Abstract
Objectives
The Alzheimer's Disease Anti-inflammatory Prevention Trial Follow-up Study (ADAPT-FS) was designed to evaluate the efficacy of naproxen and celecoxib for the primary prevention of Alzheimer's disease (AD) several years after cessation of treatment in ADAPT.
Methods
ADAPT was a randomized, double-masked, multicenter clinical trial of naproxen or celecoxib vs placebo (1:1:1.5 assignment ratio) at six U.S.-based clinics. The trial enrolled 2528 people between 2001 and 2004. Treatments were discontinued in December 2004 and participants were monitored regularly until 2007. In 2010 and 2011, ADAPT-FS screened 1537 participants by telephone and, if indicated, examined them in person using standardized clinical assessments. The primary outcome was time to diagnosis of AD. Death index searches were performed for participants not located.
Results
Eighty-nine additional AD events were identified (24 celecoxib, 25 naproxen, and 40 placebo) yielding a total of 161 events (48 [6.6% of randomized participants] celecoxib, 43 [6.0%] naproxen, and 70 [6.5%] placebo) across ADAPT and ADAPT-FS. Adjusted hazard ratios (HRs) comparing each treatment with placebo showed no overall reduction in risk of AD: HR celecoxib vs placebo, 1.03 (95% confidence interval [CI], 0.72–1.50; P = .86); HR naproxen vs placebo, 0.92 (95% CI, 0.62– 1.35; P = .66). There were 349 deaths (110 [15.2%] celecoxib, 96 [13.4%] naproxen, and 143 [13.2%] placebo). Risk of death was similar for the naproxen- and placebo-assigned groups (HR, 0.99; 95% CI, 0.76−1.28; P = .93) and slightly higher for celecoxib compared with the placebo-assigned group (HR, 1.15; 95% CI, 0.90−1.48; P = .27).
Conclusions
These results acquired during a follow-up of approximately 7 years (which included a median of less than 1.5 years of treatment) do not support the hypothesis that celecoxib or naproxen prevent AD in adults with a family history of dementia.
Keywords: Prevention, Clinical trial, Alzheimer's disease, Nonsteroidal anti-inflammatory drug, Naproxen, Celecoxib
1. Introduction
Substantial evidence from laboratory and epidemiologic studies suggests that nonsteroidal anti-inflammatory drugs (NSAIDs) can defer or prevent onset of Alzheimer's dementia (AD; for review see Szkely and colleagues [1]). NSAIDs inhibit cyclooxygenase (COX) enzymes that mediate the synthesis of prostaglandins [2,3]. As a result, they suppress synthesis of several cytokines that promote inflammatory processes that have, in turn, been implicated in the pathogenesis of AD [4,5]. Some NSAIDs have also been shown to modulate the activity of gamma-secretases and thereby to reduce the production of neurotoxic amyloid β1–42 [6,7], the principal component of amyloid plaques that accumulate in the brain of patients with AD.
The Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT) was initiated to determine whether naproxen (a nonselective COX inhibitor) or celecoxib (a selective COX-2 inhibitor) could delay the onset of dementia among cognitively normal older adults who were at risk because of advanced age and a family history of dementia [8,9]. ADAPT treatments were stopped 3.7 years after the first participant was randomized because of concerns about possible adverse cardiovascular effects of NSAIDs emerging from other studies [10]. Initial results from the curtailed trial indicated that neither celecoxib nor naproxen prevented onset of AD [11] or slowed the decline in cognitive function over time [12]. Instead, there were trends toward increased occurrence of AD with NSAID treatments. However, ADAPT data collection continued for 2 years more under double-masked conditions using a protocol identical to the original except for omission of treatment administration and the late addition of a telephone assessment battery of neuropsychological testing. This continuation phase of ADAPT suggested possible effects of naproxen on AD incidence over time, with decreased risk of AD emerging between 2 years and 3 years after randomization [13].
The ADAPT Follow-up Study (ADAPT-FS) was carried out to examine whether the latter trend toward decreased risk was sustained, thereby suggesting that NSAIDs could prevent AD over the long term.
2. Methods
2.1. Design of ADAPT
ADAPT was a randomized, placebo-controlled, primary prevention trial sponsored by the National Institute on Aging. Participants were enrolled from March 2001 to December 2004 and assigned to the following parallel treatment groups in a 1:1:1.5 ratio: (i) naproxen sodium 220 mg twice daily, (ii) celecoxib 200 mg twice daily, or (iii) placebo. Participants and personnel at the clinical sites were masked to treatment assignment using a doubleplacebo design [8]. A detailed description of the ADAPT design and methods has been published [9].
ADAPT participants were recruited at six field sites in the United States (Baltimore, MD; Boston, MA; Rochester, NY; Seattle, WA; Sun City, AZ; and Tampa, FL). The coordinating center for the study was located at the Johns Hopkins University School of Public Health. Participants were age 70 years or older and had a history of at least one firstdegree relative with Alzheimer–like dementia. Before enrollment, participants completed a cognitive screening test intended to identify and exclude those with dementia or other cognitive disorders.
After enrollment, participants were screened annually using an in-person cognitive assessment battery. In December 2004, enrollment and treatment administration were suspended following the announcement from the Adenoma Prevention with Celecoxib trial that celecoxib used in two doses (one of which was identical to that used in ADAPT) produced increased risks of cardiovascular death, myocardial infarction, and related events. The rationale for suspending both treatments in ADAPT has been discussed elsewhere [10]. A subsequent analysis of ADAPT data did not show the same level of risk for celecoxib as that of the Adenoma Prevention with Celecoxib trial [14]. The continuation phase of ADAPT ended in February 2007.
