Abstract
Quantification of the age- and gender-specific in vivo mechanical characteristics of the ascending aorta (AA) will allow for identification of abnormalities aside from changes brought on by aging alone. Multiphase clinical CT scans of 45 male patients between the ages of 30 and 79 years were analyzed to assess age-dependent in vivo AA characteristics. The three-dimensional AA geometry for each patient was reconstructed from the CT scans for 9–10 phases throughout the cardiac cycle. The AA circumference was measured during each phase and was used to determine the corresponding diameter, circumferential strain, and wall tension at each phase. The pressure-strain modulus was also determined for each patient. The mean diastolic AA diameter was significantly smaller among young (42.6±5.2 years) at 29.9±2.8 mm than old patients (69.0±5.2 years) at 33.2±3.2 mm. The circumferential AA strain from end-diastole to peak-systole decreased from 0.092±0.03 in young to 0.056±0.03 in old patients. The pressure-strain modulus increased two-fold from 68.4±30.5 kPa in young to 162.0±93.5 kPa in old patients, and the systolic AA wall tension increased from 268.5±31.3 kPa in young to 304.9±49.2 kPa in old patients. The AA dilates and stiffens with aging which increases the vessel wall tension, likely predisposing aneurysm and dissection.
Key terms: ascending aorta, aging, mechanics, imaging, elasticity
INTRODUCTION
An understanding of the ascending aorta (AA) in vivo mechanics is important for diagnostic and pre-operative evaluation. There is growing evidence that arterial stiffening is both a marker and predictor of cardiovascular disease. For instance, decreased aortic distensibility or increased stiffness may be correlated to atherosclerosis and hypertension [1, 2]. However, it is well accepted that the arteries also stiffen with age, and stiffness differences between male and female patients have been reported, where young female patients tend to have more compliant arteries, and older females have stiffer arteries than age-matched males [3, 4]. Thus, establishing the age-specific, gender-specific AA tissue characteristics will allow for identification of abnormalities aside from changes brought on by aging and gender alone. Such information becomes even more important in the current era, as mechanical evaluation of the diseased aorta is becoming part of clinical practice [5–7].
Prior investigations have assessed the effects of aging on the ascending aorta properties using in vivo imaging data [8–26]. The consistent finding in all studies is that the AA is indeed changing over time, with marked dilation (as well as elongation) and stiffening, which O’Rourke and Hashimoto [27] attribute to the fatigue of the non-living components of the tissue. Measurement accuracy is one of critical issues for the quantification of AA mechanical characteristics. The aorta is not oriented perfectly axial, and measuring the AA diameter from a stack of 2D axial computed tomography (CT) scans [8, 21, 26, 28] may yield inaccurate results [29]. Hager et al. [10] found that by using strictly axial plane CT images to measure the AA, the AA diameter can be overestimated by as much as 21%. Moreover, the AA diameter measurement distinction needs to be made between different phases of the cardiac cycle. The relative change in aortic cross-sectional area throughout the cardiac cycle may be as high as 50% in young patients [30]. Finally, the sample size should be relatively large [13, 15] and due to the known aortic size difference between male and female [9, 10, 15, 17, 21, 23], a gender-specific quantification of the AA diameter is necessary.
There have been relatively few engineering studies on the quantification of the detailed AA in vivo mechanical properties [12, 13, 15, 16, 18, 19, 25, 26]. The AA stiffness was indirectly assessed by the pulse wave velocity in [12], [18], and [22] in 162, 122, and 56 patients respectively using magnetic resonance imaging (MRI). In [15], [16], and [25] the distensibility of the ascending aorta was assessed by MRI in 26 (13 male, 13 female), 20, and 224 patients respectively. While, in [26], the AA distensibility was assessed based on diameter measurements made from axial CT slices in 293 patients. In each case, the distensibility was shown to be negatively correlated with age [15, 16, 25, 26]. In [13] the AA cyclic strain was measured in the circumferential and longitudinal directions in 14 patients from CT data, and in [19] the relative thoracic aortic strain was measured in 4 patients from MRI data. Due to the relatively low spatial resolution of MRI, and the small sample sizes of past CT studies, there remains a need to perform a more rigorous study to establish the age- and gender-specific AA stiffness norms.
Our aim in this study was to investigate the age-dependent in vivo mechanics of the AA in males with multi-phase CT scans. By analyzing images taken at different time points, the AA mechanical characteristics, such as circumferential strain, pressure-strain modulus and wall tension under physiological loading conditions, were quantified and analyzed.
MATERIALS AND METHODS
Patient Selection
A total of 45 male patients referred to Hartford Hospital Cardiac Lab for multiphase cardiac computed tomography imaging were studied. The study population was limited to male patients to isolate the effects of aging from possible gender-based differences on the AA properties. All patients underwent CT scans because of suspected coronary artery disease, but presented with no known aortopathies, e.g. aortic dilation, dissection, Marfan syndrome, bicuspid aortic valve, etc. The exam images were retrospectively analyzed in this study. The CT examination was performed on a GE LightSpeed 64-channel volume computed tomography scanner. A collimation of 25–30 × 0.625 mm and a rotation time of 375 ms were used, resulting in a temporal resolution of less than 200 ms between scans (phases). Typically 10 phases can be obtained for each cardiac cycle depending on the heart rate and pitch. There were approximately 100–150 axial CT images with thickness of 0.625 mm, containing the aortic geometry from the aortic valve to the ascending aorta for each phase [31]. Institutional Review Board approval to review these de-identified images was obtained.
Clinical data, including the age, diastolic and systolic blood pressures measured by brachial sphygmomanometer, and known cardiovascular risk factors, were obtained for each of the patients. To study the effect of age, patients were selected from five age groups: 30–39 year olds, 40–49 year olds, 50–59 year olds, 60–69 year olds, and 70–79 year olds. Because the number of younger patients was limited, only 5 patients were analyzed for the 30–39 year old group. All other groups consisted of 10 patients. The patient clinical data is summarized in Table 1.
