Abstract
Helicobacter pylori infection is associated with extragastric diseases. The thyroid may be one of the targets of chronic inflammation. Here, we sought to investigate whether H. pylori infections were associated with the presence of thyroid nodules. A total of 988 euthyroid subjects from China were included in this cross-sectional study. Four hundred thirty-five (44.0%) subjects were diagnosed as having thyroid nodules, and 486 (49.2%) were diagnosed with H. pylori infections. The thyroid nodules group had a higher proportion of H. pylori infections than the control group (P = 0.002). Free thyroxine (FT4) levels were lower and the prevalence of thyroid nodules was higher in patients with H. pylori infection compared to those without infection, even after adjustment for age, gender, and body mass index (BMI; all P < 0.05). The prevalence of H. pylori infection showed a decreasing trend as serum FT4 level increased (P trend = 0.020). Stepwise logistic regression analysis showed that H. pylori infection was significantly associated with the risk of thyroid nodules (odds ratio: 1.390, 95% confidence interval: 1.059–1.824, P = 0.018). Our results suggested that H. pylori infections were positively associated with the presence of thyroid nodules in the euthyroid population, whose thyroid functions were in the reference range.
Introduction
Helicobacter pylori is a gram-negative, spiral-shaped, pathogenic bacterium that typically colonizes and infects the gastric mucosa. H. pylori infections and H. pylori-induced chronic inflammation can cause gastric diseases, such as chronic gastritis, peptic ulcers, and gastric malignancies [1,2]. Moreover, H. pylori infection can also cause extragastric diseases. Preliminary data have shown that there is a positive relationship between H. pylori infection and extragastric diseases, such as metabolic syndrome [3], nonalcoholic fatty liver disease [4], diabetes mellitus [5], and insulin resistance (IR) [6]. In addition, some reports have shown a positive correlation between H. pylori infections and autoimmune thyroid diseases (ATDs) [7-9]. Indeed, studies have demonstrated that some bacteria and viruses are able to mimic the antigenic profile of the thyroid cell membrane, thereby playing an important role in the onset of autoimmune diseases [10-13]. Therefore, the thyroid may be attacked by autoantibodies after H. pylori infection.
Thyroid nodules are common, and benign, nodular goiters account for about 80%–90% of nodules, while thyroid cancer accounts for only about 5%–10% [14]. In the clinical setting, some patients exhibit thyroid nodules by ultrasound imaging, but have normal serum thyroid function levels. Though the vast majority of nodules are benign, the risk factors for thyroid nodules among euthyroid population have not yet been fully elucidated. Bassi et al. have demonstrated a noteworthy correlation between H. pylori infection and Graves’ disease, independent of hormonal status [8]. However, it is not know whether there is a link between H. pylori infection and the presence of thyroid nodules.
Therefore, we conducted a cross-sectional study to investigate the correlation between H. pylori infection and the presence of thyroid nodules in the euthyroid population.
Materials and Methods
Study design and subjects
From January to December 2012, we evaluated patients who underwent health screening that included thyroid ultrasounds, fasting 13C urea breath tests, and examination of laboratory data at the International Health Care Center, First Affiliated Hospital of Zhejiang University College of Medicine. Many company labor unions voluntarily organize for all their serving and retired staff to take part in annual health screening. All subjects voluntarily participated in this study. Individuals were excluded from this study if they had a history of thyroid diseases, including hyperthyroidism, hypothyroidism, or thyroid hormone replacement therapy for any reason or if they had thyroid dysfunction, defined as serum thyroid-stimulating hormone (TSH) greater than 4.34 mIU/L or less than 0.38 mIU/L and/or free thyroxine (free T4, FT4) greater than 24.38 pmol/L or less than 10.45 pmol/L. Additionally, all individuals who used drugs that may influence the results of 13C urea breath tests, such as antibiotics, histamine 2 receptor antagonists, proton pump inhibitors, or bismuths, within a 1-month period before screening, were excluded from this study. A total of 988 eligible subjects were enrolled (621 men and 367 women, with mean age of 46.88 ± 11.92 years). All subjects have not accepted eradication therapies of H. pylori infection in the past one year according to the history records. All procedures were approved by the Ethics Committee of Zhejiang University of College of Medicine. Each method and the potential risks were explained to the participants in detail, and all subjects gave written informed consent before the study.