2.2. ADAPT-FS data collection
ADAPT-FS collected information on the vital and cognitive status of ADAPT participants nearly 3 years after the close of ADAPT. We contacted these participants between February 2010 and February 2011. An introductory letter informed eligible participants of our intent to contact them by telephone. The phone contact included a brief assessment of cognitive performance. When indicated, participants were invited to participate in an in-person dementia assessment. Participants provided oral consent for the telephone assessment and written consent for any subsequent in-person assessment. The study procedures were approved by the institutional review boards at the coordinating center and each of the six field sites.
2.3. Assessment of cognitive status
Eligible participants were alive, had not refused further contact during ADAPT, and had not received a diagnosis of dementia during ADAPT. The initial telephone contact assessed cognitive status using a telephone assessment battery (TAB) designed for this purpose, as well as questions about interim medical history. The TAB comprised the Telephone Interview for Cognitive Status [15], a test of generative verbal fluency [16], and a narrative from the Rivermead Behavioral Memory Test [17]. Participants whose TAB results fell below specified criteria, or those who were otherwise thought by a study clinician to require further evaluation, were invited to participate in an in-person dementia evaluation visit (DEV). The TAB and DEV protocols have been described elsewhere [9,11,12]. The DEV involved a more extensive neuropsychological assessment, a detailed medical history, neurological examination and global mental status examination, collateral interviews, and, when appropriate, laboratory testing and neuroimaging.
The results of each DEV were reviewed by a team of physicians, nurses, and neuropsychologists. The team assigned diagnoses of dementia using Diagnostic and Statistical Manual of Mental Disorders, 4th edition, criteria [18]. Probable or possible AD was diagnosed in accordance with National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria [19]. In view of the extended interval since participants' last observation, the date of onset of dementia was assigned by convention as the date of the DEV.
2.4. Assessment of vital status
Vital status information was collected for all participants believed to be alive at the close of ADAPT. If participants could not be reached by telephone in ADAPT-FS, their friends or family members were approached for this information. If a participant had refused further contact during ADAPT, then no further information was collected from him or her or any collateral respondents. If all other attempts to obtain vital status were futile, then local newspaper obituaries, the Social Security Administration Death Master File and the National Death Index were searched for death records.
2.5. Data analyses
The primary outcome was time to AD after enrollment in ADAPT. During separate analyses, we also examined time to dementia of any cause. Analyses included all randomized ADAPT participants who had at least one cognitive assessment after enrollment. Person-time was censored after the participants' last completed cognitive assessment; participants who did not complete an ADAPT-FS assessment were censored at their last ADAPT follow-up visit.
Time to all-cause death was also compared by treatment group. Mortality analyses included all randomized participants who had any follow-up (cognitive assessment or other contact with staff). Person-time for the mortality analysis was censored after the participants' last contact. If the participant was not available to participate in ADAPT-FS but a collateral respondent reported that the participant was alive, the date of that report was used as the censoring date for the mortality analysis.
For all analyses, participants were counted in the treatment group to which they were randomized (intention to treat). By design, naproxen and celecoxib were compared with placebo, and not with one another.
Time to event of each outcome was evaluated using Kaplan-Meier plots with log-rank statistics to test for differences between treatment groups. Cox proportional hazards regression was used to test for differences between treatment groups while controlling for covariates. Cox models were adjusted for variables used in the stratification of randomization, including field site and age group. The proportional hazards assumption of the Cox model was assessed by testing for an interaction between treatment and the log of continuous person-time. Because of the time gap between ADAPT and ADAPT-FS, we also performed logistic regression comparing the proportion of participants with AD and dementia in each group (adjusting for stratification variables). Last, we created a binary composite outcome of AD or death. Treatment group differences in the composite outcome were examined using logistic regression.
Sensitivity analyses were performed to assess the robustness of the results. In addition to using the date of the DEV, we performed analyses that defined the onset of dementia as the date of the TAB that triggered the dementia evaluation and the date of dementia diagnosis. Secondary analyses also excluded participants enrolled with an existing cognitive impairment, including those who seemed normal on the screening cognitive assessment but triggered a dementia evaluation at the ADAPT baseline visit that resulted in a diagnosis of dementia or either dementia or cognitive impairment with no dementia (CIND).
The primary analysis included only participants who had been assigned a diagnosis of AD by ADAPT/ADAPT-FS clinical teams. Sometimes, however, when a participant was unable to take part in the ADAPT-FS assessments, we obtained a report of a dementia diagnosis from a friend or family member (a collateral), or we obtained a physician report of AD via authorized review of medical records. Sensitivity analyses used logistic regression with the outcome of AD that was expanded to include additional cases identified by medical record review or additional cases identified either by medical record review or by collateral report.
3. Results
3.1. Study population
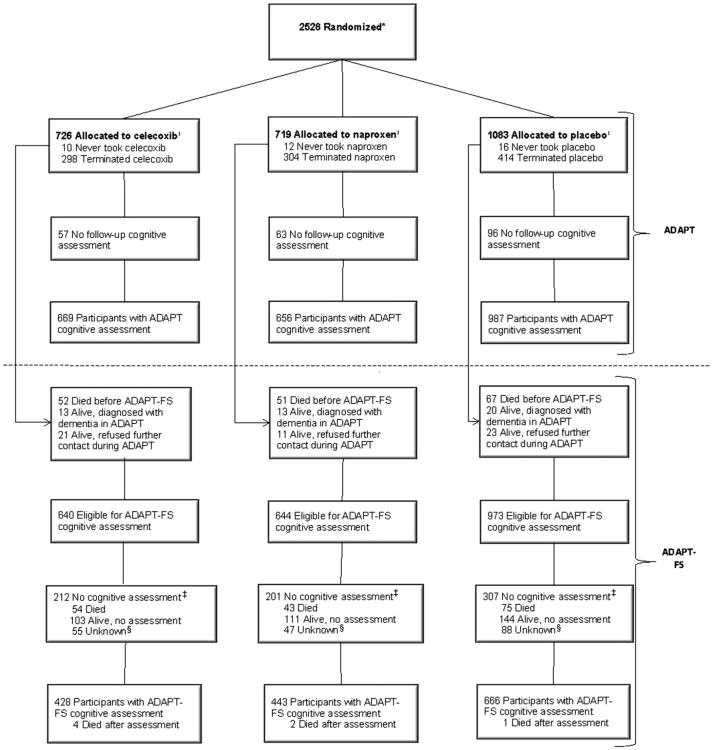
A total of 2528 participants enrolled in ADAPT. Of these, 2257 were thought to remain potentially eligible for cognitive assessment in ADAPT-FS. The proportion eligible for ADAPT-FS assessments did not differ by treatment group (χ2 = 1.38, df = 2, P = .50). However, some 720 of the 2257 potential participants did not have an ADAPT-FS cognitive assessment: 172 had died in the interim between ADAPT and ADAPT-FS, 41 were too ill to participate, 297 were unavailable or refused, 20 agreed to participate but could not be reached for administration of the TAB, and 190 could not be located. The proportion of ADAPT participants who received an ADAPT-FS cognitive assessment did not differ by treatment group (χ2 = 1.46, df = 2, P = .48). Fig. 1 shows the flow of participants through ADAPT and ADAPT-FS.
