Abstract
This review summarizes emerging information regarding the Hedgehog (Hh) signaling pathway during neoplastic transformation in the gastrointestinal tract. Although there is a role for the well-established canonical pathway in which Hedgehog ligands interact with their receptor Patched, there is sufficient evidence that downstream components of the Hh pathway, e.g., Gli1, are hijacked by non-Hh signaling pathways to promote the conversion of the epithelium to dysplasia and carcinoma. We review the canonical pathway and involvement of primary cilia, and then focus on current evidence for Hh signaling in luminal bowel cancers as well as accessory organs, i.e., liver, pancreas and biliary ducts. We conclude that targeting the Hh pathway with small molecules, nutriceuticals and other mechanisms will likely require a combination of inhibitors that target Gli transcription factors in addition to canonical modulators such as Smoothened.
Keywords: primary cilia, stroma, MDSCs, Gli1, stomach
Introduction
During the 1970s, mutagenesis screens in Drosophila uncovered a number of important developmental signaling pathways that have since been applicable to mechanisms for both mammalian development and cancer 1. The Hedgehog (Hh) signal transduction pathway is one such pathway. The initial discovery uncovered a gene locus that when mutated induced the abnormal development of hair-like projections on flies (denticles) such that its physical appearance was reminiscent of the porcupine-like mammal called hedgehog. The Hh locus was found to encode a 50 kDa precursor protein autocatalytically processed to the 19-20 kDa functional protein that was later discovered to be the ligand for a receptor encoded by another locus called Patched (Ptch). Hh ligand binding to its receptor Ptch relieves the inhibition exerted on a vast signaling cascade, which includes Smoothened (Smo), and a repressor complex (Costal 2, Fused kinase, Suppressor of Fused) that regulates the availability of the transcriptional regulator Cubitus Interruptus (Ci) 2. Details of the Drosophila pathway have been extensively reviewed elsewhere 3-5. Moreover since this review focuses on Hh signaling in the mammalian gastrointestinal tract during transformation, key components of the mammalian Hh pathway and what is known regarding canonical Hh signal transduction will be briefly summarized first for several tissue types 6-9. Second, the role of Hh signaling during neoplastic transformation for individual cancers of the gastrointestinal (GI) tract will be described with an emphasis on the response by stromal cells. Stromal cells have typically been the targets of small molecule development and natural product inhibitors (nutraceuticals) with the expectation that they will serve as adjuvant therapies for Hh responsive cancers 10, 11. The final section will discuss specific examples of how Hh signaling contributes to transformation by activating tumor associated mesenchymal and immune cell types. Investigations into the mechanism of Hh signaling has led to the discovery that some cancers are ligand independent (non-canonical signaling) 12 and will require examination of therapies that inhibit downstream signaling components such as the mammalian homolog of Ci called glioma-associated protein 1 (Gli1) 13, 14. In addition, one might query whether components of the Hh signaling pathway can be used as biomarkers 15. Interestingly, it has recently been reported that Shh circulates in plasma raising the possibility of using the ligand as a biomarker in some cancers 16.
Overview of the Hh Signaling Pathway in Mammalian Cells
Canonical Hh signaling involves epithelial expression of the ligands, which subsequently bind the 12-pass transmembrane receptors Ptch1 and Ptch2 to relieve their inhibitory influence on an adjacent 7-pass transmembrane Hh activator called Smo. Smo is located on ectodermal (neural) or mesodermal-derived cell types that respond to the Hh ligands (Fig. 1). Co-receptors that bind ligand and cooperate with Ptch to modulate the cellular response such as proliferation include members of the immunoglobulin superfamily, Growth arrest specific 1 (Gas1), CAM-related/down-regulated by oncogenes (Cdo) and brother of Cdo (Boc) 17-19. Mammalian cells express three Hh ligands--Sonic Hh (Shh), Indian Hh (Ihh) and Desert Hh (Dhh) that bind the Ptch receptor with apparently the same affinity 6 (Table 1). In the gastrointestinal tract, the major ligands expressed are Shh in the proximal gut (esophagus, stomach, liver and pancreas) and Ihh in the midgut and hindgut (small intestine and colon) 20. However, Dhh expression appears to be tissue-restricted to the nervous system and testes 21. Some tissues exhibit differential potency amongst the ligand paralogs with respect to Hh signaling (Shh>>>Ihh>Dhh). Although the luminal GI tract (stomach, small intestine and colon) constitutively expresses Hh ligands during development and after birth, parenchymal organs (liver and pancreas) express the ligands only in mature tissues and when injured 22-26.
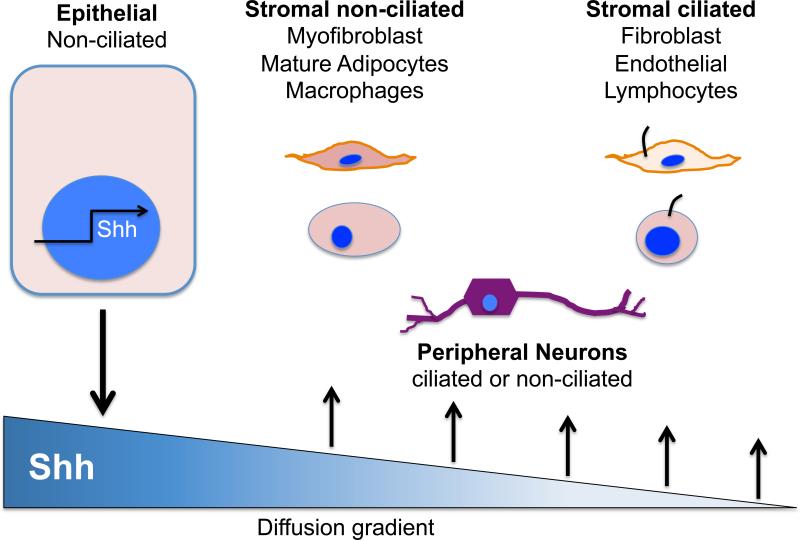
Figure 1. Canonical Hedgehog Signal Transduction.
Canonical Hedgehog signal transduction implies that the ligand, e.g., sonic hedgehog (Shh) is secreted by an epithelial cell and forms a gradient as it diffuses away from the cell. The ligand is sensed by cells in the stroma, which express Hh signaling components and primary cilia (ciliated versus non-ciliated).
Table 1. Major components of the Hedgehog signaling pathway.
Each column represents a different component of the canonical Hh signaling pathway from ligand to transmembrane receptors to signaling components and tissue specific gene targets. Thus although there are only three Hh ligands, the myriad of genes encoding modulating factors will influence which tissue-specific gene targets become activated.
| Ligands | Trans-membrane Receptors | Signaling Modulators | Signaling Adaptors | Transcrip-tion Factors | Regulatory Kinases | Gene Targets |
|---|---|---|---|---|---|---|
| Sonic Hedgehog | Patched 1 (Ptchl) 9q22.3 | Dispatched-multimerization of ligand | Costal2 (COS2) | Glil | Casein kinasel | General: Ptch, HhIP, Gli1, cyclinD |
| Indian Hedgehog | Patched 2 (Ptch2) 1p34 | Skinny Hedgehog (Skn)-lipid modification of ligand | Fused (FU) | Gli2 | Protein kinase A | Endothelial: VEGF, Ang1,2 |
| Desert Hedgehog | Smoothened (SMO) | ADAM proteases | Suppressor of Fused (SuFu) | Gli3 | Glycogen-S Kinase 3b | Bone: PTHrP, Igf1,2 |
| Hedgehog Interacting Peptide (HhIP) | β-transducin repeat-containing protein (TrCP) | Neural: N-myc | ||||
| Growth Arrest Specific (GAS)1 | Skip/Cullin1/F-box (SCF) E3 ubiquitin ligase | Gut: Lasp-1, H,K-ATPase, Gastrin | ||||
| CAM-related Down-regulated by Oncogenes (CDO) | Fibroblast: FOXL1, SFRP, SNAIL | |||||
| Brother of CDO | Stem Cells: Bmi1, Sox2, Nanog |
Unlike Ptch, Smo is a heterotrimeric guanine nucleotide binding-protein coupled receptor (GPCR) that can activate at least two intracellular signaling cascades-- a non-canonical, ligand-independent pathway that ultimately modulates the actin cytoskeleton by activating Rac1 and Rho1 GTPases 27,28 or a canonical, ligand-dependent pathway via Gli2 activation. Canonical signaling through Smo involves intracellular activation of Gli2 by limited proteolysis. Full-length Gli2 resides in the cell cytoplasm coupled to a suppressor complex comprised of Fused kinase (Fu), Suppressor of Fused (SuFu) and Costal2 (Cos2). Gli2 repression of target genes occurs after a series of phosphorylation steps by protein kinase A (PKA), glycogen synthase 3 (GSK3 ) and casein kinase I (CK1), which directs Gli2 to the proteasome for limited protein degradation after ubiquitination by the Skip-Cullin-F-box (SCF) protein/ Transducin repeat Containing Protein (TrCP) complex (Fig. 2). Limited degradation of phosphorylated Gli2 removes the C-terminal activation domain uncovering the N-terminal repressor domain. The partially degraded DNA binding protein then translocates to the nucleus and forms a complex with co-repressors such as HDAC-Sin3A to block target gene activation 29, 30. Smo activation releases Gli2 from the suppressor complex and the transcription factor preventing phosphorylation by PKA, GSK3 and CK1. Limited proteasomal degradation in the absence of specific phosphorylation removes the N-terminal repressor domain producing the Gli2 activator version of the protein, which translocates to the nucleus to bind the promoters of genes induced by Hh signaling. Examples of genes induced by Gli2-mediated Hh signaling include its receptor Ptch, Hedgehog interacting protein (Hhip) and the transcription factor Gli1 29. Thus Gli1, Ptch and Hhip are general transcriptional targets of canonical Hh signaling activity. Gli1 mediates transcriptional activation; whereas Ptch and Hhip are transmembrane receptors that block the pathway, albeit by different mechanisms. Hhip binds and sequesters ligand; whereas, ligand binding to Ptch relieves its inhibition on Smo de-repressing Hh signaling. Examples of numerous tissue-restricted genes regulated by Hh signaling include Vascular Endothelial Growth Factors (VEGF), Angiopoietin-1 and Angiopoietin-2 in endothelial cells 31, 32. Snail, Zeb1,2, Twist2, FoxF1 in fibroblasts 33-35 -smooth muscle actin, vimentin and IL-6 in myofibroblasts 36, 37, N-myc in neurons regulating development and myelination 38, 39, BMI, Sox2, Nanog in cancer stem cells 7, 40 (Table 1).