Physical examination
All subjects were required to fast overnight prior to physical examinations in the morning. After a health habit inventory was recorded, body measurement and blood pressure were measured by a trained physician. Body mass index (BMI) was then calculated as mass (kg)/height (m)2. Blood pressure was measured with an automated sphygmomanometer on the right arm of the followed-up individuals in a comfortable sitting position after a 5-min rest. Three measurements were taken. The second and third pressure readings were averaged, and systolic blood pressure (SBP) and diastolic blood pressure (DBP) readings were used for analysis.
Laboratory assessments
Peripheral venous blood samples were collected after physical examination and used for the analysis of thyroid function. Serum TSH levels, FT4, and free triiodothyronine (free T3, FT3) were assessed with an ADVIA Centaur XP system (Siemens AG, Munich, Germany). The diagnosis of H. pylori infection was based on the result of fasting 13C urea breath test (13C-UBT), 13C-UBT was performed under the following conditions: patients underwent an 8-h fast, mouth washing before dosing, drinking water (100 mL) as standard meal, and subsequent administration of 75 mg 13C urea (Boran Pharmaceutical Co., Ltd. of Beijing, China); breath samples were then collected in two 10-mL sample plastic bags for a baseline reading and at a 30-min sampling point, with patients in a sitting position. The breath samples were analyzed by infrared heterodyne ratiometry (Huaheng Anbang Company of Beijing, China). H. pylori infection was considered present if the difference between the 30-min value and baseline value divided by the baseline value exceeded 4.0‰. Reference value ranges of all indexes were based on the biochemistry criteria of Department of Clinical Laboratory, The First Affiliated Hospital, College of Medicine, Zhejiang University.
Ultrasonographic examination
Thyroid ultrasonography for all subjects was performed by a trained ultrasonographer using a Philips Ultrasound System HD11XE (Royal Dutch Philips Electronics Ltd., Amsterdam, Netherlands) with a 10-MHz linear probe. The location, size, number, shape, border, and internal echo of thyroid nodules were described in the ultrasonographic examination.
Statistical analysis
Statistical analysis was performed with SPSS 13.0 statistical package (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test was used to assess whether continuous data were normally distributed. Continuous variables are presented as the mean and standard deviation or median and interquartile range (IQR), as appropriate. Continuous data for different groups was compared using Student’s t-test or the Mann–Whitney U-test. The chi-square (χ2) test was used for comparisons of categorical variables. Adjusted analysis was used to determine the relationships between H. pylori infection and thyroid function or thyroid nodules. Stepwise multiple regression analysis was used to evaluate the risk factors for thyroid nodules (backward: Wald; cutoff for entry: 0.05, for removal: 0.10). Differences with P-values less than 0.05 were considered statistically significant.
Results
Subject characteristics
Of the 988 subjects enrolled in this study, 435 (44.0%) were diagnosed with thyroid nodules, and 486 (49.2%) were diagnosed with H. pylori infections. Characteristics of the subjects according to thyroid nodule status are illustrated in Table 1. The average age of subjects in the thyroid nodules group was higher than that in the control group. SBP, DBP, BMI, and the presence of H. pylori infection were unfavorable and the proportion of women was higher in the thyroid nodules group when compared to the control group. Additionally, FT4 and FT3 levels were significantly lower in subjects with thyroid nodules than in control subjects (all P < 0.05).
Table 1. Characteristics of study subjects according to thyroid nodule (TN) status.
| Variables | Subjects with TNs (n = 435) | Subjects without TNs (n = 553) | t-value | P-value |
|---|---|---|---|---|
| Age (years) | 50 (43–59) | 43 (36–50) | 9.771a | < 0.001 |
| Gender (male/female, n) | 240/195 | 381/172 | 19.643b | < 0.001 |
| Systolic blood pressure (mmHg) | 129.2 ± 18.8 | 124.0 ± 16.4 | 4.673 | < 0.001 |
| Diastolic blood pressure (mmHg) | 78.1 ± 10.7 | 76.1 ± 10.9 | 2.906 | 0.004 |
| Body mass index (kg/m2) | 24.1 ± 3.0 | 23.5 ± 3.1 | 3.094 | 0.002 |
| Thyroid- stimulating hormone (mIU/L) | 1.67 (1.13–2.28) | 1.68 (1.20–2.39) | 0.689a | 0.491 |
| Free T4 (pmol/L) | 15.70 ± 2.22 | 16.06 ± 2.28 | 2.420 | 0.016 |
| Free T3 (pmol/L) | 4.74 ± 0.54 | 4.86 ± 0.54 | 3.251 | 0.001 |
| 13C urea breath test (positive/negative, n) | 238/197 | 248/305 | 9.483b | 0.002 |
Data are expressed as mean (SD) or median (IQR). a Z value; b χ2 value.