Table 1.
Patient characteristics
| Known Cardiovascular Risk Factors | ||||||||
|---|---|---|---|---|---|---|---|---|
| Patient Group | Smoker | prior MI | Diabetes | CAD | Hypercholesterolemia | Obesity |
Family history |
Hypertension |
| 30–39 years (n=5) | n=1 | N/A | N/A | N/A | n=1 | N/A | n=1 | n=0 |
| 40–49 years (n=10) | n=2 | N/A | n=1 | n=2 | n=2 | n=1 | n=2 | n=0 |
| 50–59 years (n=10) | N/A | n=1 | N/A | n=1 | N/A | N/A | N/A | n=3 |
| 60–69 years (n=10) | n=2 | n=2 | n=1 | n=1 | N/A | N/A | n=2 | n=4 |
| 70–79 years (n=10) | N/A | n=1 | n=1 | n=1 | n=1 | N/A | n=1 | n=2 |
| Total (n=45) | n=5 | n=4 | n=3 | n=6 | n=4 | n=1 | n=6 | n=9 |
MI=myocardial infarction, CAD=coronary artery disease, Family history refers to family history of cardiovascular disease.
3D ascending aorta reconstruction
The 3D AA and aortic root geometry was reconstructed for each patient for each of the phases using Avizo 6.3 software (Burlington, MA). Figure 1a shows a representative axial CT slice of the aortic valve. The AA and aortic root were segmented semi-automatically in Avizo by adjusting the pixel intensity thresholds to isolate the AA geometry from the neighboring tissues (Figure 1b&c).
Figure 1.
(a) A cross-sectional image of the aortic valve and (b) the same image with the 3D aortic root geometry (shown in pink) projected into the y-plane. (c) A long axis view of the reconstructed aortic root geometry with the same cross-section from b and c shown in the x-z plane. (d) Aortic root geometry overlaid for each phase, with each color representing a different phase and a dashed line indicating the cross-sectional measurement plane. (e) The aortic root geometries after bisection at the measurement plane. (f) A close-up of the measurement plane where the circumference measurements were made and (g) the measurement plane during a single phase with points along the surface that were used to create a spline curve around the circumference.
Analysis of in vivo tension and strain state
Following the 3D reconstruction, the AA surface geometry for each phase was then imported into Altair HyperMesh 11.0 (Altair Engineering Inc, MI) for subsequent analysis. Figure 1d displays the overlaid AA and aortic root geometry for one representative patient, where each color represents the geometry during a single phase. Cardiac movement was evident from the small displacement of the coronary arteries. The measurement plane was then created in the ascending aorta at the level of the right pulmonary artery by bisecting the overlaid geometries from each phase orthogonal to the direction of flow. From Figure 1e&f it can be clearly seen that manipulation of the 3D geometries, such as overlapping and dissecting using engineering software, facilitates the accurate measurement of AA dimensions. The AA circumference for each phase was obtained by measuring a closed smooth curve generated from multiple points selected around the AA circumference (Fig. 1g).
The circumferential aortic strain, ε, was defined as the ratio of the change in aortic circumference with respect to the aortic circumference at end-diastole [13], εi = (Ci − Cdias)/Cdias, where Ci is the AA circumference for phase i, while is Cdias for diastole. The use of circumference in the strain calculation, rather than diameter, eliminates the assumption of a circular cross-section [13]. The diastolic and systolic blood pressures were obtained for each patient. The CT images corresponding to the intermediate phases were assumed to be taken at equal CT scan time intervals. The AA pressure waveform [32] between peak-systole and end-diastole was assumed to be the same for each patient. The pressure-strain modulus [33–35], PSmod, was then calculated as a measure of the physiological AA stiffness by, PSmod = (Cdias (Psys − Pdias))/(Csys − Cdias), where Psys and Pdias are the systolic and diastolic pressures. The PSmod is an attractive metric because it can be easily calculated from non-invasive clinical measurements. As in other studies [36–39], we assumed that a small segment of the AA can be treated as a thin-walled cylinder and thus the Laplace equation can be used to assess wall stresses. The Laplace equation, σ = (PR)/t, expresses the stress, σ, along the circumference of a cylindrical pressure vessel as a function of the luminal pressure, P, the vessel radius, R, and the wall thickness, t. Because the AA wall thickness could not be discerned from the imaging data, the circumferential AA wall tension, T, was calculated for each phase, Ti = (PiDi)/2, where Pi is the associated pressure and Di is the AA diameter for phase i determined from the measured circumference (Di = Ci/π).
Statistical analysis
The linear correlation between the AA mechanical characteristics, i.e., systolic circumferential strain, mean AA diameter, systolic wall tension, and pressure-strain modulus, versus the patient ages were tested using SigmaPlot 11.0 (San Jose, California) via the Pearson Product Moment correlation test. One-way ANOVA and rank-sum tests were used to compare the mean values between age groups using Minitab16 (State College, PA). In all cases, p-values less than 0.05 were considered to indicate a significant difference between the means. In the proceeding sections, all results are presented as the mean value ± the standard deviation.