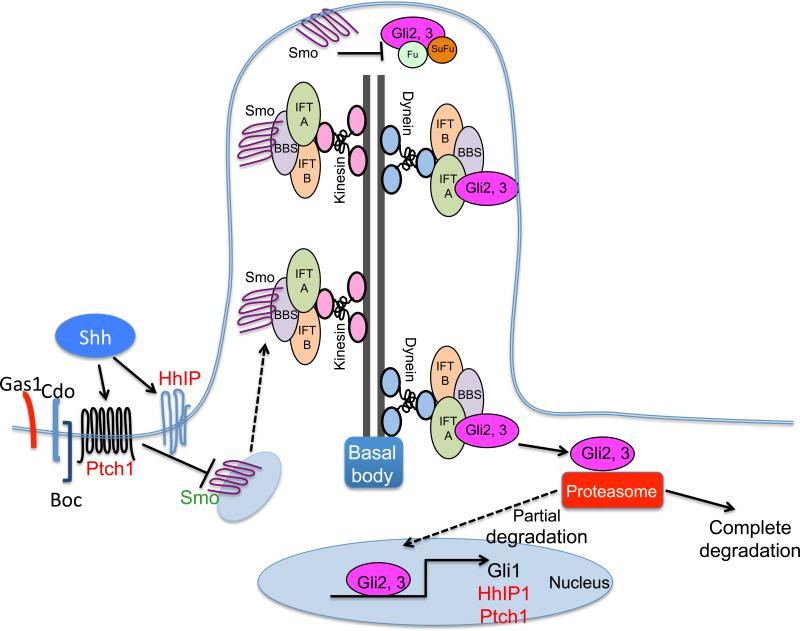
Figure 2. Primary cilia and Hedgehog signal transduction.
Sonic Hedgehog (Shh) ligand binds its receptor Patch (Ptch), which subsequently releases its inhibitory effect on quiescent Smoothened (Smo) contained in cytoplasmic vesicles. Activated Smo moves antegrade into the cilia along tracks comprised of acetylated tubulin coupled to ciliary proteins Bardet-Biedl syndrome proteins (BBS), intraflagellar transport protein (IFT) and kinesin proteins. At the vesicle tip activated Smo induces the release of Gli2 from the cilia. Ciliary proteins (BBS and IFT) plus dynein move cargo proteins retrograde into the cytoplasm. Once cytoplasmic, limited degradation directs the transcription factor to the nucleus to bind and induce Hh-regulated genes Gli1, Ptch and Hedgehog interacting protein (HhIP).
The Hh pathway has evolved to represent crosstalk between the endodermally-derived epithelium that produces the ligand and responding cells derived from ectoderm (neural cells) and mesoderm (fibroblasts, immune cells, myocytes) (Fig. 1). In the adult gastrointestinal tract, it is the stromal cells derived from meso- or ectodermal cell layers that typically express the Hh signaling receptor and transcription factors. However, immunohistochemical staining of adenocarcinomas from the gut and other cancer tissue types suggest that these epithelial-derived human tumors are capable of both ligand and receptor expression 26. This observation implies autocrine regulation of neoplastic cell growth that emerges during transformation since Ptch, Smo and Gli1, Gli2, Gli3 mRNA expression is essentially never observed in the normal gut epithelium 41.
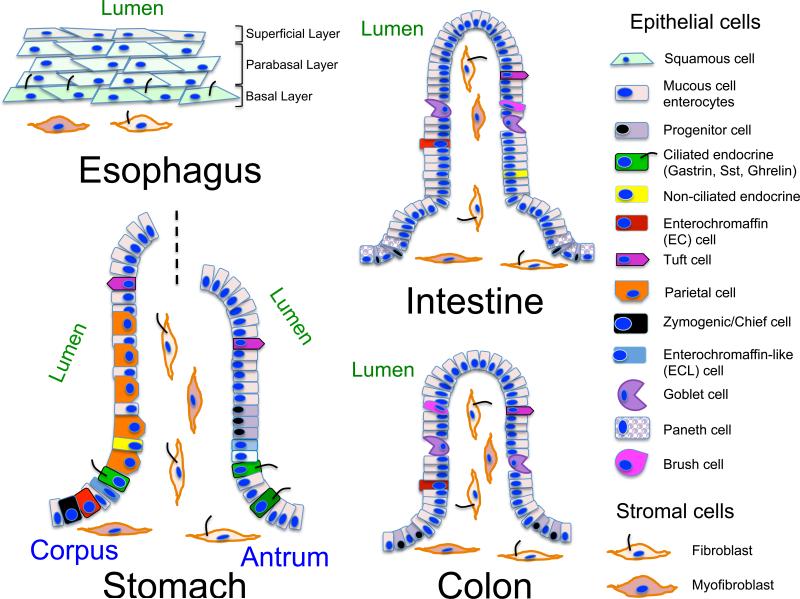
In addition to expression of specific signaling components, some Hh responding cells also produce an extracellular organelle called the primary cilium, which is required to properly transduce canonical Hh signaling 42, 43. Primary cilia are collections of acetylated -tubulin arranged as nine dimers in a cylindrical configuration with (9+2) or without a central dimer (9+0) encased within a plasma membrane sheath. Regardless of the arrangement, primary cilia are motile or non-motile sensory organelles. Although some chemosensory cells such as tuft cells contain bundles of acetylated -tubulin at the cell apex, these bundles do not associate with basal bodies and therefore are not considered primary cilia. A notable characteristic of primary cilia is that there is only one cilium per cell 44. Acetylated -tubulin dimers form tracks that move diverse signaling molecules, e.g., Smo, Gli2 or Gli3, bidirectionally via the ciliary transport complex comprised of proteins affected in the human obesity-prone Bardet-Biedl syndrome (BBS), intraflagellar transport proteins (IFT), kinesin (Kif) and dynein 45, 46 (Fig. 2). All cells are generally thought to produce primary cilia. Nevertheless, these extracellular organelles have been best characterized for a subset of stromal cells, e.g., fibroblasts, myofibroblasts, myocytes, T lymphocytes and neurons. Some lamina propria-residing stromal cells throughout the entire gastrointestinal tract produce primary cilia, as well as a subset of epithelial cells lining the stomach and biliary ducts; whereas intestinal and colonic epithelial cells do not 47 (Fig. 3). Moreover, the gastric epithelial cells expressing primary cilia are mostly endocrine cells that secrete gastrin, ghrelin or somatostatin 47. Pancreatic duct cells and endocrine cells in the islet express primary cilia, but normal acinar cells do not 48-50. Bile duct cholangiocytes express primary cilia but hepatocytes and endothelial cells lining hepatic sinusoids do not 51, 52. The canals of Hering are lined by hepatocytes that segue into cilia-expressing cholangiocytes as they carry bile from the central portion of the hepatic lobule to the terminal bile ducts in the periphery 53. The canals of Hering are distinguished by characteristic immunohistochemical staining for biliary cytokeratins 19 and 7. Both Hh ligands and Hh-regulated transcription factors are expressed in the canals of Hering, which coincides with the location of liver progenitor cells, suggesting possible autocrine Hh regulation within this distinct site. This observation also raises the possibility that if the hepatic progenitor cell produces a primary cilium that it could be regulated by autocrine Hh signaling 52.
Figure 3. Primary cilia in the luminal gastrointestinal tract.
Schematic depiction of cells lining the esophagus, stomach, intestine and colon. The cells in the esophagus are squamous with the exception of the basal cell layer, which are cuboidal. The cuboidal cells of the basal cell layer produce primary cilia. The stomach, small intestine and colon all have columnar epithelia that sit on a basement membrane. Only the stomach contains a few epithelial cells that express primary cilia. Most of these are enteroendocrine cells. None of the epithelial cells in the intestine or colon express primary cilia. There are several cell types within the lamina propria including neurons, fibroblast, immune cells and myocytes.