Associations between H. pylori infection and thyroid function or thyroid nodules
When subjects were assessed relative to their H. pylori infection status, the results showed that the FT4 levels were significantly lower, and the prevalence of thyroid nodules was significantly higher in subjects with H. pylori infection compared to control subjects, even after adjustment for age, gender and BMI (all P < 0.05). However, there were no differences in TSH or FT3 levels between H. pylori-infected subjects and controls (Table 2).
Table 2. Associations between H. pylori infection and thyroid function or thyroid nodules (TNs).
| Variables | Unadjusted | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Crude OR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| TSH | 0.966 (0.837-1.115) | 0.953 (0.825-1.101) | 0.977 (0.843-1.132) | 0.961 (0.828-1.115) |
| FT4 | 0.916 (0.866-0.969)* | 0.930 (0.878-0.985)* | 0.912 (0.859-0.968)* | 0.912 (0.859-0.969)* |
| FT3 | 1.158 (0.919-1.459) | 1.220 (0.965-1.544) | 1.153 (0.896-1.485) | 1.116 (0.865-1.439) |
| TNs | 1.486 (1.154-1.912)* | 1.367 (1.049-1.780)* | 1.432 (1.094-1.874)* | 1.388 (1.059-1.819)* |
Model 1: adjusted for age Model 2: adjusted for age and gender.
Model 3: adjusted for age, gender and body mass index.
P < 0.05
Abbreviations: TSH, thyroid-stimulating hormone; FT4, free thyroxine; FT3, free triiodothyronine; OR, odds ratio; CI, confidence interval.
Association between thyroid function and the prevalence of H. pylori infection
To investigate the relationship between thyroid function and the prevalence of H. pylori infection, all subjects were classified into average quartiles according to their TSH, FT4, and FT3 levels. For TSH, quartile 1 (Q1): TSH ≤ 1.16 mIU/L; quartile 2 (Q2): TSH 1.17–1.67 mIU/L; quartile 3 (Q3): TSH 1.68–2.34 mIU/L; quartile 4 (Q4): TSH ≥ 2.35 mIU/L. For FT4, Q1: FT4 ≤ 14.31 pmol/L; Q2: FT4 14.32–15.79 pmol/L; Q3: FT4 15.80–17.26 pmol/L; Q4: FT4 ≥ 17.27 pmol/L. For FT3, Q1: FT3 ≤ 4.45 pmol/L; Q2: FT3 4.46–4.80 pmol/L; Q3: FT3 4.81–5.11 pmol/L; Q4: FT3 ≥ 5.12 pmol/L. The prevalence of H. pylori infection in subjects with different quartile levels of TSH, FT4, and FT3 was analyzed.
As shown in Figure 1, the prevalence of H. pylori infection showed a decreasing trend as serum FT4 levels increased. Compared to subjects with serum FT4 levels in Q1, the prevalence ratios for subjects in Q2, Q3, and Q4 were 0.88, 0.87, and 0.80, respectively. This trend was significant after adjustment for age, gender and BMI (Figure 1; P for trend = 0.020). However, the trends for TSH and FT3 were not statistically significant (Figure 1). These results suggested that lower FT4 levels were more likely to be related to H. pylori infection.
Figure 1. Prevalence of Helicobacter pylori infection according to thyroid function.