RESULTS
Analysis of in vivo AA characteristics
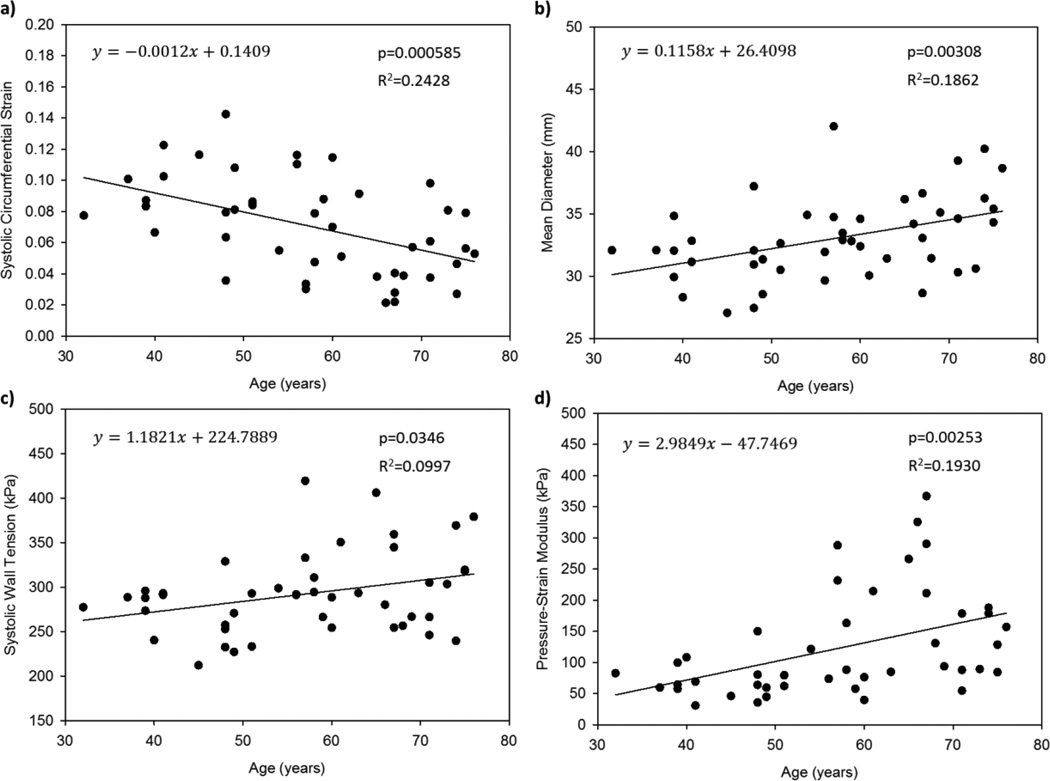
Figure 2 shows a significant negative linear correlation between the peak circumferential strain and patient age (Fig.2a), as well as significant positive linear correlations between the mean AA diameter, systolic wall tension, and pressure-strain modulus and the patient age (Fig.2b–d). The most statistically significant correlation was between strain and age with a p-value of 0.0006. The results of the linear correlation tests are given in Figure 2, where y is the dependent measured variable and x is the patient age.
Figure 2.
Raw (a) peak circumferential AA strain, (b) mean diameter, (c) systolic wall tension, and (d) pressure-strain modulus data plotted versus age with linear regression lines.
Comparison between age groups
The mean peak strains were significantly higher in the 30–39 and 40–49 groups at 0.088±0.018 and 0.087±0.039, than in the 60–69 group at 0.051±0.026, with p-values of 0.009 and 0.027 respectively, as well as in the 70–79 group at 0.052±0.022, with p-values of 0.003 and 0.018. There was essentially no difference in the peak strain values between the 30–39 and 40–49 groups (p=0.954) or the 60–69 and 70–79 groups (p=0.951). The mean peak strain in the 50–59 group (0.072±0.030) was smaller than those of the 30–39 and 40–49 groups and larger than in the 60–69 and 70–79 groups, although these differences were not significant. For this reason, the original 5 patient age groups were condensed to 3 for the subsequent analysis: 30–49, 50–59, and 60–79 year olds. The mean peak strains were significantly higher in the 30–49 at 0.092±0.03 than in the 60–79 group at 0.056±0.03 with p <0.001 (Figure 3a). There was no significant difference between the 30–49 and 50–59 year old groups, or between the 50–59 and 60–79 year old groups; however, the mean peak strain was consistently larger in the younger patients.
Figure 3.
(a) The mean systolic circumferential strain for each patient age group with standard deviation bars. (b) Physiological pressure versus ascending aorta diameter for the 3 age groups, where the low pressure points represent the diastolic condition, the high pressure points represent the systolic condition, and the middle pressure point was taken from an intermediate phase, plotted as a mean with bi-directional standard error bars.
The mean diastolic diameter was significantly smaller among the young patients (30–49 years) at 29.9±2.8 mm than the old patients (60–79 years) at 33.2±3.2 mm (p=0.003). The difference between the mean diastolic diameter in young patients compared to that of the middle-aged (50–59 years) patients at 32.4±3.8 mm was approaching statistical significance (p=0.062). There was no difference in the mean diastolic diameters between the middle-aged and old groups (p=0.542). The same trends were observed while comparing the mean systolic diameters between the groups; however the differences between the means were less drastic. The mean systolic diameter of the young patients (32.5±2.5 mm) was smaller than that of the middle-aged patients (34.7±3.2 mm) although not significantly (p=0.067) and of the old patients (35.1±3.3 mm) at statistical significance (p=0.017). Again, there was no difference between the mean systolic diameters of the middle-aged and old groups (p=0.783).