Hedgehog in GI Cancers
Hedgehog signaling initiates cancer in some organ systems, e.g., skin (basal cell), brain (medulloblastoma) 54, 55, but a clear etiologic role has not been described for gastrointestinal cancers in the luminal GI tract such as esophagus, colon and stomach 56. Nevertheless since there are several Hh signaling inhibitors currently used in clinical trials, it will be important to better define whether and how they might be used to treat gastrointestinal cancers, all of which have a poor prognosis if diagnosed in the late stages. Beachy and coworkers were the first to demonstrate widespread expression of Hh signaling proteins in gastrointestinal tract tumors and implicate their role in cancer progression 57. Subsequent studies confirmed the expression of Hh pathway components in cancer tissue and cell lines, putative cancer stem cells and the stroma 26, 58-60. The pattern of Hh ligand expression and its signaling components have been used to determine the direction of signaling with implications for developing anti-cancer strategies 61. Typically canonical Hh signaling indicates that the ligand is produced in an epithelial cell and received by a stromal cell (Fig. 1). However there are several other signaling mechanisms that neoplastic cells use to promote unregulated growth 8, 62. During neoplastic transformation, canonical signaling indicates ligand expression from the adenocarcinoma to the supporting stroma (paracrine signaling). However, reverse paracrine signaling suggests ligand expression by stromal cells that regulate epithelial-derived adenocarcinomas. Autocrine signaling has been described for both epithelial-derived cancer cells as well as cells within the tumor microenvironment, with the obvious phenotype being expansion of either the glandular adenocarcinoma cell compartment or the stromal cell compartment 63. Finally, a number of reports have implicated a role for non-ligand dependent tumor regulation in which mutant Ptch (Gorlin's syndrome) and SuFu or constitutively active Smo and Gli proteins initiate the cancer phenotype 64-67. Smo and Gli proteins are druggable targets within the Hh pathway currently in clinical trials 68-71. More recent observations show that tumor resistance emerges in the presence of Smo inhibition underscoring the importance of better understanding non-canonical regulation, especially through Gli1 activation 72. It was previously reported that both the TGF and Ras-Map kinase pathways directly activate Gli1 independent of Smo 73, 74. Moreover, it is now evident that cytokines activate Gli1 75. Das et al. reported that the inflammatory cytokine osteopontin (OPN) secreted by epithelial cancers into the tumor microenvironment modulates AKT-GSK3 signaling 64. Gli1 binds to the OPN promoter to induce its expression, while OPN, a phosphoglycoprotein inhibits GSK3 , which allows Gli1 to accumulate in the nucleus. cMyc binding and induction of Gli1 in Burkitt lymphoma cell lines represents another example of non-canonical Gli1 transcriptional activation. The authors found that the Gli inhibitor GANT 61 increases apoptosis and reduces the viability of Burkitt lymphoma cell lines, while exposure to Hh ligands or the Smo inhibitor cyclopamine had no effect 13.
Esophageal cancers
Although squamous cell carcinoma is the most frequent type of esophageal cancer, the prevalence of adenocarcinoma of the esophagus is rapidly increasing, especially in developed countries where the 5 year survival ranges from 15 to 25 percent 76. Obesity and esophageal reflux disease are the major risk factors for Barrett's esophagus, a precursor lesion and risk factor for adenocarcinoma of the esophagus especially if features of intestinal metaplasia and dysplasia are present. By contrast, cigarette smoke is the major risk factor for squamous cell carcinoma, suggesting that there are genetic differences that distinguish these two histologic subtypes. Gene amplification events in basal progenitor cells of stratified epithelium were frequently observed for squamous cell cancer compared to adenocarcinoma at gene loci such as paired homeobox 9 (PAX9) on chromosome 14 and Sry-related HMG box 2 (Sox2) 77, 78. Downstream regulators of Hh signaling are overexpressed in both adenocarcinoma and squamous esophageal cancers 79. However, the most consistent observation was the overexpression of Gli1 in Barrett's and adenocarcinoma of the esophagus 80, 81. Cdx2 is a transcription factor and marker for intestinal metaplasia and cell proliferation in gastric lesions and Barrett's esophagus 82-85. Using chromatin immunoprecipitation, Rizvi et al. showed that Gli1 binds to the Cdx2 promoter in Barrett's-associated adenocarcinoma cell lines (SKGT4 and FLO-1) and that the activation occurs independently of Smo 81. Thus non-canonical signaling appears to mediate transition from squamous to columnar epithelium creating an environment suitable for Barrett's intestinal metaplasia 86.
Recently, two genome-wide analyses of esophageal adenocarcionmas revealed polymorphisms at several gene loci including TP53, CDKN2A, SMAD4, the Major Histocompatibility Complex locus and chromosome 16q24.1, which associates with predisposition to Barrett's esophagus 86, 87. Chromosome 16q24.1 is closest to the locus for FOXF1, which is an Hh signaling target gene. The Forkhead homeobox (FOX) transcription factor gene cluster (FOXF1, FOXC2, FOXL1, MTHFSD) has been implicated in foregut development (lung, esophagus and stomach) but also micro-deletions in the FOX locus generate a phenotype resembling VACTERL (vertebral anomalies, gastrointestinal atresias, congenital heart malformations, urinary tract malformations, lung anomalies and alveolar capillary dysplasia) 88. At present, it is not clear whether the polymorphisms found in esophageal adenocarcinomas result in elevated or reduced expression of FOX transcription factors. However, prior work from Kaestner and co-workers showed that the FOXF1 and FOXL1 homeobox genes in stromal cells mediate Hh ligand-mediated suppression of Wnt signaling. More recently, Ihh regulation of Foxf2 was shown to inhibit intestinal polyp development, consistent with the prior observation that canonical activation of these Forkhead homeobox genes in stromal cells suppresses epithelial proliferation 89, 90. Collectively, these studies suggest that FOX transcription factors activate a program of genes in stromal cells that inhibits epithelial proliferation.
Gastric cancer
Gastric cancers are the 2nd leading cause of cancer deaths worldwide (www.globocan.iarc.fr) and like esophageal cancer, the 5 year survival rate is about 20 percent 91. Most gastric cancers are intestinal-type or diffuse adenocarcinomas that arise from the epithelium. Compared to the intestinal-type, diffuse gastric cancers are characterized by rapidly proliferating epithelial cells that do not form glandular structures and are highly invasive. By contrast, gastrointestinal stromal tumors (GIST), which arise from the interstitial cells of Cajal are positive for tyrosine kinase receptors, e.g., cKit + (CD117) inhibited by imatinib or the platelet-derived growth factor (PDGF) receptor alpha+ 92, 93; while diffuse gastric cancers exhibit inactivating mutations in the E-cadherin gene (CDH1), an inhibitor of the pro-proliferative Wnt- catenin pathway 94. Until the late 1980s, gastric adenocarcinomas typically arose in the distal stomach (antrum). Interestingly, a switch in the primary cancer site from distal to proximal stomach has coincided with the increased use of gastric acid blocking agents or proton pump inhibitors. Using the SEER (Surveillance Epidemiology & End Results) database (www.seer.cancer.gov), Camargo et al. reported that cancers in the distal stomach (antrum) have decreased, while more proximal gastric cancers in the corpus and cardia have increased 95. Extrapolating from these epidemiologic trends, adenocarcinomas of the stomach appear to exhibit a different behavior depending on their location suggesting that different molecular mechanisms regulate neoplastic transformation in the proximal and distal gastric sub-sites 95,96.
Over the past several years, we have characterized a substantive role for Hh signaling in the homeostasis of the stomach 6, 97-99. Apparently a substantial proportion of Shh ligand in circulation during Helicobacter infection originates from the gastric parietal cells and functions as a chemoattractant for myeloid cells 100. Gastric cancer initiated by chronic inflammation, e.g., Helicobacter infection, typically progresses to atrophic gastritis, metaplasia, dysplasia then adenocarcinoma 101. Recently, we reported that Gli1 is required for the transition from chronic gastritis to corpus metaplasia or spasmolytic polypeptide-expressing metaplasia (SPEM) 6 months after Helicobacter infection in mice 102. However, ectopic expression of Shh ligand in the corpus was not sufficient to induce metaplasia unless infected with Helicobacter. Although super-physiologic levels of Shh expression increased the intensity of the inflammation and induced metaplasia, our study underscores the importance of Gli1+ inflammatory cells in initiating preneoplastic changes. Treating the macrophage-like RAW 264.7 cells with Shh ligand did not induce Gli1 expression. This result was consistent with prior studies suggesting that mature myeloid cells do not respond to Hh ligand despite the expression of Ptch1 and Smo mRNA 103, 104. Indeed, mutations in Ptch1 and Smo are infrequent in gastric cancers 105. Acutely, Shh ligand acts as a chemoattractant recruiting inflammatory cells to the stomach 100, but chronically myeloid cells do not appear to be responsive to canonical signaling indicating that non-canonical Hh signaling modulates Gli1 activity during chronic gastritis 6, 75, 102. Thus the use of Hh inhibitors in human subjects chronically infected with Helicobacter might effectively prevent pre-neoplastic mucosal changes, i.e., SPEM, from which gastric adenocarcinomas can arise 106.
