signals during propofol-induced anaesthesia. PLoS One. 2012;7:e29072. doi: 10.1371/journal.pone.0029072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicolaou N, Hourris S, Alexandrou P, Georgiou J. EEG-based automatic classification of “awake” versus “anesthetized” state in general anesthesia using Granger causality. PLoS One. 2012;7:e33869. doi: 10.1371/journal.pone.0033869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boly M, Moran R, Murphy M, Boveroux P, Bruno MA, Noirhomme Q, Ledoux D, Bonhomme V, Brichant JF, Tononi G, Laureys S, Friston K. Connectivity changes underlying spectral EEG changes during propofol-induced loss of consciousness. J Neurosci. 2012;32:7082–90. doi: 10.1523/JNEUROSCI.3769-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee U, Ku SW, Noh GJ, Baek SH, Choi BM, Mashour GA. Disruption of frontal-parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology. 2013;118:1264–75. doi: 10.1097/ALN.0b013e31829103f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boveroux P, Vanhaudenhuyse A, Bruno MA, Noirhomme Q, Lauwick S, Luxen A, Degueldre C, Plenevaux A, Schnakers C, Phillips C, Brichant JF, Bonhomme V, Maquet P, Greicius MD, Laureys S, Boly M. Breakdown of within- and between-network resting state during propofol-induced loss of consciousness. Anesthesiology. 2010;113:1038–53. doi: 10.1097/ALN.0b013e3181f697f5. [DOI] [PubMed] [Google Scholar]
- 10.Imas OA, Ropella KM, Wood JD, Hudetz AG. Isoflurane disrupts anterio-posterior phase synchronization of flash-induced field potentials in the rat. Neurosci Lett. 2006;402:216–21. doi: 10.1016/j.neulet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Moore JT, Chen J, Han B, Meng QC, Veasey SC, Beck SG, Kelz MB. Direct activation of sleep-promoting VLPO neurons by volatile anesthetics contributes to anesthetic hypnosis. Curr Biol. 2012;22:2008–16. doi: 10.1016/j.cub.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]