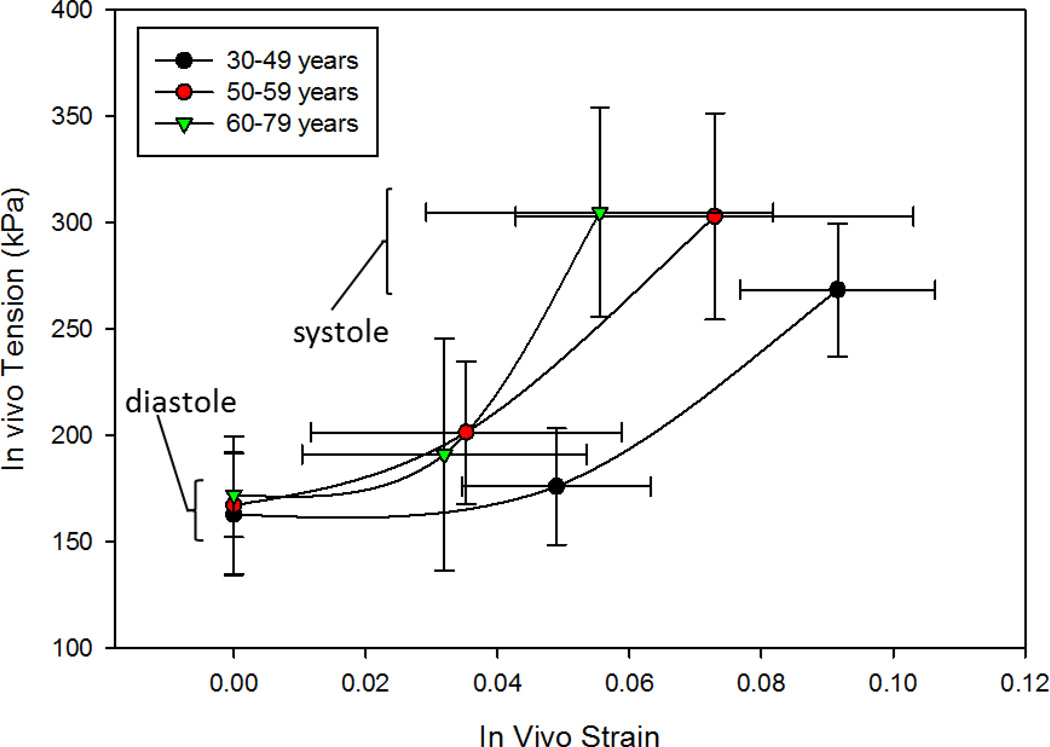
The in vivo AA pressure-diameter and tension-strain responses were evaluated for each patient group. Figure 3b shows that the pressure-diameter response is clearly shifted to the right with age, indicating larger diameters at each phase. This figure also shows that end-diastolic pressures were lower and peak-systolic pressures were higher for the older patients although not significantly. Moreover, the pressure-diameter response represented the AA structural compliance. The figure demonstrated steeper curves with aging indicating increased stiffness. The mean tension-strain responses for each group in Figure 4 show that the tissue modulus increased with age, and suggest that while the diastolic tension remains relatively constant throughout life, the systolic wall tension is higher in the middle-aged (303.0±48.5 kPa) and old (304.9±49.2 kPa) patient groups compared to the young (268.5±31.3 kPa) patient group with p-values of 0.041 and 0.017 respectively.
Figure 4.
In vivo tension versus strain responses of the 3 age groups with lower points representing the diastolic condition, the upper points representing the systolic condition, and the middle points representing an intermediate phase, plotted as the mean value with bi-directional standard deviation bars.
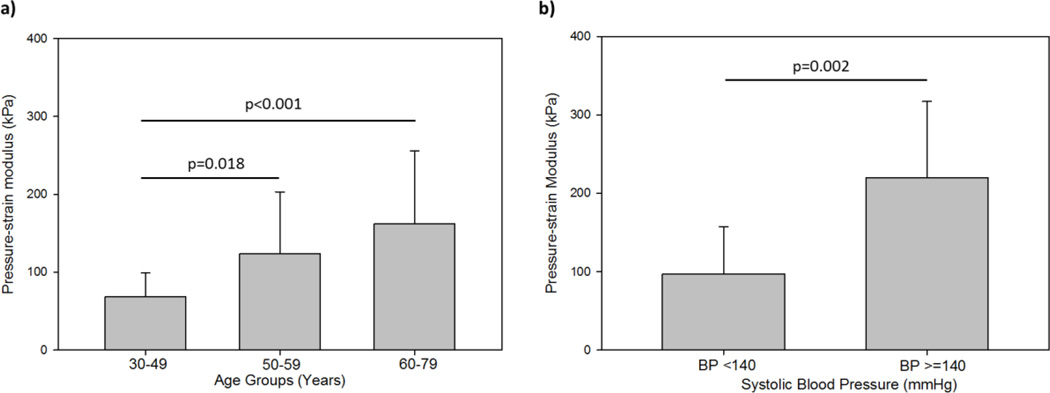
The mean pressure-strain modulus was compared between patient age groups (Figure 5a). The mean pressure-strain modulus for the young patients was significantly less than that of both the middle-aged (p=0.018) and old patients (p<0.001). The mean pressure-strain modulus of the middle-aged patients group was also less than that of the old patients group, although not significantly (p=0.276). Figure 5b shows that the pressure-strain modulus was also found to be significantly lower among normotensive patients of all ages as compared to the hypertensive patients (p=0.002).
Figure 5.
Comparison of the mean pressure- strain modulus value between (a) patients in each of the 3 age groups, and (b) normotensive and hypertensive patients, each plotted with standard deviation bars.
DISCUSSION
Ascending aorta age-related changes
Overall, the age-dependent trends observed in this study for the ascending aorta are in line with findings from other studies on the in vivo mechanics of the abdominal [1, 12, 22, 34, 40–42] and descending aorta [12, 22, 40] as well as the aortic arch [12]. The aortic tissue stiffness was shown to increase with aging; however the degree of aortic stiffening may differ along the length of the aorta [12, 22, 40].
The mean AA diameter measurement for all of our patients (i.e., non-age -phase specific) was 33.0±3.3 mm, which is similar to the mean AA diameters measured by CT as reported in [10] (32.0±4.2 mm), [13] (~32 mm), [11] (32±3 mm), [8] (33.6±4.1 mm), [17] (32±4 mm), and [21] (33.5±3.6mm). In addition, the AA diameter in this study was positively correlated with the patient age, as reported by other studies [9–15, 17, 21]. According to the linear regression presented in Fig. 2b, the mean AA diameter dilates 0.12 cm per decade, whereas the corresponding value reported for male patients is 0.15 cm per decade in [11], and 0.1 cm per decade in [14].
The mean circumferential strain between diastole and systole for our young group (42.6±5.3 years) was 0.092± 0.03 and for our old group (68.9±5.2 years) was 0.056±0.03. Morrison et al. [13] reported a similar value of 0.103±0.038 for young patients (41 years), and a much smaller value of 0.026±0.012 for old patients (68 years). The difference between our results and those of Morrison et al. [13] may be due to their inclusion of both male and female patients (5 men and 2 women), and relatively small sample sizes (n=7 for both young and old groups). Although not quantified here, similar age-related changes to the female AA mechanical properties are expected. Waddell et al. [4] suggest that age-related arterial stiffening in women is even more pronounced than in men. Young women tend to have lower, while older women have higher, arterial stiffness than age-matched men [4], which may explain the more extreme strain values measured in [13].