In contrast to inflammation-dependent regulation of Gli1 in the corpus 102, we found using LacZ reporter mice that Gli2 but not Gli1 expression increased in hyperplastic antrums of gastrin null mice 75. Loss of gastrin predisposes to tumor formation in the antrum but not the corpus 107. Further, the pro-inflammatory cytokine IL-1β stimulates expression of Gli2 instead of Gli1 in the AGS gastric cell line. Thus, the Hh-related transcription factor in the antrum initiating preneoplastic changes appears to be epithelial Gli2 and not Gli1, whose expression remained exclusively in the stroma 75. This study uncovered the possibility that transformation in the gut can be mediated by epithelial expression of Gli2 independent of Hh ligand.
Colon Cancer
In the colon, several studies have implicated Hh signaling in proliferation and neoplastic transformation. Qualtrough et al. examined the expression of Hh signaling components in colorectal cell lines and found expression of Shh and Ihh ligands as well as Ptch, Smo and Gli1 108. Since cell lines expressed both ligands and signaling components, the authors concluded that Hh-mediated tumorigenesis in the colon is autocrine. Studies by Varnat et al. showed by lineage-tracing with the ShhCre;ROSA26LacZ reporter mice that there is strong Shh gene expression in the epithelium of adult mouse colons 109. Conditional deletion of Smo (SmoFL/FL) decreased polyp formation in APCFL/FL; ActinCreERT2 chimeric mice 109. Clinically, this result might be related to non-canonical activation of Smo due to a Ptch mutation (Gorlin's syndrome) or Erk activation of Gli1 via growth factor induction of Ras. Indeed, Houghton and coworkers showed that the Gli inhibitor GANT61 inhibits colon cell line proliferation 110, 111. Vitamin D, a hydroxysteroid, inhibits Hh signaling by facilitating the interaction of Ptch with Smo 112, 113 and is likely to have relevance since the steroid exhibits efficacy against colon cancer 114. Moreover, Vitamin D suppression of Hh signaling has been recently shown to be an effective treatment for clear cell renal and basal cell carcinomas 115, 116. Thus the current evidence supports a significant role for Hh signaling-mediated tumorigenesis in the colon through both the canonical and non-canonical pathways.
Pancreatic Cancer
Pancreatic cancer is the 4th leading cause of cancer deaths in the US primarily due to the lack of early symptoms, biomarkers and advanced stage once discovered 117. The 5-year survival rate is less than 5 percent if un-resected. Hh signaling is required for pancreatic development and re-emerges in the adult pancreas when tissue regeneration is required, such as after an inflammatory or toxic injury 118. Hh ligand expression increases during pancreatic tumorigenesis suggesting that canonical Hh signaling mediates tumor progression. Pancreatic cancer generates a robust fibrotic response that has recently been shown to be the result of epithelial to mesenchymal transition (EMT) 119. Using lineage tracing in genetically-engineered mouse models, Stanger and coworkers showed that pancreatic duct epithelial cells exhibit a mesenchymal phenotype before they enter the circulation and seed the liver. This invasive phenotype was accelerated by inflammation. Although the authors did not examine Hh signaling directly in their report, pancreatic duct cells are known to express Shh ligand when inflamed or with transformation 16. Recent studies show that curcumin-mediated inhibition of Shh-Gli signaling will block EMT 120. Moreover, Gli1 deletion in a mouse model of pancreatic cancer generated by expressing oncogenic KRAS impedes cancer progression 22. Although Shh levels in the pre-neoplastic pancreas increase locally, we observed a decrease rather than an increase in circulating levels of the ligand, which we concluded correlated with the increase in fibrosis accompanying cells expressing the Ptch receptor 16. Thus both canonical and non-canonical signaling appear to participate in the progression of pancreatic cancer. However, the robust stromal response is apparently related to ligand- and Smo-independent components of the Hh pathway 22.
Hepatocellular carcinoma
Hepatocellular carcinoma or hepatomas arise within a chronically damaged organ primarily in response to viral hepatitis or toxins such as alcohol 121. Alcohol-induced liver cancer primarily proceeds through an intermediate lesion called cirrhosis in which stromal cells proliferate and secrete collagen replacing the normal hepatocyte. By contrast, viral hepatitis can either result in carcinogenesis in the hepatocyte or trigger the cirrhotic response prior to transformation. Low levels of Hh signaling are observed in the normal liver due to overexpression of HhIP, which can prevent any ligand from binding Ptch 52. In addition, Hh signaling components decrease as the liver matures 52. Additional evidence of Hh signaling in the liver include ciliopathies that result in cystic malformations of the intrahepatic biliary tree and liver fibrosis 43. However, Ptch and Smo become overexpressed in human hepatomas 122-124, suggesting a ramping-up of Hh signaling with injury and transformation. Injured hepatocytes release Hh ligands as they undergo apoptosis coincident with a decrease in HhIP expression. Hh ligands are then able to bind Ptch to initiate canonical signaling of resident quiescent cells, e.g., hepatic stellate cells, immune cells and myofibroblasts. These stromal cells then release factors such as TGF and PDGF that potentiate Hh signaling via Smo-independent activation 52. Diehl and coworkers have nicely shown that induction of Hh ligand expression in hepatocytes stimulates the accumulation of Hh-responsive myofibroblasts, which produce lactate and fuels the proliferation of adjacent malignant hepatocytes 37. Clinical trials in which Gli1 is targeted by a nanoparticle-encapsulated Gli1 inhibitor are underway 125. Moreover, inhibition of Hh signaling by the Smo inhibitor GDC-0449 in a mouse model of liver fibrosis and cancer reduced liver tumors and metastatic lesions 126. Thus once fibrosis has been initiated, it will likely be important to inhibit Smo independent regulators, i.e., Gli1.
Cholangiocarcinoma
As in the pancreas and liver, Hh signaling in the epithelial cells lining of the biliary tree known as cholangiocytes is primarily active during development 127, 128. However, recent studies by LaRusso and colleagues have demonstrated that the loss of primary cilia contributes to aberrant activation of both Erk and Hh signaling pathways 129. Apparently, the mechano-shear forces of bile flow in the duct are sensed by primary cilia on the cholangiocytes, which in turn regulate normal functions, such as proliferation and transport 130. It is known that protrusion of the cilia varies inversely with cell proliferation 131, 132. Consistent with this concept, the authors found that loss of primary cilia by HDAC6 overexpression or preventing cilia regeneration with IFT88-shRNA induces cell proliferation, anchorage-independent growth and activation of the Hh pathway 129. By contrast, inhibition of HDAC6 expression by shRNA or by tubastatin-A treatment decreased cholangiocarcinoma tumor growth in a xenograft mouse model. Although the authors do not examine whether activation of Hh signaling in the cholangiocytes is Hh ligand-dependent, the observation that Erks are activated suggests non-canonical signaling activated by loss of primary cilia. This concept is consistent with the observation that primary cilia suppress Hh signaling in cartilage tumors 133.
Hedgehog Signaling in the Mesenchyme
Adenocarcinoma development is a sequential process (metaplasia, dysplasia and neoplasia) generated by the accumulation of genomic aberrations in the epithelial cell and sustained by different immune and stromal cell types in the tumor microenvironment. A prominent feature of the tumor microenvironment for example in breast, lung and pancreatic cancers is the recruitment of cancer associated fibroblasts (CAFs), tumor-associated myeloid and T regulatory cells (Tregs) that retard the access of cytotoxic killer cells to the tumor but also secrete cytokines and growth factors that amplify the fibrotic (stromal) reaction in the tumor 134, 135. In pancreatic cancer, this “stromal reaction” is a prominent feature of cancer development and significantly contributes to the difficulty in developing effective treatments for this deadly cancer. Hedgehog inhibitors targeting Smo decrease proliferation of CAFs in pancreatic cancer and cholangiocarcinomas 136, 137. Moreover, CAFs orchestrate tumor-promoting inflammation 138 and produce the oncogenic cytokine IL-6 in gastric cancer 139. Myeloid-derived suppressor cells (MDSCs) are a subset of immune regulatory cells that play a prominent role in modulating the tumor microenvironment 140. Hallmarks of these cells include expression of the CD11b and Gr1 surface markers and their ability to inhibit T cell proliferation 141-143. In inflammation-driven gastric cancer, cells in the tumor microenvironment include MDSCs and CAFs, which represent a major source of pro-inflammatory cytokines 144. TAMs and M1/M2-polarized macrophages are also an essential component of the tumor microenvironment 145, 146, which have not been thoroughly investigated in the stomach 147, 148. A prior study demonstrated that MDSCs induce gastric carcinoma on a murine Rag2−/− background in the absence of T-cells 144. This result suggested that MDSCs promote gastric cancer in a T-cell independent manner. Therefore MDSCs might play a dual role by promoting pro-tumorigenic cytokines to initiate carcinogenesis, but also mediate cytotoxic T-cell tolerance of tumor cells 149. Collectively these immune cell types likely exhibit some phenotypic overlap, and therefore constitute an attractive target for therapy. However, until now the pathways driving emergence of these cell types have not been defined.