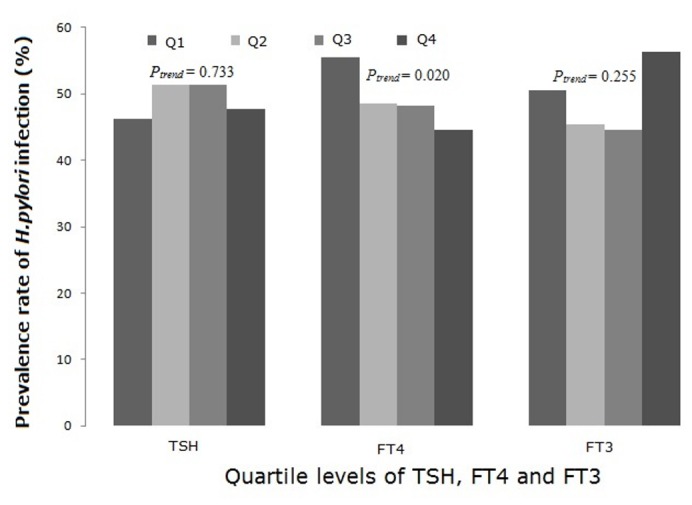
Subjects were classified into different groups according to their TSH, FT4, and FT3 quartiles. The prevalence of H. pylori showed a decreasing trend as serum FT4 increased, while the trend was not significantly associated with serum TSH or FT3 levels. P-values were adjusted for age, gender and BMI status.
Risk factor analysis for thyroid nodules
Univariate logistic-regression analysis was performed to evaluate the relationship between nine variables and thyroid nodules. Nine variables included age, female gender, SBP, DBP, BMI, H. pylori infection, TSH levels, FT4 levels, and FT3 levels. The results showed that age, female gender, SBP, DBP, BMI, FT4, FT3 and H. pylori infection were significantly associated with the risk for thyroid nodules (Table 3, all P < 0.05). Stepwise logistic regression analysis was performed to evaluate the relationship between H. pylori infection and thyroid nodules. The results showed that age, female gender, BMI, and H. pylori infection were significantly and positively associated with the risk for thyroid nodules, while TSH was inversely correlated with the risk for thyroid nodules (Table 4).
Table 3. Relationship between nine variables and thyroid nodules (TNs) in euthyroid population using univariate logistic-regression.
| Variables | β | SE | Wald χ2 | P-value | OR | 95% CI of OR |
|---|---|---|---|---|---|---|
| Age | 0.056 | 0.006 | 80.641 | 0.000 | 1.057 | 1.045-1.070 |
| Female | 0.588 | 0.133 | 19.474 | 0.000 | 1.800 | 1.386-2.337 |
| SBP | 0.017 | 0.004 | 20.966 | 0.000 | 1.017 | 1.010-1.025 |
| DBP | 0.017 | 0.006 | 8.327 | 0.004 | 1.017 | 1.006-1.029 |
| BMI | 0.064 | 0.021 | 9.372 | 0.002 | 1.067 | 1.023-1.111 |
| TSH | -0.070 | 0.074 | 0.905 | 0.342 | 0.932 | 0.806-1.077 |
| FT4 | -0.069 | 0.029 | 5.796 | 0.016 | 0.933 | 0.882-0.987 |
| FT3 | -0.391 | 0.122 | 10.316 | 0.001 | 0.677 | 0.533-0.859 |
| H. pylori infection | 0.396 | 0.129 | 9.451 | 0.002 | 1.486 | 1.154-1.912 |
Abbrevations: β, partial regression coefficient; SE, standard error of partial regression coefficient; OR, odds ratio; CI, confidence interval.
Table 4. Risk factors for thyroid nodules (TNs) in the euthyroid population.
| Variables | β | SE | Wald χ2 | P-value | OR | 95% CI of OR |
|---|---|---|---|---|---|---|
| Age | 0.055 | 0.006 | 73.581 | 0.000 | 1.056 | 1.043–1.070 |
| Female | 0.947 | 0.158 | 35.953 | 0.000 | 2.578 | 1.892–3.514 |
| BMI | 0.073 | 0.024 | 8.851 | 0.003 | 1.075 | 1.025–1.128 |
| TSH | -0.243 | 0.083 | 8.630 | 0.003 | 0.784 | 0.667–0.922 |
| H. pylori infection | 0.329 | 0.139 | 5.633 | 0.018 | 1.390 | 1.059–1.824 |
Abbrevations: β, partial regression coefficient; SE, standard error of partial regression coefficient; OR, odds ratio; CI, confidence interval.