Overall, the AA mechanics seem to go through a transition period during a patient’s fifties, when mechanical properties start to decline. There were no significant differences between the 30–39 year old and 40–49 year old groups; however, the AA diameter was larger, the circumferential strain was smaller, and the pressure-strain modulus was larger in the 50–59 year old patients than in patients under 50 years. By the age of 60 years, the AA properties had further declined. However, beyond 60 years the AA properties declined only slightly, with no significant differences between the 60–69 year old and 70–79 year old groups. Incidentally, 60 years is the approximate onset age for thoracic aortic aneurysm [43]. Given that reduced elasticity is also characteristic of the dilated aorta [44], the reduced compliance of the AA beyond age 60 years may predispose dilation.
Possible mechanisms of age related changes
In the ascending aorta, the collagen and elastin fibers are wavy in young patients, and it has been hypothesized that they become straightened out [45] and more aligned [46] over time contributing to higher stiffness. In one of our prior studies [47], the histology of aged (~90 years) human ascending aorta samples were studied and the collagen and elastin fibers were nearly straight [47]. Collagen fiber un-crimping and elastin fiber fracture may explain the concurrent positive correlations observed in this study between diameter and age, as well as pressure-strain modulus versus age. The slightly larger and more significant increase in diastolic diameter per year (0.13 mm, p=0.0014) compared to the systolic diameter (0.10 mm, p=0.0081) also suggests collagen fiber straightening and elastin fiber fracture, because these changes would be realized by significant dilation of the diastolic diameter with little change to the systolic diameter, due to the concurrent reduction in elasticity.
As the aging aorta dilates and stiffens, the systolic pressure may increase and the diastolic pressure may decrease [48], which were both observed in this study (Fig. 4b). We also observed that on average, patients with hypertension have an AA pressure-strain modulus which is over twice that of normotensive patients (Fig. 5b). Ganten et al. [1] suggest that increased arterial stiffness may be both the cause and the effect of the concurrent increase in hypertension amongst aging patients. A recent study by Craiem et al. [24], shows that the mean AA diameter in age- and gender-matched individuals is also higher in hypertensive patients compared to normotensive patients. Regardless of the cause, the increase in systolic blood pressure and dilation of the AA will increase the AA wall tension, which may render older patients more susceptible to aneurysm formation and dissection. O’Rourke et al.[49] also attributes arteriosclerotic changes in the microvasculature of the end organs (e.g., heart, kidneys, brain, etc.) to increased aortic stiffness.
Limitations
The aorta moves during the cardiac cycle, so it was difficult to measure the strain at the exact same location of the aorta in each phase. Others have adopted a local reference frame defined by anatomical markers to avoid this problem [13]; however, using 3D reconstruction of AA geometries and overlaying the geometries at different phases offers a good approximation of using the same measurement location, with little post-processing required. Furthermore, the 3D reconstruction method utilizes CT data with superior resolution to MRI, and allowed for AA circumference measurements, which eliminated the circular cross-section assumption for the strain calculations. Therefore, we assume the strain measurements presented here, accurately describe the in vivo AA behavior. The mechanical properties in the axial direction were not measured. Morrison et al. [13] measured the length of the aorta along the centerline path from the left coronary artery to the third intercostal artery to measure the axial mechanics, however the imaging data available in our study often did not include the aortic arch; thus, leaving no anatomical markers for consistent length measurements.
The AA luminal pressure at diastole and systole were not available in this study, and, were thus, assumed to be equivalent to the corresponding pressure measurements taken by sphygmomanometer at the brachial artery. It has been shown that peripheral blood pressure may overestimate the pressure-strain modulus and wall tension in young patients [49]. Furthermore, the AA pressure waveform for each patient was assumed to follow the same shape although increased tissue stiffness has been shown to amplify pressure spikes in the AA pressure waveform [50]. Therefore, for these reasons, the increase in pressure-strain modulus with aging is likely underestimated in this analysis. The interpretation of our results should consider these limitations. The use of simultaneous pressure recordings with gated CT images would have eliminated the need for this assumption.
Finally, because all of the patients studied were referred for cardiac CT, some displayed cardiovascular risk factors such as hypertension and coronary artery disease. Therefore, this patient sample may have slightly more extreme AA tissue properties than the normal healthy age-matched patient population. However, because the clinical characteristics are similar for each group, the gender-specific, age-based comparison is valid.
Conclusions
In this study, the 3D AA geometry at multiple phases throughout the cardiac cycle was reconstructed for 45 male patients between the ages of 30 and 79 years. From the reconstructed geometries, the AA circumference, diameter, and strain were measured, and the pressure-strain modulus and physiological tension-strain response were determined. This study resulted in valuable information on AA mechanical characteristics which add to our understanding of aortic mechanics. To summarize, our key findings are as follows:
-
◦
The mean ascending aorta diameter increases by 0.12 cm per decade.
-
◦
The ascending aorta expands by 9.2% between diastole and systole in young male patients, and this value decreases with age to about 5.6% in old male patients.
-
◦
Ascending aortic mechanics deteriorate with aging, i.e. diameter increases, systolic expansion decreases, and pressure-strain modulus increases.
These changes all lead to increased wall tension with age, likely predisposing to aneurysm and dissection. In the future, these methods will be implemented to assess AA mechanics in female patients, who undergo even more pronounced AA mechanical changes with aging, and are more likely to experience AA dissection and rupture than men [43, 51–53].