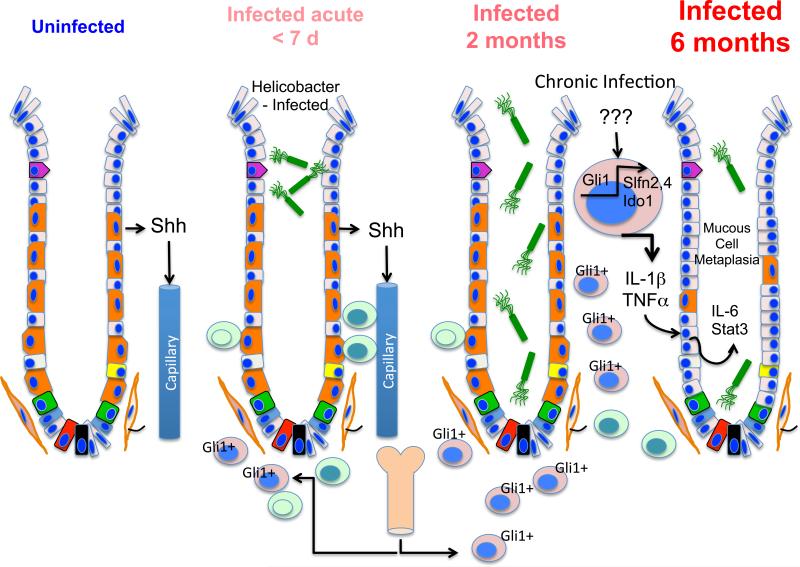
To study the role of Hh signaling during Helicobacter-induced gastritis, we infected C57BL/6 wild type and Gli1−/− mice for two and six months 102. Chronic gastritis was observed in both groups. However, only wild type mice developed SPEM at 6 months whereas, the infected Gli1 null mice did not progress to exhibit the metaplastic changes (Fig. 4). Moreover, we found that Helicobacter infection induced a subset of myeloid cells expressing CD45+MHCII+CD11b+CD11c+ surface markers suggestive of the MDSC phenotype 102. A microarray analysis of Gli1-dependent genes up-regulated during Helicobacter-induced metaplasia revealed several genes that regulate myeloid cell recruitment and differentiation, e.g., serum amyloid protein A3 (SAA3), indoleamine 2,3 dioxygenase 1 (Ido1), Schlafen-2 and Schlafen-4 (Slfn-2, Slfn-4). SAA3 regulates myeloid cell recruitment 150. Ido1 initiates tryptophan catabolism and produces bioactive kynurenine metabolites 151. Elevated levels of Ido1 in colon cancers predict a poor prognosis. Moreover, Ido1 and subsequently an increase in the kynurenine/tryptophan ratio favors suppression of effector T cell function and differentiation of regulatory T cells, which in turn facilitate immune escape by the cancer 152. Slfn 2 and 4 are myeloid differentiation factors 153 that were found to mark the myeloid subpopulation exhibiting MDSC-like surface markers (CD45+MHCII+CD11b+CD11c+) 102. The MDSC population we identified was not present in the bone marrow and only appeared in the gastric microenvironment in response to chronic inflammation from the Helicobacter infection at 6-month suggesting that these cells were “educated” in the inflamed gastric microenvironment (Fig. 4). Despite the robust inflammation in the mouse corpus from Helicobacter felis infection, the antrum remained unaffected, consistent with the notion that the corpus and antrum respond differently to inflammation.
Figure 4. Paradigm for gastritis-induced pre-neoplastic changes.
Shh secreted by gastric parietal cells recruits inflammatory cells to the stomach during the acute phase of Helicobacter infection. By 6 months, there is a switch in the quality of myeloid cells present in the stomach that is mediated by Gli1 target genes, e.g., Ido1, Schlafen 2 and Schlafen 4. These cells also express MHCII CD45, Cd11b and Cd11c surface markers suggestive of an myeloid-derived suppressor cell type (MDSC). The cells expressing additional targets of Gli1, i.e., Slfn 2, Slfn4 also produce proinflammatory cytokines IL-1 and TNF . IL-1 stimulates epithelial mucous neck cells to produce IL-6 and the proto-oncogene STAT3 coincident with the development of SP-expressing metaplasia (SPEM). The Slfn2+, Sfln4+ cells apparently develop within the microenvironment of the inflamed gastric mucosa since these markers were not observed in the bone marrow.
We characterized the mechanism by which IL-1 induces mucous cell metaplasia (SPEM) and found that IL-6 was expressed in proliferating mucous neck epithelial cells in the gastric corpus but was absent in the Gli1−/−-infected mice (Fig. 4). This result was in contrast to reports that IL-6 is secreted primarily from fibroblasts and myeloid cells 154. Correlating with the increased IL-6 production by mucous cells, SPEM cells expressed high levels of pSTAT3 and were Ki67+. SPEM was rarely observed in the Shh-overexpressing mice unless the mice were infected with Helicobacter. Therefore, we concluded that Shh is not sufficient to drive gastric metaplasia in the absence of chronic inflammation. However, the acute inflammatory response initiated by Helicobacter infection was diminished with gastric epithelial deletion of the Shh ligand or Smo loci demonstrating a role for canonical signaling during acute gastric inflammation 100. In summary, our study shows that Hedgehog-dependent gastric metaplasia depends on a MDSC-like population (Fig. 4). Thus therapies targeting this aberrant myeloid-derived cell population could be essential in preventing the irreversible progression to dysplasia not only for gastric cancer but also for other epithelial-derived neoplasia triggered by chronic inflammation.
Concluding Remarks
In summary, there are several consistent patterns regarding the Hh signaling pathway and carcinogenesis that continue to emerge. First, non-canonical signaling appears to be a significant signaling mechanism used by a variety of tumors to uncouple expression of proliferative signals from the normal regulatory mechanisms. Both Gli1 and Gli2 appear to be important targets of pro-proliferative pathways independent of Smo as well as Hh ligands (non-canonical Hh signaling). Second, multiple components of the Hh signaling pathway will need to be targeted to effectively block neoplastic transformation 155. Third, the signaling pathways capable of activating non-canonical Gli1 signaling is ever expanding and includes TGF , growth factor activation of the Ras-ERK pathway and more recently inflammatory cytokines. Finally, what will this mean for biomarker and small molecule development? The Shh ligand clearly circulates in human plasma, but whether it is a reliable marker of tumorigenesis or merely increases its abundance in the setting of receptor uncoupling remains to be determined. A corollary to this concept is the notion that while targeting the ligand with humanized antibodies, nutriceuticals or small molecules is accessible, resistance is likely to emerge since Gli proteins appear to be regulated by multiple pathways.
Acknowledgments
Grant Support: Public Health Service Grant P01 DK62041-10
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Goodman RH, Smolik SM. Cubitus interruptus requires drosophila CREB-binding protein to activate wingless expression in the drosophila embryo. Mol Cell Biol. 2000;20:1616–25. doi: 10.1128/mcb.20.5.1616-1625.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87:1343–75. doi: 10.1152/physrev.00054.2006. [DOI] [PubMed] [Google Scholar]
- 4.Perrimon N, Pitsouli C, Shilo BZ. Signaling mechanisms controlling cell fate and embryonic patterning. Cold Spring Harb Perspect Biol. 2012;4:a005975. doi: 10.1101/cshperspect.a005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan KE, Chiang C. Hedgehog secretion and signal transduction in vertebrates. J Biol Chem. 2012;287:17905–13. doi: 10.1074/jbc.R112.356006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merchant JL. Hedgehog signalling in gut development, physiology and cancer. J Physiol. 2012;590:421–32. doi: 10.1113/jphysiol.2011.220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahi MH, Rey JA, Castresana JS. The sonic hedgehog-GLI1 signaling pathway in brain tumor development. Expert Opin Ther Targets. 2012;16:1227–38. doi: 10.1517/14728222.2012.720975. [DOI] [PubMed] [Google Scholar]
- 8.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30:303–12. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Kar S, Deb M, Sengupta D, et al. Intricacies of hedgehog signaling pathways: a perspective in tumorigenesis. Exp Cell Res. 2012;318:1959–72. doi: 10.1016/j.yexcr.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Hadden MK. Hedgehog pathway inhibitors: a patent review (2009--present). Expert Opin Ther Pat. 2013;23:345–61. doi: 10.1517/13543776.2013.757304. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar FH, Li Y, Wang Z, et al. The role of nutraceuticals in the regulation of Wnt and Hedgehog signaling in cancer. Cancer Metastasis Rev. 2010;29:383–94. doi: 10.1007/s10555-010-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marini KD, Payne BJ, Watkins DN, et al. Mechanisms of Hedgehog signalling in cancer. Growth Factors. 2011;29:221–34. doi: 10.3109/08977194.2011.610756. [DOI] [PubMed] [Google Scholar]
- 13.Yoon JW, Gallant M, Lamm ML, et al. Non-canonical regulation of the Hedgehog mediator GLI1 by c-MYC in Burkitt Lymphoma. Mol Cancer Res. 2013 doi: 10.1158/1541-7786.MCR-12-0441. [DOI] [PubMed] [Google Scholar]