Fig. 1.
Participant flow in the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT) and the Alzheimer's Disease Anti-inflammatory Prevention Trial Follow-up Study (ADAPT-FS). *Numbers available only for those randomized, not those screened for eligibility. †Participants considered to have terminated study drug if study drug had been started but was no longer issued prior to December 17, 2004. Does not include temporary interruptions. The number of participants who never took the study drug is updated from previous publications. ‡Participants were eligible but did not have an assessment. §Participants' status was considered unknown after final death sweep.
Table 1 provides baseline characteristics for all ADAPT participants as well as for the participants who completed cognitive assessment in ADAPT-FS. As reported previously [9], a highly educated, mostly white group of participants enrolled in ADAPT. The median age at randomization in ADAPT was 75 years. The participants who completed an ADAPT-FS cognitive assessment were similar to the original ADAPT sample, and the characteristics at randomization of those who completed an ADAPT-FS assessment did not differ by treatment group. Characteristics at randomization for ADAPT participants who did not complete an ADAPT-FS cognitive assessment are provided in Supplementary Table 1.
Table 1. Characteristics at randomization for ADAPT participants.
| Randomized in ADAPT | Participated in ADAPT-FS cognitive assessments | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Characteristics | Total | Cel | Nap | Plb | Total | Cel | Nap | Plb |
| n | 2528 | 726 | 719 | 1083 | 1537 | 428 | 443 | 666 |
| Age, median | ||||||||
| 74.5 | 74.5 | 74.5 | 74.5 | 74.0 | 74.0 | 74.0 | 73.0 | |
| Age, years, % | ||||||||
| 70–74 | 55.5 | 55.4 | 55.8 | 55.3 | 59.0 | 58.9 | 56.4 | 60.7 |
| 75–79 | 31.5 | 31.4 | 31.7 | 31.5 | 31.0 | 30.1 | 33.6 | 29.7 |
| 80–84 | 11.3 | 11.6 | 10.6 | 11.5 | 9.1 | 9.6 | 8.8 | 9.0 |
| >85 | 1.7 | 1.7 | 1.9 | 1.7 | 1.0 | 1.4 | 1.1 | 0.6 |
| Sex, % | ||||||||
| Male | 54.1 | 52.9 | 54.1 | 54.9 | 53.8 | 52.6 | 53.5 | 54.8 |
| Female | 45.9 | 47.1 | 45.9 | 45.1 | 46.2 | 47.4 | 46.5 | 45.2 |
| Race/ethnic origin, % | ||||||||
| White | 97.0 | 96.1 | 97.1 | 97.4 | 96.8 | 95.6 | 97.3 | 97.2 |
| Black | 1.5 | 1.8 | 1.8 | 1.0 | 1.6 | 1.9 | 2.0 | 1.2 |
| Hispanic | 0.7 | 1.4 | 0.3 | 0.6 | 0.7 | 1.4 | 0 | 0.6 |
| Other | 0.8 | 0.6 | 0.7 | 0.9 | 0.9 | 0.9 | 0.5 | 1.1 |
| Did not answer | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0 |
| Education, % | ||||||||
| Less than high school | 4.0 | 3.9 | 4.9 | 3.6 | 3.5 | 3.3 | 3.6 | 3.5 |
| High school degree | 19.9 | 20.8 | 17.5 | 20.9 | 17.6 | 20.0 | 14.9 | 18.0 |
| College, no degree | 27.5 | 27.7 | 28.4 | 26.8 | 25.4 | 25.5 | 26.6 | 24.5 |
| College degree | 19.1 | 19.1 | 17.0 | 20.6 | 19.6 | 18.7 | 17.6 | 21.5 |
| Postgraduate | 29.4 | 28.5 | 32.3 | 28.2 | 34.0 | 32.7 | 37.3 | 32.6 |
| Marital status, % | ||||||||
| Married | 71.9 | 70.2 | 75.0 | 70.9 | 72.3 | 72.4 | 71.6 | 72.7 |
| Widowed | 18.2 | 19.7 | 16.1 | 18.7 | 17.6 | 17.8 | 18.3 | 17.1 |
| Separated | 0.5 | 0.4 | 0.3 | 0.7 | 0.7 | 0.5 | 0.5 | 0.9 |
| Divorced | 6.8 | 6.9 | 5.8 | 7.3 | 6.5 | 6.3 | 6.3 | 6.8 |
| Never married | 2.6 | 2.8 | 2.8 | 2.3 | 2.9 | 3.0 | 3.4 | 2.4 |
| Not reported | 0 | 0 | 0 | 0.1 | 0.1 | 0 | 0.2 | 0 |