Discussion
Thyroid nodules represent a common medical problem, and the majority (> 95%) of thyroid nodules are benign [15]. Thorough histories and physical examinations, serum TSH levels, thyroid ultrasounds, and fine need aspirations (FNAs) comprise the standard evaluation of patients with thyroid nodules. Individuals with nodules demonstrating suspicious features on thyroid ultrasound in particular should undergo FNA, and/or individuals with thyroid dysfunction should undergo further examinations to identify the reason for such dysfunction; however, such individuals were not considered in this study. Thyroid nodules refer to one or more lumpy tissues representing structural abnormalities induced by various causes in thyroid tissue [16], and nodular goiters account for about 80%–90%, while thyroid cancer accounts for only about 5%–10% [14]. However, the mechanism of thyroid nodule development remains unclear. The potential for chronic infectious agents to be a causal factors for autoimmune disease has long been recognized and has recently been receiving increased attention [17,18]. Bassi et al found that there was a positive correlation between H. pylori infections and Grave’s disease [7,8]. Nodule formation is a type of reactive hyperplastic lesion arising from chronic inflammation. In this study, our data showed that H. pylori infection was significantly associated with the presence of thyroid nodules in the euthyroid population. First, the proportion of subjects with H. pylori infection was higher in the thyroid nodules group than in the control group. Second, the prevalence of thyroid nodules was significantly higher in subjects with H. pylori infection than in control subjects, even after adjustment for age, gender, and BMI. Third, logistic regression analysis further showed that H. pylori infection significantly contributed to the risk for thyroid nodules. Then, how can we explain the relationship between H. pylori infection and thyroid nodules? Molecular modeling has indicated that one type of bacteria produces a substance capable of disabling the vitamin D receptor (VDR); then, bacteria-induced VDR dysfunction could lead to low 25-D and high 1,25-D levels [19]. 1,25-D has a very high affinity for the α-thyroid receptor, and if transcription by the α-thyroid receptor is dysregulated, a cascade of metabolic dysfunction will result [20]. Further studies in animal models are needed to clarify the mechanisms mediating the relationship between H. pylori infection and thyroid nodules.
In this study, our data showed that FT4 levels were negatively related to H. pylori infection in both unadjusted and adjusted models. Additionally, our results showed that the prevalence of H. pylori infection decreased gradually as serum FT4 levels increased. Triantafillidis et al. assessed the relationship between H. pylori infection and serum thyroid hormone levels in 110 normal volunteers and found that FT4 levels were significantly different (1.04 ± 0.2 ng/dL vs. 1.17 ± 0.3 ng/dL, P = 0.025) in subjects who were positive and negative for H. pylori [21]. The results were the same as our study, but the specific mechanism was not clear.
Actually, age is an important factor influencing the prevalence of H. pylori infection, and age was also a significant factor for the prevalence of thyroid nodules in our study. As to describe the characteristics of the prevalence of thyroid nodules, but also to avoid selection bias of age, we assess the relationship between thyroid nodules and H. pylori infection by the adjusted models which are adjusted for age. Additionally, multivariate analysis found that age and female gender were significant risk factors for thyroid nodules in our study. Kim et al. reported that thyroid nodules increase with age and that their frequency is higher among women [22]. Moreover, our data showed that BMI was also significantly and positively associated with the risk for thyroid nodules, consistent with the study by Kim et al.[22] Recent studies have also shown that insulin resistance was associated with thyroid functional and morphological abnormalities [23,24]. The insulin/insulin-like growth factor-1 (IGF-1) signaling pathway has long been known to modulate thyroid gene expression and may be considered an additional important factor in thyrocyte proliferation and differentiation [25-28]. H. pylori infection may have a pathogenic role in the development of insulin resistance [6]. Thus, activation of the insulin pathway may be a plausible explanation for our results. Junik et al. observed the presence of negative linear correlations between thyroid volume and TSH concentration in the group of type 2 diabetics, and the authors explained that this alteration could contribute to its role in the insulin pathway [24]. According to our results, TSH was inversely correlated with the risk for thyroid nodules, and this could also be caused by the insulin pathway.
In summary, our results showed that H. pylori infection was positively associated with the presence of thyroid nodules in the euthyroid population. However, it is a pity that the size and number of thyroid nodules of all subjects hadn’t been provided in the study, as it is an annual health screening, a part of records on the size or number of thyroid nodules were recorded unclearly. Further studies are necessary in order to fully understand the potential mechanisms mediating this association and may help to identify the key point of disease prevention.