- 14.Ok CY, Singh RR, Vega F. Aberrant activation of the hedgehog signaling pathway in malignant hematological neoplasms. Am J Pathol. 2012;180:2–11. doi: 10.1016/j.ajpath.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzelepi V, Karlou M, Wen S, et al. Expression of hedgehog pathway components in prostate carcinoma microenvironment: shifting the balance towards autocrine signalling. Histopathology. 2011;58:1037–47. doi: 10.1111/j.1365-2559.2011.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Zaatari M, Daignault S, Tessier A, et al. Plasma shh levels reduced in pancreatic cancer patients. Pancreas. 2012;41:1019–28. doi: 10.1097/MPA.0b013e31824a0eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenzen T, Allen BL, Cole F, et al. The cell surface membrane proteins Cdo and Boc are components and targets of the Hedgehog signaling pathway and feedback network in mice. Dev Cell. 2006;10:647–56. doi: 10.1016/j.devcel.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Allen BL, Tenzen T, McMahon AP. The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development. Genes Dev. 2007;21:1244–57. doi: 10.1101/gad.1543607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grachtchouk M, Pero J, Yang SH, et al. Basal cell carcinomas in mice arise from hair follicle stem cells and multiple epithelial progenitor populations. J Clin Invest. 2011;121:1768–81. doi: 10.1172/JCI46307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–72. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 21.Makela JA, Saario V, Bourguiba-Hachemi S, et al. Hedgehog signalling promotes germ cell survival in the rat testis. Reproduction. 2011;142:711–21. doi: 10.1530/REP-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills LD, Zhang Y, Marler RJ, et al. Loss of the Transcription Factor GLI1 Identifies a Signaling Network in the Tumor Microenvironment Mediating KRAS-Induced Transformation. J Biol Chem. 2013 doi: 10.1074/jbc.M112.438846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi SS, Syn WK, Karaca GF, et al. Leptin promotes the myofibroblastic phenotype in hepatic stellate cells by activating the hedgehog pathway. J Biol Chem. 2010;285:36551–60. doi: 10.1074/jbc.M110.168542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syn WK, Jung Y, Omenetti A, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478–1488. e8. doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fendrich V, Esni F, Garay MV, et al. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology. 2008;135:621–31. doi: 10.1053/j.gastro.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukaya M, Isohata N, Ohta H, et al. Hedgehog signal activation in gastric pit cell and in diffuse-type gastric cancer. Gastroenterology. 2006;131:14–29. doi: 10.1053/j.gastro.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Polizio AH, Chinchilla P, Chen X, et al. Sonic Hedgehog activates the GTPases Rac1 and RhoA in a Gli-independent manner through coupling of smoothened to Gi proteins. Sci Signal. 2011;4:pt7. doi: 10.1126/scisignal.2002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang LH, Zhang L, Tobe RY, et al. Cost-effectiveness analysis of neonatal hearing screening program in China: should universal screening be prioritized? BMC Health Serv Res. 2012;12:97. doi: 10.1186/1472-6963-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol. 2008;85:225–60. doi: 10.1016/S0070-2153(08)00809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez-Zapico ME. Primers on molecular pathways GLI: more than just Hedgehog? Pancreatology. 2008;8:227–9. doi: 10.1159/000134271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao C, Feng R, Engevik AC, et al. Sonic Hedgehog contributes to gastric mucosal restitution after injury. Lab Invest. 2013;93:96–111. doi: 10.1038/labinvest.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Tuyl M, Groenman F, Wang J, et al. Angiogenic factors stimulate tubular branching morphogenesis of sonic hedgehog-deficient lungs. Dev Biol. 2007;303:514–26. doi: 10.1016/j.ydbio.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 33.Katoh M, Igarashi M, Fukuda H, et al. Cancer genetics and genomics of human FOX family genes. Cancer Lett. 2013;328:198–206. doi: 10.1016/j.canlet.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Katoh Y, Katoh M. Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med. 2009;9:873–86. doi: 10.2174/156652409789105570. [DOI] [PubMed] [Google Scholar]
- 35.Saito RA, Micke P, Paulsson J, et al. Forkhead box F1 regulates tumor-promoting properties of cancer-associated fibroblasts in lung cancer. Cancer Res. 2010;70:2644–54. doi: 10.1158/0008-5472.CAN-09-3644. [DOI] [PubMed] [Google Scholar]
- 36.Shaker A, Binkley J, Darwech I, et al. Stromal cells participate in the murine esophageal mucosal injury response. Am J Physiol Gastrointest Liver Physiol. 2013;304:G662–72. doi: 10.1152/ajpgi.00225.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan IS, Guy CD, Chen Y, et al. Paracrine Hedgehog signaling drives metabolic changes in hepatocellular carcinoma. Cancer Res. 2012;72:6344–50. doi: 10.1158/0008-5472.CAN-12-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferent J, Zimmer C, Durbec P, et al. Sonic Hedgehog signaling is a positive oligodendrocyte regulator during demyelination. J Neurosci. 2013;33:1759–72. doi: 10.1523/JNEUROSCI.3334-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haag D, Zipper P, Westrich V, et al. Nos2 inactivation promotes the development of medulloblastoma in Ptch1(+/−) mice by deregulation of Gap43-dependent granule cell precursor migration. PLoS Genet. 2012;8:e1002572. doi: 10.1371/journal.pgen.1002572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santini R, Vinci MC, Pandolfi S, et al. Hedgehog-GLI signaling drives self-renewal and tumorigenicity of human melanoma-initiating cells. Stem Cells. 2012;30:1808–18. doi: 10.1002/stem.1160. [DOI] [PubMed] [Google Scholar]
- 41.Kolterud A, Grosse AS, Zacharias WJ, et al. Paracrine Hedgehog signaling in stomach and intestine: new roles for hedgehog in gastrointestinal patterning. Gastroenterology. 2009;137:618–28. doi: 10.1053/j.gastro.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003;15:105–10. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 43.Pedersen LB, Veland IR, Schroder JM, et al. Assembly of primary cilia. Dev Dyn. 2008;237:1993–2006. doi: 10.1002/dvdy.21521. [DOI] [PubMed] [Google Scholar]
- 44.Saqui-Salces M, Keeley TM, Grosse AS, et al. Gastric tuft cells express DCLK1 and are expanded in hyperplasia. Histochem Cell Biol. 2011;136:191–204. doi: 10.1007/s00418-011-0831-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei Q, Zhang Y, Li Y, et al. The BBSome controls IFT assembly and turnaround in cilia. Nat Cell Biol. 2012;14:950–7. doi: 10.1038/ncb2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pazour GJ, Agrin N, Leszyk J, et al. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–13. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saqui-Salces M, Dowdle WE, Reiter JF, et al. A high-fat diet regulates gastrin and acid secretion through primary cilia. FASEB J. 2012;26:3127–39. doi: 10.1096/fj.11-197426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fendrich V, Oh E, Bang S, et al. Ectopic overexpression of Sonic Hedgehog (Shh) induces stromal expansion and metaplasia in the adult murine pancreas. Neoplasia. 2011;13:923–30. doi: 10.1593/neo.11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto M, Kataoka K. Electron microscopic observation of the primary cilium in the pancreatic islets. Arch Histol Jpn. 1986;49:449–57. doi: 10.1679/aohc.49.449. [DOI] [PubMed] [Google Scholar]
- 50.Cervantes S, Lau J, Cano DA, et al. Primary cilia regulate Gli/Hedgehog activation in pancreas. Proc Natl Acad Sci U S A. 2010;107:10109–14. doi: 10.1073/pnas.0909900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang BQ, Masyuk TV, Muff MA, et al. Isolation and characterization of cholangiocyte primary cilia. Am J Physiol Gastrointest Liver Physiol. 2006;291:G500–9. doi: 10.1152/ajpgi.00064.2006. [DOI] [PubMed] [Google Scholar]
- 52.Omenetti A, Choi S, Michelotti G, et al. Hedgehog signaling in the liver. J Hepatol. 2011;54:366–73. doi: 10.1016/j.jhep.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saxena R, Theise N. Canals of Hering: recent insights and current knowledge. Semin Liver Dis. 2004;24:43–8. doi: 10.1055/s-2004-823100. [DOI] [PubMed] [Google Scholar]
- 54.Yun JI, Kim HR, Park H, et al. Small molecule inhibitors of the hedgehog signaling pathway for the treatment of cancer. Arch Pharm Res. 2012;35:1317–33. doi: 10.1007/s12272-012-0801-8. [DOI] [PubMed] [Google Scholar]
- 55.Sahebjam S, Siu LL, Razak AA. The utility of hedgehog signaling pathway inhibition for cancer. Oncologist. 2012;17:1090–9. doi: 10.1634/theoncologist.2011-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merchant AA, Matsui W. Targeting Hedgehog--a cancer stem cell pathway. Clin Cancer Res. 2010;16:3130–40. doi: 10.1158/1078-0432.CCR-09-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berman DM, Karhadkar SS, Maitra A, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–51. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 58.Liu H, Gu D, Xie J. Clinical implications of hedgehog signaling pathway inhibitors. Chin J Cancer. 2011;30:13–26. doi: 10.5732/cjc.010.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruiz i Altaba A. Hedgehog signaling and the Gli code in stem cells, cancer, and metastases. Sci Signal. 2011;4:pt9. doi: 10.1126/scisignal.2002540. [DOI] [PubMed] [Google Scholar]
- 60.Li X, Ma Q, Duan W, et al. Paracrine sonic hedgehog signaling derived from tumor epithelial cells: a key regulator in the pancreatic tumor microenvironment. Crit Rev Eukaryot Gene Expr. 2012;22:97–108. doi: 10.1615/critreveukargeneexpr.v22.i2.20. [DOI] [PubMed] [Google Scholar]
- 61.Raju GP, Pham D. Hedgehog inhibition as an anti-cancer strategy. Vitam Horm. 2012;88:507–22. doi: 10.1016/B978-0-12-394622-5.00023-7. [DOI] [PubMed] [Google Scholar]