| Karnofsky score, % | ||||||||
| 100% | 82.3 | 84.3 | 80.1 | 82.5 | 83.7 | 83.4 | 81.9 | 85.1 |
| 90% | 15.3 | 13.5 | 18.2 | 14.6 | 14.9 | 14.7 | 17.1 | 13.5 |
| 80% | 2.2 | 2.1 | 1.4 | 2.8 | 1.2 | 1.9 | 0.7 | 1.2 |
| 60–70% | 0.2 | 0.1 | 0.3 | 0.2 | 0.1 | 0 | 0.2 | 0.2 |
| Cognitive score, median | ||||||||
| Adjusted 3MS-E | 95.0 | 95.0 | 95.0 | 95.0 | 95.0 | 95.0 | 95.0 | 95.0 |
| GVF | 25.0 | 24.0 | 24.0 | 25.0 | 25.0 | 25.0 | 25.0 | 26.0 |
| RBMT delayed recall | 6.0 | 6.5 | 6.0 | 6.0 | 6.5 | 6.5 | 7.0 | 6.0 |
| BVMT-R delayed recall | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 |
| Adjusted HVLT-R trial 4 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 10.0 |
| Digit Span, forward | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 |
| Digit Span, backward | 7.0 | 7.0 | 6.0 | 7.0 | 7.0 | 8.0 | 8.0 | 8.0 |
Abbreviations: ADAPT, Alzheimer's Disease Anti-inflammatory Prevention Trial; ADAPT-FS, Alzheimer's Disease Anti-inflammatory Prevention Trial Follow-up Study; Cel, celecoxib; Nap, naproxen; Plb, placebo; 3MS-E, Modified Mini-Mental State Examination; GVF, Generative Verbal Fluency; RBMT, Rivermead Behavioral Memory Test; BVMT-R, Brief Visuospatial Memory Test - revised; HVLT-R, Hopkins Verbal Learning Test- revised.
Characteristics at the time of ADAPT-FS enrollment for participants with an ADAPT-FS cognitive assessment are provided in Supplementary Table 2. Their median age was 82 years. A large majority (85%) of these participants still lived in their own home. The proportion of participants who reported use of a nonaspirin NSAID 4 days or more per week for 6 months or longer during the 3 years prior to ADAPT-FS enrollment did not differ by treatment groups (12% celecoxib, 12% naproxen, 10% placebo; χ2 = 1.85, df = 2, P =.40).
3.2. Alzheimer's disease, dementia, and death
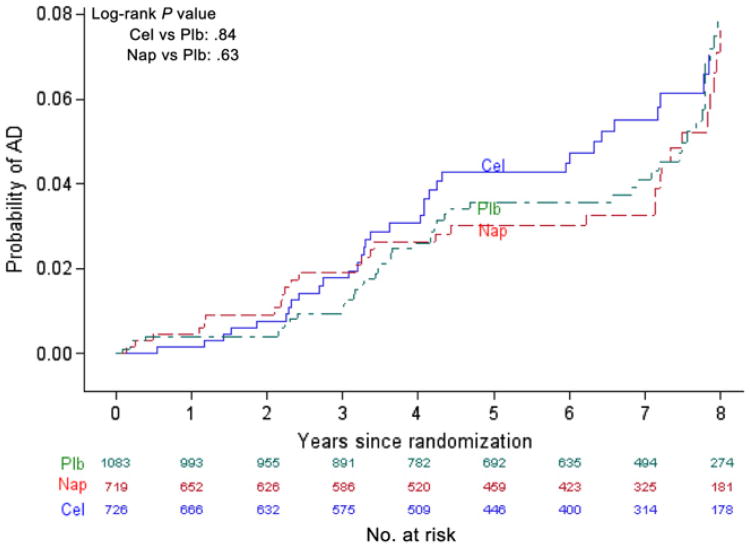
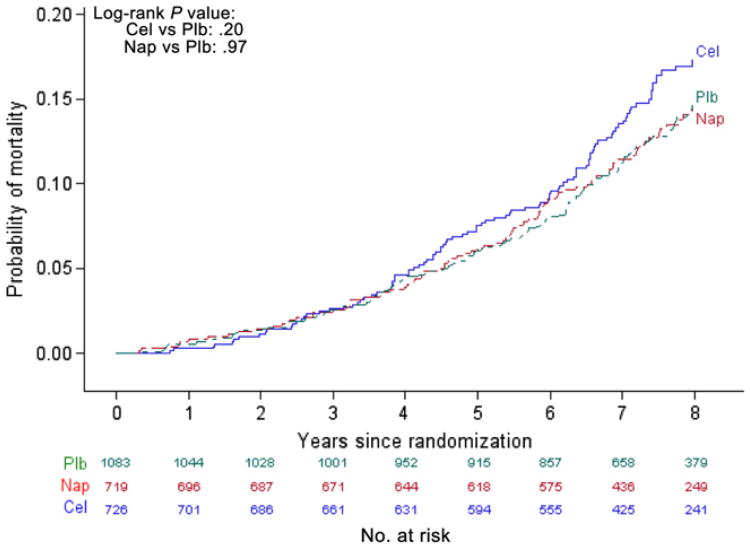
Table 2 shows the number of events, follow-up person-time, and rates of AD, all-cause dementia, and death during ADAPT, ADAPT-FS, and in total. An additional 89 cases of AD were diagnosed during ADAPT-FS (24 celecoxib, 25 naproxen, 40 placebo) yielding a total of 161 events in all (48 [6.6% of those randomized to] celecoxib, 43 [6.0%] naproxen, 70 [6.5%] placebo). The cumulative incidence of AD in ADAPT and ADAPT-FS is shown in Figure 2. The incidence of AD during the entire follow-up period did not differ by treatment group (celecoxib vs placebo log-rank χ2 = 0.04, df = 1, P = .84; naproxen vs placebo log-rank χ2 = 0.24, df = 1, P = .63). Of the 181 cases of dementia, 161 (89%) were characterized as AD. The cumulative incidence of all-cause dementia in ADAPT and ADAPT-FS, shown in Supplementary Figure 1, did not differ appreciably by treatment group (celecoxib vs placebo log-rank χ2 = 0.09, df = 1, P = .76; naproxen vs placebo logrank χ2 = 0.22, df = 1, P = .64). Cumulative rate of death is shown in Fig. 3. Starting around year 4 after randomization, the risk of death was higher in the celecoxib-assigned group; however, the overall difference was not statistically significant (celecoxib vs placebo log-rank χ2 = 1.64, df = 1, P = .20). The risk of death was almost identical for participants assigned to naproxen and to placebo (naproxen vs placebo log-rank χ2 < 0.00, df = 1, P = .97).