ACKNOWLEDGEMENTS
This work was supported in part by the NIH HL108239 and HL104080 grants. Caitlin Martin is supported by NIH NRSA pre-doctoral fellowship HL112632. The authors would also like to thank Qian Wang and Alexander Werne for collecting and processing the CT image data.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
REFERENCES
- 1.Ganten M, Krautter U, Hosch W, Hansmann J, von Tengg-Kobligk H, Delorme S, Kauczor H-U, Kauffmann G, Bock M. Age related changes of human aortic distensibility: evaluation with ECG-gated CT. European Radiology. 2007;17(3):701–708. doi: 10.1007/s00330-006-0309-z. [DOI] [PubMed] [Google Scholar]
- 2.Metafratzi Z, Efremidis S, Skopelitou A, De Roos A. The clinical significance of aortic compliance and its assessment with magnetic resonance imaging. J Cardiovasc Magn Reson. 2002;4(4):481–491. doi: 10.1081/jcmr-120016386. [DOI] [PubMed] [Google Scholar]
- 3.Vlachopoulos C, Aznaouridis K, Stefanadis C. Clinical appraisal of arterial stiffness: the Argonauts in front of the Golden Fleece. Heart. 2006;92(11):1544–1550. doi: 10.1136/hrt.2005.067025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waddell TK, Dart AM, Gatzka CD, Cameron JD, Kingwell BA. Women exhibit a greater age-related increase in proximal aortic stiffness than men. Journal of Hypertension. 2001;19(12):2205–2212. doi: 10.1097/00004872-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Venkatasubramaniam AK, Fagan MJ, Mehta T, Mylankal KJ, Ray B, Kuhan G, Chetter IC, McCollum PT. A Comparative Study of Aortic Wall Stress Using Finite Element Analysis for Ruptured and Non-ruptured Abdominal Aortic Aneurysms. European Journal of Vascular and Endovascular Surgery. 2004;28(2):168–176. doi: 10.1016/j.ejvs.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Fillinger MF, Marra SP, Raghavan ML, Kennedy FE. Prediction of rupture risk in abdominal aortic aneurysm during observation: Wall stress versus diameter. Journal of Vascular Surgery. 2003;37(4):724–732. doi: 10.1067/mva.2003.213. [DOI] [PubMed] [Google Scholar]
- 7.Li Z-Y, Sadat U, U-King-Im J, Tang TY, Bowden DJ, Hayes PD, Gillard JH. Association Between Aneurysm Shoulder Stress and Abdominal Aortic Aneurysm Expansion / Clinical Perspective. Circulation. 2010;122(18):1815–1822. doi: 10.1161/CIRCULATIONAHA.110.939819. [DOI] [PubMed] [Google Scholar]
- 8.Mao SS, Ahmadi N, Shah B, Beckmann D, Chen A, Ngo L, Flores FR, Gao Yl, Budoff MJ. Normal Thoracic Aorta Diameter on Cardiac Computed Tomography in Healthy Asymptomatic Adults: Impact of Age and Gender. Academic Radiology. 2008;15(7):827–834. doi: 10.1016/j.acra.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcier JM, Petitcolin V, Filaire M, Mofid R, Azarnouch K, Ravel A, Vanneuville G, Boyer L. Normal diameter of the thoracic aorta in adults: a magnetic resonance imaging study. Surgical and Radiologic Anatomy. 2003;25(3):322–329. doi: 10.1007/s00276-003-0140-z. [DOI] [PubMed] [Google Scholar]
- 10.Hager A, Kaemmerer H, Rapp-Bernhardt U, Blücher S, Rapp K, Bernhardt TM, Galanski M, Hess J. Diameters of the thoracic aorta throughout life as measured with helical computed tomography. The Journal of Thoracic and Cardiovascular Surgery. 2002;123(6):1060–1066. doi: 10.1067/mtc.2002.122310. [DOI] [PubMed] [Google Scholar]
- 11.Craiem D, Chironi G, Redheuil A, Casciaro M, Mousseaux E, Simon A, Armentano R. Aging Impact on Thoracic Aorta 3D Morphometry in Intermediate-Risk Subjects: Looking Beyond Coronary Arteries with Non-Contrast Cardiac CT. Annals of Biomedical Engineering. 2011;40(5):1028–1038. doi: 10.1007/s10439-011-0487-y. [DOI] [PubMed] [Google Scholar]
- 12.Hickson SS, Butlin M, Graves M, Taviani V, Avolio AP, McEniery CM, Wilkinson IB. The Relationship of Age With Regional Aortic Stiffness and Diameter. JACC: Cardiovascular Imaging. 2010;3(12):1247–1255. doi: 10.1016/j.jcmg.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Morrison TM, Choi G, Zarins CK, Taylor CA. Circumferential and longitudinal cyclic strain of the human thoracic aorta: Age-related changes. Journal of Vascular Surgery. 2009;49(4):1029–1036. doi: 10.1016/j.jvs.2008.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aronberg DJ, Glazer HS, Madsen K, Sagel SS. Normal thoracic aortic diameters by computed tomography. Journal of computer assisted tomography. 1984;8(2):247–250. [PubMed] [Google Scholar]
- 15.Rose JL, Lalande A, Bouchot O, Bourennane EB, Walker PM, Ugolini P, Revol-Muller C, Cartier R, Brunotte F. Influence of age and sex on aortic distensibility assessed by MRI in healthy subjects. Magnetic Resonance Imaging. 2010;28(2):255–263. doi: 10.1016/j.mri.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Nelson AJ, Worthley SG, Cameron JD, Willoughby SR, Piantadosi C, Carbone A, Dundon BK, Leung MC, Hope SA, Meredith IT, Worthley MI. Cardiovascular magnetic resonance-derived aortic distensibility: Validation and observed regional differences in the elderly. Journal of Hypertension. 2009;27(3):535–542. doi: 10.1097/hjh.0b013e32831e4599. [DOI] [PubMed] [Google Scholar]