- 62.Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–33. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 63.Saqui-Salces M, Merchant JL. Hedgehog signaling and gastrointestinal cancer. Biochim Biophys Acta. 2010;1803:786–95. doi: 10.1016/j.bbamcr.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Das S, Samant RS, Shevde LA. Non-Classical Activation of Hedgehog Signaling Enhances Multidrug Resistance and Makes Cancer Cells Refractory to SMOH-Targeting Hedgehog Inhibition. J Biol Chem. 2013 doi: 10.1074/jbc.M112.432302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watkins DN, Peacock CD. Hedgehog signalling in foregut malignancy. Biochem Pharmacol. 2004;68:1055–60. doi: 10.1016/j.bcp.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 66.Nolan-Stevaux O, Lau J, Truitt ML, et al. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23:24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robbins DJ, Fei DL, Riobo NA. The Hedgehog signal transduction network. Sci Signal. 2012;5:re6. doi: 10.1126/scisignal.2002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kiselyov AS. Targeting the hedgehog signaling pathway with small molecules. Anticancer Agents Med Chem. 2006;6:445–9. doi: 10.2174/187152006778226495. [DOI] [PubMed] [Google Scholar]
- 69.Rudin CM, Hann CL, Laterra J, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–8. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stanton BZ, Peng LF. Small-molecule modulators of the Sonic Hedgehog signaling pathway. Mol Biosyst. 2010;6:44–54. doi: 10.1039/b910196a. [DOI] [PubMed] [Google Scholar]
- 71.Mazumdar T, DeVecchio J, Agyeman A, et al. The GLI genes as the molecular switch in disrupting Hedgehog signaling in colon cancer. Oncotarget. 2011;2:638–45. doi: 10.18632/oncotarget.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Atwood SX, Chang AL, Oro AE. Hedgehog pathway inhibition and the race against tumor evolution. J Cell Biol. 2012;199:193–7. doi: 10.1083/jcb.201207140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson RW, Nguyen MP, Padalecki SS, et al. TGF-beta promotion of Gli2-induced expression of parathyroid hormone-related protein, an important osteolytic factor in bone metastasis, is independent of canonical Hedgehog signaling. Cancer Res. 2011;71:822–31. doi: 10.1158/0008-5472.CAN-10-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lauth M, Toftgard R. Non-Canonical Activation of GLI Transcription Factors: Implications for Targeted Anti-Cancer Therapy. Cell Cycle. 2007;6:2458–63. doi: 10.4161/cc.6.20.4808. [DOI] [PubMed] [Google Scholar]
- 75.Saqui-Salces M, Coves-Datson E, Veniaminova NA, et al. Inflammation and Gli2 suppress gastrin gene expression in a murine model of antral hyperplasia. PLoS One. 2012;7:e48039. doi: 10.1371/journal.pone.0048039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2013;381:400–12. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 77.Bandla S, Pennathur A, Luketich JD, et al. Comparative genomics of esophageal adenocarcinoma and squamous cell carcinoma. Ann Thorac Surg. 2012;93:1101–6. doi: 10.1016/j.athoracsur.2012.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu K, Jiang M, Lu Y, et al. Sox2 cooperates with inflammation-mediated stat3 activation in the malignant transformation of foregut Basal progenitor cells. Cell Stem Cell. 2013;12:304–15. doi: 10.1016/j.stem.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang L, Wang LS, Chen XL, et al. Hedgehog signaling activation in the development of squamous cell carcinoma and adenocarcinoma of esophagus. Int J Biochem Mol Biol. 2012;3:46–57. [PMC free article] [PubMed] [Google Scholar]
- 80.Clemons NJ, Wang DH, Croagh D, et al. Sox9 drives columnar differentiation of esophageal squamous epithelium: a possible role in the pathogenesis of Barrett's esophagus. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1335–46. doi: 10.1152/ajpgi.00291.2012. [DOI] [PubMed] [Google Scholar]
- 81.Rizvi S, Demars CJ, Comba A, et al. Combinatorial chemoprevention reveals a novel smoothened-independent role of GLI1 in esophageal carcinogenesis. Cancer Res. 2010;70:6787–96. doi: 10.1158/0008-5472.CAN-10-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stairs DB, Kong J, Lynch JP. Cdx genes, inflammation, and the pathogenesis of intestinal metaplasia. Prog Mol Biol Transl Sci. 2010;96:231–70. doi: 10.1016/B978-0-12-381280-3.00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sugano K. Premalignant conditions of gastric cancer. J Gastroenterol Hepatol. 2013 doi: 10.1111/jgh.12209. [DOI] [PubMed] [Google Scholar]
- 84.Barros R, Freund JN, David L, et al. Gastric intestinal metaplasia revisited: function and regulation of CDX2. Trends Mol Med. 2012;18:555–63. doi: 10.1016/j.molmed.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 85.Quante M, Bhagat G, Abrams JA, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell. 2012;21:36–51. doi: 10.1016/j.ccr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Su Z, Gay LJ, Strange A, et al. Common variants at the MHC locus and at chromosome 16q24.1 predispose to Barrett's esophagus. Nat Genet. 2012;44:1131–6. doi: 10.1038/ng.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dulak AM, Stojanov P, Peng S, et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet. 2013 doi: 10.1038/ng.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shaw-Smith C. Genetic factors in esophageal atresia, tracheo-esophageal fistula and the VACTERL association: roles for FOXF1 and the 16q24.1 FOX transcription factor gene cluster, and review of the literature. Eur J Med Genet. 2010;53:6–13. doi: 10.1016/j.ejmg.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van den Brink GR, Rubin DC. Foxf2: a mesenchymal regulator of intestinal adenoma development. Gastroenterology. 2013;144:873–6. doi: 10.1053/j.gastro.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nik AM, Reyahi A, Ponten F, et al. Foxf2 in intestinal fibroblasts reduces numbers of lgr5(+) stem cells and adenoma formation by inhibiting wnt signaling. Gastroenterology. 2013;144:1001–11. doi: 10.1053/j.gastro.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 91.Nagini S. Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol. 2012;4:156–69. doi: 10.4251/wjgo.v4.i7.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Edris B, Willingham SB, Weiskopf K, et al. Anti-KIT monoclonal antibody inhibits imatinib-resistant gastrointestinal stromal tumor growth. Proc Natl Acad Sci U S A. 2013;110:3501–6. doi: 10.1073/pnas.1222893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813–25. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 94.Chun N, Ford JM. Genetic testing by cancer site: stomach. Cancer J. 2012;18:355–63. doi: 10.1097/PPO.0b013e31826246dc. [DOI] [PubMed] [Google Scholar]
- 95.Camargo MC, Anderson WF, King JB, et al. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut. 2011;60:1644–9. doi: 10.1136/gut.2010.236737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Anderson WF, Camargo MC, Fraumeni JF, Jr., et al. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303:1723–8. doi: 10.1001/jama.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stepan V, Ramamoorthy S, Nitsche H, et al. Regulation and function of the sonic hedgehog signal transduction pathway in isolated gastric parietal cells. J Biol Chem. 2005;280:15700–8. doi: 10.1074/jbc.M413037200. [DOI] [PubMed] [Google Scholar]
- 98.Zavros Y, Waghray M, Tessier A, et al. Reduced pepsin a processing of sonic hedgehog in parietal cells precedes gastric atrophy and transformation. J Biol Chem. 2007;282:33265–33274. doi: 10.1074/jbc.M707090200. [DOI] [PubMed] [Google Scholar]
- 99.Waghray M, Zavros Y, Saqui-Salces M, et al. Interleukin-1beta promotes gastric atrophy through suppression of Sonic Hedgehog. Gastroenterology. 2010;138:562–72. doi: 10.1053/j.gastro.2009.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schumacher MA, Donnelly JM, Engevik AC, et al. Gastric Sonic Hedgehog acts as a macrophage chemoattractant during the immune response to Helicobacter pylori. Gastroenterology. 2012;142:1150–1159. e6. doi: 10.1053/j.gastro.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Correa P, Haenszel W, Cuello C, et al. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 102.El-Zaatari M, Kao JY, Tessier A, et al. Gli1 deletion prevents helicobacter-induced gastric metaplasia and expansion of myeloid cell subsets. PLoS One. 2013;8:e58935. doi: 10.1371/journal.pone.0058935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Merchant A, Joseph G, Wang Q, et al. Gli1 regulates the proliferation and differentiation of HSCs and myeloid progenitors. Blood. 2010;115:2391–6. doi: 10.1182/blood-2009-09-241703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bhardwaj G, Murdoch B, Wu D, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172–80. doi: 10.1038/84282. [DOI] [PubMed] [Google Scholar]
- 105.Wang XD, Inzunza H, Chang H, et al. Mutations in the hedgehog pathway genes SMO and PTCH1 in human gastric tumors. PLoS One. 2013;8:e54415. doi: 10.1371/journal.pone.0054415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.El-Zimaity HM, Ota H, Graham DY, et al. Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer. 2002;94:1428–36. doi: 10.1002/cncr.10375. [DOI] [PubMed] [Google Scholar]
- 107.Zavros Y, Eaton KA, Kang W, et al. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene. 2005;24:2354–66. doi: 10.1038/sj.onc.1208407. [DOI] [PubMed] [Google Scholar]