Table 2. AD, dementia, and death in ADAPT and ADAPT-FS.
| Outcome | Total | Cel | Nap | Plb |
|---|---|---|---|---|
| AD* | ||||
| ADAPT | ||||
| No. of events | 72 | 24 | 18 | 30 |
| Person-time† | 9431 | 2672 | 2685 | 4074 |
| Incidence rate‡ | 0.76 | 0.90 | 0.67 | 0.74 |
| ADAPT-FS | ||||
| No. of events | 89 | 24 | 25 | 40 |
| Person-time | 4911 | 1371 | 1410 | 2130 |
| Incidence rate | 1.81 | 1.75 | 1.77 | 1.88 |
| Total | ||||
| No. of events | 161 | 48 | 43 | 70 |
| Person-time | 14,342 | 4043 | 4094 | 6204 |
| Incidence rate | 1.12 | 1.19 | 1.05 | 1.13 |
| Dementia* | ||||
| ADAPT | ||||
| No. of events | 82 | 25 | 22 | 35 |
| Person-time | 9427 | 2671 | 2683 | 4073 |
| Incidence rate | 0.87 | 0.94 | 0.82 | 0.86 |
| ADAPT-FS | ||||
| No. of events | 99 | 27 | 28 | 44 |
| Person-time | 4917 | 1373 | 1412 | 2133 |
| Incidence rate | 2.01 | 1.97 | 1.98 | 2.06 |
| Total | ||||
| No. of events | 181 | 52 | 50 | 79 |
| Person-time | 14,345 | 4044 | 4095 | 6206 |
| Incidence rate | 1.26 | 1.29 | 1.22 | 1.27 |
| Death§ | ||||
| ADAPT | ||||
| No. of deaths | 130 | 38 | 39 | 53 |
| Person-time | 10,555 | 3002 | 3028 | 4525 |
| Incidence rate | 1.23 | 1.26 | 1.29 | 1.17 |
| ADAPT-FS | ||||
| No. of deaths | 219 | 72 | 57 | 90 |
| Person-time | 6745 | 1895 | 1947 | 2903 |
| Incidence rate | 3.24 | 3.79 | 2.92 | 3.10 |
| Total | ||||
| No. of deaths | 349 | 110 | 96 | 143 |
| Person-time | 17,300 | 4897 | 4975 | 7428 |
| Incidence rate | 2.02 | 2.25 | 1.93 | 1.92 |
Abbreviations: AD, Alzheimer's disease; ADAPT, Alzheimer's Disease Anti-inflammatory Prevention Trial; ADAPT-FS, Alzheimer's Disease Anti-inflammatory Prevention Trial Follow-up Study; Cel, celecoxib; Nap, naproxen; Plb, placebo.
Event date is dementia evaluation visit date.
Person-time is in years.
Incidence rate is per 100 person-years.
Four participants had missing day and month for date of death. Middle of the year is taken as death date for these participants (if after the last known in-person contact). Three participants had missing values for date of death. Death date is taken as last known in-person contact +150 days.
Fig. 2.
Cumulative incidence of Alzheimer's disease (AD) over the Alzheimer's Disease Anti-inflammatory Prevention Trial and the Alzheimer's Disease Anti-inflammatory Prevention Trial Follow-up Study. Cel, celecoxib; Plb, placebo; Nap, naproxen.
Fig. 3.
Cumulative incidence of death the Alzheimer's Disease Anti-inflammatory Prevention Trial and the Alzheimer's Disease Anti-inflammatory PreventionTrial Follow-up Study. Cel, celecoxib; Plb, placebo; Nap, naproxen.
Table 3 shows the proportional hazards models for AD, all-cause dementia, and death adjusted for the stratification variables (age at randomization and field site). The results from these models are consistent with the cumulative incidence comparisons. The adjusted AD hazard ratio (HR) for celecoxib vs placebo was 1.03 (95% confidence interval [CI], 0.72–1.50; P = .86) and the adjusted HR for naproxen vs placebo was 0.92 (95% CI, 0.62–1.35; P = .66). HRs for all-cause dementia and for mortality did not differ significantly with assignment to either naproxen or celecoxib vs placebo. There was no evidence for a treatment by person-time interaction using AD as the outcome (celecoxib vs placebo, P = .69; naproxen vs placebo, P = .40). This finding affirmed that the proportional hazards assumption over the entire study period was not violated. The results from the logistic regression models shown in Supplementary Table 3 were nearly identical to those from the proportional hazard models. The odds ratios (ORs) for the composite outcome of AD or death did not differ by treatment group (celecoxib vs placebo: OR, 1.09; 95% CI, 0.85–1.38; P = .51; naproxen vs placebo: OR, 0.99; 95% CI, 0.77–1.26; P = .92).