Acknowledgments
The authors are grateful to Tian’An Jiang from the Department of Ultrasound, the First Affiliated Hospital, College of Medicine, Zhejiang University, for helping with the ultrasonographic examination in this study.
Funding Statement
This study was supported by Zhejiang Provincial Laboratory Animal Science Technology Program of China (number 2011C37088); Zhejiang Provincial Natural Science Foundation of China (numbers LY12H03011 and LY13H030003); Natural Science Foundation of Ningbo City (number 2012A610244). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG (1991) Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 338: 1175-1176. doi: 10.1016/0140-6736(91)92035-Z. PubMed: 1682595. [DOI] [PubMed] [Google Scholar]
- 2. Herrera V, Parsonnet J (2009) Helicobacter pylori and gastric adenocarcinoma. Clin Microbiol Infect 15: 971-976. doi: 10.1111/j.1469-0691.2009.03031.x. PubMed: 19874380. [DOI] [PubMed] [Google Scholar]
- 3. Shin DW, Kwon HT, Kang JM, Park JH, Choi HC et al. (2012) Association between metabolic syndrome and Helicobacter pylori infection diagnosed by histologic status and serological status. J Clin Gastroenterol 46: 840-845. doi: 10.1097/MCG.0b013e3182522477. PubMed: 23064216. [DOI] [PubMed] [Google Scholar]
- 4. Polyzos SA, Kountouras J, Papatheodorou A, Patsiaoura K, Katsiki E et al. (2013) Helicobacter pylori infection in patients with nonalcoholic fatty liver disease. Metabolism 62: 121-126. doi: 10.1016/j.metabol.2012.06.007. PubMed: 22841522. [DOI] [PubMed] [Google Scholar]
- 5. Gasbarrini A, Ojetti V, Pitocco D, De Luca A, Franceschi F et al. (1998) Helicobacter pylori infection in patients affected by insulin-dependent diabetes mellitus. Eur J Gastroenterol Hepatol 10: 469-472. doi: 10.1097/00042737-199806000-00006. PubMed: 9855061. [DOI] [PubMed] [Google Scholar]
- 6. Polyzos SA, Kountouras J, Zavos C, Deretzi G (2011) The association between Helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter 16: 79-88. doi: 10.1111/j.1523-5378.2011.00822.x. PubMed: 21435084. [DOI] [PubMed] [Google Scholar]
- 7. Bassi V, Marino G, Iengo A, Fattoruso O, Santinelli C (2012) Autoimmune thyroid diseases and Helicobacter pylori: the correlation is present only in Graves's disease. World J Gastroenterol 18: 1093-1097. doi: 10.3748/wjg.v18.i10.1093. PubMed: 22416184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bassi V, Santinelli C, Iengo A, Romano C (2010) Identification of a correlation between Helicobacter pylori infection and Graves' disease. Helicobacter 15: 558-562. doi: 10.1111/j.1523-5378.2010.00802.x. PubMed: 21073613. [DOI] [PubMed] [Google Scholar]
- 9. Stechova K, Pomahacova R, Hrabak J, Durilova M, Sykora J et al. (2009) Reactivity to Helicobacter pylori antigens in patients suffering from thyroid gland autoimmunity. Exp Clin Endocrinol Diabetes 117: 423-431. doi: 10.1055/s-0029-1214385. PubMed: 19472102. [DOI] [PubMed] [Google Scholar]
- 10. Rapoport B, McLachlan SM (2001) Thyroid autoimmunity. J Clin Invest 108: 1253-1259. doi: 10.1172/JCI200114321. PubMed: 11696565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valtonen VV, Ruutu P, Varis K, Ranki M, Malkamäki M et al. (1986) Serological evidence for the role of bacterial infections in the pathogenesis of thyroid diseases. Acta Med Scand 219: 105-111. PubMed: 3754083. [DOI] [PubMed] [Google Scholar]
- 12. Joasoo A, Robertson P, Murray IPC (1975) Viral antibodies and thyrotoxicosis. Lancet 2: 125-131. [DOI] [PubMed] [Google Scholar]
- 13. Tomer Y, Davies TF (1993) Infection, thyroid disease, and autoimmunity. Endocr Rev 14: 107-120. doi: 10.1210/edrv-14-1-107. PubMed: 8491150. [DOI] [PubMed] [Google Scholar]