- 17.Biaggi P, Matthews F, Braun J, Rousson V, Kaufmann PA, Jenni R. Gender, Age, and Body Surface Area are the Major Determinants of Ascending Aorta Dimensions in Subjects With Apparently Normal Echocardiograms. Journal of the American Society of Echocardiography. 2009;22(6):720–725. doi: 10.1016/j.echo.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Redheuil A, Yu WC, Wu CO, Mousseaux E, De Cesare A, Yan R, Kachenoura N, Bluemke D, Lima JAC. Reduced ascending aortic strain and distensibility: Earliest manifestations of vascular aging in humans. Hypertension. 2010;55(2):319–326. doi: 10.1161/HYPERTENSIONAHA.109.141275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wedding KL, Draney MT, Herfkens RJ, Zarins CK, Taylor CA, Pelc NJ. Measurement of vessel wall strain using cine phase contrast MRI. Journal of Magnetic Resonance Imaging. 2002;15(4):418–428. doi: 10.1002/jmri.10077. [DOI] [PubMed] [Google Scholar]
- 20.Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age-Associated Elongation of the Ascending Aorta in Adults. JACC: Cardiovascular Imaging. 2008;1(6):739–748. doi: 10.1016/j.jcmg.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Wolak A, Gransar H, Thomson LEJ, Friedman JD, Hachamovitch R, Gutstein A, Shaw LJ, Polk D, Wong ND, Saouaf R, Hayes SW, Rozanski A, Slomka PJ, Germano G, Berman DS. Aortic Size Assessment by Noncontrast Cardiac Computed Tomography: Normal Limits by Age, Gender, and Body Surface Area. JACC: Cardiovascular Imaging. 2008;1(2):200–209. doi: 10.1016/j.jcmg.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Taviani V, Hickson SS, Hardy CJ, McEniery CM, Patterson AJ, Gillard JH, Wilkinson IB, Graves MJ. Age-related changes of regional pulse wave velocity in the descending aorta using Fourier velocity encoded M-mode. Magnetic Resonance in Medicine. 2010;65(1):261–268. doi: 10.1002/mrm.22590. [DOI] [PubMed] [Google Scholar]
- 23.Mirea O, Maffessanti F, Gripari P, Tamborini G, Muratori M, Fusini L, Claudia C, Fiorentini C, Plesea IE, Pepi M. Effects of aging and body size on proximal and ascending aorta and aortic arch: Inner edge-to-inner edge reference values in a large adult population by two-dimensional transthoracic echocardiography. Journal of the American Society of Echocardiography. 2013;26(4):419–427. doi: 10.1016/j.echo.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Craiem D, Chironi G, Casciaro ME, Redheuil A, Mousseaux E, Simon A. Three-dimensional evaluation of thoracic aorta enlargement and unfolding in hypertensive men using non-contrast computed tomography. Journal of Human Hypertension. 2013 doi: 10.1038/jhh.2012.69. [DOI] [PubMed] [Google Scholar]
- 25.Chue CD, Edwards NC, Ferro CJ, Townend JN, Steeds RP. Effects of age and chronic kidney disease on regional aortic distensibility: A cardiovascular magnetic resonance study. International Journal of Cardiology. 2013 doi: 10.1016/j.ijcard.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Siegel E, Thai WE, Techasith T, Major G, Szymonifka J, Tawakol A, Nagurney JT, Hoffmann U, Truong QA. Aortic distensibility and its relationship to coronary and thoracic atherosclerosis plaque and morphology by MDCT: Insights from the ROMICAT Trial. International Journal of Cardiology. 2013 doi: 10.1016/j.ijcard.2012.04.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Rourke MF, Hashimoto J. Mechanical Factors in Arterial Aging: A Clinical Perspective. Journal of the American College of Cardiology. 2007;50(1):1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 28.Roman MJ, Devereux RB, Kramer-Fox R, O'Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. The American Journal of Cardiology. 1989;64(8):507–512. doi: 10.1016/0002-9149(89)90430-x. [DOI] [PubMed] [Google Scholar]
- 29.Elefteriades JA, Farkas EA. Thoracic Aortic Aneurysm: Clinically Pertinent Controversies and Uncertainties. J Am Coll Cardiol. 2010;55(9):841–857. doi: 10.1016/j.jacc.2009.08.084. [DOI] [PubMed] [Google Scholar]
- 30.Wilson KA, Hoskins PR, Lee AJ, Fowkes FGR, Ruckley CV, Bradbury AW. Ultrasonic measurement of abdominal aortic aneurysm wall compliance: A reproducibility study. Journal of Vascular Surgery. 2000;31(3):507–513. [PubMed] [Google Scholar]
- 31.Wang Q, Book G, Contreras Ortiz S, Primiano C, McKay R, Kodali S, Sun W. Dimensional Analysis of Aortic Root Geometry During Diastole Using 3D Models Reconstructed from Clinical 64-Slice Computed Tomography Images. Cardiovascular Engineering and Technology. 2011;2(4):324–333. [Google Scholar]
- 32.Klocke R, Cockcroft JR, Taylor GJ, Hall IR, Blake DR. Arterial stiffness and central blood pressure, as determined by pulse wave analysis, in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2003;62(5):414–418. doi: 10.1136/ard.62.5.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunter KS, Albietz JA, Lee P-F, Lanning CJ, Lammers SR, Hofmeister SH, Kao PH, Qi HJ, Stenmark KR, Shandas R. In vivo measurement of proximal pulmonary artery elastic modulus in the neonatal calf model of pulmonary hypertension: development and ex vivo validation. Journal of Applied Physiology. 108(4):968–975. doi: 10.1152/japplphysiol.01173.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lanne T, Sonesson B, Bergqvist D, Bengtsson H, Gustafsson D. Diameter and compliance in the male human abdominal aorta: Influence of age and aortic aneurysm. European Journal of Vascular Surgery. 1992;6(2):178–184. doi: 10.1016/s0950-821x(05)80237-3. [DOI] [PubMed] [Google Scholar]