Table 3. Adjusted hazard ratios* for AD, dementia, and death.
| Outcome | Hazard ratio | 95% Lowerconfidence limit | 95% Upper confidence limit | P value |
|---|---|---|---|---|
| AD | ||||
| Cel vs Plb | 1.03 | 0.72 | 1.50 | .86 |
| Nap vs Plb | 0.92 | 0.62 | 1.35 | .66 |
| Dementia | ||||
| Cel vs Plb | 1.03 | 0.72 | 1.46 | .88 |
| Nap vs Plb | 0.94 | 0.65 | 1.35 | .72 |
| Death | ||||
| Cel vs Plb | 1.15 | 0.90 | 1.48 | .27 |
| Nap vs Plb | 0.99 | 0.76 | 1.28 | .93 |
Abbreviations: AD, Alzheimer's disease; Cel, celecoxib; Plb, placebo; Nap, naproxen.
Hazard ratios calculated using Cox proportional hazard regression, adjusting for strata (age and clinic). AD and dementia event date is dementia evaluation visit date.
3.3. Sensitivity analyses
The sensitivity analyses using different definitions for the onset of dementia showed no significant difference in the log-rank estimates or the HRs for either treatment when compared with results that relied on date of the DEV (data not shown).
There were eight individuals with AD and 57 with CIND, which was diagnosed after a DEV that had been triggered during the baseline cognitive assessment of ADAPT. Excluding these eight prevalent AD cases from the analysis yielded the following HRs for AD: celecoxib vs placebo, 1.08 (95% CI, 0.74–1.57; P = .69); naproxen vs placebo, 0.90 (95% CI, 0.60–1.33; P =.30). Excluding both the prevalent AD and CIND cases yielded an HR for celecoxib vs placebo of 1.00 (95% CI, 0.67–1.50; P =.99); for naproxen vs placebo, the HR was 0.87 (95% CI, 0.57–1.33; P = .52).
There were an nine additional AD events identified through medical records without a corroborating ADAPT-FS dementia evaluation. The date of diagnosis was not recorded for these events. When these dementia cases were included in the logistic regression analysis, the ORs were as follows: celecoxib vs placebo, 0.95 (95% CI, 0.65–1.39; P = .80); naproxen vs placebo, 0.94 (95% CI, 0.64–1.37; P = .75). An additional 37 dementia cases were identified by collateral report that was not confirmed by a dementia evaluation or by medical records. Including these cases as well as those identified by medical record review yielded an OR for celecoxib vs placebo of 1.13 (95% CI, 0.80–1.59; P = .48); for naproxen vs placebo, the OR was 1.08 (95% CI, 0.77–1.53; P = .65).
4. Discussion
In ADAPT-FS we assessed the vital and cognitive status of participants approximately 3 years after the close-out of ADAPT. Analysis of combined data from ADAPT and ADAPT-FS failed to confirm a previously reported decrease in AD risk in participants assigned to naproxen beginning some 2.5 years after randomization [13]. Instead, throughout the entire period of ADAPT and ADAPT-FS there were no notable differences in the cumulative risk of AD or all-cause dementia after earlier assignment to either naproxen or celecoxib vs placebo. There was a trend toward increased mortality in those assigned to celecoxib during this interval, but the difference in rates failed to reach conventional criteria for statistical significance.
The aggregate results here may appear to contradict our recent report summarizing results of ADAPT through its continuation phase [13]. In fact, however, Fig. 2 is consistent with results reported earlier through year 5 after randomization. There are no data to evaluate treatment effects between years 5 and 7 (the Kaplan-Meier method simply maintains previous ordinate values until new events are observed). Fig. 2 then suggests that any neuroprotective effect of naproxen, as originally hypothesized by ADAPT and possibly suggested during the time of its continuation, is no longer evident with the additional 1 to 1.5 years of ADAPT-FS follow-up.
Numerous epidemiologic studies and several meta-analyses have examined the relationship between NSAIDs and AD. A number of these studies suggested that NSAID use is associated with a reduced risk of AD [20,21]. Motivated in part by these findings, at least seven randomized treatment trials have been carried out to test whether NSAIDs can slow the progression of symptomatic AD [22–27]. Results showed no benefit of NSAID treatment. One randomized, secondary prevention trial was conducted to test whether NSAIDs could delay the progression of mild cognitive impairment to AD [28]. That study reported that rofecoxib, a selective COX-2 inhibitor, was associated with an increased rate of progression to AD when compared with placebo. ADAPT is the only primary prevention trial of NSAIDs among dementia-free individuals. We initially hypothesized that previous trials of NSAIDs had administered treatments too late during the disease process to have any noteworthy effect, but that a primary prevention trial would reveal a neuroprotective effect consistent with earlier observational studies. These results from ADAPT and ADAPT-FS do not support that hypothesis.
Several factors merit consideration when reviewing the results from ADAPT and ADAPT-FS. First, the interval of NSAID treatments in ADAPT was far shorter than originally intended. The treatment was planned to last up to 7 years, but no participant received more than 4 years of treatment. In fact, the median time from enrollment to cessation of treatment was only 14.8 months (15.6 months for those who completed an ADAPT-FS cognitive assessment). It is unlikely that this duration of treatment would have sufficed to produce a sustained protective effect.
Second, when planning the trial we assumed the incidence of AD would be 2.5% in the first year with a 10% proportional increase in each subsequent year (ie, 2.75% in the second year, 3.03% in the third year, etc). Based on this assumption, the trial was designed to have 80% power to detect a 30% reduction in the incidence of AD over 7 years of follow-up. As reported in Table 2, the actual observed incidence rate over the duration of the trial was only 1.12%. The participants in the trial were volunteers, who tended to be white, highly educated, and generally healthy. Thus, despite being at elevated risk because of a family history of Alzheimer–like dementia, this was a select population that did not develop dementia at the anticipated rates. The result was a reduction in statistical power below that originally projected. Foreseeing this difficulty, we had sought additional funds in 2004 to expand the cohort and to extend the period of observations, but that initiative was preempted in December 2004 by widely publicized evidence regarding the possible cardiotoxicity of celecoxib. As it was, we observed 161 cases of AD yielding a 95% CI around the HR estimates for AD that suggested no reduction in risk below an HR of 0.62 for naproxen compared with placebo (0.72 for celecoxib). However, given the CIs around point estimates for each intervention, we cannot confidently rule out a 30% reduction in AD incidence with naproxen, which the trial was initially designed to detect.