- 108.Qualtrough D, Buda A, Gaffield W, et al. Hedgehog signalling in colorectal tumour cells: induction of apoptosis with cyclopamine treatment. Int J Cancer. 2004;110:831–7. doi: 10.1002/ijc.20227. [DOI] [PubMed] [Google Scholar]
- 109.Varnat F, Zacchetti G, Ruiz i Altaba A. Hedgehog pathway activity is required for the lethality and intestinal phenotypes of mice with hyperactive Wnt signaling. Mech Dev. 2010;127:73–81. doi: 10.1016/j.mod.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 110.Shi T, Mazumdar T, Devecchio J, et al. cDNA microarray gene expression profiling of hedgehog signaling pathway inhibition in human colon cancer cells. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mazumdar T, DeVecchio J, Shi T, et al. Hedgehog signaling drives cellular survival in human colon carcinoma cells. Cancer Res. 2011;71:1092–102. doi: 10.1158/0008-5472.CAN-10-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bijlsma MF, Spek CA, Zivkovic D, et al. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006;4:e232. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hoff M. Inhibiting hedgehog: new insights into a developmentally important signaling pathway. PLoS Biol. 2006;4:e258. doi: 10.1371/journal.pbio.0040258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cross HS, Nittke T, Peterlik M. Modulation of vitamin D synthesis and catabolism in colorectal mucosa: a new target for cancer prevention. Anticancer Res. 2009;29:3705–12. [PubMed] [Google Scholar]
- 115.Dormoy V, Beraud C, Lindner V, et al. Vitamin D3 triggers antitumor activity through targeting hedgehog signaling in human renal cell carcinoma. Carcinogenesis. 2012;33:2084–93. doi: 10.1093/carcin/bgs255. [DOI] [PubMed] [Google Scholar]
- 116.Tang JY, Xiao TZ, Oda Y, et al. Vitamin D3 inhibits hedgehog signaling and proliferation in murine Basal cell carcinomas. Cancer Prev Res (Phila) 2011;4:744–51. doi: 10.1158/1940-6207.CAPR-10-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim EJ, Simeone DM. Advances in pancreatic cancer. Curr Opin Gastroenterol. 2011;27:460–6. doi: 10.1097/MOG.0b013e328349e31f. [DOI] [PubMed] [Google Scholar]
- 118.Thayer SP, Di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–6. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–61. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun XD, Liu XE, Huang DS. Curcumin reverses the epithelial-mesenchymal transition of pancreatic cancer cells by inhibiting the Hedgehog signaling pathway. Oncol Rep. 2013;29:2401–7. doi: 10.3892/or.2013.2385. [DOI] [PubMed] [Google Scholar]
- 121.Shiraha H, Yamamoto K, Namba M. Human hepatocyte carcinogenesis (review). Int J Oncol. 2013;42:1133–8. doi: 10.3892/ijo.2013.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pereira Tde A, Witek RP, Syn WK, et al. Viral factors induce Hedgehog pathway activation in humans with viral hepatitis, cirrhosis, and hepatocellular carcinoma. Lab Invest. 2010;90:1690–703. doi: 10.1038/labinvest.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kappler R, von Schweinitz D. A better way forward: targeting hedgehog signaling in liver cancer. Front Biosci (Schol Ed) 2012;4:277–86. doi: 10.2741/268. [DOI] [PubMed] [Google Scholar]
- 124.Patil MA, Zhang J, Ho C, et al. Hedgehog signaling in human hepatocellular carcinoma. Cancer Biol Ther. 2006;5:111–7. doi: 10.4161/cbt.5.1.2379. [DOI] [PubMed] [Google Scholar]
- 125.Xu Y, Chenna V, Hu C, et al. Polymeric nanoparticle-encapsulated hedgehog pathway inhibitor HPI-1 (NanoHHI) inhibits systemic metastases in an orthotopic model of human hepatocellular carcinoma. Clin Cancer Res. 2012;18:1291–302. doi: 10.1158/1078-0432.CCR-11-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Philips GM, Chan IS, Swiderska M, et al. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer. PLoS One. 2011;6:e23943. doi: 10.1371/journal.pone.0023943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Omenetti A, Syn WK, Jung Y, et al. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production. Hepatology. 2009;50:518–27. doi: 10.1002/hep.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Omenetti A, Diehl AM. Hedgehog signaling in cholangiocytes. Curr Opin Gastroenterol. 2011;27:268–75. doi: 10.1097/MOG.0b013e32834550b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gradilone SA, Radtke BN, Bogert PS, et al. HDAC6 Inhibition Restores Ciliary Expression and Decreases Tumor Growth. Cancer Res. 2013;73:2259–70. doi: 10.1158/0008-5472.CAN-12-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gradilone SA, Masyuk AI, Splinter PL, et al. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci U S A. 2007;104:19138–43. doi: 10.1073/pnas.0705964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102:11325–30. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–78. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 133.Ho L, Ali SA, Al-Jazrawe M, et al. Primary cilia attenuate hedgehog signalling in neoplastic chondrocytes. Oncogene. 2012 doi: 10.1038/onc.2012.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lu T, Ramakrishnan R, Altiok S, et al. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121:4015–29. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Clark CE, Hingorani SR, Mick R, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–27. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 136.Hwang RF, Moore TT, Hattersley MM, et al. Inhibition of the hedgehog pathway targets the tumor-associated stroma in pancreatic cancer. Mol Cancer Res. 2012;10:1147–57. doi: 10.1158/1541-7786.MCR-12-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2012;9:44–54. doi: 10.1038/nrgastro.2011.222. [DOI] [PubMed] [Google Scholar]
- 138.Raz Y, Erez N. An inflammatory vicious cycle: Fibroblasts and immune cell recruitment in cancer. Exp Cell Res. 2013 doi: 10.1016/j.yexcr.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 139.Kinoshita H, Hirata Y, Nakagawa H, et al. Interleukin-6 Mediates Epithelial-Stromal Interactions and Promotes Gastric Tumorigenesis. PLoS One. 2013;8:e60914. doi: 10.1371/journal.pone.0060914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Goedegebuure P, Mitchem JB, Porembka MR, et al. Myeloid-derived suppressor cells: general characteristics and relevance to clinical management of pancreatic cancer. Curr Cancer Drug Targets. 2011;11:734–51. doi: 10.2174/156800911796191024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lindau D, Gielen P, Kroesen M, et al. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–15. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Haverkamp JM, Crist SA, Elzey BD, et al. In vivo suppressive function of myeloid-derived suppressor cells is limited to the inflammatory site. Eur J Immunol. 2011;41:749–59. doi: 10.1002/eji.201041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68:2561–3. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- 144.Tu S, Bhagat G, Cui G, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–19. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sica A, Schioppa T, Mantovani A, et al. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–27. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 146.Solinas G, Germano G, Mantovani A, et al. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 147.Ishigami S, Natsugoe S, Tokuda K, et al. Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer Res. 2003;23:4079–83. [PubMed] [Google Scholar]
- 148.Quiding-Jarbrink M, Raghavan S, Sundquist M. Enhanced M1 macrophage polarization in human helicobacter pylori-associated atrophic gastritis and in vaccinated mice. PLoS One. 2010;5:e15018. doi: 10.1371/journal.pone.0015018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nagaraj S, Schrum AG, Cho HI, et al. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol. 2010;184:3106–16. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Han CY, Subramanian S, Chan CK, et al. Adipocyte-derived serum amyloid A3 and hyaluronan play a role in monocyte recruitment and adhesion. Diabetes. 2007;56:2260–73. doi: 10.2337/db07-0218. [DOI] [PubMed] [Google Scholar]
- 151.Ciorba MA. Indoleamine 2,3 dioxygenase in intestinal disease. Curr Opin Gastroenterol. 2013;29:146–52. doi: 10.1097/MOG.0b013e32835c9cb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cesario A, Rocca B, Rutella S. The interplay between indoleamine 2,3-dioxygenase 1 (IDO1) and cyclooxygenase (COX)-2 in chronic inflammation and cancer. Curr Med Chem. 2011;18:2263–71. doi: 10.2174/092986711795656063. [DOI] [PubMed] [Google Scholar]
- 153.van Zuylen WJ, Garceau V, Idris A, et al. Macrophage activation and differentiation signals regulate schlafen-4 gene expression: evidence for Schlafen-4 as a modulator of myelopoiesis. PLoS One. 2011;6:e15723. doi: 10.1371/journal.pone.0015723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–69. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 155.Bahra M, Kamphues C, Boas-Knoop S, et al. Combination of hedgehog signaling blockage and chemotherapy leads to tumor reduction in pancreatic adenocarcinomas. Pancreas. 2012;41:222–9. doi: 10.1097/MPA.0b013e31822896dd. [DOI] [PubMed] [Google Scholar]