- 35.Peterson LH, Jensen RE, Parnell J. Mechanical Properties of Arteries in Vivo. Circulation Research. 1960;8(3):622–639. [Google Scholar]
- 36.Koullias G, Modak R, Tranquilli M, Korkolis DP, Barash P, Elefteriades JA. Mechanical deterioration underlies malignant behavior of aneurysmal human ascending aorta. The Journal of Thoracic and Cardiovascular Surgery. 2005;130(3):677.e671–677.e679. doi: 10.1016/j.jtcvs.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 37.García-Herrera CM, Atienza JM, Rojo FJ, Claes E, Guinea GV, Celentano DJ, García-Montero C, Burgos RL. Mechanical behaviour and rupture of normal and pathological human ascending aortic wall. Medical and Biological Engineering and Computing. 2012:1–8. doi: 10.1007/s11517-012-0876-x. [DOI] [PubMed] [Google Scholar]
- 38.Duprey A, Khanafer K, Schlicht M, Avril S, Williams D, Berguer R. In Vitro Characterisation of Physiological and Maximum Elastic Modulus of Ascending Thoracic Aortic Aneurysms Using Uniaxial Tensile Testing. European Journal of Vascular and Endovascular Surgery. 2010;39(6):700–707. doi: 10.1016/j.ejvs.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Khanafer K, Duprey A, Zainal M, Schlicht M, Williams D, Berguer R. Determination of the elastic modulus of ascending thoracic aortic aneurysm at different ranges of pressure using uniaxial tensile testing. The Journal of Thoracic and Cardiovascular Surgery. 2011;142(3):682–686. doi: 10.1016/j.jtcvs.2010.09.068. [DOI] [PubMed] [Google Scholar]
- 40.Gillessen T, Gillessen F, Sieberth H, Hanrath P, Heintz B. Age-related changes in the elastic properties of the aortic tree in normotensive patients: Investigation by intravascular ultrasound. Eur J Med Res. 1995;1(3):144–148. [PubMed] [Google Scholar]
- 41.Astrand H, Stalhand J, Karlsson J, Karlsson M, Sonesson B, Länne T. In vivo estimation of the contribution of elastin and collagen to the mechanical properties in the human abdominal aorta: Effect of age and sex. Journal of Applied Physiology. 2011;110(1):176–187. doi: 10.1152/japplphysiol.00579.2010. [DOI] [PubMed] [Google Scholar]
- 42.Ahlgren AR, Hansen F, Sonesson B, Lanne T. Stiffness and diameter of the common carotid artery and abdominal aorta in women. Ultrasound in Medicine and Biology. 1997;23(7):983–988. doi: 10.1016/s0301-5629(97)00082-3. [DOI] [PubMed] [Google Scholar]
- 43.Davies RR, Gallo A, Coady MA, Tellides G, Botta DM, Burke B, Coe MP, Kopf GS, Elefteriades JA. Novel Measurement of Relative Aortic Size Predicts Rupture of Thoracic Aortic Aneurysms. Ann Thorac Surg. 2006;81(1):169–177. doi: 10.1016/j.athoracsur.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 44.Sokolis DP, Kritharis EP, Giagini AT, Lampropoulos KM, Papadodima SA, Iliopoulos DC. Biomechanical response of ascending thoracic aortic aneurysms: association with structural remodelling. Computer Methods in Biomechanics and Biomedical Engineering. 2012;15(3):231–248. doi: 10.1080/10255842.2010.522186. [DOI] [PubMed] [Google Scholar]
- 45.Wuyts FL, Vanhuyse VJ, Langewouters GJ, Decraemer WF, Raman ER, Buyle S. Elastic properties of human aortas in relation to age and atherosclerosis: A structural model. Physics in Medicine and Biology. 1995;40(10):1577–1597. doi: 10.1088/0031-9155/40/10/002. [DOI] [PubMed] [Google Scholar]
- 46.Haskett D, Johnson G, Zhou A, Utzinger U, Vande Geest J. Microstructural and biomechanical alterations of the human aorta as a function of age and location. Biomechanics and Modeling in Mechanobiology. 9(6):725–736. doi: 10.1007/s10237-010-0209-7. [DOI] [PubMed] [Google Scholar]
- 47.Martin C, Pham T, Sun W. Significant differences in the material properties between aged human and porcine aortic tissues. European Journal of Cardio-Thoracic Surgery. 2010;40(1):28–34. doi: 10.1016/j.ejcts.2010.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karamanoglu M, O'Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. European Heart Journal. 1993;14(2):160–167. doi: 10.1093/eurheartj/14.2.160. [DOI] [PubMed] [Google Scholar]
- 49.O'Rourke MF, Safar ME, Dzau V. The Cardiovascular Continuum extended: Aging effects on the aorta and microvasculature. Vascular Medicine. 2010;15(6):461–468. doi: 10.1177/1358863X10382946. [DOI] [PubMed] [Google Scholar]
- 50.O'Rourke MF. Arterial aging: pathophysiological principles. Vascular Medicine. 2007;12(4):329–341. doi: 10.1177/1358863X07083392. [DOI] [PubMed] [Google Scholar]
- 51.Vorp DA, Schiro BJ, Ehrlich MP, Juvonen TS, Ergin MA, Griffith BP. Effect of aneurysm on the tensile strength and biomechanical behavior of the ascending thoracic aorta. Annals of Thoracic Surgery. 2003;75(4):1210–1214. doi: 10.1016/s0003-4975(02)04711-2. [DOI] [PubMed] [Google Scholar]
- 52.Coady MA, Rizzo JA, Hammond GL, Mandapati D, Darr U, Kopf GS, Elefteriades JA. What is the appropriate size criterion for resection of thoracic aortic aneurysms? The Journal of Thoracic and Cardiovascular Surgery. 1997;113(3):476–491. doi: 10.1016/S0022-5223(97)70360-X. [DOI] [PubMed] [Google Scholar]
- 53.Davies R, Goldstein L, Coady M, Tittle S, Rizzo J, Kopf G, Elefteriades J. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73(1):17–27. doi: 10.1016/s0003-4975(01)03236-2. [DOI] [PubMed] [Google Scholar]