Third, around 30% of eligible ADAPT participants did not participate in the ADAPT-FS cognitive assessments. As a result, it is possible the AD event rates in ADAPT-FS were underestimated. However, nonparticipation rates were similar across treatment groups. Moreover, sensitivity analyses incorporating different levels of information about missed outcome events did not change the results meaningfully.
Fourth, nearly 12% of the participants in ADAPT-FS reported taking nonaspirin NSAIDs 4 days or more per week for 6 months or longer during the 3 years prior to enrollment in ADAPT-FS. There were no apparent differences in the proportions of these extended NSAID users among treatment groups. However, even a nondifferential exposure to NSAIDs could tend to bias comparisons of AD risk between treatment groups toward the null.
In summary, the results of ADAPTand ADAPT-FS do not support the use of NSAIDs for prevention of AD in the elderly. The contrasts in results between the observational and randomized studies of NSAIDs and AD have been the subject of much debate [29]. Given practical and ethical concerns about the safety of NSAIDs in elderly participants, it seems unlikely that further large-scale, randomized studies of NSAIDs for AD will be carried out. As a result, explanations for the differences between observational and randomized studies will need to come from other types of studies.
Supplementary Material
Research in Context.
Systematic review: We have published widely on the relationship between nonsteroidal anti-inflammatory drugs (NSAIDs) and Alzheimer's disease (AD), including primary data analyses from two observational studies (PubMed identification [PMID] 12297571; 17636065; 18003940), a mega-analysis of pooled data from multiple observational studies (PMID: 18509093), and several systematic reviews of published observational and randomized studies (PMID: 15279021; 17612054; 20205647).
Interpretation: The Alzheimer's Disease Anti-Inflammatory Prevention Trial (ADAPT) and Alzheimer's Disease Anti-Inflammatory Prevention Trial Follow-up Study (ADAPT-FS) were designed to test the hypothesis supported by observational research that naproxen or celecoxib could delay the onset of AD among cognitively normal older adults. The results of ADAPT and ADAPT-FS do not support this hypothesis.
Future directions: Given practical and ethical concerns, additional large, randomized trials of NSAIDs for the prevention of AD seem unlikely. Explanations for the differences between observational and randomized studies will need to come from observational studies examining if and how genetic and other risk factors interact with NSAIDs in the prevention of AD.
Acknowledgments
John Breitner, MD, MPH (Study Chair), Centre for Studies on Prevention of Alzheimer's Disease, Douglas Mental Health University Institute and McGill University, Montreal; Laura Baker, PhD, Veteran Affairs Puget Sound Health Care System and University of Washington School of Medicine, Seattle, WA; Lea Drye, PhD, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD; Denis Evans, MD, Rush-Presbyterian-St. Luke's Medical Center, Chicago, IL; Constantine Lyketsos, MD, MHS, Johns Hopkins School of Medicine, Baltimore, MD; Laurie Ryan, PhD, National Institute on Aging, Bethesda, MD; and Peter Zandi, PhD, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD. Research group: Resource Centers: Chairman's Office, Veteran Affairs Puget Sound Health Care System and University of Washington School of Medicine, Seattle, WA: Laura Baker, PhD (Director); John Breitner, MD, MPH (Co-Director, affiliated with Centre for Studies on Prevention of Alzheimer's Disease, Douglas Mental Health University Institute, and McGill University, Montreal); Hector Hernandez Saucedo (Lead Coordinator); Jane Anau; Brenna Cholerton, PhD; and Kirise Kramer. Coordinating Center, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD: Peter Zandi, PhD (Director); Lea Drye, PhD (Associate Director); Anne Shanklin Casper, MA, CCRC (Lead Coordinator); Curtis Meinert, PhD; Barbara Martin, PhD; Gabrielle Jenkins; Lee McCaffrey, MA; Jill Meinert; Vijay Vaidya, MPH; Alka Ahuja, MS; and Pat May, MS. Project Office, National Institute on Aging, Bethesda, MD: Laurie Ryan, PhD (Project Officer). Field sites: Johns Hopkins School of Medicine, Baltimore, MD: Constantine G. Lyketsos, MD, MHS (Director); Martin Steinberg, MD (Associate Director); Jason Brandt, PhD (Neuropsychologist); Julia J. Pedroso, RN, MA (Lead Coordinator); Alyssa Bergey, MA; Carol Gogel, RN; Lynn Smith, MA; and Jennifer Kraus. Boston University School of Medicine, Boston, MA: Robert A. Stern, PhD (Director); Robert C. Green, MD (Previous Director); Brandon Gavett, PhD (Neuropsychologist); Jane Mwicigi, MBChB, MPH (Lead Coordinator); Lorraine Baldwin; Theresa McGowan; Patricia Johnson; Wendy Qiu, MD; Jamie Frederick; Sumati Raghavan, MBBS; Carol Rossi, RN; Alan Mandell, MD; Daniella Dinizo, MPH; and Mary Tara Roth, RN, MSN, MPH. University of Rochester School of Medicine, Rochester, NY: Anton Porsteinsson, MD (Director); M. Saleem Ismail, MD (Previous Director); Miriam Weber, PhD (Neuropsychologist); Connie Brand, RN (Lead Coordinator); Jennifer Richard; Kelly Stear; Sue Schepp; Kelly Cosman; and Kimberly Martin, RN. Veteran Affairs Puget Sound Health Care System and University of Washington School of Medicine, Seattle, WA: Suzanne Craft, PhD (Director); Laura Baker, PhD (Neuropsychologist); Deborah Dahl, RN, BSN (Lead Coordinator); Grace Garrett; Jamie Tidwell; Stephen Thielke, MD, MSPH; Lauren Smith; Matthew Arbuckle; William Strong; Juliet Ladenberg; Maureen Callaghan, MD; Stennis Watson, MD; Jeannine Skinner; and Kaysha Bowton. Sun Health Research Institute, Sun City, AZ: Marwan Sabbagh, MD (Director); Christine Belden, PsyD (Neuropsychologist); Carolyn Liebsack, RN, BSN, CCRC (Lead Coordinator); Kathryn Davis; Lauren Arnieri; Michael Malek-Ahmadi; Lisa Nicholson, RN; Sandra Jacobson, MD; and Elliot Schwartz. Roskamp Institute Memory Clinic, Tampa, FL: Michael Mullan, MBBS, PhD (Director); Cheryl Luis, PhD, ABPP-CN (Neuropsychologist); Julia Parrish, LPN, CCRC (Lead Coordinator); Marlee Faircloth; Terry Ervin; Janette Girard; Deborah Burke, MD; and Andrew Keegan, MD. Collaborator: Denis Evans, MD, Rush-Presbyterian-St. Luke's Medical Center, Chicago, IL.