- 14. Iyer NG, Shaha AR (2010) Management of thyroid nodules and surgery for differentiated thyroid cancer. Clin Oncol R Coll Radiol 22: 405-412. doi: 10.1016/j.clon.2010.03.009. PubMed: 20381323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stojadinovic A, Peoples GE, Libutti SK, Henry LR, Eberhardt J et al. (2009) Development of a clinical decision model for thyroid nodules. BMC Surg 9: 12. doi: 10.1186/1471-2482-9-12. PubMed: 19664278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Thyroid Association (ATA) Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT et al. (2009) Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19: 1167-1214. [DOI] [PubMed] [Google Scholar]
- 17. Fredricks DN, Relman DA (1998) Infectious agents and the etiology of chronic idiopathic diseases. Curr Clin Top Infect Dis 18: 180-200. PubMed: 9779355. [PubMed] [Google Scholar]
- 18. Pordeus V, Szyper-Kravitz M, Levy RA, Vaz NM, Shoenfeld Y (2008) Infections and autoimmunity: a panorama. Clin Rev Allergy Immunol 34: 283-299. doi: 10.1007/s12016-007-8048-8. PubMed: 18231878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marshall TG (2008) Vitamin D discovery outpaces FDA decision making. Bioessays 30: 173-182. doi: 10.1002/bies.20708. PubMed: 18200565. [DOI] [PubMed] [Google Scholar]
- 20. Proal AD, Albert PJ, Marshall TG (2009) Dysregulation of the vitamin D nuclear receptor may contribute to the higher prevalence of some autoimmune diseases in women. Ann N Y Acad Sci 1173: 252-259. doi: 10.1111/j.1749-6632.2009.04672.x. PubMed: 19758159. [DOI] [PubMed] [Google Scholar]
- 21. Triantafillidis JK, Georgakopoulos D, Gikas A, Merikas E, Peros G et al. (2003) Relation between Helicobacter pylori infection, thyroid hormone levels and cardiovascular risk factors on blood donors. Hepatogastroenterology 50(Suppl. 2): cccxviii-ccccccxx PubMed: 15244214. [PubMed] [Google Scholar]
- 22. Kim JY, Jung EJ, Park ST, Jeong SH, Jeong CY et al. (2012) Body size and thyroid nodules in healthy Korean population. J Korean Surg Soc 82: 13-17. doi: 10.4174/jkss.2012.82.1.13. PubMed: 22324041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rezzonico J, Rezzonico M, Pusiol E, Pitoia F, Niepomniszcze H (2008) Introducing the thyroid gland as another victim of the insulin resistance syndrome. Thyroid 18: 461–464. doi: 10.1089/thy.2007.0223. PubMed: 18346005. [DOI] [PubMed] [Google Scholar]
- 24. Junik R, Kozinski M, Debska-Kozinska K (2006) Thyroid ultrasound in diabetic patients without overt thyroid disease. Acta Radiol 47: 687–691. doi: 10.1080/02841850600806308. PubMed: 16950706. [DOI] [PubMed] [Google Scholar]
- 25. Kimura T, Van Keymeulen A, Golstein J, Fusco A, Dumont JE et al. (2001) Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr Rev 22: 631–656. doi: 10.1210/er.22.5.631. PubMed: 11588145. [DOI] [PubMed] [Google Scholar]
- 26. Riedemann J, Macaulay VM (2006) IGF1R signalling and its inhibition. Endocr Relat Cancer 13(Suppl. 1): S33–S43. doi: 10.1677/erc.1.01280. PubMed: 17259557. [DOI] [PubMed] [Google Scholar]
- 27. Mohan S, Baylink DJ, Pettis JL (1996) Insulin-like growth factor (IGF)-binding proteins in serum–do they have additional roles besides modulating the endocrine IGF actions? J Clin Endocrinol Metab 81: 3817–3820. doi: 10.1210/jc.81.11.3817. PubMed: 8923818. [DOI] [PubMed] [Google Scholar]
- 28. Santisteban P, Acebrón A, Polycarpou-Schwarz M, Di Lauro R (1992) Insulin and insulin-like growth factor I regulate a thyroid-specific nuclear protein that binds to the thyroglobulin promoter. Mol Endocrinol 6: 1310–1317. doi: 10.1210/me.6.8.1310. PubMed: 1406708. [DOI] [PubMed] [Google Scholar]