Funding for this study was provided by the National Institute on Aging (NIA) U01 AG15477 and 2U01 AG015477-06A2, the N. Bud Grossman Center for Memory Research and Care, and the Minnesota Medical Foundation.
Footnotes
Trial registered at www.clinicaltrials.gov. Trial Registration Identifiers: NCT00007189 and NCT01417130.
References
- 1.Szekely CA, Town T, Zandi PP. NSAIDs for the chemoprevention of Alzheimer's disease. Subcell Biochem. 2007;42:229–48. doi: 10.1007/1-4020-5688-5_11. [DOI] [PubMed] [Google Scholar]
- 2.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–5. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 3.Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993;268:6610–4. [PubMed] [Google Scholar]
- 4.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGeer EG, McGeer PL. Inflammatory processes in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:741–9. doi: 10.1016/S0278-5846(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 6.Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–6. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 7.Eriksen JL, Sagi SA, Smith TE, Weggen S, Das P, McLendon DC, et al. NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J Clin Invest. 2003;112:440–9. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin BK, Meinert CL, Breitner JC. Double placebo design in a prevention trial for Alzheimer's disease. Control Clin Trials. 2002;23:93–9. doi: 10.1016/s0197-2456(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 9.Meinert CL, McCaffrey LD, Breitner JC. Alzheimer's Disease Anti-inflammatory Prevention Trial: design, methods, and baseline results. Alzheimers Dement. 2009;5:93–104. doi: 10.1016/j.jalz.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ADAPT Steering Committee. Statement from the Steering Committee of the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT) for communication to the FDA Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee. 2005 Feb 18; [Google Scholar]
- 11.ADAPT Research Group. Lyketsos CG, Breitner JC, Green RC, Martin BK, Meinert C, et al. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68:1800–8. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- 12.ADAPT Research Group. Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC. Cognitive function over time in the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65:896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breitner JC, Baker LD, Montine TJ, Meinert CL, Lyketsos CG, Ashe KH. Extended results of the Alzheimer's Disease Anti-inflammatory Prevention Trial. Alzheimers Dement. 2011;7:402–11. doi: 10.1016/j.jalz.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ADAPT Research Group. Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer's Disease Anti-Inflammatory Prevention Trial (ADAPT) PLoS Clin Trials. 2006;1:e33. doi: 10.1371/journal.pctr.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–7. [Google Scholar]
- 16.Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I: clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 17.Wilson BA. The development and validation of a test of everyday memory behavior. J Clin Exp Psychol. 1989;11:855–87. doi: 10.1080/01688638908400940. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–32. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- 21.Szekely CA, Thorne JE, Zandi PP, Ek M, Messias E, Breitner JC, et al. Nonsteroidal anti-inflammatory drugs for the prevention of Alzheimer's disease: a systematic review. Neuroepidemiology. 2004;23:159–69. doi: 10.1159/000078501. [DOI] [PubMed] [Google Scholar]
- 22.Scharf S, Mander A, Ugoni A, Vajda F, Christophidis N. A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer's disease. Neurology. 1999;53:197–201. doi: 10.1212/wnl.53.1.197. [DOI] [PubMed] [Google Scholar]
- 23.Rogers J, Kirby LC, Hempelman SR, Berry DL, McGeer PL, Kaszniak AW, et al. Clinical trial of indomethacin in Alzheimer's disease. Neurology. 1993;43:1609–11. doi: 10.1212/wnl.43.8.1609. [DOI] [PubMed] [Google Scholar]
- 24.Aisen PS, Schmeidler J, Pasinetti GM. Randomized pilot study of nimesulide treatment in Alzheimer's disease. Neurology. 2002;58:1050–4. doi: 10.1212/wnl.58.7.1050. [DOI] [PubMed] [Google Scholar]
- 25.Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819–26. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 26.Reines SA, Block GA, Morris JC, Liu G, Nessly ML, Lines CR, et al. Rofecoxib: no effect on Alzheimer's disease in a 1-year, randomized, blinded, controlled study. Neurology. 2004;62:66–71. doi: 10.1212/wnl.62.1.66. [DOI] [PubMed] [Google Scholar]
- 27.Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, et al. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;302:2557–64. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thal LJ, Ferris SH, Kirby L, Block GA, Lines CR, Yuen E, et al. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005;30:1204–15. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- 29.Szekely CA, Zandi PP. Non-steroidal anti-inflammatory drugs and Alzheimer's disease: the epidemiological evidence. CNS Neurol Disord Drug Targets. 2010;9:132–9. doi: 10.2174/187152710791012026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.